Abstract
OBJECTIVE
Disruptions in stress response system development have been posited as mechanisms linking child maltreatment (CM) to psychopathology. Existing theories predict elevated sympathetic nervous system (SNS) reactivity following CM, but evidence for this is inconsistent. We present a novel framework for conceptualizing stress reactivity following CM using the biopsychosocial model of challenge and threat. We predicted that in the context of a social-evaluative stressor, maltreated adolescents would exhibit a threat pattern of reactivity, involving SNS activation paired with elevated vascular resistance and blunted cardiac output (CO) reactivity.
METHODS
A sample of 168 adolescents (mean age=14.9 years) participated. Recruitment targeted maltreated adolescents; 38.2% qualified as maltreated. Electrocardiogram, impedance cardiography, and blood pressure were acquired at rest and during an evaluated social stressor (Trier Social Stress Test). Pre-ejection period (PEP), CO, and total peripheral resistance (TPR) reactivity were computed during task preparation, speech-delivery, and verbal mental-arithmetic. Internalizing and externalizing symptoms were assessed.
RESULTS
Maltreatment was unrelated to PEP reactivity during preparation or speech, but maltreated adolescents had reduced PEP reactivity during math. Maltreatment exposure (F(1,145)=3.8-9.4, p=.053-<.001) and severity (β=−.10-.12, p=.030-.007) were associated with significantly reduced CO reactivity during all components of the stress-task and marginally associated with elevated TPR reactivity (F(1,145)=3.8-9.4, p=.053-<.001; β=.07-.11, p=.11-.009, respectively). Threat reactivity was negatively associated with externalizing symptoms.
CONCLUSIONS
Child maltreatment is associated with a dysregulated pattern of physiological reactivity consistent with theoretical conceptualizations of threat but not previously examined in relation to maltreatment, suggesting a more nuanced pattern of stress reactivity than predicted by current theoretical models.
Keywords: child maltreatment, childhood adversity, adverse childhood experiences, autonomic nervous system, stress reactivity, internalizing, externalizing
Adverse childhood experiences are potent risk factors for psychopathology in childhood and adolescence. (1-4) Child maltreatment (CM) has particularly strong associations with both internalizing and externalizing disorders. (1, 3) Disruptions in the development of stress response systems—including the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS)—have frequently been posited to be a central neurodevelopmental mechanism underlying these associations. (5-7) Specifically, exposure to traumatic stress during HPA and SNS development is thought to lead to lasting alterations in the functioning of these systems, culminating in heightened risk for psychopathology.
The effects of early-life adversity on the development of physiological systems have been well characterized in animals. In rodent and non-human primate models the primary method used to experimentally induce early-life adversity has been prolonged separation of the animal from its mother. (8, 9) Exposure to this type of adversity is associated with hyper-reactivity of the HPA axis and SNS in adolescence and adulthood, and elevations in anxiety, fearful behaviors, and aggression. (8-11)
The consistency of evidence from animal models contrasts with human studies, where a remarkably mixed set of findings have emerged. Most human studies have focused on HPA axis activation. Although some studies document elevated cortisol and adrenocorticotropic hormone reactivity following CM, (12-14) numerous studies find that children and adults who were maltreated exhibit blunted cortisol reactivity.(15-19) Fewer studies have examined CM and SNS reactivity. Of these, some report heightened SNS reactivity following high levels of family adversity, whereas others observe no association. (20-23) More complicated patterns of stress reactivity following CM have also been found. For example, one study reported a strong association between HPA axis and SNS responses to an active stressor in non-maltreated children, but no association of responses across systems in maltreated children. (19)
Multiple theories have been developed to account for patterns of physiological reactivity following exposure to adverse early-life environments. Biological sensitivity to context theory (24) argues that high reactivity can emerge in the context of extreme adverse environments and in environments that are nurturing and supportive, and that elevated physiological reactivity should be associated with negative outcomes in adverse environments and positive adaptation in supportive environments. (20, 25, 26) An extension of this theory, the adaptive calibration model, describes a wider variety of stress response system profiles that may emerge depending on the severity and chronicity of early-life adversity.(27, 28) Although existing theories are supported by evidence from studies of children exposed to less extreme forms of adversity, (20, 26, 28) they do not explain the disparate findings with regard to CM specifically. Biological sensitivity to context argues that extreme adverse environments should lead to elevated physiological reactivity,(24) and adaptive calibration posits that, due to evolutionary sex differences in optimal reproductive strategies in environments characterized by extreme threat, elevated physiological reactivity among females and blunted reactivity among males should be observed following traumatic stressors. (27) Neither of these models explains the diversity of findings regarding physiological reactivity in maltreated children.
We propose that inconsistency of current models with stress reactivity patterns following CM may be accounted for by a lack of specificity in existing descriptions of SNS responses (i.e., as either elevated or blunted). To overcome this limitation, we examine the association between CM and physiological reactivity using a theoretical approach that differentiates between adaptive and maladaptive SNS responses to acute stressful situations. Specifically, we apply the biopsychosocial model of challenge and threat, (29) a theory built upon prior animal and human work on physiological “toughness”(30) and Lazarus and Folkman’s coping theory(31) and supported by substantial evidence in social and health psychology, (32-34) to the study of CM. This theory argues that patterns of appraisal and cardiovascular responses during tasks that require instrumental cognitive responses (i.e., active tasks) can be used to distinguish between approach (i.e., challenge) and withdrawal (i.e., threat) responses. (29, 33) Challenge responses are characterized by appraisals that one’s resources exceed situational demands and a pattern of cardiovascular reactivity involving increased SNS activation paired with low levels of vascular resistance and increased cardiac output (CO). (29, 33) Conversely, threat responses are associated with appraisals that situational demands outweigh one’s resources and a cardiovascular pattern characterized by increased SNS activation (though typically less than observed in challenge states), increased vascular resistance, and less cardiac efficiency (i.e., little to no increases in CO). (33, 35) The threat response is viewed as maladaptive because vascular resistance interferes with the delivery of oxygenated blood to the brain and peripheral tissues to facilitate performance in demanding situations. Indeed, threat responses are associated with negative cognitive and affective responses to stress and poor behavioral performance in a variety of active stress tasks. (36-38)
The purpose of the current study was to determine whether CM is associated with a threat pattern of physiological reactivity in adolescence during a social-evaluative stressor, the Trier Social Stress Test (TSST). (39) We anticipated that adolescents exposed to physical, sexual, or emotional abuse would be more likely to exhibit a threat pattern of cardiovascular reactivity during the stressor. We further predicted that CM would be associated with cognitive appraisals indicating greater demands—that the task was more stressful, more difficult, and required more effort. Given that sex differences in stress reactivity emerge during adolescence (40) and are expected following traumatic stress based on prevailing theoretical models, (27) we examined whether these associations varied by sex. Finally, we examined whether cardiovascular reactivity (CO and TPR) were associated with internalizing and externalizing symptoms. We anticipated that threat reactivity (low CO and high TPR reactivity) would be associated with greater symptoms.
Methods
Sample
A community-based sample of 168 adolescents aged 13-17 was recruited for participation at schools, after-school programs, medical clinics, and the general community in Boston and Cambridge, MA between July 2010 and November 2012. Recruitment efforts were targeted at recruiting a sample with high variability in exposure to CM. To do so, we recruited heavily from neighborhoods with high levels of violence and from clinics that served a predominantly low-SES catchment area. Adolescents taking medications known to influence cardiovascular functioning were excluded (n=4). The sample was 56.0% female (n=94) and had a mean age of 14.9 years (SD=1.36). All females were post-menarchal. Racial/ethnic composition of the sample was as follows: 40.8% White (n=69), 18.34% Black (n=31), 17.8% Hispanic (n=30), 7.7% Asian (n=13), and 14.8% Biracial or Other (n=25). Approximately one-third of the sample (40.1%, n=63) was from single-parent households; 26.8% (n=42) were living below the poverty line. Poverty was assessed with parent-reported information on family income and size. Poverty thresholds were defined according to U.S. Census Bureau guidelines for 2011. Equipment malfunctions resulted in loss of autonomic data from 8 participants. An additional 3 participants were excluded from analysis due to heart murmur (n=1), severe cognitive impairment (n=1), and pervasive developmental disorder (n=1). The final analytic sample included 157 participants. All procedures were approved by the Institutional Review Board at Harvard University and Boston Children’s Hospital.
Child Maltreatment
Child abuse was assessed using a self-report questionnaire, the Childhood Trauma Questionnaire (CTQ), (41) and an interview, the Childhood Experiences of Care and Abuse (CECA) interview.(42, 43) The CTQ is a 28-item scale that assesses the frequency of maltreatment during childhood and adolescence. Three types of abuse are assessed: physical, sexual, and emotional. The CTQ has excellent psychometric properties including internal consistency, test-retest reliability, and convergent and discriminant validity with interviews and clinician reports of maltreatment.(41, 44) We created a maltreatment severity composite by summing items from each of the abuse sub-scales. This composite demonstrated good reliability in our sample (α=0.88). The CECA assesses multiple aspects of caregiving experiences, including physical and sexual abuse. Inter-rater reliability for maltreatment reports is excellent, and multiple validation studies suggest high agreement between siblings on reports of maltreatment. (42, 43)
We used the CECA and the CTQ to create a dichotomous indicator of abuse exposure. Participants who reported physical or sexual abuse during the interview or who had a score on any of the three CTQ abuse sub-scales above a previously-identified threshold(45) were classified as having experienced abuse. A total of 38.2% of the sample experienced abuse using this threshold as compared to population-based estimates of 12.5-20.0%, (1, 46) reflecting our efforts to recruit maltreated children. No participant was currently experiencing maltreatment, and the proper authorities were contacted in cases where we had safety concerns.
Physiological measures
Electrocardiogram (ECG) recordings were obtained with a Biopac ECG amplifier (Goleta, CA) using a modified Lead II configuration (right clavicle, left lower torso, and right leg ground). Cardiac impedance recordings were obtained with a Bio-Impedance Technology model HIC-2500 impedance cardiograph (Chapel Hill, NC). One pair of mylar tapes encircled the neck and another pair encircled the torso. A continuous 500μA AC 95 kHz current was passed through the two outer electrodes, and basal thoracic impedance (z0) and the first derivative of basal impedance (dz/dt) was measured from the inner electrodes. A Biopac MP150 integrated the ECG and impedance cardiography (ICG) signals, sampled at 1.0 kHz, using Acqknowledge software. A Colin Prodigy II oscillometric blood pressure machine (Colin Medical Instruments, San Antonio, TX) was used to obtain blood pressure recordings at predetermined times during the study.
ECG and ICG data were scored by raters unaware of maltreatment status. Signals were averaged into one-minute epochs using Mindware Software (Mindware Technologies, Gahanna, OH). The combination of this equipment allowed us to estimate the target physiological variables. Stroke volume (SV), estimated from the dz/dt signal, provides an estimate of the amount of blood ejected from the heart on each cardiac cycle. CO for each minute was calculated as SV × HR. Because accurate scoring of ICG data requires manual placement of the B point (opening of the aortic valve),(47) these data were scored by two independent raters. SV differences of more than 5% (present in 8.2% of minutes scored) were reviewed and adjudicated by the first author (KM). We calculated TPR using the standard formula (Mean Arterial Pressure/CO) × 80.(48) Pre-ejection period (PEP), a measure of SNS activation representing the amount of time that elapses from the beginning of ventricular depolarization to the opening of the aortic valve (electrical systole), was calculated based on the ECG and ICG signals. The Q-onset in the ECG was placed using a validated automated scoring algorithm(49) that was visually inspected to ensure accurate placement and adjusted if needed.
Cognitive Appraisals
Participants completed appraisals of the degree of demands and resources they anticipated and experienced before and after the TSST using a measure of cognitive appraisals utilized in studies of challenge and threat. (37, 50) Item wording was modified slightly from pre-task (e.g., “The upcoming task will take a lot of effort to complete”) to post-task (e.g., “The task took a lot of effort to complete”). Each item was rated on a one-to seven-point scale. Items representing situational demands (e.g., “The upcoming task is difficult”) and personal resources (e.g., “I have the abilities to perform the upcoming task successfully”) were summed separately and demonstrated good reliability (α=0.77 and 0.81, respectively). Previous experimental studies of adults have shown that greater pre-task demand appraisals are associated with a threat pattern of cardiovascular reactivity and that instructing participants to engage in re-appraisal to generate resource rather than demand appraisals results in a more adaptive pattern of cardiovascular reactivity.(33, 37)
Internalizing and Externalizing Symptoms
Internalizing and externalizing symptoms were assessed using the Youth Self Report form of the Child Behavior Checklist (CBCL).(51) The CBCL scales are among the most widely used measures of youth emotional and behavioral problems and use extensive normative data to generate age-standardized estimates of severity of internalizing and externalizing symptoms. These scales have demonstrated validity in discriminating between youths with and without psychiatric disorders.(51-53)
Procedure
Baseline physiological data were collected during a five-minute period in which participants were asked to sit quietly. Adolescents then completed questionnaire and interview measures assessing CM and psychopathology. Informed consent was obtained from the parent/guardian who attended the session, and assent was provided by adolescents.
Participants completed the TSST, (39) a widely-used stress induction procedure that has been used with children and adolescents. (54, 55) The TSST involves three periods. After being told they would be delivering a speech in front of trained evaluators who would judge their performance, participants completed measures of pre-task appraisals and were given five-minutes to prepare their speech. In the current study, participants were asked to talk about the qualities of a good friend and which of those characteristics they did and did not possess. Next, participants delivered a five-minute speech in front of two evaluators. Evaluators were trained to provide neutral and mildly negative feedback (e.g., appearing bored) during the speech. Finally, participants completed mental subtraction out loud in front of the evaluators for five-minutes. Specifically, participants were asked to count backwards in steps of seven from a three-digit number and were stopped and asked to start again each time they made a mistake. Post-task appraisals were assessed immediately following the end of the math task. ECG and ICG recordings were measured continuously across each period; blood pressure recordings were sampled during the first and fourth minutes of each period.
Statistical Analysis
We examined the associations of CM with cardiovascular reactivity and appraisals using univariate ANCOVAs with CM as a between-subject factor. Cardiovascular reactivity scores were created by subtracting the baseline value of each physiological parameter from the first minute of each task (preparation, speech, math), which is standard practice.(33, 37, 47) Each model controlled for baseline values of the physiological parameter of interest (to control for baseline differences between groups), and covariates included age, gender, single-parent household, and poverty. Next we examined the continuous measure of maltreatment severity as a predictor of cardiovascular reactivity using linear regression and the same covariates described above. Primary analysis focused on the two parameters that consistently differentiate challenge and threat responses: CO and TPR. TPR values were skewed and were log-transformed for analysis. We also examined differences in PEP reactivity, which differentiates threat and challenge profiles—greater PEP reactivity in challenge states than threat states—although less consistently than CO and TPR.(56) Analysis of appraisals reported before and after the TSST was also conducted. We evaluated whether the associations of CM with cardiovascular reactivity and appraisals varied by gender by creating interaction terms between gender and CM exposure and severity. Finally, we evaluated whether cardiovascular reactivity was associated with internalizing and externalizing symptoms using linear regression.
Results
Descriptive Statistics
Table 1 provides socio-demographics and baseline physiological characteristics of the sample for adolescents with and without maltreatment exposure. Maltreated adolescents were more likely to be female (63.6%) and older (mean age = 15.2 years) than non-maltreated adolescents (51.0% female, mean age = 14.7 years). CM was unassociated with baseline physiological characteristics, with the exception of diastolic blood pressure (Table S1, Supplemental Digital Content 1).
Table 1.
Distribution of socio-demographics and baseline physiological characteristics by maltreatment status (N=157)
| Maltreated (n=60) | Controls (n=97) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| % | (n) | % | (n) | χ 2 | p-value | |
| Female | 63.6 | 42 | 51.0 | 52 | 2.60 | .11 |
| Race/Ethnicity | 6.94 | .14 | ||||
| White | 28.8 | 19 | 49.0 | 50 | ||
| Black | 22.7 | 15 | 14.7 | 15 | ||
| Latino | 21.1 | 14 | 15.7 | 16 | ||
| Asian/Pacific Islander | 7.6 | 5 | 7.8 | 8 | ||
| Other/Biracial | 18.2 | 12 | 12.7 | 13 | ||
| Single Parent Family | 53.0 | 35 | 27.5 | 28 | 11.19* | .001 |
| Poverty | 33.3 | 22 | 19.6 | 20 | 4.34* | .037 |
| Mean | (SD) | Mean | (SD) | t-value | p-value | |
|
|
||||||
| Age | 15.24 | (1.31) | 14.69 | (1.36) | −2.63* | .009 |
| Baseline SBP (mm Hg) | 114.45 | (13.02) | 113.14 | (11.50) | −0.67 | .49 |
| Baseline DBP (mm Hg) | 60.92 | (7.59) | 57.86 | (7.59) | −2.46* | .015 |
| Baseline MAP (mm Hg) | 78.77 | (9.05) | 76.36 | (7.84) | −1.82 | .070 |
| Baseline HR (bpm) | 75.23 | (11.69) | 75.13 | (11.69) | −0.49 | .96 |
| Baseline PEP (ms) | 102.83 | (16.43) | 102.47 | (13.64) | −0.15 | .88 |
| Baseline SV (mL) | 75.80 | (24.70) | 77.91 | (35.73) | 0.40 | .69 |
| Baseline CO (L/min) | 5.47 | (1.74) | 5.53 | (2.16) | 0.19 | .87 |
| Baseline TPR (resistance units) |
1250.20 | (380.66) | 1299.85 | (570.04) | 0.60 | .55 |
p < .05, 2-sided test
Abbreviations: SBP, systolic blood pressure; DPB, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; PEP, pre-ejection period; SV, stroke volume; CO, cardiac output; TPR, total peripheral resistance.
Child Maltreatment and Cardiovascular Reactivity
Our primary hypothesis was that CM would be associated with a profile of cardiovascular reactivity consistent with threat. We first examined changes in PEP to determine if participants experienced SNS activation—a requirement for examining the challenge/threat distinction. Significant increases in SNS activation (i.e., lower PEP than baseline based on paired-samples t-tests) were observed during the preparation (t = 9.35, p < .001), speech (t = 13.78, p < .001), and math (t = 11.52, p < .001), periods (Table 2; see Table S2, Supplemental Digital Content 1, for reactivity values for all physiological parameters). During the math period only and consistent with the challenge and threat distinction, maltreated adolescents exhibited significantly less PEP reactivity than non-maltreated adolescents, F(1,145) = 4.56, p = .034.
Table 2.
Child maltreatment and cardiovascular reactivity1 (N=157)
| Maltreated (n=60) | Controls (n=97) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean | (SD) | Mean | (SD) | F-value2 | p-value | |
|
|
||||||
| Pre-ejection period (ms) | ||||||
| Preparation | −7.68 | (13.31) | −11.52 | (13.09) | 2.78 | .097 |
| Speech | −13.42 | (12.63) | −17.96 | (15.42) | 3.65 | .058 |
| Math | −9.13 | (11.56) | −13.26 | (12.67) | 4.56* | .034 |
| Cardiac Output (L/min) | ||||||
| Preparation | 0.11 | (0.90) | 0.60 | (1.16) | 9.49* | .002 |
| Speech | 0.56 | (0.96) | 0.85 | (1.27) | 3.80 | .053 |
| Math | 0.34 | (0.87) | 0.65 | (1.10) | 5.88* | .017 |
| Total Peripheral Resistance (resistance units)3 |
||||||
| Preparation | 151.64 | (208.78) | 79.47 | (290.35) | 2.33* | .049 |
| Speech | 167.22 | (215.95) | 133.95 | (276.39) | 2.96 | .138 |
| Math | 157.16 | (236.06) | 110.28 | (218.58) | 2.40 | .265 |
p < .05, 2−sided test
Values represent reactivity scores calculated by subtracting the values from the first minute of each task from the baseline period.
Univariate ANCOVA controlling for age, gender, single parent household, and poverty; degrees of freedom for F−tests: (1,145).
Mean TPR reactivity values are shown for untransformed TPR values to facilitate interpretation; ANCOVAs were estimated on log−transformed TPR values due to the skewed distribution in our sample.
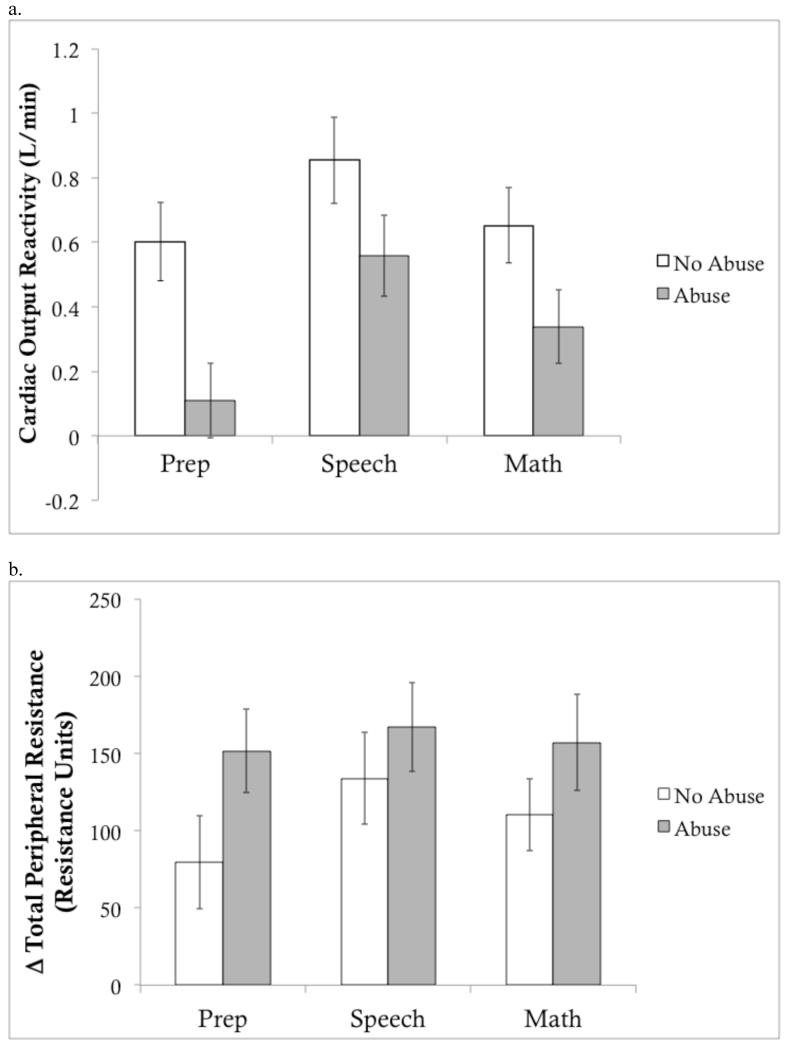
We next turned to the variables that consistently differentiate challenge and threat (Figure 1). Maltreated adolescents exhibited a significantly different pattern of CO reactivity than controls. Specifically, maltreatment exposure was associated with smaller increases in CO during the preparation, F(1,145) = 9.94, p = .002, speech, F(1,145) = 3.80, p = .053, and math, F(1,145) = 5.88, p = .017, portions of the TSST (Table 2). Effects of CM with TPR reactivity were weaker. CM was significantly associated with TPR increases during the preparation period, F(1,145) = 3.94, p = .049, but was unrelated to TPR increases during the speech, F(1,145) = 2.23, p = .13, and math, F(1,145) = 1.26, p = .27.
Figure 1. Cardiac output and total peripheral resistance reactivity according to child maltreatment status.
Change in a) cardiac output (L/min) and b) total peripheral resistance (resistance units) during each component of the Trier Social Stress Test relative to baseline (unadjusted means). Error bars represent within-group standard error (SE).
We then examined these same cardiovascular reactivity variables using a continuous indicator of CM severity. Higher severity of CM was associated with smaller increases in CO during the preparation, β = -0.12, p = .007, speech, β = -0.10, p = .030, and math, β = -0.11, p = .012, periods, controlling for baseline CO (Table 2). Higher maltreatment severity was significantly associated with TPR increases during the preparation, β = 0.11, p = .009, and marginally associated with TPR reactivity during the speech, β = 0.07, p = .11, and math, β = 0.08, p = .053, periods.
Interactions between sex and maltreatment were added to these models. None of these interactions were significant.
Child Maltreatment and Demand Appraisals
CM exposure was unrelated to demand appraisals prior to the TSST, F(1,148) = 0.78, p = .38, or following the speech, F(1,148) = 0.08, p = .78, but was associated with demand appraisals related to math, F(1,148) = 5.41, p = .025. CM severity was unrelated to demand appraisals. No associations were observed in predicting resource appraisals or interactions between sex and maltreatment in predicting appraisals.
Cardiovascular Reactivity and Symptoms
CM was associated strongly with both the internalizing and externalizing symptoms (Table 3). We then examined if psychopathology was related to CV reactivity. CO reactivity was related to externalizing, but not internalizing, symptoms (Table 4). CO reactivity was negatively related to externalizing symptoms and TPR reactivity was positively related to externalizing symptoms—including conduct disorder, oppositional defiant disorder (ODD), and ADHD symptoms. This pattern was observed for CO during all three portions of the TSST and for TPR reactivity during speech and math.
Table 3.
Child maltreatment and internalizing and externalizing symptoms (N=157)
| Maltreated (n=60) | Controls (n=97) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean | (SD) | Mean | (SD) | F-value1 | p-value | |
|
|
||||||
| YSR Internalizing | 56.97 | (9.55) | 50.30 | (9.83) | 14.88* | <.001 |
| Anxious/Depressed | 58.48 | (7.86) | 54.31 | (5.56) | 15.27* | <.001 |
| Depressed/Withdrawn | 58.28 | (6.87) | 54.61 | (5.84) | 9.02* | .003 |
| YSR Externalizing | 57.03 | (8.89) | 48.92 | (8.68) | 22.95* | <.001 |
| Conduct | 57.66 | (6.92) | 53.71 | (5.08) | 12.79* | <.001 |
| ODD | 57.22 | (7.08) | 53.42 | (5.34) | 12.86* | <.001 |
| ADHD | 59.66 | (7.63) | 56.12 | (6.99) | 4.29* | .040 |
p < .05, 2-sided test
Univariate ANCOVA controlling for age, gender, single parent household, and poverty; degrees of freedom for F-tests: (1,153).
Table 4.
Cardiovascular reactivity and internalizing and externalizing symptoms (N=157)
| Internalizing | Anxious/ Depressed |
Depressed/ Withdrawn |
Externalizing | Conduct Disorder |
Oppositional Defiant Disorder |
ADHD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | |
|
|
||||||||||||||
| CO Reactivity | ||||||||||||||
| Preparation | −.13 | .483 | −.44 | .659 | −.01 | .967 | −.43* | .015 | −.53* | .002 | −.37* | .037 | −.38* | .030 |
| Speech | −.22 | .223 | −.25 | .161 | −.19 | .280 | −.38* | .032 | −.44* | .012 | −.45* | .011 | −.41* | .020 |
| Math | −.11 | .560 | −.17 | .396 | .03 | .886 | −.50* | .008 | −.65* | <.001 | −.59* | .002 | −.46* | .013 |
| TPR Reactivity1 | ||||||||||||||
| Preparation | .09 | .658 | .02 | .936 | .05 | .785 | .31 | .094 | .27 | .150 | .32 | .092 | .50* | .006 |
| Speech | .14 | .492 | .10 | .625 | .10 | .630 | .42* | .035 | .31 | .121 | .46* | .024 | .47* | .019 |
| Math | .01 | .978 | −.09 | .672 | −.11 | .606 | .49* | .018 | .61* | .003 | .56* | .008 | .55* | .007 |
p<.05, 2-sided test
TPR values were log-transformed for analysis
Discussion
Disruptions in the development of stress response systems have been posited to be a central mechanism underlying the association between CM and psychopathology.(5, 7) However, patterns of HPA axis reactivity observed among youths exposed to maltreatment have varied widely across studies, (13-16, 18) and relatively few investigations have examined associations of maltreatment and SNS reactivity. As a result, considerable gaps exist between prevailing theories and existing evidence of how CM influences physiological reactivity. We extend this literature in several important ways. First, we applied a well-validated theoretical model that differentiates between approach and withdrawal responses to psychosocial stress that has not previously been utilized in the study of CM. Specifically, we expected that CM would be associated with a threat pattern of cardiovascular reactivity characterized by heightened peripheral resistance and blunted CO reactivity.(29, 33) Our findings supported these hypotheses. Second, we evaluated whether this threat pattern of reactivity was related to adolescent internalizing and externalizing symptoms. Threat reactivity was negatively associated with externalizing but not internalizing symptoms. Finally, we examined patterns of cardiovascular reactivity to a social stressor during adolescence, a developmental period that has been studied less frequently with regards to CM and stress reactivity.
Why might CM be associated with this physiological response pattern? Experiences of abuse are often associated with the potential for physical harm and low control over the situation. Chronic exposure to this type of environment is likely to influence perceptions of control, which have been shown to predict stress responses in youths. (57) Low perceived control associated with CM exposure might lead to greater threat perceptions during social stress situations and a physiological response that more closely resembles freezing or immobilization than a fight-or-flight response. Threat responses have been linked to freezing behavior in previous studies,(50) reflecting avoidance in a situation where escape is not possible and a fight-or-flight response is unlikely to promote safety. (58, 59) Freezing is characterized by withdrawal, disengagement, and lower levels of sympathetic activation and CO than a fight-or-flight response,(58, 60) consistent with the pattern we observed among maltreated adolescents. The reduced CO and higher TPR even in the context of a robust SNS response to the TSST, as evidenced by a decrease in PEP, provides strong evidence for a threat, or withdrawal, pattern of cardiovascular responding to social stress following CM.(29, 32-35) To our knowledge, this is the first study to assess and document this type of dysregulated physiological response among maltreated youths. Thus, exposure to CM might result in heightened perceptions of danger or under-estimations of one’s own capabilities, which trigger a dysregulated physiological response, even in the context of relative safety.
What are the implications of this type of autonomic response? Although such a response might be adaptive in the context of legitimate threats to survival, the pattern of threat responses associated with CM in the current study has been linked to a variety of adverse functional outcomes, including poor decision-making in emotional situations, (61) accelerated aging, (62) and low levels of positive approach-oriented behavior.(33) This pattern of physiological reactivity is also associated with other markers of negative emotional reactivity, such as resting frontal EEG asymmetry in the alpha frequency band favoring the right hemisphere.(63) Physiological threat responses are related to heightened negative affect in response to stress and poor behavioral performance in multiple types of situations—particularly cognitively demanding tasks. (36-38) Together, these findings suggest that this profile of reactivity may have negative downstream effects on multiple aspects of cognitive, affective, and behavioral functioning. Indeed, CO and TPR reactivity were associated with multiple types of externalizing symptoms. To our knowledge, these findings are novel and suggest that threat responses might have implications for mental health. Disruptions in perceptions of threat are common in conduct disorder and ODD. (64, 65) This is primarily true for cases in which symptoms are associated with negative developmental environments but not genetic risk or callous-unemotional traits.(66) It is possible that persistent threat appraisals paired with low confidence in one’s capabilities and dysregulated physiological responses increase risk for reactive forms of aggression. Prior research has documented reduced PEP reactivity among children with disruptive behavior disorders, (67) which is compatible with the higher TPR reactivity seen in the threat response as an increase in TPR tends to blunt PEP reactivity through afterload effects. The degree to which this pattern of reactivity represents a vulnerability factor versus consequence of psychopathology remains to be determined.
We provide novel evidence linking CM to maladaptive patterns of autonomic reactivity as defined in the biopsychosocial model of challenge and threat. This model provides a theoretical framework for identifying maladaptive patterns of stress reactivity across a variety of contexts, (29, 33) which could inform other theories such as the biological sensitivity to context(24) or adaptive calibration models(27) in terms of describing more specific patterns of physiological dysregulation associated with environmental adversity. The pattern of findings observed here differs from the predictions of these theories. In regards to the biological sensitivity to context, we found no evidence for elevated PEP reactivity among maltreated adolescents—in fact, CM was associated with reduced PEP reactivity during the math task. This is consistent with evidence of lower PEP reactivity in threat than challenge states,(33) likely reflecting the fact that increases in peripheral resistance are associated with increases in PEP. With regards to the adaptive calibration model, we found no sex differences in the association of CM with cardiovascular reactivity. One difference between our work and other studies supporting these models is that previous investigations have focused on younger children.(28) Given the developmental changes in stress reactivity that occur during adolescence, it will be important for future studies to replicate our findings in younger samples.
Although the pattern of physiological reactivity among maltreated adolescents was consistent with our hypotheses, some predictions were only partially supported. Vascular resistance patterns were only marginally different between groups. This likely occurred because we used a non-continuous blood pressure machine, which obtained blood pressure readings at pre-specified intervals during the task rather than continuously. Continuous blood pressure readings would have allowed us to obtain hemodynamic responses more unobtrusively than the method we used. Furthermore, maltreated adolescents reported greater demand appraisals regarding the math task, but not in anticipation or in response to the speech. Since the speech always preceded the math task, this may be due to the expectation that evaluators would be kinder or more encouraging than they actually were, and the math task appraisals thus reflect the experience of a rejecting audience combined with a novel math task. Although associations between cognitive appraisals and physiological reactivity have been documented in adults,(33, 37) evidence suggests that this relationship is absent in adolescents.(68) This might be related to a delay in the development of higher-order cognitive processes that facilitate emotional awareness relative to the developmental increases in physiological reactivity to social/evaluative stressors during adolescence.(55) Replication of our findings in samples of adults is an important goal for future research.
This study is also limited by a cross-sectional design that does not allow us to determine whether patterns of hemodynamic reactivity are associated prospectively with symptoms. Thus, it is possible that elevated peripheral resistance and lower CO are a consequence rather than determinant of externalizing symptoms. Future prospective studies of the biopsychosocial model are needed to determine the direction of effect. Second, symptoms were assessed using the CBCL scales rather than a diagnostic interview. Determining whether the patterns of cardiovascular reactivity observed here are related to psychiatric disorders is another important goal for future research. Finally, the effect sizes for associations of CM with CO and TPR were moderate in magnitude. Replication of the patterns observed here in future studies is therefore warranted.
CM is associated with maladaptive patterns of cardiovascular reactivity to psychosocial stress in adolescence. The biopsychosocial model of challenge and threat is a useful theoretical framework through which to interpret patterns of cardiovascular reactivity following CM and may help to reconcile inconsistent findings in previous studies. Our finding that these maladaptive responses are one mechanism linking CM to externalizing symptoms is consistent with previous neuroimaging and behavioral findings of enhanced threat perceptions in children with externalizing disorders. We extend this literature by documenting a psychophysiological signature associated with enhanced threat perception that might prove useful in future studies of both CM and externalizing psychopathology.
Supplementary Material
Acknowledgments
Source of Funding: This research was supported by grants from the National Institutes of Health (K01-MH092526 to McLaughlin and K01-MH092555 to Sheridan).
Abbreviations
- CM
child maltreatment
- HPA
hypothalamic-pituitary-adrenal
- SAM
sympathetic-adrenal-medullary
- SNS
sympathetic nervous system
- CO
cardiac output
- TPR
total peripheral resistance
- TSST
Trier Social Stress Test
- ECG
electrocardiogram
- ICG
impedance cardiographic
- PEP
pre-ejection period
Footnotes
Conflict of Interest: The authors have no financial disclosures or conflicts of interest to report.
References
- 1.McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky A, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a national sample of adolescents. Archives of General Psychiatry. 2012;69:1151–60. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green JG, McLaughlin KA, Berglund P, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;62:113–23. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen P, Brown J, Smailes E. Child abuse and neglect and the development of mental disorders in the general population. Development and Psychopathology. 2001;13:981–99. [PubMed] [Google Scholar]
- 4.Scott KM, McLaughlin KA, Smith DAR, Ellis PM. Childhood maltreatment and DSM-IV adult mental disorders: comparison of prospective and retrospective findings. British Journal of Psychiatry. 2012;200:469–75. doi: 10.1192/bjp.bp.111.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–9. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 7.Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 8.Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2012;22:82–91. doi: 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- 9.Lyons DM, Wang OJ, Lindley SE, Levine S, Kalin NH, Schatzberg AF. Separation induced changes in squirrel monkey hypothalamic-pituitary-adrenal physiology resemble aspects of hypercortisolism in humans. Psychoneuroendocrinology. 1999;24:131–42. doi: 10.1016/s0306-4530(98)00065-1. [DOI] [PubMed] [Google Scholar]
- 10.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 11.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 12.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA: Journal of the American Medical Association. 2000;284:592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Wells W, Ryan ND. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry. 1997;42:669–79. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- 14.Fries ABW, Shirtcliff EA, Pollak SD. Neuroendocrine dysregulation following early social deprivation in children. Developmental Psychobiology. 2008;50:588–99. doi: 10.1002/dev.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, Trickett PK. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. Journal of Clinical Endocrinology and Metabolism. 1994;78:249–55. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- 16.MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van Der Meulen J, Boyle MH, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the Youth Mood Project. Biological Psychiatry. 2009;66:62–8. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher PA, Kim HK, Bruce J, Pears KC. Cumulative effects of prenatal substance exposure and early adversity on foster children’s HPA-axis reactivity during a psychosocial stressor. International Journal of Behavioral Development. 2011;36:29–35. doi: 10.1177/0165025411406863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunnar MR, Frenn K, Wewerka S, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10-12 year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–87. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Development and Psychopathology. 2005;17:303–28. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- 21.Oosterman M, de Schipper JC, Fisher P, Dozier M, Schuengel C. Autonomic reactivity in relation to attachment and early adversity among foster children. Development and Psychopathology. 2010;22:109–18. doi: 10.1017/S0954579409990290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Sheikh M. The role of emotional responses and physiological reactivity in the marital conflict-child functioning link. Journal of Child Psychology and Psychiatry. 2005;46:1191–9. doi: 10.1111/j.1469-7610.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- 23.Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: A study among healthy young subjects Psychoneuroendocrinology. 2008;33:227–37. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 25.Obradovic J, Bush NR, Boyce WT. The interactive effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: The role of laboratory stressors. Development and Psychopathology. 2011;23:101–14. doi: 10.1017/S0954579410000672. [DOI] [PubMed] [Google Scholar]
- 26.Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81:270–89. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35:1562–92. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Giudice M, Hinnant JB, Ellis BJ, El-Sheikh M. Adaptive patterns of stress responsivity: A preliminary investigation. Developmental Psychology. 2012;48:775–90. doi: 10.1037/a0026519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blascovich J. Challenge and threat. In: Elliot AJ, editor. Handbook of approach and avoidance motivation. Psychology Press; New York: 2008. pp. 431–45. [Google Scholar]
- 30.Dienstbier RA. Arousal and physiological toughness: Implications for physical and mental health. Psychological Review. 1991;96:84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- 31.Folkman S, Lazarus RS, Gruen RJ, DeLongis A. Appraisal, coping, health status, and psychological symptoms. Journal of Personality and Social Psychology. 1986;50:571–9. doi: 10.1037//0022-3514.50.3.571. [DOI] [PubMed] [Google Scholar]
- 32.Mendes WB, Blascovich J, Major B, Seery M. Challenge and threat during downward and upward social comparisons. European Journal of Social Psychology. 2001;31:477–97. [Google Scholar]
- 33.Mendes WB, Major B, McCoy S, Blascovich J. How attributional ambiguity shapes physiological and emotional responses to social rejction and acceptance. Journal of Personality and Social Psychology. 2008;94:278–91. doi: 10.1037/0022-3514.94.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blascovich J, Mendes WB. Social “facilitation” as challenge and threat. Journal of Personality and Social Psychology. 1999;77:68–77. doi: 10.1037//0022-3514.77.1.68. [DOI] [PubMed] [Google Scholar]
- 35.Blascovich J, Mendes WB. Social psychophysiology and embodiment. In: Fiske ST, Gilbert DT, Lindzey G, editors. The Handbook of Social Psychology. 5th Edition Wiley; New York: 2010. pp. 194–227. [Google Scholar]
- 36.Tomaka J, Blascovich J, Kelsey RM. Subjective, physiological, and behavioral effects of threat and challenge appraisal. Journal of Personality and Social Psychology. 1993;65:248–60. [Google Scholar]
- 37.Jamieson JP, Nock MK, Mendes WB. Mind over matter: Reappraising arousal improves cardiovascular and cognitive responses to stress. Journal of Experimental Psychology: General. 2012;141:417–22. doi: 10.1037/a0025719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drach-Zahavy A, Erez M. Challenge versus threat effects on the goal-performance relationship. Organizational Behavior and Human Decision Processes. 2002;88:667–82. [Google Scholar]
- 39.Kirschbaum C, Pirke K-M, Hellhammer D. The ‘Trier Social Stress Test’ -a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 40.Stroud LR, Papandonatos GD, Williamson D, Dahl R. Sex differences in the effects of pubertal development on responses to corticotropinreleasing hormone challenge. Annal of the New York Academy of Sciences. 2004;1021:348–51. doi: 10.1196/annals.1308.043. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:340–8. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Bifulco A, Brown GW, Harris TO. Childhood Experiences of Care and Abuse (CECA): a retrospective interview measure. Journal of Child Psychology and Psychiatry. 1994;35:1419–35. doi: 10.1111/j.1469-7610.1994.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 43.Bifulco A, Brown GW, Lillie A, Jarvis J. Memories of childhood neglect and abuse: Corroboration in a series of sisters. Journal of Child Psychology and Psychiatry. 1997;38:365–74. doi: 10.1111/j.1469-7610.1997.tb01520.x. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein DP, Fink L, Hondelsman L, Foote J, Lovejoy M. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 45.Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, Koss MP, Katon W. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Archives of General Psychiatry. 1999;56:609–13. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- 46.Finkelhor D, Ormrod R, Turner H, Hamby SL. The victimization of children and youth: A comprehensive, national survey. Child Maltreatment. 2005;10:5–25. doi: 10.1177/1077559504271287. [DOI] [PubMed] [Google Scholar]
- 47.Blascovich J, Mendes WB, Vanman E, Dickerson S. Social psychophysiology for social and personality psychology. Sage; Los Angeles: 2011. [Google Scholar]
- 48.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Dooren LJP. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 49.Berntson GG, Lozano DL, Chen Y-J, Cacioppa JT. Where to Q in PEP. Psychophysiology. 2004;41:333–7. doi: 10.1111/j.1469-8986.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 50.Mendes WB, Gray H, Mendoza-Denton R, Major B, Epel ES. Why egalitarianism might be good for your health: Physiological thriving during intergroup interactions. Psychological Science. 2007;18:991–8. doi: 10.1111/j.1467-9280.2007.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Achenbach TM. Integrative guide for the 1991 CBCL/4-18, YSR and TRF Profiles. Department of Psychiatry, University of Vermont; Burlington, VT: 1991. [Google Scholar]
- 52.Chen W, Faraone SV, Biederman J, Tsuang MT. Diagnostic accuracy of the Children Behavior Checklist scales for attention-deficit hyperactivity disorder. Journal of Consulting and Clinical Psychology. 1994;62:1017–25. doi: 10.1037/0022-006X.62.5.1017. [DOI] [PubMed] [Google Scholar]
- 53.Seligman L, Ollendick T, Langley AK, Baldacci B. The utility of measures of child and adolescent anxiety: a meta-analytic review of the Revised Children’s Manifest Anxiety Scale, the State-Trait Anxiety Inventory for Children, and the Child Behavior Checklist. Journal of Clinical Child and Adolescent Psychology. 2004;33:557–65. doi: 10.1207/s15374424jccp3303_13. [DOI] [PubMed] [Google Scholar]
- 54.Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–26. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jamieson JP, Mendes WB, Blackstock E, Schmader T. Turning the knots in your stomach into bows: Reappraising arousal improves performance on the GRE. Journal of Experimental Social Psychology. 2010;46:208–12. doi: 10.1016/j.jesp.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Compas BE, Banez GA, Malcarne V, Worsham M. Perceived control and coping with stress: A developmental perspective. Journal of Social Issues. 1991;47:23–34. [Google Scholar]
- 58.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neuroscience and Biobehavioral Reviews. 2001;25:669–78. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 59.Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Research Bulletin. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 60.Porges SW. Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. Annals of the New York Academy of Sciences. 1997;807:62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x. [DOI] [PubMed] [Google Scholar]
- 61.Kassam KS, Koslov K, Mendes WB. Decisions under distress: Stress profiles influence anchoring and adjustment. Psychological Science. 2009;20:1394–9. doi: 10.1111/j.1467-9280.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O’Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging. Circulation. 2010;122:690–7. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koslov K, Mendes WB, Pajtas PE, Pizzagalli DA. Asymmetry in resting intracortical activity as a buffer to social threat. Psychological Science. 2011;22:641–9. doi: 10.1177/0956797611403156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crowe SL, Blair RJR. The development of antisocial behavior: what can we learn from functional neuroimaging studies? Development and Psychopathology. 2008;20:1145–59. doi: 10.1017/S0954579408000540. [DOI] [PubMed] [Google Scholar]
- 65.Viding E, Fontaine NMG, McCrory EJ. Antisocial behaviour in children with and without callous-unemotional traits. Journal of the Royal Society of Medicine. 2012;105:195–200. doi: 10.1258/jrsm.2011.110223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viding E, Fontaine NMG, Oliver BR, Plomin R. Negative parental discipline, conduct problems and callous-unemotional traits: monozygotic twin differences study. British Journal of Psychiatry. 2009;195:414–9. doi: 10.1192/bjp.bp.108.061192. [DOI] [PubMed] [Google Scholar]
- 67.Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology. 2001;110:610–24. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- 68.Rith-Najarian L, McLaughlin KA, Sheridan MA, Nock MK. The biopsychosocial model of stress in adolescence: Self-awareness of performance versus stress reactivity. Stress. 2014;17:193–203. doi: 10.3109/10253890.2014.891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.