Abstract
It is often assumed that the promise of a monetary bonus improves cognitive control. We show that in fact appetitive motivation can also impair cognitive control, depending on baseline levels of dopamine synthesis capacity in the striatum. These data demonstrate not only that appetitive motivation can have paradoxical detrimental effects for cognitive control, but also provide a mechanistic account of these effects.
Keywords: dopamine, motivation, cognition, striatum, PET, Attention
Introduction
Cognitive performance is generally thought to benefit from a promised bonus (Pessoa & Engelmann, 2010). In folk terms, being motivated implies being goal-driven, that is, having purely beneficial consequences for our ability to direct our behavior at our cognitive goals. In line with this intuition, appetitive motivation has been shown to improve a wide range of cognitive control functions (Jimura, Locke, & Braver, 2010; Krawczyk, Gazzaley, & D'Esposito, 2007; Pessoa & Engelmann, 2010), suggesting general enhancing effects on cognition. Nevertheless, inconsistent results have been obtained in studies investigating the effects of reward on cognitive control functions like focused attention (Braem, Verguts, Roggeman, & Notebaert, 2012; Padmala & Pessoa, 2011; Sasaki, Nanez, & Watanabe, 2010; van Steenbergen, Band, & Hommel, 2009). Individual differences in levels of the neurotransmitter dopamine have been speculated to play an important role in the effects of motivation on cognitive control (Pessoa & Engelmann, 2010; van Steenbergen et al., 2009). Dopamine has long been known to play an important role in reward and motivation (Berridge & Robinson, 1998; Robbins & Everitt, 1992). However, none of the studies above have actually taken dopamine processing into account, which might explain the inconsistencies observed.
Here, fourteen young healthy participants underwent positron emission tomography (PET) to assess 6-[(18)F]-fluoro-L-m-tyrosine (FMT) binding, a stable measure of baseline dopamine synthesis capacity (Jordan et al., 1997), in different striatal subregions (nucleus accumbens, caudate nucleus and putamen) (Fig. 1a). Subsequently, participants performed a Stroop-like task (Aarts, Roelofs, & van Turennout, 2008) following high and low monetary reward cues to assess the effects of a promised reward on cognitive control; that is, the ability to focus attention on currently relevant goals while ignoring irrelevant information (Fig. 1b). The Stroop targets were either incongruent (i.e. a conflicting distractor), requiring focusing of attention, or congruent (i.e. a non-conflicting irrelevant dimension), benefiting from less focus. The reward cues were followed by cues informing the participants about the upcoming Stroop target congruency.
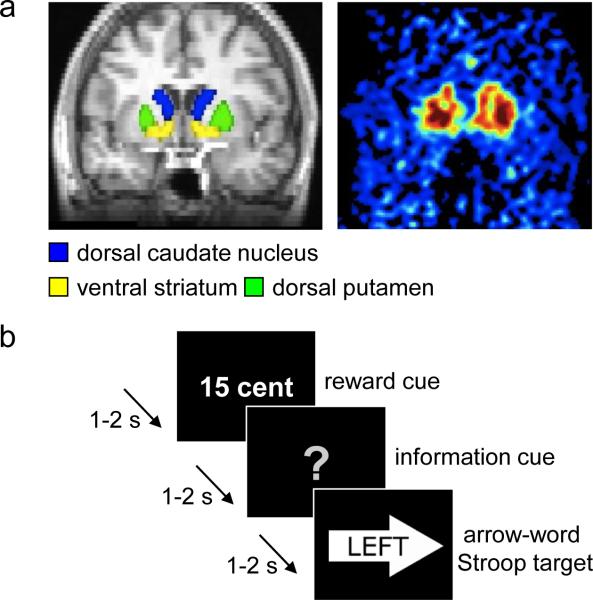
Figure 1.
Methods. (a) Left. Six striatal regions of interest (ROIs) were drawn on an anatomical MR image: left and right dorsal caudate nucleus, ventral striatum, and dorsal putamen. The MR image was subsequently coregistered to the PET images. Right. Averaged Ki values extracted from the ROIs were associated with performance in the task described in b. (b) In this example trial of the rewarded Stroop paradigm, the participant could earn a high reward (i.e., 15 cents) for a correct and fast enough answer on the incongruent arrow-word Stroop target. Participants were instructed to respond to the word of the targets (the correct response was a left button press in this trial). In this example, the information cue did not provide any information about the congruency of the upcoming target (i.e., uninformed trial).
Methods
Participants
Data from 14 participants (6 men) aged 24-34 years (mean=28, SD=2.7) are presented. Thirty-three neurologically and psychiatrically healthy, right-handed participants who previously participated in a PET-FMT study were invited to participate in this behavioral study. Sixteen participants accepted, but two participants were excluded due to an inability to perform the task (error rate: 34% and 40%; overall mean=18%, SD=9%). The time between the PET scans and behavioral measurements was on average 2.3 years (SD=1.1; range: 1.0-4.2), similar to previous studies with this stable PET ligand (Cools et al., 2009). In the Results, we report an analysis that explicitly addresses the question whether our results could be explained by this delay. All participants gave written informed consent and were paid for participation according to institutional guidelines of the local ethics committee (University of California Berkeley Committee for the Protection of Human Participants).
PET data acquisition
PET imaging and 6-[(18)F]-fluoro-L-m-tyrosine (FMT) synthesis were performed at Lawrence Berkeley National Laboratory. FMT synthesis has been described previously (VanBrocklin et al., 2004). FMT is comparable with [18F]fluorodopa, with the exception that it is not a substrate for O-methylation in the periphery and therefore provides higher signal-to-noise images (Jordan et al., 1997). FMT is a substrate of aromatic L-amino acid decarboxylase (AADC), a dopamine-synthesizing enzyme whose activity provides an estimate of the ability of dopaminergic neurons to synthesize dopamine when provided with optimal substrate (DeJesus, 2003). AADC activity is shown to be stable in young healthy adults (Kish, Zhong, Hornykiewicz, & Haycock, 1995), and its measurement with PET is highly reproducible (Vingerhoets et al., 1994). FMT is metabolized by AADC to 6-[18F]fluorometatyramine, which is oxidized to 6-[18F]fluorohydroxyphenylacetic acid (FPAC). FPAC remains in the dopaminergic terminals and is visible on PET-FMT scans. Signal intensity on PET-FMT scans is thus indicative of pre-synaptic dopamine synthesis capacity (Jordan et al., 1997).
PET scans were performed using the Siemens (Knoxville, TN) ECAT-HR PET camera, as described elsewhere (Landau, Lal, O'Neil, Baker, & Jagust, 2009). Approximately 2.5 mCi of high specific activity FMT was injected as a bolus into an antecubital vein and a dynamic acquisition sequence in 3D mode was obtained for a total of 89 min scan time.
Structural MRI
Two high-resolution anatomical images (MPRAGE) were acquired in each participant on a Siemens 1.5T Magnetom Avanto MRI system (Siemens, Erlangen, Germany), using a 12-channel head coil (TE/TR = 3.58/2120 ms; voxel size = 1.0 × 1.0 × 1.0 mm, 160 axial slices; FOV = 256 mm; scanning time ~9 minutes). The two MPRAGEs were averaged to obtain one high-resolution structural image, which was used to generate individual striatal regions of interest (ROI).
Regions of interest (ROIs)
Striatal sub-regions and the cerebellum were manually drawn on each participant's anatomical MRI scan using FSLView (http://www.fmrib.ox.ac.uk/fsl/). The striatal ROIs were drawn according to guidelines described previously(Mawlawi et al., 2001). Both inter- and intra-rater reliability were above 95% (from ratings made by two lab members). We used the cerebellum as the reference region for calculating FMT signal intensity. To avoid contamination of FMT signal from the dopaminergic nuclei, only the posterior three-fourths of the gray matter were included in the cerebellar reference region. After coregistration to PET-FMT space, we only included the voxels with an above 50% chance to lie in a certain ROI to ensure high grey matter probability. This procedure resulted in six participant-specific striatal ROIs: left and right ventral striatum, dorsal caudate nucleus, and dorsal putamen (Fig. 1a).
PET data analysis
FMT images were reconstructed with an ordered subset expectation maximization algorithm with weighted attenuation, scatter corrected, and smoothed with a 4mm full width half maximum kernel. To correct for motion during scanning, all FMT frames were realigned to the middle (12th) frame using SPM8 (www.fil.ion.ucl.ac.uk/spm/). The anatomical MRI scan was coregistered to the mean image of all realigned frames in the FMT scan using FSL-FLIRT (http://www.fmrib.ox.ac.uk/fsl/, version 4.1.2). Using an in-house graphical analysis program implementing Patlak plotting (Logan, 2000; Patlak & Blasberg, 1985), Ki images were created representing the amount of tracer accumulated in the brain relative to the reference region (cerebellum). We extracted average Ki values from the 6 ROIs and computed associations between regional FMT binding (Ki values) and the measures on the task described below.
Rewarded Stroop paradigm
Behavioral responses were assessed with a Stroop-like conflict task with high and low reward conditions. The task resembled a previously used Stroop paradigm (Aarts et al., 2008), with in addition reward cues at the beginning of each trial and smaller inter-stimulus intervals (1-2 sec). Participants responded with a left (right index finger) or right (right middle finger) button press to the words “left” or “right” (relevant dimension) positioned in a left or right pointing arrow (irrelevant dimension). The direction denoted by the word was either congruent or incongruent with the direction indicated by the arrow (Fig. 1b). All trials began with a cue predicting high or low reward for correct performance. Following the reward cue, an information cue (100% valid) explicitly informed participants about the congruency of the upcoming Stroop target (Aarts et al., 2008). Reward cues [1/15 cents], information cues [informative: red cross (incongruent) or green circle (congruent) / uninformative: gray question mark], and target congruency [incongruent/congruent] were equally distributed across the 240 trials (~30min).
Three practice blocks (~15min in total) preceded the actual experiment: one to familiarize the participants with the use of the information cues (12 trials; no reward cues); one to familiarize the participants with the use of reward cues (32 trials, immediate feedback); and one to set the initial response window (average correct RT per trial-type; 48 trials). In the main experiment, reward was only obtained when an answer was correct and within an individually determined response window. The response window was adapted per trial type after correct responses on time (−25 ms) or too late responses (+25 ms). Hence, reward receipt did not vary with difficulty and was similar across participants. Both errors and misses were punished with no reward (0 cents). After every 30 trials, participants were informed about the amount of a) reward obtained in the previous block, b) reward that could have been obtained, c) total reward earned so far, d) misses and e) errors made in the previous block. The bonus money (mean=9.33 USD, SD=0.67) was added to the participants’ compensation.
Behavioral data analyses
Reaction times (RTs) and error rates were analyzed with IBM SPSS statistics version 19. RTs of all correct trials were analyzed, including those of ‘too late’ responses. We ran six different analyses of variance (ANOVA) with within-subjects factors REWARD (high, low), CONGRUENCY (congruent, incongruent), and INFORMATION (informed, uninformed), and one covariate of interest: FMT binding in the left or right ventral striatum, caudate nucleus, or putamen. We corrected for multiple comparisons (Bonferroni corrected alpha-value of .0042). Upon significant effects, we assessed the detrimental effects of motivation (high > low anticipated reward) on Stroop performance (incongruent > congruent) for informed and uninformed trials separately and, finally, the effects of motivation on congruent and incongruent trials separately. Post-hoc simple Pearson's correlations were calculated between FMT binding and these behavioral measures.
In a supplementary analysis, the six covariates were put together in one model to infer unique contributions of FMT binding in each ROI, while correcting for the effects in the other ROIs. Moreover, a multiple regression analysis was performed to demonstrate that the observed effects could not be attributed to the time between PET and behavioral measurements or to age.
Results
Participants performed more poorly on incongruent than congruent Stroop trials (CONGRUENCY, RT: (F(1,13)=66.47, p<.001; errors: F(1,13)=50.23, p<.001) and when uninformed versus informed about the upcoming congruency (INFORMATION, RT: F(1,13)=19.02, p=.001; errors: F(1,13)=3.95, p=.068) (Table 1).
Table 1.
Raw data on the rewarded Stroop paradigm.
| low reward |
high reward |
|||
|---|---|---|---|---|
| congruent | incongruent | congruent | incongruent | |
| Response times (ms) | ||||
| uninformed | 391.9 (9.4) | 433.6 (9.6) | 397.7 (9.5) | 439.5 (9.9) |
| informed | 370.3 (8.0) | 403.1 (10.6) | 369.8 (10.1) | 401.1 (10.8) |
| Error rates (%) | ||||
| uninformed | 10.2 (2.7) | 31.0 (3.4) | 11.3 (3.0) | 30.2 (4.3) |
| informed | 10.0 (2.5) | 24.6 (4.5) | 7.7 (2.4) | 20.0 (2.9) |
Note. Values represent mean response times and mean proportion of incorrect responses (standard errors of the mean).
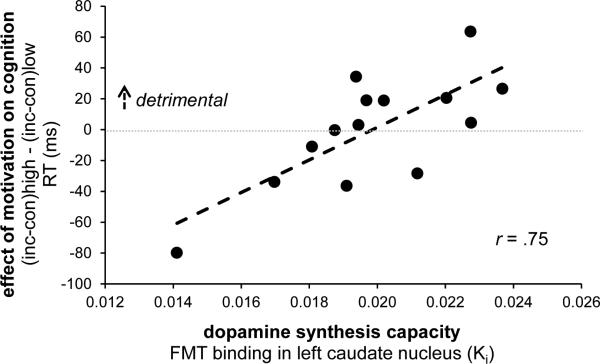
The effects of promised reward on Stroop performance depended on individual differences in dopamine synthesis capacity, specifically in the left caudate nucleus, as indexed by the degree of FMT binding (REWARD × CONGRUENCY × INFORMATION × lCaud-FMT, RT: F(1,12)=12.8, p=.004). This interaction in RTs was significant after Bonferroni correction for multiple striatal subregions and was driven by the uninformed trials (REWARD × CONGRUENCY × lCaud-FMT, F(1,12)=15.3, p=.002). Thus, greater dopamine synthesis capacity was associated with enhanced Stroop interference (incongruent > congruent trials) when anticipating high versus low reward (r=0.75; Fig. 2). This effect was present only when participants were uninformed, that is, could not prepare for the type of cognitive control (more versus less focusing) that was required by the (incongruent versus congruent) target.
Figure 2.
Dopamine-dependent detrimental effects of reward on cognitive control. Dopamine synthesis capacity in the left caudate nucleus (on x-axis) predicted a detrimental effect of anticipated reward (high > low) on Stroop performance (RT: incongruent > congruent). Both measures were normally distributed. The correlation is still significant (r=.57, t(11)=2.3, p=.04) without the participant with the least FMT binding and the least detrimental effect of reward (lower left data point). This participant's x- or y-values do not represent outliers according to the Grubbs’ test (Barnett & Lewis, 1994), i.e. they do not differ more than 2.51*SD from the mean. The reported results are from uninformative trials only (see Fig. 1b).
RT = response time; inc = incongruent; con = congruent; FMT = 6-[(18)F]-fluoro-L-m-tyrosine PET ligand; Ki = binding potential; r = Pearson's correlation coefficient.
The nature of the relationship between the RT effects of reward and FMT binding in the left caudate nucleus was different for the congruent and incongruent trials. Increased dopamine synthesis capacity predicted beneficial effects of reward on congruent trials (reward × lCaud-FMT, F(1,12)=9.12, p=.011; r=−0.66), whereas it tended to predict detrimental effects of reward on incongruent trials (reward × lCaud-FMT, F(1,12)=3.81, p=.075; r=.49).
No main effects of FMT binding on speed or accuracy, or interactions between FMT binding and CONGRUENCY without the factor REWARD were observed. Moreover, no significant FMT binding effects were obtained with other striatal ROIs or in error rates. The unique effect of FMT binding in the left caudate nucleus was confirmed in a supplementary analysis including FMT binding in all six ROIs as covariates (Table 2).
Table 2.
Interaction effects in response times obtained from the ANOVA with FMT binding in only the left caudate nucleus ROI as single covariate (left) and from the ANOVA with FMT binding in all ROIs as six covariates (right).
| ANOVA, response times | incl. only lCaud | incl. all | ||
|---|---|---|---|---|
| ROI F(1,12) | p | ROIs F(1,7) | p | |
| REWARD x CONGRUENCY x INFORMATION x lCaud-FMT | 12.8 | 0.004 | 10.4 | 0.015 |
| uninformed: REWARD x CONGRUENCY x lCaud-FMT | 15.3 | 0.002 | 9.8 | 0.017 |
| informed: REWARD x CONGRUENCY x lCaud-FMT | < 1 | ns | < 1 | ns |
| uninformed | ||||
| congruent: REWARD x lCaud-FMT | 9.12 | 0.011 | 1.2 | ns |
| incongruent: REWARD x lCaud-FMT | 3.81 | 0.075 | 6.5 | 0.038 |
Note. The interaction effects in the ANOVA with a single covariate survived Bonferroni correction for multiple striatal subregions (corrected alpha-value of .0042). The ANOVAs with the other striatal ROIs as single covariates did not reveal significant effects. In the ANOVA including all striatal ROIs, effects only significantly co-varied with FMT binding in the left caudate nucleus. No significant effects were observed in terms of error rates.
incl. = included as covariate(s); lCaud-FMT = FMT binding in the left caudate nucleus ROI.
A multiple regression analysis confirmed a significant interaction between FMT binding in the left caudate nucleus and the effect of reward on congruency (beta=.88, t(10)=3.34, p=.008). Moreover, this analysis showed that the time between PET and behavioral measurements did not explain our observed effect of reward on congruency (t(10)<1 and >−1), and neither did age (beta=−.24, t(10)=−1.07, p=.31) .
Discussion
The present data demonstrate not only that appetitive motivation can have detrimental effects for cognitive control depending on individual differences, but also provide a mechanistic account of these effects. Specifically, in individuals with high baseline dopamine synthesis capacity, a promised bonus might ‘overdose’ their dopaminergic system, resulting in cognitive impairment rather than improvement (Cools & D'Esposito, 2011). We speculate that this effect reflects motivation-induced increases in striatal dopamine that amplify the direct Go-pathway and inhibit the indirect NoGo-pathway in the striatum, resulting in an overall Go bias and a ‘gating in’ of cognitive representations (Hazy, Frank, & O'Reilly, 2006). Such gating in of multiple representations would lead to processing of both relevant and irrelevant features of a stimulus, hence causing unselective attention and, thus, impaired Stroop performance in individuals with high dopamine synthesis capacity. In contrast, increased dopamine processing by anticipated reward would lead to more optimal cognitive control in low baseline dopamine individuals, in accordance with inverted-U shaped dopamine action (Cools & D'Esposito, 2011).
Our effects were uniquely predicted by dopamine synthesis capacity in the left dorsal caudate nucleus (see Table 2), which is in line with previously observed interactions between motivation and cognition in the same region depending on variation in a dopaminergic gene (Aarts et al., 2010). One important implication of the present observation is that motivation does not necessarily contribute to greater cognitive control.
Our observation is remarkable in the context of the intuition that increased motivation should help us attain our goals. However, psychologists have long recognized that behavior is motivated not only by the goals that we set ourselves, but also by generalized drives that do not necessarily contribute to adaptive, optimized behavior (Dayan, Niv, Seymour, & Daw, 2006; Dickinson & Balleine, 2002). Stimuli that elicit motivation can modify behavior without accessing goal representations, that is, in a manner that is not goal-directed (Aarts, van Holstein, & Cools, 2011). This is illustrated most clearly by the role of reward-predictive stimuli in the – dopamine-mediated – compulsive seeking and taking of drugs of abuse or other targets of addiction, despite negative consequences (Everitt et al., 2008; Robinson & Berridge, 2008; Volkow et al., 2006). In this context, it is perhaps not surprising that there is large individual variability in the effects of appetitive motivation on cognition as a function of striatal dopamine synthesis capacity.
Acknowledgements
We thank Mieke van Holstein for piloting the experiment. This work was supported by the Niels Stensen foundation (to E.A.), National Institute of Health (R01-DA20600 to M.D.) and National Institute on Drug Abuse (F32DA027684 to D.L.W.). R.C. is supported by a James McDonnell scholar award. She is a consultant for Abbott Laboratories as well as Pfizer, but is not an employee or stock shareholder.
Footnotes
All authors contributed to the study design. Data acquisition was performed by E. Aarts, D.L. Wallace, and L.C. Dang. The analyses were performed by E. Aarts and L.C. Dang. All authors contributed to writing the paper, and all authors approved the final version of the paper for submission.
The authors declare no competing financial interests.
References
- Aarts E, Roelofs A, Franke B, Rijpkema M, Fernandez G, Helmich RC, et al. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology. 2010;35(9):1943–1951. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts E, Roelofs A, van Turennout M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. J Neurosci. 2008;28(18):4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts E, van Holstein M, Cools R. Striatal Dopamine and the Interface between Motivation and Cognition. Front Psychol. 2011;2:163. doi: 10.3389/fpsyg.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett V, Lewis T. Outliers in Statistical Data. 3 ed. John Wiley & Sons Ltd.; West Sussex: 1994. [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Braem S, Verguts T, Roggeman C, Notebaert W. Reward modulates adaptations to conflict. Cognition. 2012;125(2):324–332. doi: 10.1016/j.cognition.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69(12):e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29(5):1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Niv Y, Seymour B, Daw ND. The misbehavior of value and the discipline of the will. Neural Netw. 2006;19(8):1153–1160. doi: 10.1016/j.neunet.2006.03.002. [DOI] [PubMed] [Google Scholar]
- DeJesus OT. Positron-labeled DOPA analogs to image dopamine terminals. Drug Development Research. 2003;59(2):249–260. [Google Scholar]
- Dickinson A, Balleine BW. The role of learning in the operation of motivational systems. In: Gallistel CR, editor. Stevens' Handbook of Experimental Psychology Vol. 3: Learning, Motivation and Emotion. 3 ed. Vol. 3. Wiley; New York: 2002. pp. 497–533. [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O'Reilly RC. Banishing the homunculus: making working memory work. Neuroscience. 2006;139(1):105–118. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proc Natl Acad Sci U S A. 2010;107(19):8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S, Eberling JL, Bankiewicz KS, Rosenberg D, Coxson PG, VanBrocklin HF, et al. 6-[18F]fluoro-L-m-tyrosine: metabolism, positron emission tomography kinetics, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine lesions in primates. Brain Res. 1997;750(1-2):264–276. doi: 10.1016/s0006-8993(96)01366-2. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Zhong XH, Hornykiewicz O, Haycock JW. Striatal 3,4-dihydroxyphenylalanine decarboxylase in aging: disparity between postmortem and positron emission tomography studies? Ann Neurol. 1995;38(2):260–264. doi: 10.1002/ana.410380220. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D'Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19(2):445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. 2000;27(7):661–670. doi: 10.1016/s0969-8051(00)00137-2. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. J Cogn Neurosci. 2011;23(11):3419–3432. doi: 10.1162/jocn_a_00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Functions of dopamine in the dorsal and ventral striatum. seminars in The Neurosciences. 1992;4:119–127. [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Nanez JE, Watanabe T. Advances in visual perceptual learning and plasticity. Nat Rev Neurosci. 2010;11(1):53–60. doi: 10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenbergen H, Band GP, Hommel B. Reward Counteracts Conflict Adaptation: Evidence for a Role of Affect in Executive Control. Psychol Sci. 2009;20(12):1473–1477. doi: 10.1111/j.1467-9280.2009.02470.x. [DOI] [PubMed] [Google Scholar]
- VanBrocklin HF, Blagoev M, Hoepping A, O'Neil JP, Klose M, Schubiger PA, et al. A new precursor for the preparation of 6-[18F]Fluoro-L-m-tyrosine ([18F]FMT): efficient synthesis and comparison of radiolabeling. Appl Radiat Isot. 2004;61(6):1289–1294. doi: 10.1016/j.apradiso.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Vingerhoets FJ, Snow BJ, Schulzer M, Morrison S, Ruth TJ, Holden JE, et al. Reproducibility of fluorine-18-6-fluorodopa positron emission tomography in normal human subjects. J Nucl Med. 1994;35(1):18–24. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]