Summary
The combination of variable telomere length in cancer cells combined with shorter telomere length in cancer-associated stromal cells, strongly correlate with progression to prostate cancer metastasis and cancer death. The implications are that telomere length measurements not only have the potential as a prognostic indicator of prostate cancer outcomes but also as a risk stratification enrichment biomarker for individualized therapeutic interventions.
Telomeres are the repetitive TTAGGG DNA ends of human linear chromosomes. Telomeres do not encode genes but have a specialized structure including shelterin proteins to hide/cap/protect the DNA ends from exonuclease activity and DNA damage recognition. Telomeres progressively shorten throughout life. This includes stem cells and highly proliferative transit amplifying cells due to failure of lagging DNA strand synthesis to be completed to the very end, often referred to as the end replication problem. In addition, oxidative damage responses may accelerate the loss of telomeres. Almost all in situ pre-neoplastic lesions (sometimes referred to as indolent lesions of epithelial origin) have critically shortened telomeres which may be an initial protective mechanism limiting the maximum number of divisions human cells can undergo. Since a large number of genetic and epigenetic alterations are required for a normal cell to become malignant, limiting the number of cellular divisions in human cells results in a pre-neoplastic proliferative growth arrest state referred to as senescence. Replicative senescence may have evolved as an initial potent anti-cancer molecular mechanism (1). Pre-malignant cells expressing viral oncoproteins can bypass senescence, move into an extension of cell growth phase, and finally enter a state termed “crisis” or what we now know as terminal telomere shortening. In crisis telomeres are so short that end-end fusions occur followed by bridge-breakage-fusion cycles and only rarely in humans does a cell engage a mechanism to escape from crisis. The relationship of shortened telomeres in the pre-neoplastic cells in crisis compared to the contribution of short telomeres in the cancer associated stromal cell compartment including inflammatory cells is much less clearly understood.
In ∼85-90% of all carcinomas, the molecular mechanism to bypass crisis is by activating the gene TERT or telomerase reverse transcriptase (2). The mechanisms of activation of telomerase are still controversial but include mutations in the TERT promoter, engagement of TERT alternative splicing, TERT gene amplification, and epigenetic changes. Another intriguing possibility is that the human TERT gene may autoregulate itself since it is located very close to the telomere end of chromosome 5. In most large long-lived species TERT is also close to a telomere but in small short-lived species such as mice TERT is not located near a telomere. Interestingly, telomerase is more promiscuous in mice and inbred strains of mice have very long telomeres compared to humans but the reasons for this are not understood. One could speculate that the TERT gene being located near a telomere in long-lived species may have been selected for over evolutionary time to regulate telomerase and thus the maximal telomere length (3). Telomerase is active during early human fetal development, then becomes silenced in most tissues. Thus, when telomeres reach a certain length (∼15-20 kb) during human development, chromatin modifications involving telomere position effects (TPE) may silence the hTERT gene (3). As part of cancer progression, as telomeres shorten the chromatin silencing effects may become relaxed making a permissive environment for telomerase reactivation. This is consistent with the observation that almost 70% of all cancers are in the 65 and older segment of the population.
Mice deleted in the TERT gene after several generations develop short telomeres and phenocopy many of the hallmarks of human aging. In humans having rare disorders of telomere maintenance (called telomeropathies) there is an early onset of disease such as bone marrow failure, idiopathic pulmonary fibrosis and dyskeratosis congenita (a disease demonstrating age-associated tissue dysfunctions and a modest increase in cancer in highly proliferative tissues). These diseases suggest that short telomeres in combination with additional genetic and epigenetic alterations contribute to malignant cell transformation. There is no convincing evidence that shortened telomeres without other alterations leads to genomic instability or cancer. In a large population study, a statistically significant inverse relationship between telomere length and both cancer incidence and mortality has been reported (4). In addition to short telomeres correlating with poor prognosis (4), short telomeres in both the epithelial and stromal cell compartments have been reported to have a senescence-associated secretory pathway (SASP) making the microenvironment more permissive for cancer progression (5). Senescent cells may also promote inflammation, which is a common feature of all major age-related diseases including cancer, and proliferation in the setting of chronic inflammation predispose to cancer.
Thus, we come to the present study by Alan Meeker's lab in this issue of Cancer Discovery(6). Heaphy et al (6) show that the combination of variable telomere length in cancer cells combined with shorter telomere length in cancer associated stromal cells, strongly correlate with progression to metastasis and cancer death. The study included ∼600 men 65 years old on average at diagnosis who were enrolled in the Health Professionals Follow-up Study. These men had a prostatectomy with pathologically organ-confined disease (Gleason sum of 7, clinically localized disease). The mean follow-up times were ∼13 years for lethal prostate cancer. The cumulative incidences for biochemical recurrence 33%; lethal prostate cancer 19%; prostate cancer death 17%; and non-prostate cancer death 56%. The results showed that men with more variable telomere length in prostate cancer cells and shorter cancer adjacent stromal cell telomere length had 8 times the risk of progressing to lethal prostate cancer and 14 times the risk of dying of their prostate cancer. Men with the less variable/longer combinations of telomere length were much less likely to die of their prostate cancer than expected, and their time to advanced disease was much longer. Importantly, these associations were independent of currently used prognostic indicators in prostate cancer. This provocative study suggests the translation potential of telomere length measurements as prognostic indicators and potentially as a risk stratification biomarker for individualized therapeutic interventions. The telomere biomarker adds to the predictive capability of the currently used prognostic indicators and may also result in less aggressive therapy for those men at low risk of recurrence of disease.
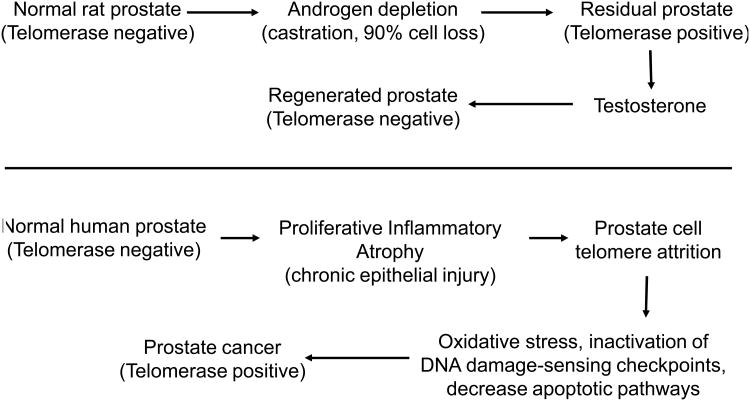
Previous studies on prostate cancer and telomeres/telomerase biology have been consistent (7, 8). Using high resolution in situ methods, extensive telomere shortening has been observed in cancer cells compared with normal epithelial cells in the vast majority of prostate cancers and in high-grade prostatic intraepithelial neoplasias (PIN). In normal prostate tissue or benign prostatic hyperplasia (BPH) there is very little or no detectable telomerase activity (8). In greater than 60% of micro-dissected high grade PIN lesions there is detectable telomerase activity. 90% of all prostate carcinomas have telomerase activity and 98% of patients with a Gleason's score >7 have telomerase activity (8). There is mounting evidence that telomerase may be present in some rare human prostate stem cells (9, 10). In one study (Figure, top panel) the rat prostate had no detectable telomerase activity. After androgen depletion via castration, there was 90% cell loss from the prostate. In the residual prostate there was some detectable telomerase activity. Then testosterone was added, the prostate regenerated and telomerase activity was silent again. This study (8) suggests that turnover of stem cells may lead to progressive telomere shortening.
Figure. Evidence for a stem cell population in the rat prostate (top, based on data from reference 8). Evidence for the role of localized proliferative inflammatory atrophy in the human prostate (bottom, based on data from reference 11).
Another mechanism to increase turnover in the non-cancerous human prostate is termed focal prostatic atrophy (11), which is associated with chronic inflammation resulting in increased proliferation, cell turnover, and ultimately shortened telomeres (Figure, bottom panel). Inflammation is frequently present in BPH biopsies and in radical prostatectomy specimens. Inflammatory infiltrates are often observed in areas of prostatic atrophy that are characterized by an increased proliferation (11). Chronic inflammation may result in increased oxidative stress, leading to increased cell proliferation, and to telomere attrition. The end result is inactivation of DNA damage checkpoint pathways, accumulation of driver genetic and epigenetic oncolytic changes, and emerging prostate cancer cells expressing telomerase.
One way to progress these observations into translational opportunities is to use telomerase inhibitors in selective cohorts of men with the highest risk factor for recurrence of disease (12). Following prostatectomy, the small pool of residual cancer cells with greatly shortened telomeres may be removed by a period of anti-telomerase treatment. The telomerase enzyme seems like a perfect cancer target as it is only expressed in a small subset of proliferative stem cells and cancers. Telomerase expression is essential for the proliferation of most advanced cancer cells, but the enzyme is inactive in the vast majority of normal human tissues (2). This suggests that inhibiting the telomere maintenance enzyme should, in theory, be a relatively safe and effective way to drive cancer cells back into crisis before or after treating with cytolytic agents. The results of the first anti-telomerase clinical trials have just been completed (Geron Corp, Menlo Park, CA). Subgroup analyses from a Phase 2 advanced non-small cell lung cancer maintenance trial, following standard induction chemotherapy, showed that imetelstat (telomerase competitive inhibitor) was most effective in patients whose lung tumors at baseline had the shortest telomeres. While not reaching statistical significance, the early analysis suggested a modest trend of efficacy in favor of the imetelstat arm. In a retrospective measurement of tumor telomere length, the analyses suggested that patients whose tumors had short telomeres at baseline experienced an increase in progression-free survival when treated with imetelstat in comparison to patients in the control arm. The treatment effect was not observed in imetelstat-treated patients whose tumors had medium-to-long telomeres.
Overall, these new results (6) could be interpreted to indicate that prostate cancer patients with short/variable telomeres in their tumor cells and short telomeres in their associated stromal cells at the time of diagnosis may be an enrollment enrichment biomarker for more aggressive treatments. Future studies should address the utility of short or variable telomere length as a prognostic tool at the time of biopsy and in risk stratification to individualize treatment and surveillance strategies. Tissue-based markers are urgently needed to improve treatment and surveillance decision-making in men with prostate cancer and this study is an important advance.
Acknowledgments
Funding: The JWS laboratory is supported in part by the Southland Financial Corporation Distinguished Chair in Geriatric Research, Simmons Cancer Center Support Grant (5P30 CA 142543-03); a grant from National Institutes of Health (C06 RR30414); grants from the National Aeronautics and Space Administration (NNX11AC15G, NNJ05HD36G, NNX09AU95G); and the National Cancer Institute SPORE Grant (P50CA70907).
References
- 1.Gomes NMV, Ryder OA, Houck ML, Charter SJ, Walker W, Forsyth NR, et al. The comparative biology of mammalian telomeres: ancestral states and functional transitions. Aging Cell. 2011;10:761–68. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim NW, Harley CB, Prowse KR, Weinrich SL, Piatyszek MA, et al. Telomeres, telomerase and cancer. Science. 1995;268:1115–17. [Google Scholar]
- 3.Shay JW, Wright WE. Implications of mapping the human telomerase genes (hTERT) as the most distal gene on chromosome 5p. Neoplasia. 2000;2:195–96. doi: 10.1038/sj.neo.7900093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstätter A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 5.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:3 966–72. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heaphy CM, Yoon GS, Peskoe SB, Joshu CE, Lee TK, Giovannuci E, et al. Cancer Discovery (XX) doi: 10.1158/2159-8290.CD-13-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeker AK, Hicks JL, Platz EA, March GE, Bennett CJ, Delannoy MJ, et al. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–09. [PubMed] [Google Scholar]
- 8.Sommerfeld H, Meeker AK, Piatyszek MA, Bova GS, Shay JW, Coffey DS. Telomerase activity: A prevalent marker of malignant human prostate tissue. Cancer Res. 1996;56:218–22. [PubMed] [Google Scholar]
- 9.Marian CO, Shay JW. Telomerase inhibition therapy to target prostate tumor-initiating cells. BBA, Molecular Basis of Disease. 2009;1792:289–96. doi: 10.1016/j.bbadis.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Marian CO, Wright WE, Shay JW. The effect of telomerase inhibition on prostate tumor-initiating cells. International J Cancer. 2010;127:321–31. doi: 10.1002/ijc.25043. [DOI] [PubMed] [Google Scholar]
- 11.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155(6):1985–92. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canales BK, Li Y, Thompson MG, Gleason JM, Chen Z, Malaeb B, Corey DR, Herbert BS, Shay JW, Koeneman KS. Small molecule, oligonucleotide-based telomerase template inhibition in combination with cytolytic therapy in an in-vitro androgen-independent prostate cancer model. Urologic Oncology. 2006;24:141–151. doi: 10.1016/j.urolonc.2005.11.003. [DOI] [PubMed] [Google Scholar]