Abstract
The polyglutamine expansion within huntingtin is the causative factor in the pathogenesis of Huntington’s disease (HD). Although the underlying mechanisms by which mutant huntingtin causes neuronal dysfunction and degeneration have not been fully elucidated, the compelling evidence suggests that mitochondrial dysfunction and compromised energy metabolism are key players in HD pathogenesis. Longitudinal studies of HD subjects have shown that reductions in glucose utilization before the disease clinical onset. Preferential striatal neurodegeneration, a hallmark of HD pathogenesis, has also been associated with interrupted energy metabolism. Data from genetic HD models indicate that mutant huntingtin disrupts mitochondrial bioenergetics and prevents ATP generation, implying altered energy metabolism as an important component of HD pathogenesis. Here we revisit the evidence of abnormal energy metabolism in the central nervous system of HD patients, overview our current understanding of the molecular mechanisms underlying abnormal metabolism induced by mutant huntingtin, and discuss the promising therapeutic development by halting abnormal metabolism in HD.
Keywords: Energy metabolism, mitochondria, AMPK, PGC-1α, Sirtuins, Huntington’s disease
Introduction
Huntington disease (HD) is an autosomal dominant neurodegenerative disease which is caused by a CAG repeat expansion in the first exon of the huntingtin gene that encodes huntingtin (Htt) protein1,2. Individuals who have 36 CAG repeats or more in the huntingtin gene develop the clinical symptoms, including motor, psychiatric, and cognitive abnormalities that cause a progressive loss of functional capacity and shortened life span. HD patients also exhibit profound metabolic abnormalities3,4. At present, no pharmacological interventions are available to delay the onset or reverse progression of this devastating disease.
Despite remarkable progress in the understanding of the process underlying HD pathogenesis, the molecular mechanisms by which mutant Htt (mHtt) causes disease progression remain uncertain. Compelling data from studies in human HD subjects and experimental HD models suggest that deficits in energy metabolism, due to mitochondrial dysfunction, play a critical role in promoting HD pathogenesis 5–7. The nature and cause of mitochondria dysfunction in HD is multifactorial, involving direct mHtt-mitochondria interactions and indirect effects via transcriptional dysregulation and trafficking impairment, which compromise mitochondria bioenergetics and dynamics 7. Neurons are highly dependent on mitochondria ATP production and Ca2+ buffering to maintain excitability and normal synaptic communication 8. Moreover, neurons require efficient biogenesis and mitophagy to renew or adapt mitochondria levels throughout their lifespan 9,10. Therefore, neurons are very sensitive to disturbed energy metabolism.
Here we provide an overview on evidence of metabolism deficits in HD brain and discuss the possible molecular mechanisms underlying the abnormal metabolism in the context of both a toxic gain-of-function from mHtt and the loss of function of normal Htt. The understanding the molecular mechanisms that drive to abnormal metabolism and neurodegeneration should open new avenues for developing promising therapeutic approaches to preserve neuronal function and prevent neurodegeneration in HD.
Deficits in energy metabolism are detected in human HD brain
Studies of cerebral glucose metabolism using F-18 fluorodeoxyglucose positron emission tomography (FDG-PET) provide strong evidence for an impairment of energy metabolism in the caudate putamen and cortex of presymptomatic HD patients 11,12. Powers and colleagues investigated mitochondrial oxidative metabolism in the striatum of pre-symptomatic HD subjects by direct measurements of the molar ratio of cerebral oxygen metabolism to cerebral glucose metabolism with positron emission tomography and observed selective defect of glycolysis in early HD striatum13, these data suggest that metabolic deficit is an early event in HD and metabolic impairment precedes neuropathology and clinical symptoms in HD patients. A further study showed that impaired basal ganglia metabolism is highly correlated with the functional capacity of HD patients and the degree of their motor dysfunction14. Using magnetic resonance spectroscopy (MRS) imaging, increased lactate levels were observed in the striatum and occipital cortex of HD patients 15, it may reflect inefficient oxidative phosphorylation which leads to accumulation of lactate from pyruvate via lactate dehydrogenase. In contrast, a MRS study of cerebrospinal fluid from HD patients showed reduced levels of both lactate and citrate may indicate an impairment of both glycolysis and tricarboxylic acid cycle function in HD patients16.. Various mechanisms that underlie the energy metabolic deficits in HD brain have been proposed, including inhibition of mitochondrial complex II, compromised mitochondrial calcium handling 17,18, increased oxidative stress 19, dysfunctional mitochondrial bioenergetics 20–22, abnormal mitochondria trafficking22–24, deregulation of key factors controlling mitochondrial biogenesis 25, and deregulated kinases such AMP-activated protein kinase26 and creatine kinase27.
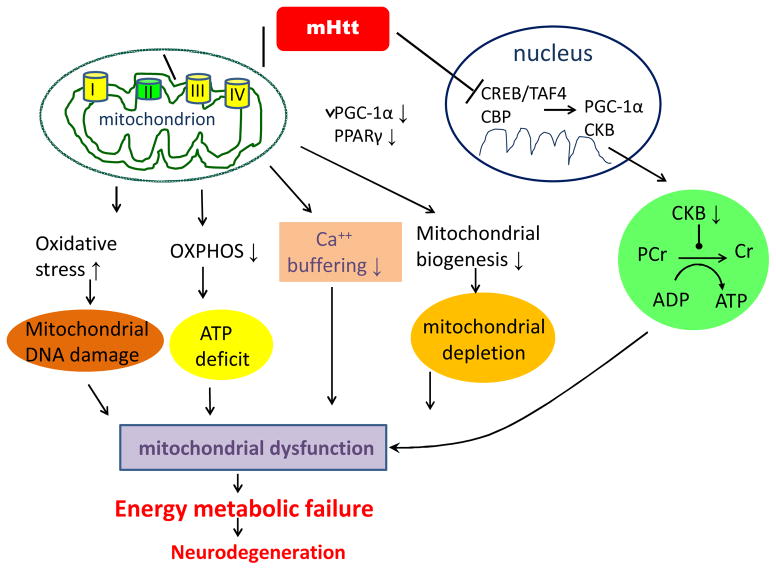
Molecular mechanisms underlying abnormal metabolism in HD (Summarized in Figure 1)
Figure 1.
Summary figure indicating the putative molecular mechanism by which mutant huntingtin (mHtt) induces energy metabolic failure and neurodegeneration in HD.
Inhibition of mitochondrial complex II by mHtt
Postmortem studies show marked deficiency of mitochondrial complex II in the striatum of HD subjects28–30. Cultured striatal neurons transfected with N-terminus mHtt showed decreased complex II enzymatic activity associated with selective depletion of SDH, and overexpression of complex II/SDH subunits protected cells in this model31. Furthermore, expression of full-length mHtt in immortalized striatal progenitor cells (derived from the HdhQ111 knock-in mouse model) decreases complex II activity and increases the sensitivity of cells to calcium-induced decreases in oxygen consumption and mitochondrial membrane potential, whereas overexpression of complex II prevents mitochondrial dysfunction and cell death 31,32.
mHtt disrupts Ca 2+ buffering capacity in mitochondria
Mitochondria play an important role in buffering changing cytoplasmic calcium levels in response to neuronal activity 33. Mitochondrial calcium transport is powered by the mitochondrial proton gradient, and increased neuronal calcium modifies mitochondrial ATP production by uncoupling oxidative phosphorylation (OXPHOS). Calcium overload may result in discharge of the mitochondrial membrane potential, opening of the mitochondrial permeability transition (MPT) pore, release of cytochrome c, and activation of cell death pathways 33. Mitochondria isolated from lymphoblast cells of HD patients and HD mouse brain have a reduced membrane potential and depolarize at lower calcium concentrations than control mitochondria20. The reduced mitochondrial ATP levels and decreased ATP/ADP ratio found in mHtt-containing striatal cells is linked to increased calcium influx through NMDA receptors, and cell ATP/ADP ratio is normalized by blocking calcium influx34. It was reported that both wild Htt and mHtt bind to the outer mitochondrial membrane in human neuroblastoma cells and cultured striatal cells18. Mitochondria incubated with mHtt had increased sensitivity to calcium-induced opening of the MPT and release of cytochrome c, consistent with a direct effect of mHtt on mitochondrial calcium handling18. It is intriguing to note that striatal mitochondria contain more cyclophilin D than cortical mitochondria and are more sensitive to calcium-induced MPT pore opening35.
mHtt increases oxidative stress
Evidence of enhanced oxidative stress in HD brain includes an increase in accumulation of lipofuscin, a product of unsaturated fatty acid peroxidation that is most prominent in vulnerable striatal neurons30. A significantly increased 8-hydroxydeoxyguanosine (8-OHdG), an oxidized DNA marker, has also been shown in the caudate of HD patients30. In addition, a significant increase in 8-OHdG in mitochondrial DNA of the parietal cortex was found in late stage (Vonsattel grade 3–4) of HD patients. Similarly, 8-OHdG was higher in forebrain tissue and striatum of R6/2 mice at 12 and 14 weeks of age36,37. Increased oxidative damage to DNA is also detected outside the brain of HD patients38,39. Oxidative modification of proteins and lipids is increased in the brain of HD subjects and animal models30,38. Another indicator of increased oxidative stress is the finding that oxidative defense mechanisms including mitochondrial and cytoplasmic superoxide dismutase (MnSOD and Cu-Zn SOD, respectively) are induced in HD brains40 and transgenic HD mice41. Oxidative stress could promote mHtt aggregation and mHtt-induced cell death by impairing proteasome function42. These results support the hypothesis on increased oxidative damage in HD.
mHtt impairs mitochondrial bioenergetics
Reduced ATP/ADP ratio is a consequence of mHtt, which has been shown in the striatum of mHtt knock-in mice43, HD postmortem brains44, and the lymphoblasts of HD patients34. Increased carbonylation of the mitochondrial enzymes results in decreased mitochondrial enzyme activity and then impaired energy production has been evident in the striatum of HD mice45. Further evidence supporting defective mitochondrial bioenergetics in HD is revealed by the direct interaction of mHtt with brain mitochondria18,20–22. The localization of mHtt to brain mitochondria correlates with age and disease progression in an HD knock-in mouse model22, and results in reduction of mitochondrial ATP generation22.
Mitochondrial spare respiratory capacity is a measure of the ability of mitochondria to generate energy beyond that required for sustaining the basic metabolic needs of the cells, and is important for maintaining homeostasis and survival of neurons. A significant reduction in mitochondrial spare respiratory capacity was reported in human HD fibroblasts and immortalized mHtt expressing mouse striatal cells compared to wild-type cells46, indicating that mitochondrial bioenergetics is compromised by mHtt, and supporting a toxic role of mHtt on mitochondrial bioenergetics. Induced pluripotent cells (iPSc) from HD subjects also have reduced mitochondrial spare respiratory capacity and ATP production (unpublished data, personal communication with Dr. Leslie Thompson). Despite these significant observations, it remains unclear how mHtt leads to impaired mitochondrial bioenergetics.
mHtt interrupts mitochondrial trafficking and dynamics
Altered mitochondrial trafficking precedes neuronal dysfunction in both in vitro and in vivo models of HD23. Full-length mHtt may impair mitochondrial motility in mammalian neurons through both a toxic gain of function from the polyglutamine tract and a loss of function of normal Htt 23. In addition, it was found that mHtt altered the distribution and reduced the transport rate of mitochondria in the synaptosomes isolated from mHtt knock-in mouse brain 22. However, the mechanisms by which mHtt affects intracellular mitochondrial trafficking are not fully understood 24. A study of HD brains identified a reduced number and altered distribution of mitochondria within vulnerable, calbindin-positive striatal neurons that was more pronounced with disease progression47. Reductions of mitochondria were seen preferentially in large- and medium-size mitochondria in conjunction with an increase in levels of dynamin-related protein 1 (Drp1) protein, a mediator of mitochondrial fission, and a decrease in levels of mitofusion 1 (Mfn1), a mediator of mitochondrial fusion 47. Kim and colleagues also demonstrated that reductions in peroxisome proliferator-activated receptor (PPAR) gamma coactivator 1 alpha (PGC-1α), a principal regulator of energy metabolism, in HD postmortem brain tissue correlate with reductions in numbers of mitochondria 47. These results support the view that altered mitochondrial dynamics represent an important mechanism of mitochondrial dysfunction, and these abnormalities contribute to the mismatch between energy supply and demand that is a recurring event in HD. Interestingly, mHtt has been shown to interact with Drp1, leading to defective mitochondrial axonal transport and synaptic degeneration in postmortem HD brains and primary neurons from transgenic HD mice48,49.
mHtt reduces PGC-1α and mitochondrial biogenesis
PGC-1α regulates the expression of genes involved in mitochondrial biogenesis and energy homeostasis25,50. PGC-1α interacts with a number of transcription factors, including NRF-1 and NRF-2 which regulate the expression of mitochondrial respiratory genes 51. PGC-1α knockout mice exhibit defects in energy metabolism52, indicating that PGC-1α plays a central role in modulating energy metabolism. The expression of PGC-1α is repressed by mHtt, due in part to the fact that mHtt interferes with the TAF4/CREB signaling pathway25. In addition, reduced CREB phosphorylation and CRE signaling in mHtt-expressing striatal cells may also contribute to the downregulation of PGC-1α expression 43. PGC-1α target genes are decreased in the postmortem human HD brain 25 and myoblasts from HD patients53. Moreover, PGC-1α mRNA and protein levels are significantly decreased in the brain, liver, brown adipose tissue, muscle of mHtt knock-in mice or HD transgenic mice53,54, as well in the STHdhQ111/111 cell line25. Crossing of PGC-1α knockout mice with HD knock-in mice resulted in increased neurodegeneration of striatal neurons and motor abnormalities in the HD mice25. Additionally, expression of PGC-1α partially protects against the toxic effects of mHtt in cultured striatal neurons25. Overall, these data indicate that there is defective PGC-1α functioning and therefore downstream events are likely impaired in HD. mHtt could interfere with transcription of PGC-1α-regulated genes including PGC-1α itself and its target genes which has been shown in the striatum of HD N171-82Q mice and human HD patients55. The transcript of PPARγ, a transcription factor that is critical for energy homeostasis, was also downregulated in multiple tissues of an HD mouse model and HD patients56. Recently, Soyal and colleagues identified brain- specific isoforms of PGC-1α, including full-length and truncated isoforms, this study indicated differences in regulation of isoform-specific transcripts of PGC-1α as well as potential functional differences between full-length and truncated isoforms, suggesting that newer treatments may be fine-tuned to target the brain-specific isoforms57.
mHtt alters AMPK activity
AMPK is a Ser/Thr kinase and major energy sensor that maintains cellular metabolic homeostasis and stimulates pathways that promote energy production or inhibit energy expenditure in response to metabolic stress 58,59. AMPK comprises three subunits (α, β, and γ) 60,61, α subunit is the catalytic subunit and has two different isoforms (α1 and α2) 62. AMPK activation is known to be associated with a pro-survival role against certain stresses, but the roles and regulation of AMPK in HD pathogenesis are not fully understood. It has been shown that systemic activation of AMPK by anti-diabetic drug metformin extended the shortened lifespan and reduced hindlimb clasping in an HD mouse model63. We recently found that activation of AMPK exhibited a dynamic pattern in an HD cell model, the cells expressing full-length mHtt displayed increased activation of AMPK at early pathogenic phase and then decreased activation of AMPK at late pathogenic phase; moreover, metformin activated AMPK and protected these cells from mHtt toxicity (Jin. et al unpublished data). Additional experiments are needed to evaluate whether the protective effects of metformin are due to AMPK activation in HD models. This is an important issue because AMPK activation may provide distinct functions in different disease stages. It has been reported that overactivation of AMPK-α1 was found in brains of postmortem HD brain and a fragment HD mouse model26,64, and intrastriatal infusion of AMPK agonist AICAR worsened the motor impairment and neurodegeneration of R6/2 mice 26. However, AMPK activation might be a double-sided sword in HD, early activation may represent a compensatory response to energy deficits induced by mHtt and promote cell survival, but overactivation of AMPK, particularly AMPK-α1, at late stage might be detrimental to cells.
mHtt decreases creatine kinase
The creatine kinase (CK)/phosphocreatine (PCr) system is one of the major machineries controlling proper energy utilization in cells. There are two cytosolic CKs, brain-type CK (CKB) and muscle-type CK (CKM)65. Increased PCr concentrations and decreased CKB activities were demonstrated in brains of several HD mouse models (R6/2, N171-82Q, and HdhQ111) and HD patients44,66. Downregulation of CKB transcripts in the brain of HD mice was also demonstrated by a microarray analysis67, suggesting that mHtt might also regulate CKB at the transcriptional level. Moreover, a poor CK/PCr system in HD brains is associated with a reduced ATP/ADP ratio and impaired energy homeostasis, using a microwave fixation method, accumulation of PCr and depletion of ATP were demonstrated in brains of HD mice at an early disease stage44.
Besides affecting cellular energy homeostasis, suppression of CKB in HD might also compromise functions of its interacting proteins. One intriguing example is that K-Cl co-transporter 2 (KCC2), which directly binds to CKB and is highly expressed in GABAergic neurons which are selectively vulnerable in HD, was reported to promote spine formation68. It would be of great interest to investigate whether inhibition of CKB might account for the loss of spine density 69 in HD mice. The detailed mechanism that mediates the suppression of CKB by mHtt is largely uncharacterized. Because CKB is very sensitive to oxidative stress27,70,71, mHtt enhance ROS production 30,72, and ROS are likely to mediate suppression of CKB in HD.
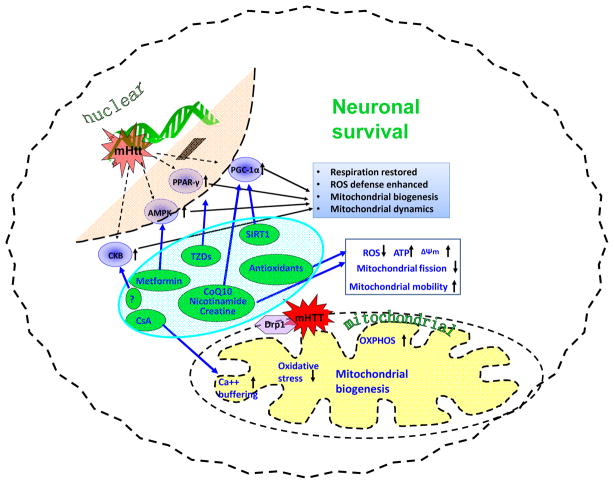
Therapeutic approaches by targeting defective metabolism in HD (Summarized in Figure 2)
Figure 2.
Potential therapeutic approaches by targeting mitochondrial dysfunction and improving energy metabolism in HD.
Targeting impaired bioenergetics and metabolism is a beneficial strategy to delay onset given before symptoms begin and prevent/slow disease progression given after onset of disease in HD. A number of promising therapeutics with a particular emphasis on halting mitochondrial dysfunction and improving energy metabolism are developing for treatment of HD, including compounds in preclinical development stage, such as mPTP opening inhibitor, sirtuins modulators, and PPAR activators; and compounds in clinical trials, such as CoQ10 and creatine.
mPTP opening inhibitor
It has been suggested that the neuroprotective properties of cyclosporine A (CsA) are due in part to its ability to prevent mitochondrial permeability transition pore (mPTP) opening in response to high levels of calcium or oxidative stress 73,74. Exposure to high levels of calcium or oxidative stress results in the mPTP opening of the inner mitochondrial membrane, causing disruption and swelling of mitochondria74–76. CsA significantly attenuated NMDA-induced calcium peak and loss in the medium-size spiny neurons of YAC128 HD mice77. Treatment with CsA protected striatal neurons toxicity induced by 3-NP in vitro and in vivo 75. Although CsA is beneficial in HD models, its may not be an idea candidate for chronic treatment in HD due to its powerful immune suppressing effect. Therefore, developing more specific mPTP opening inhibitors is of potential therapeutic benefit by protecting vulnerable neurons populations affected in HD.
Sirtuin modulators
Sirtuins represent a promising new class of conserved deacetylases that play an important role in regulating metabolism78. Seven members (SIRT1-7) were identified in mammalian sirtuin family; the role of a few sirtuin members has being explored in HD. SIRT1 deacetylates PGC-1α at specific lysine residues79, resulting in increased transcriptional activity of PGC-1α and expression of its target genes. We and others have shown that genetically increasing SIRT1 is neuroprotective in several HD transgenic mouse models, whereas a deficiency of SIRT1 exacerbates the HD phenotype80,81. An approach to activating SIRT1 is to increase nicotinamide adenine dinucleotide (NAD+) levels by administration of nicotinamide precursors 82.
Interestingly, nicotinamide improved the motor phenotype in HD mice83. Activation of SIRT3 increases superoxide dismutase 2 activity and enhances antioxidant efficiency. We previously reported that trans-(−)-ε-Viniferin, a compound isolated from natural product, increased SIRT3 levels and protected cells from mHtt toxicity 84. Resveratrol (a SIRT1 activator and antioxidant) rescued early neuronal dysfunction induced by mHtt expression in Caenorhabditis elegans85 and protected against peripheral deficits in HD mice86. In others studies, treatment with resveratrol significantly increased the aerobic capacity in mice87. These effects were explained by the fact that in addition to being an antioxidant, resveratrol activates SIRT1 and thus induction of mitochondrial biogenesis which improved mitochondrial function87. These and other findings suggest that increase in SIRT1 activity in HD could improve mitochondrial function and maintain metabolism homeostasis in HD. However, the specificity of resveratrol on SIRT1 activity was questioned. Recently, it was reported that SIRT1 can be directly activated through an allosteric mechanism common to chemically diverse sirtuin-activating compounds (STACs), and more specific STACs has been identified by using this system88,89. We found that a specific STAC identified by this new system prolonged survival and improved motor function in an HD mouse model (Jiang et al., unpublished data). In contrast, Smith and colleagues reported that Selisistat, a SIRT1/Sir2 inhibitor, alleviates pathology in Drosophila and mammalian cell and mouse models of HD90. It is intriguing that NF-κB p65 subunit, a reported SIRT2 substrate91, was deacetylated by Selisistat at the concentrations used in this HD study90. It is interesting to know whether Selisistat at the concentrations used in this study also inhibits SIRT2, because SIRT2 inhibition was reported to provide protective effects in an HD model 92. The controversial results urge us to further explore the role of sirtuin(s) in different disease stage and develop more specific sirtuin modulators.
PPAR activators
PGC-α is a potent co-activator of the type II nuclear receptor PPAR. Administration of the pan-PPAR agonist, bezafibrate, was efficacious in improving rotarod performance and survival, and atrophy of the striatal medium spiny neurons in HD mice 93. Bezafibrate treatment produced a significant increase in numbers of mitochondria in the striatal spiny neurons in R6/2 HD mice 93. In addition, PPAR γ agonist rosiglitazone penetrates blood-brain barrier and improves mitochondrial biogenesis in the brain94. The PPARγ signaling pathway was significantly impaired in the mHtt expressing striatal cells95. Pretreatment of mHtt expressing cells with the rosiglitazone prevented the loss of mitochondrial calcium deregulation and oxidative stress overproduction in response to thapsigargin95. We demonstrated that rosiglitazone improved motor function in HD mice96. These findings suggest that PPAR agonists could be considered in the treatment of HD.
coenzyme Q10 (CoQ10) is critically involved in the electron transport chain97 and exerts beneficial effects in mouse models of HD98–101, though controversial results were also reported in HD models102 In patients with manifest HD, 600 mg daily of CoQ10 combined with remacemide (CARE-HD) showed a trend toward slowing HD progression with CoQ10 treatment103. Subsequently, another study of CoQ10 (Pre2CARE) in manifest HD and healthy controls demonstrated a relative plateau in plasma CoQ10 levels above 2,400 mg daily104. The shorter duration of trials, compared with typical duration of disease progression, might affect the clinical effects. Moreover, it would be better to identify at-risk subjects and start the treatments earlier. The failure of the CoQ10 treatment may, in part, be due to difficulty reversing the considerable damage needed to cause clinically significant symptoms. As a consequence, a second phase III clinical trial examining the efficacy of CoQ10 at a dose of 2,400 mg daily (2CARE) is presently being carried out by the Huntington Study Group. This trial is enrolling Six hundred eight research participants randomized to CoQ10 or placebo followed for 5 years, with the primary outcome of change in total functional capacity. The 2CARE study will be the largest therapeutic clinical trial to date in HD.
Creatine
Dietary creatine supplementation (1–3%) was shown to delay disease progression by improving aggregate formation, weight loss, impaired motor coordination, brain atrophy, lifespan, and hearing loss in HD mice 105,106. Nonetheless, results from human trials on dietary creatine supplementation (5–10 g/day) in HD patients have not been very promising to date 107. Considering the low permeability of the blood-brain barrier to creatine108, one possible solution is to further increase dosages of dietary creatine in human trials. A phase II prevention and biomarker trials of creatine in at risk HD, neuroimaging demonstrated treatment-related slowing of cortical and striatal atrophy at 6 and 18 months, but the slowing of brain atrophy with creatine treatment was not associated with any clinical benefit109. A phase III clinical trial of high-dose creatine (40 g/day) for HD patients is currently recruiting participants (CREST-E), the results from the trial will provide further information
Concluding remarks
A deficit in energy metabolism is a prominent feature of HD. The early onset of metabolism-related manifestations in the presymptomatic stage suggests that metabolic deficit occurs in the early cascade of events leading to HD pathogenesis. There is substantial evidence that metabolic alterations associated with mHtt play an important role in HD pathogenesis. Eventually, the ability to monitor disease progression may allow us to treat patients before disease onset and intervene with neuroprotective treatments in order to slow or prevent disease progression. Studies of energy metabolism in HD are therefore having high potential to identify therapeutic targets as well as develop biomarkers. A deeper understanding of mitochondrial biology and correspondingly impaired energy metabolism induced by mHtt will be necessary to develop meaningful therapies to treat HD. As it has been more than 20 years since the discovery of mHtt, an important objective for the next decade of research on HD is to develop interventions that will prevent or slow the progression of these diseases. A full understanding of how mHtt induces compromised metabolism could be an important step in unlocking novel targets and pathways amenable to directed therapy development. Treatments designed to improve energy metabolism are likely to delay onset and slow the pace of disease progression significantly.
Acknowledgments
We thank the following for financial support: NIH NS074196, NS082338 and CHDI Foundation (to WD)
Footnotes
The authors declare no conflict of interest.
References
- 1.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Gusella JF, et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature. 1983;306:234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- 3.Djousse L, et al. Weight loss in early stage of Huntington’s disease. Neurology. 2002;59:1325–1330. doi: 10.1212/01.wnl.0000031791.10922.cf. [DOI] [PubMed] [Google Scholar]
- 4.Martin JB, Gusella JF. Huntington’s disease. Pathogenesis and management. The New England journal of medicine. 1986;315:1267–1276. doi: 10.1056/NEJM198611133152006. [DOI] [PubMed] [Google Scholar]
- 5.Damiano M, Galvan L, Deglon N, Brouillet E. Mitochondria in Huntington’s disease. Biochimica et biophysica acta. 2010;1802:52–61. doi: 10.1016/j.bbadis.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira JM. Mitochondrial bioenergetics and dynamics in Huntington’s disease: tripartite synapses and selective striatal degeneration. Journal of bioenergetics and biomembranes. 2010;42:227–234. doi: 10.1007/s10863-010-9287-6. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira JM. Nature and cause of mitochondrial dysfunction in Huntington’s disease: focusing on huntingtin and the striatum. Journal of neurochemistry. 2010;114:1–12. doi: 10.1111/j.1471-4159.2010.06741.x. [DOI] [PubMed] [Google Scholar]
- 8.Kann O, Kovacs R. Mitochondria and neuronal activity. American journal of physiology Cell physiology. 2007;292:C641–657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Human molecular genetics. 2009;18:R169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochimica et biophysica acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feigin A, et al. Metabolic network abnormalities in early Huntington’s disease: an [(18)F]FDG PET study. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2001;42:1591–1595. [PubMed] [Google Scholar]
- 12.Ciarmiello A, et al. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2006;47:215–222. [PubMed] [Google Scholar]
- 13.Powers WJ, et al. Selective defect of in vivo glycolysis in early Huntington’s disease striatum. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2945–2949. doi: 10.1073/pnas.0609833104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young AB, et al. PET scan investigations of Huntington’s disease: cerebral metabolic correlates of neurological features and functional decline. Annals of neurology. 1986;20:296–303. doi: 10.1002/ana.410200305. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins BG, Koroshetz WJ, Beal MF, Rosen BR. Evidence for impairment of energy metabolism in vivo in Huntington’s disease using localized 1H NMR spectroscopy. Neurology. 1993;43:2689–2695. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- 16.Garseth M, et al. Proton magnetic resonance spectroscopy of cerebrospinal fluid in neurodegenerative disease: indication of glial energy impairment in Huntington chorea, but not Parkinson disease. Journal of neuroscience research. 2000;60:779–782. doi: 10.1002/1097-4547(20000615)60:6<779::AID-JNR10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Lim D, et al. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. The Journal of biological chemistry. 2008;283:5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 18.Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Human molecular genetics. 2004;13:1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- 19.Tabrizi SJ, et al. Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Annals of neurology. 1999;45:25–32. doi: 10.1002/1531-8249(199901)45:1<25::aid-art6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Panov AV, et al. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nature neuroscience. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 21.Petrasch-Parwez E, et al. Cellular and subcellular localization of Huntingtin [corrected] aggregates in the brain of a rat transgenic for Huntington disease. The Journal of comparative neurology. 2007;501:716–730. doi: 10.1002/cne.21272. [DOI] [PubMed] [Google Scholar]
- 22.Orr AL, et al. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trushina E, et al. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Molecular and cellular biology. 2004;24:8195–8209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XJ, Orr AL, Li S. Impaired mitochondrial trafficking in Huntington’s disease. Biochimica et biophysica acta. 2010;1802:62–65. doi: 10.1016/j.bbadis.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui L, et al. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Ju TC, et al. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington’s disease. The Journal of cell biology. 2011;194:209–227. doi: 10.1083/jcb.201105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi H, et al. Creatine kinase B is a target molecule of reactive oxygen species in cervical cancer. Molecules and cells. 2001;12:412–417. [PubMed] [Google Scholar]
- 28.Stahl WL, Swanson PD. Biochemical abnormalities in Huntington’s chorea brains. Neurology. 1974;24:813–819. doi: 10.1212/wnl.24.9.813. [DOI] [PubMed] [Google Scholar]
- 29.Gu M, et al. Mitochondrial defect in Huntington’s disease caudate nucleus. Annals of neurology. 1996;39:385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 30.Browne SE, et al. Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Annals of neurology. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 31.Benchoua A, et al. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Molecular biology of the cell. 2006;17:1652–1663. doi: 10.1091/mbc.E05-07-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milakovic T, Quintanilla RA, Johnson GV. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. The Journal of biological chemistry. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- 33.Nicholls DG. Mitochondrial calcium function and dysfunction in the central nervous system. Biochimica et biophysica acta. 2009;1787:1416–1424. doi: 10.1016/j.bbabio.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seong IS, et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Human molecular genetics. 2005;14:2871–2880. doi: 10.1093/hmg/ddi319. [DOI] [PubMed] [Google Scholar]
- 35.Brustovetsky N, et al. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:4858–4867. doi: 10.1523/JNEUROSCI.23-12-04858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabrizi SJ, et al. Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Annals of neurology. 2000;47:80–86. doi: 10.1002/1531-8249(200001)47:1<80::aid-ana13>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 37.Bogdanov MB, Andreassen OA, Dedeoglu A, Ferrante RJ, Beal MF. Increased oxidative damage to DNA in a transgenic mouse model of Huntington’s disease. Journal of neurochemistry. 2001;79:1246–1249. doi: 10.1046/j.1471-4159.2001.00689.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen CM, et al. Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington’s disease patients. Biochemical and biophysical research communications. 2007;359:335–340. doi: 10.1016/j.bbrc.2007.05.093. [DOI] [PubMed] [Google Scholar]
- 39.Hersch SM, et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2′dG. Neurology. 2006;66:250–252. doi: 10.1212/01.wnl.0000194318.74946.b6. [DOI] [PubMed] [Google Scholar]
- 40.Sorolla MA, et al. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free radical biology & medicine. 2008;45:667–678. doi: 10.1016/j.freeradbiomed.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Santamaria A, et al. Comparative analysis of superoxide dismutase activity between acute pharmacological models and a transgenic mouse model of Huntington’s disease. Neurochemical research. 2001;26:419–424. doi: 10.1023/a:1010911417383. [DOI] [PubMed] [Google Scholar]
- 42.Goswami A, et al. Oxidative stress promotes mutant huntingtin aggregation and mutant huntingtin-dependent cell death by mimicking proteasomal malfunction. Biochemical and biophysical research communications. 2006;342:184–190. doi: 10.1016/j.bbrc.2006.01.136. [DOI] [PubMed] [Google Scholar]
- 43.Gines S, et al. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington’s disease knock-in mice. Human molecular genetics. 2003;12:497–508. doi: 10.1093/hmg/ddg046. [DOI] [PubMed] [Google Scholar]
- 44.Mochel F, et al. Early alterations of brain cellular energy homeostasis in Huntington disease models. The Journal of biological chemistry. 2012;287:1361–1370. doi: 10.1074/jbc.M111.309849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorolla MA, et al. Protein oxidation in Huntington disease affects energy production and vitamin B6 metabolism. Free radical biology & medicine. 2010;49:612–621. doi: 10.1016/j.freeradbiomed.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Siddiqui A, et al. Mitochondrial DNA damage is associated with reduced mitochondrial bioenergetics in Huntington’s disease. Free radical biology & medicine. 2012;53:1478–1488. doi: 10.1016/j.freeradbiomed.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, et al. Mitochondrial loss, dysfunction and altered dynamics in Huntington’s disease. Human molecular genetics. 2010;19:3919–3935. doi: 10.1093/hmg/ddq306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song W, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nature medicine. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shirendeb UP, et al. Mutant huntingtin’s interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington’s disease. Human molecular genetics. 2012;21:406–420. doi: 10.1093/hmg/ddr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell metabolism. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. Journal of cellular biochemistry. 2006;97:673–683. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- 52.Lin J, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Chaturvedi RK, et al. Impaired PGC-1alpha function in muscle in Huntington’s disease. Human molecular genetics. 2009;18:3048–3065. doi: 10.1093/hmg/ddp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaturvedi RK, et al. Impairment of PGC-1alpha expression, neuropathology and hepatic steatosis in a transgenic mouse model of Huntington’s disease following chronic energy deprivation. Human molecular genetics. 2010;19:3190–3205. doi: 10.1093/hmg/ddq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weydt P, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell metabolism. 2006;4:349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Chiang MC, et al. Modulation of energy deficiency in Huntington’s disease via activation of the peroxisome proliferator-activated receptor gamma. Human molecular genetics. 2010;19:4043–4058. doi: 10.1093/hmg/ddq322. [DOI] [PubMed] [Google Scholar]
- 57.Soyal SM, et al. A greatly extended PPARGC1A genomic locus encodes several new brain-specific isoforms and influences Huntington disease age of onset. Human molecular genetics. 2012;21:3461–3473. doi: 10.1093/hmg/dds177. [DOI] [PubMed] [Google Scholar]
- 58.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. The Journal of clinical investigation. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. The Journal of physiology. 2006;574:85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews Molecular cell biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiological reviews. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 62.Kemp BE, et al. Dealing with energy demand: the AMP-activated protein kinase. Trends in biochemical sciences. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 63.Ma TC, et al. Metformin therapy in a transgenic mouse model of Huntington’s disease. Neuroscience letters. 2007;411:98–103. doi: 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 64.Chou SY, et al. CGS21680 attenuates symptoms of Huntington’s disease in a transgenic mouse model. Journal of neurochemistry. 2005;93:310–320. doi: 10.1111/j.1471-4159.2005.03029.x. [DOI] [PubMed] [Google Scholar]
- 65.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiological reviews. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 66.Zhang SF, et al. Impaired brain creatine kinase activity in Huntington’s disease. Neuro-degenerative diseases. 2011;8:194–201. doi: 10.1159/000321681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luthi-Carter R, et al. Dysregulation of gene expression in the R6/2 model of polyglutamine disease: parallel changes in muscle and brain. Human molecular genetics. 2002;11:1911–1926. doi: 10.1093/hmg/11.17.1911. [DOI] [PubMed] [Google Scholar]
- 68.Li H, et al. KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron. 2007;56:1019–1033. doi: 10.1016/j.neuron.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 69.Spires TL, et al. Dendritic spine pathology and deficits in experience-dependent dendritic plasticity in R6/1 Huntington’s disease transgenic mice. The European journal of neuroscience. 2004;19:2799–2807. doi: 10.1111/j.0953-816X.2004.03374.x. [DOI] [PubMed] [Google Scholar]
- 70.Aksenov M, Aksenova M, Butterfield DA, Markesbery WR. Oxidative modification of creatine kinase BB in Alzheimer’s disease brain. Journal of neurochemistry. 2000;74:2520–2527. doi: 10.1046/j.1471-4159.2000.0742520.x. [DOI] [PubMed] [Google Scholar]
- 71.Mekhfi H, et al. Creatine kinase is the main target of reactive oxygen species in cardiac myofibrils. Circulation research. 1996;78:1016–1027. doi: 10.1161/01.res.78.6.1016. [DOI] [PubMed] [Google Scholar]
- 72.Firdaus WJ, et al. Huntingtin inclusion bodies are iron-dependent centers of oxidative events. The FEBS journal. 2006;273:5428–5441. doi: 10.1111/j.1742-4658.2006.05537.x. [DOI] [PubMed] [Google Scholar]
- 73.Morris M. Dementia and cognitive changes in Huntington’s disease. Advances in neurology. 1995;65:187–200. [PubMed] [Google Scholar]
- 74.Petersen A, Castilho RF, Hansson O, Wieloch T, Brundin P. Oxidative stress, mitochondrial permeability transition and activation of caspases in calcium ionophore A23187-induced death of cultured striatal neurons. Brain research. 2000;857:20–29. doi: 10.1016/s0006-8993(99)02320-3. [DOI] [PubMed] [Google Scholar]
- 75.Leventhal L, et al. Cyclosporin A protects striatal neurons in vitro and in vivo from 3-nitropropionic acid toxicity. The Journal of comparative neurology. 2000;425:471–478. doi: 10.1002/1096-9861(20001002)425:4<471::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 76.Merlini L, et al. Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5225–5229. doi: 10.1073/pnas.0800962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fernandes HB, Baimbridge KG, Church J, Hayden MR, Raymond LA. Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA-induced apoptosis in YAC128 model of Huntington’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:13614–13623. doi: 10.1523/JNEUROSCI.3455-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duan W. Sirtuins: from metabolic regulation to brain aging. Frontiers in aging neuroscience. 2013;5:36. doi: 10.3389/fnagi.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 80.Jiang M, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nature medicine. 2012;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jeong H, et al. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nature medicine. 2012;18:159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang T, Chan NY, Sauve AA. Syntheses of nicotinamide riboside and derivatives: effective agents for increasing nicotinamide adenine dinucleotide concentrations in mammalian cells. Journal of medicinal chemistry. 2007;50:6458–6461. doi: 10.1021/jm701001c. [DOI] [PubMed] [Google Scholar]
- 83.Hathorn T, Snyder-Keller A, Messer A. Nicotinamide improves motor deficits and upregulates PGC-1alpha and BDNF gene expression in a mouse model of Huntington’s disease. Neurobiology of disease. 2011;41:43–50. doi: 10.1016/j.nbd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu J, et al. trans-(−)-epsilon-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. The Journal of biological chemistry. 2012;287:24460–24472. doi: 10.1074/jbc.M112.382226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parker JA, et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nature genetics. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- 86.Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Experimental neurology. 2010;225:74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 87.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Sinclair DA, Guarente L. Small-molecule allosteric activators of sirtuins. Annual review of pharmacology and toxicology. 2014;54:363–380. doi: 10.1146/annurev-pharmtox-010611-134657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hubbard BP, et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith MR, et al. A potent and selective Sirtuin 1 inhibitor alleviates pathology in multiple animal and cell models of Huntington’s disease. Human molecular genetics. 2014 doi: 10.1093/hmg/ddu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO. SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. Journal of cell science. 2010;123:4251–4258. doi: 10.1242/jcs.073783. [DOI] [PubMed] [Google Scholar]
- 92.Chopra V, et al. The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington’s disease mouse models. Cell reports. 2012;2:1492–1497. doi: 10.1016/j.celrep.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johri A, et al. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Human molecular genetics. 2012;21:1124–1137. doi: 10.1093/hmg/ddr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strum JC, et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. Journal of Alzheimer’s disease: JAD. 2007;11:45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- 95.Quintanilla RA, Jin YN, Fuenzalida K, Bronfman M, Johnson GV. Rosiglitazone treatment prevents mitochondrial dysfunction in mutant huntingtin-expressing cells: possible role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in the pathogenesis of Huntington disease. The Journal of biological chemistry. 2008;283:25628–25637. doi: 10.1074/jbc.M804291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin J, et al. Neuroprotective effects of PPAR-gamma agonist rosiglitazone in N171–82Q mouse model of Huntington’s disease. Journal of neurochemistry. 2013;125:410–419. doi: 10.1111/jnc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beal MF, Shults CW. Effects of Coenzyme Q10 in Huntington’s disease and early Parkinson’s disease. BioFactors. 2003;18:153–161. doi: 10.1002/biof.5520180218. [DOI] [PubMed] [Google Scholar]
- 98.Hickey MA, et al. Evidence for behavioral benefits of early dietary supplementation with CoEnzymeQ10 in a slowly progressing mouse model of Huntington’s disease. Molecular and cellular neurosciences. 2012;49:149–157. doi: 10.1016/j.mcn.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spindler M, Beal MF, Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatric disease and treatment. 2009;5:597–610. doi: 10.2147/ndt.s5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang L, et al. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. Journal of neurochemistry. 2009;109:1427–1439. doi: 10.1111/j.1471-4159.2009.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schilling G, Coonfield ML, Ross CA, Borchelt DR. Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington’s disease transgenic mouse model. Neuroscience letters. 2001;315:149–153. doi: 10.1016/s0304-3940(01)02326-6. [DOI] [PubMed] [Google Scholar]
- 102.Menalled LB, et al. Comprehensive behavioral testing in the R6/2 mouse model of Huntington’s disease shows no benefit from CoQ10 or minocycline. PloS one. 2010;5:e9793. doi: 10.1371/journal.pone.0009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huntington Study, G. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology. 2001;57:397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]
- 104.Huntington Study Group Pre, C.I., et al. Safety and tolerability of high-dosage coenzyme Q10 in Huntington’s disease and healthy subjects. Movement disorders: official journal of the Movement Disorder Society. 2010;25:1924–1928. doi: 10.1002/mds.22408. [DOI] [PubMed] [Google Scholar]
- 105.Lin YS, et al. Dysregulated brain creatine kinase is associated with hearing impairment in mouse models of Huntington disease. The Journal of clinical investigation. 2011;121:1519–1523. doi: 10.1172/JCI43220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferrante RJ, et al. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain research bulletin. 2008;76:329–343. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 108.Perasso L, et al. Kinetics of creatine in blood and brain after intraperitoneal injection in the rat. Brain research. 2003;974:37–42. doi: 10.1016/s0006-8993(03)02547-2. [DOI] [PubMed] [Google Scholar]
- 109.Rosas HD, et al. PRECREST: A phase II prevention and biomarker trial of creatine in at-risk Huntington disease. Neurology. 2014;82:850–857. doi: 10.1212/WNL.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]