Abstract
The extent to which angina pectoris (AP) predicts secondary cardiovascular events beyond independent of measures of disease severity is unknown. We evaluated the association between AP frequency and secondary events in patients with stable coronary heart disease (CHD). We administered the Seattle Angina Questionnaire to 1023 participants with stable CHD enrolled between 9/2000-12/2002 and followed for a median of 8.9 years. We used Cox proportional hazards to evaluate the association of AP frequency with death and subsequent hospitalization for AP, revascularization, myocardial infarction (MI), or heart failure. At enrollment, 633 (62%) participants reported no AP, 279 (27%) reported monthly AP, and 111 (11%) reported daily or weekly AP. During follow-up, 396 participants died, 204 were hospitalized for AP, 194 for revascularization, 140 for MI, and 188 for heart failure. Compared to participants without AP, participants with daily or weekly AP were more likely to be hospitalized for AP (Hazard Ratio 3.3; 95% Confidence Interval 2.3-4.7; p<.001), revascularization (2.0; 1.3-2.9; p=0.001), or heart failure (1.6; 1.0-2.5; p=0.03), and more likely to die (1.5; 1.1-2.0; p=0.01). AP was not independently associated with MI (1.3; 0.8-2.3; p=0.29). After adjusting for demographics, comorbidities, treadmill exercise capacity, ejection fraction, and inducible ischemia, frequency of AP remained independently associated with hospitalization for AP (2.4; 1.6-3.6; p<.001), revascularization (1.7; 1.1-2.7; p=0.02), and death (1.4; 1.0-2.0; p=0.045). In conclusion, in outpatients with stable CHD, AP frequency predicts higher rates of secondary cardiovascular events and death, independent of objective measures of disease severity.
Keywords: angina pectoris, symptoms, coronary artery disease, secondary prevention
Introduction
We sought to determine whether the frequency of angina pectoris (AP) was associated with long-term risk of cardiovascular events in a prospective cohort study of 1,023 outpatients with stable coronary heart disease (CHD). We also evaluated whether the association between AP frequency and cardiovascular events was explained by other baseline risk factors and objective measures of CHD.
Methods
We evaluated 1023 participants from the Heart and Soul Study, a prospective cohort study originally designed to investigate the effects of psychosocial factors on health outcomes in patients with stable CHD. Detailed methods of this study have been previously described [1]. In brief, participants were eligible if they had at least 1 of the following: history of myocardial infarction (MI), angiographic evidence of ≥50% stenosis in ≥1 coronary vessels, evidence of exercise-induced ischemia by treadmill electrocardiogram or stress nuclear perfusion imaging, or a history of coronary revascularization. Participants were excluded if they were unable to walk 1 block, had an acute coronary syndrome within the previous 6 months, or were likely to move out of the area within 3 years.
Between September 11, 2000 and December 20, 2002, 1024 participants were enrolled from 12 outpatient clinics in the San Francisco Bay Area, including 549 (54%) with a history of MI, 237 (23%) with a history of revascularization but not MI, and 238 (23%) with a diagnosis of coronary disease that was documented by their physician, based on a positive angiogram or treadmill test in over 98% of cases. All study participants completed a full-day evaluation including medical history, extensive questionnaires, blood tests, and an exercise treadmill test with baseline and stress echocardiograms. The analytic cohort for this investigation included the 1023 participants who completed the AP Frequency domain of the SAQ. All participants provided informed consent. This study was approved by the institutional committee on human research.
The primary predictor was AP frequency measured with the Seattle Angina Questionnaire (SAQ) [2]. The SAQ is a 19-item, self-administered questionnaire that has been validated for use in patients with CHD. The questionnaire is divided into several domains, including AP frequency, quality of life, treatment satisfaction, and physical limitation. The AP frequency domain includes 2 questions with Likert-scale responses. The questions are “Over the past 4 weeks, on average, how many times have you had chest pain, chest tightness, or AP?” and “How many times have you had to take nitroglycerin for your chest pain, chest tightness, or AP?”. Scores for AP frequency are translated into a score on a 100 point scale, with 100 representing no AP and 0 representing AP occurring 4 or more times per day. Scores for the physical limitation domain of the SAQ were also calculated on a 100-point scale, with 100 representing no limitation and 0 representing severe physical limitations due to AP.
Participants were divided into categories of AP frequency based on SAQ scores, defined as absent (score 100), monthly (score 61-99), weekly (score 31-60), and daily (score 0-30). Because only 10 participants reported daily AP, those with daily or weekly AP were combined into a single category for analysis.
Annual telephone interviews were conducted with participants or their proxy to inquire about interval hospitalization or death. For any reported event, medical records, electrocardiograms, death certificates, autopsy, and coroner’s reports were obtained. Each event was adjudicated by 2 independent and blinded reviewers. In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third, blinded adjudicator, if needed.
Hospitalization for AP was strictly defined as hospitalization for definite or probable AP on the basis of symptoms, physician diagnosis, medical treatment, documented coronary heart disease, revascularization during admission, stenosis >70% documented during admission, ischemia by electrocardiogram, or ischemia by stress testing. Hospitalization for chest pain was not considered AP without objective evidence of cardiac ischemia. Revascularization was defined as percutaneous coronary intervention or coronary artery bypass graft surgery. MI was defined using standard diagnostic criteria [3]. Heart failure was defined as hospitalization for signs and symptoms of heart failure. Death was verified by death certificates.
Demographic characteristics, medical history, and smoking status were collected by self-report questionnaire. Depressive symptoms were assessed using the 9-item Patient Health Questionnaire, a self-report instrument that measures the frequency of depressive symptoms, with a score of 10 or higher being classified as having depressive symptoms [4]. We measured weight and height to calculate body mass index (kg/m2). Resting supine blood pressure was measured with a standard sphygmomanometer. Participants were asked to bring their medications to the study appointment, and research personnel recorded all current medications and categorized using Epocrates Rx (Epocrates, Inc., San Mateo, CA). Total and high-density lipoprotein cholesterol were determined from 12-hour fasting serum samples.
Participants underwent symptom-limited treadmill exercise testing according to a standard Bruce protocol (those unable to complete the standard protocol underwent operator-modified grade and speed adjustments) with continuous 12-lead electrocardiogram monitoring. Exercise capacity was estimated as the total metabolic equivalents (METs) achieved at peak exercise [5]. Prior to exercise, participants underwent complete resting 2-dimensional echocardiograms with all standard views using an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, CA) with a 3.5-MHz transducer. Standard 2-dimensional parasternal short-axis and apical 2- and 4-chamber views were used to calculate chamber sizes (indexed to body surface area) and left ventricular ejection fraction [6]. At peak exercise, precordial long- and short-axis and apical 2- and 4-chamber views were obtained to ascertain wall motion abnormalities. We defined exercise-induced ischemia as the presence of 1 or more new wall motion abnormalities at peak exercise that were not present at rest. A single experienced cardiologist, who was blinded to the results of questionnaires and clinical histories, interpreted all echocardiograms.
Baseline characteristics were compared across categories of AP frequency using the X2 test for categorical variables and one-way analysis of variance for continuous variables. Fisher exact test was performed for categorical variables with fewer than 5 participants in a cell. Event rates per 100 person-years were calculated by category of AP frequency. Cox proportional hazards models were used to compare event rates between participants without AP to participants with daily or weekly AP. We adjusted models for all investigated baseline characteristics (demographics, comorbidities, medications, and treadmill exercise capacity) associated with AP frequency with p<0.10, as well as EF and inducible ischemia. We constructed Cox proportional hazards models representing AP frequency as a continuous variable by numerical SAQ AP frequency score and for the association of SAQ physical limitation scale with outcomes. We tested the proportional hazards assumption by evaluating Schoenfeld residuals, and found no violations of the proportional hazards assumption (all p>0.05 for association between residuals and time). Multiple imputation was performed using iterative chained equations for covariates with missing data, including smoking (n=3), hypertension (n=3), history of heart failure (n=6), diastolic blood pressure (n=10), beta-blocker use (n=13), exercise capacity (n=80), EF (n=27), and inducible ischemia (n=86). All analyses were performed using Stata (Version 12, StataCorp LP).
Results
Among 1023 participants completing the SAQ, 633 (61.9%) reported no AP, 279 (27.3%) reported monthly AP, 101 (9.9%) reported weekly AP, and 10 (1.0%) reported daily AP. Participants with daily or weekly AP were less likely to be male, more likely to be current smokers, more likely to have a history of hypertension or heart failure, more likely to have depressive symptoms, and more likely to take beta-blockers, calcium channel blockers, and nitrates (Table 1). Compared with participants who had no AP or weekly AP, those with monthly AP were younger, had higher body mass index, and had higher diastolic blood pressure.
Table 1. Baseline characteristics of 1023 participants reporting angina frequency.
| Characteristic | Angina Frequency |
P-value | ||
|---|---|---|---|---|
| Absent (N = 633 |
Monthly N = 279 |
Daily or Weekly N = 111 |
||
| Age (years), mean ± SD | 68 ± 11 | 65 ± 11 | 66 ± 12 | <.001 |
| Male | 534 (84%) | 221 (79%) | 85 (77%) | 0.048 |
| Caucasian | 391 (62%) | 156 (56%) | 67 (60%) | 0.28 |
| Smoking | 100 (16%) | 67 (24%) | 34 (31%) | <.001 |
| Hypertension | 421 (67%) | 213 (76%) | 89 (80%) | 0.001 |
| Myocardial Infarction | 330 (52%) | 151 (54%) | 66 (59%) | 0.28 |
| Heart Failure | 89 (14%) | 63 (23%) | 27 (24%) | 0.001 |
| Diabetes | 155 (24%) | 81 (29%) | 29 (26%) | 0.37 |
| Revascularization | 382 (60%) | 155 (56%) | 65 (59%) | 0.42 |
| Depressive Symptoms | 79 (12%) | 75 (27%) | 45 (41%) | <.001 |
| Body Mass Index (kg/m2) | 28 ± 5 | 29 ±6 | 28 ± 7 | 0.04 |
| Systolic blood pressure (mmHg) | 133 ± 20 | 135 ± 23 | 130 ± 20 | 0.16 |
| Diastolic blood pressure (mmHg) | 74 ± 11 | 77 ± 12 | 72 ± 10 | 0.001 |
| Total cholesterol (mg/dL) | 176 ± 40 | 182 ± 48 | 175 ± 41 | 0.13 |
| HDL cholesterol (mg/dL) | 46 ± 14 | 46 ± 15 | 45 ± 14 | 0.71 |
| LV ejection fraction (%) | 62 ± 9 | 62 ± 10 | 61 ± 10 | 0.69 |
| LV mass index (g/m2) | 97 ± 25 | 100 ± 30 | 101 ± 26 | 0.11 |
| Medications | ||||
| Beta-blockers | 352 (56%) | 162 (58%) | 79 (71%) | 0.01 |
| ACE-inhibitors or ARBs | 319 (50%) | 139 (50%) | 66 (59%) | 0.19 |
| Statins | 421 (67%) | 168 (60%) | 68 (61%) | 0.12 |
| Aspirin | 449 (71%) | 206 (74%) | 86 (77%) | 0.34 |
| Calcium Channel Blockers | 146 (23%) | 64 (23%) | 37 (33%) | 0.06 |
| Nitrates | 116 (18%) | 103 (37%) | 78 (70%) | <.001 |
| Seattle Angina Questionnaire, median (IQR) | ||||
| Angina Frequency | 100 | 80 (80-90) | 50 (40-60) | <.0 01 |
| Quality of Life | 92 (70-100) | 67 (50-75) | 42 (25-58) | <.001 |
| Physical Limitation | 86 (67-100) | 67 (50-86) | 50 (33-67) | <.001 |
Abbreviations: ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; HDL: high-density lipoprotein; IQR: interquartile range; SD: standard deviation.
Participants with daily or weekly AP were less likely to be physically active, had lower exercise capacity, and were more likely to experience AP with treadmill testing, but not more likely to have inducible ischemia on stress echocardiogram (Table 2). During or immediately following the exercise treadmill test, AP was reported by 21% of participants (21/101) who reported daily or weekly AP on the SAQ and by 2% of participants (12/589) who did not report AP on the SAQ.
Table 2. Angina frequency, physical activity, and treadmill exercise testing in 944 participants.
| Characteristic | Angina Frequency |
P- value |
||
|---|---|---|---|---|
| Absent (N=593) |
Monthly (N = 250) |
Daily or Weekly (N = 101) |
||
| Physically inactive | 177/592 (30%) | 100/249 (40%) | 49/101 (49%) | <.001 |
| Treadmill exercise capacity (METs), mean ± SD |
7.6 ± 3.5 | 7.0 ± 2.8 | 6.3 ± 3.4 | <.001 |
| Angina reported during exercise testing |
<.001 | |||
| None | 581 (98%) | 220 (88%) | 80 (79%) | |
| Non-limiting | 8 (1%) | 14 (6%) | 9 (9%) | |
| Limiting | 4 (1%) | 16 (6%) | 12 (12%) | |
| Inducible myocardial ischemia |
141/589 (24%) | 59/247 (24%) | 28/101 (28%) | 0.70 |
Abbreviations: METs: Metabolic equivalents; SD: standard deviation.
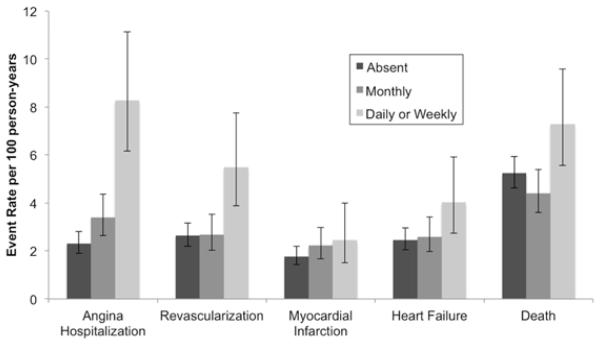
Participants were followed for a median of 8.9 years (interquartile range 5.4 to 9.9 years, longest follow-up 11.4 years). Of participants surviving to 5 years, 667/780 (86%) completed a follow-up exam at 5 years. During follow-up, 204 (19.9%) participants were hospitalized for AP, 194 (19.0%) underwent coronary revascularization, 140 (13.7%) experienced MI, 188 (18.4%) were hospitalized for heart failure, and 396 (38.7%) died. Compared to participants without AP, participants with daily or weekly AP were more likely to be hospitalized for AP, undergo revascularization, be hospitalized for heart failure, and die (Figure 1 and Table 3). Participants with daily or weekly AP were also more likely to be hospitalized for MI, but this was not statistically significant.
Figure 1.
Cardiovascular events by angina pectoris frequency in 1023 participants.
Table 3. Risk of subsequent cardiovascular events among participants with daily or weekly (vs. no) reported angina.
| Hospitalization
for Angina |
Revascularization |
Myocardial Infarction |
Heart Failure |
Death |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI)a |
P- value |
HR (95%CI)a |
P- value |
HR (95%CI)a |
P- value |
HR (95%CI)a |
P- value |
HR (95%CI)a |
P- value |
|
| Unadjusted | 3.3 (2.3, 4.7) | <.001 | 2.0 (1.3, 2.9) | 0.001 | 1.3 (0.8, 2.3) | 0.29 | 1.6 (1.0, 2.5) | 0.03 | 1.5 (1.1, 2.0) | 0.01 |
| Model 1 b | 2.5 (1.7, 3.7) | <.001 | 1.8 (1.1, 2.7) | 0.01 | 1.0 (0.5, 1.7) | 0.87 | 1.3 (0.8, 2.1) | 0.25 | 1.5 (1.1, 2.0) | 0.02 |
| Model 2 c | 2.4 (1.6, 3.6) | <.001 | 1.7 (1.1, 2.7) | 0.01 | 1.0 (0.5, 1.7) | 0.88 | 1.2 (0.7, 2.0) | 0.47 | 1.4 (1.0, 1.9) | 0.08 |
| Model 3 d | 2.4 (1.6, 3.6) | <.001 | 1.7 (1.1, 2.7) | 0.02 | 0.9 (0.5, 1.7) | 0.85 | 1.3 (0.8, 2.1) | 0.32 | 1.4 (1.0, 2.0) | 0.045 |
Hazard Ratio (HR) and 95% confidence interval (CI) for cardiovascular events in participants with daily or weekly angina compared to participants without angina at baseline.
Model 1 adjusts for age, sex, smoking, hypertension history, heart failure history, body mass index, diastolic blood pressure, beta-blocker use, calcium-channel blocker use, and nitrate use.
Model 2 adjusts for the factors in Model 1 + depressive symptoms.
Model 3 adjusts for the factors in Model 2 + treadmill exercise capacity, left ventricular ejection fraction, and inducible ischemia.
After adjusting for clinical risk factors (age, sex, smoking, hypertension history, heart failure history, body mass index, diastolic blood pressure, beta-blocker use, calcium-channel blocker use, and nitrate use), depressive symptoms, treadmill exercise capacity, ejection fraction, and inducible ischemia, participants with daily or weekly angina had more than twice the risk of hospitalization for AP, 70% higher risk of revascularization, and 40% higher risk of death (Table 3). However, AP was not independently associated with MI or heart failure after adjustment for objective measures of CHD and depressive symptoms.
When AP frequency by SAQ was considered as a continuous variable (score from 0 to 100, with 100 representing no AP), higher AP frequency was associated with higher rates of AP hospitalization (HR 1.6, 95%CI 1.4-1.8, p<.001), revascularization (HR 1.4, 95%CI 1.2-1.6, p<.001), and heart failure (HR 1.2, 95%CI 1.0-1.4, p=0.02), but not significantly associated with myocardial infarction (HR 1.2, 95%CI 1.0-1.4, p=0.07) or death (HR 1.1, 95%CI 1.0-1.4, p=0.09). After adjusting for clinical risk factors, depressive symptoms, treadmill exercise capacity, ejection fraction, and inducible ischemia, each 20 unit worsening in AP frequency score predicted a 40% higher risk of AP hospitalization (adjusted HR 1.4, 95%CI 1.2-1.7, p<.001) and a 30% higher risk of revascularization (adjusted HR 1.3, 95%CI 1.1-1.5, p=0.002), but was not significantly independently associated with myocardial infarction, heart failure, or death.
Greater physical limitation due to AP, measured by the SAQ physical limitation scale (score from 0 to 100, with 100 representing no physical limitation due to AP), was also associated with higher rates of cardiovascular events (Table 4). After adjusting for clinical risk factors, depressive symptoms, treadmill exercise capacity, EF, and inducible ischemia, each 20 unit worsening in physical limitation due to AP predicted a 20% higher risk of AP hospitalization, a 20% higher risk of revascularization, a 20% higher risk of heart failure hospitalization, and a 10% higher risk of death (Table 4).
Table 4. Risk of cardiovascular events by physical limitation entered as a continuous variable.
| Hospitalization
for Angina |
Revascularization |
Myocardial Infarction |
Heart Failure |
Death |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI)a |
P- value |
HR (95%CI)a |
P- value |
HR (95%CI)a |
P- value |
HR (95%CI)a |
P- value |
HR (95%CI)a |
P- value |
|
| Unadjusted | 1.4 (1.2, 1.6) | <.001 | 1.2 (1.1, 1.4) | 0.001 | 1.2 (1.1, 1.4) | 0.003 | 1.5 (1.4, 1.7) | <.001 | 1.3 (1.2, 1.4) | <.001 |
| Model 1 b | 1.3 (1.1, 1.4) | 0.001 | 1.2 (1.0, 1.4) | 0.008 | 1.1 (1.0, 1.3) | 0.11 | 1.4 (1.2, 1.6) | <.001 | 1.3 (1.2, 1.4) | <.001 |
| Model 2 c | 1.3 (1.1, 1.4) | 0.001 | 1.2 (1.0, 1.4) | 0.02 | 1.2 (1.0, 1.4) | 0.09 | 1.3 (1.2, 1.6) | <.001 | 1.3 (1.2, 1.5) | <.001 |
| Model 3 d | 1.2 (1.1, 1.4) | 0.004 | 1.2 (1.0, 1.4) | 0.03 | 1.1 (0.9, 1.3) | 0.46 | 1.2 (1.0, 1.4) | 0.04 | 1.1 (1.0, 1.3) | 0.01 |
Hazard Ratio (HR) and 95% confidence interval (CI) for cardiovascular events per 20 unit decrease (worsening) in Seattle Angina Questionnaire Physical Limitation scale (1-100)
Model 1 adjusts for age, sex, smoking, hypertension history, heart failure history, body mass index, diastolic blood pressure, beta-blocker use, calcium-channel blocker use, and nitrate use.
Model 2 adjusts for the factors in Model 1 + depressive symptoms.
Model 3 adjusts for the factors in Model 2 + treadmill exercise capacity, left ventricular ejection fraction, and inducible ischemia.
We conducted a sensitivity analysis for the association of SAQ AP frequency and SAQ physical limitation with outcomes by including physical inactivity in the fully adjusted model, but found that the additional inclusion of physical inactivity did not meaningfully change the point estimates or conclusions.
Discussion
In this study of 1023 patients with CHD, we found that self-reported AP was predictive of future AP hospitalization, revascularization, and death during 8.9 years of follow-up. This association was independent of clinical risk factors, depressive symptoms, and objective measures of disease severity, including treadmill exercise capacity, ejection fraction, and inducible ischemia. These findings underscore the importance of considering patient-reported symptoms in the care of CHD patients because self-reported AP frequency is not only important to patients but also captures elements of risk not otherwise identified by objective measures.
Our results extend the findings of prior reports in several important ways. First, we showed that daily or weekly AP was predictive of future hospitalization for AP, revascularization, and death, independent of objective measures of disease severity. Prior studies have found that AP is associated with mortality [7-9] but have not been able to quantify the risk of future hospitalization for AP or revascularization independent of objective measures of cardiac disease severity.
Second, we found that exercise treadmill testing did not induce AP or ischemia in most participants who reported frequent AP, and most participants with inducible ischemia did not report frequent AP on the SAQ. Exercise stress testing has imperfect sensitivity and specificity for obstructive coronary artery disease[10], and also appears to have limited correlation with patient-reported symptoms. This demonstrates the importance of assessing both patient-reported and objective measures of cardiac disease severity. We have demonstrated that measurement of patient-reported AP frequency, an inexpensive measure, provides independent prognostic information about AP hospitalization, revascularization, and death. Measurement of patient-reported health status is increasingly recognized as an important tool in the care of patients with CHD [11, 12], and our findings provide additional evidence for the importance of patient-reported symptoms.
Third, we noted that AP frequency was associated with depressive symptoms, and that adjusting for clinical risk factors and depressive symptoms reduced the association between AP frequency and cardiovascular events. Self-reported AP frequency is known to be associated with depressive symptoms [13-15], and depressive symptoms are associated with adverse cardiovascular events, largely due to behavioral factors and physical inactivity [1]. However, few previous studies have been able to account for the effects of depressive symptoms. Our findings suggest that while depressive symptoms explain some of the association between AP frequency and outcomes, that AP frequency remains predictive of outcomes beyond the influence of depressive symptoms.
Finally, many patients with AP limit their physical activity in response to symptoms of AP. Physical limitation due to AP is associated with mortality [16]. We demonstrated that in our cohort, physical limitation due to AP is associated with cardiovascular events. In addition, we demonstrated that adjusting for objective measures of cardiac disease severity, including exercise capacity, EF, and inducible ischemia did not diminish the association between AP frequency and death or hospitalization for AP or revascularization. Together, this suggests that while physical limitation due to AP does impact outcomes, that considering the results of formal exercise testing does not diminish the association between patient-reported AP frequency and outcomes
Our findings have limitations. First, most participants were urban men, which limits generalizability. AP frequency has also been associated with poorer outcomes in women [8], and women and men with stable AP have similar overall event rates of events [17]. Second, this study was not designed to determine the effects of treatment strategies on outcomes. The effectiveness of specific treatment strategies for improving outcomes by reducing AP frequency merits further study. Third, we analyzed outcomes with regard to AP measured at the beginning of the study, which does not account for the influence of subsequent changes in AP symptoms. Finally, as with all observational studies, we cannot exclude the possibility of residual confounding by unmeasured factors. However, we had very detailed data about the patients.
Research Highlights.
In patients with stable ischemic heart disease, patient-reported angina frequency predicts hospitalization for angina, revascularization, and death.
This association persists after accounting for clinical risk factors and objective measures of disease severity, including exercise stress testing.
This supports the inclusion of patient-reported angina frequency in research and clinical practice.
Acknowledgements
Alexis Beatty is supported by Award Number KL2TR000143 from the National Center for Advancing Translational Sciences. The Heart and Soul Study was supported by grants from the Department of Veterans Affairs (Epidemiology Merit Review Program), the National Heart, Lung and Blood Institute (R01 HL079235), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program) and the Ischemia Research and Education Foundation. This research is supported by an investigator-initiated grant from Gilead Sciences. None of these funding sources had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest
Dr. Beatty has no conflicts of interest to disclose. Relevant to this project, Dr. Spertus discloses that he owns the copyright to the Seattle Angina Questionnaire and has received research grant support from Gilead Sciences. Dr. Whooley has received research support from Gilead Sciences, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 3.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 4.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American College of Sports Medicine Guidelines for Exercise Testing and Prescription. 6 th ed. Lippincott Williams & Wilkins; Baltimore, MD: 2000. p. 380. [Google Scholar]
- 6.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 7.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 8.Berecki-Gisolf J, Humphreyes-Reid L, Wilson A, Dobson A. Angina symptoms are associated with mortality in older women with ischemic heart disease. Circulation. 2009;120:2330–2336. doi: 10.1161/CIRCULATIONAHA.109.887380. [DOI] [PubMed] [Google Scholar]
- 9.Califf RM, Mark DB, Harrell FE, Jr., Hlatky MA, Lee KL, Rosati RA, Pryor DB. Importance of clinical measures of ischemia in the prognosis of patients with documented coronary artery disease. J Am Coll Cardiol. 1988;11:20–26. doi: 10.1016/0735-1097(88)90160-x. [DOI] [PubMed] [Google Scholar]
- 10.Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG, American Society of E American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Jr., Smith SC, Jr., Spertus JA, Williams SV, Anderson JL, American College of Cardiology Foundation/American Heart Association Task F ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;2012;126:e354–471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 12.Rumsfeld JS, Alexander KP, Goff DC, Jr., Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat-Jacobson D, Zerwic JJ, American Heart Association Council on Quality of C. Outcomes Research CoC. Stroke Nursing CoE. Prevention CoPVD. Stroke C. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–2249. doi: 10.1161/CIR.0b013e3182949a2e. [DOI] [PubMed] [Google Scholar]
- 13.Rumsfeld JS, Magid DJ, Plomondon ME, Sales AE, Grunwald GK, Every NR, Spertus JA. History of depression, angina, and quality of life after acute coronary syndromes. Am Heart J. 2003;145:493–499. doi: 10.1067/mhj.2003.177. [DOI] [PubMed] [Google Scholar]
- 14.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold SV, Spertus JA, Ciechanowski PS, Soine LA, Jordan-Keith K, Caldwell JH, Sullivan MD. Psychosocial modulators of angina response to myocardial ischemia. Circulation. 2009;120:126–133. doi: 10.1161/CIRCULATIONAHA.108.806034. [DOI] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Bryson CL, Spertus JA, McDonell MB, Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015–1022. doi: 10.1016/S0002-8703(03)00436-8. [DOI] [PubMed] [Google Scholar]
- 17.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–1411. doi: 10.1001/jama.295.12.1404. [DOI] [PubMed] [Google Scholar]