Abstract
Background
Transfusion-transmitted babesiosis caused by Babesia microti has emerged as a significant risk to the US blood supply. This study estimated the prevalence of B. microti antibodies in blood donors using an investigational enzyme immunoassay (EIA).
Study Design and Methods
A peptide-based EIA that detects both immunoglobulin (Ig)G and IgM antibodies to B. microti was developed and validated. Donor samples randomly selected from areas defined as high-risk endemic, lower-risk endemic, and nonendemic for B. microti were deidentified and tested using the investigational EIA. Samples that were EIA repeat reactive were further tested by B. microti immunofluorescent assay (IFA), polymerase chain reaction (PCR) on red blood cell lysates, and peripheral blood smear examination. A random subset of 1272 samples from high-risk endemic areas was tested by IFA, PCR, and peripheral blood smear in parallel with EIA.
Results
Among 15,000 donations tested with the investigational B. microti EIA, EIA repeat-reactive rates were 1.08% (54/5000) in a high-risk endemic area, 0.74% (37/5000) in a lower-risk area, and 0.40% (20/5000) in a nonendemic area. After application of a revised cutoff, these values were reduced to 0.92%, (46/5000), 0.54% (27/5000), and 0.16% (8/5000). Overall concordance between EIA and IFA among donor samples was 99.34%. One seropositive sample was positive by PCR.
Conclusion
The seroprevalence of B. microti in blood donors in a high-risk area measured by an investigational EIA was approximately 1%. The EIA shows promise as an efficient high-throughput blood donor screening assay for B. microti.
Babesiosis is a malaria-like illness caused by infection by members of the genus Babesia, a group of tick-borne intraerythrocytic protozoan parasites.1 Babesia microti is responsible for the overwhelming majority of human Babesia infections reported in the United States, where it is endemic in parts of the Northeast and upper Midwest. The parasite is primarily transmitted to humans through exposure to Ixodes scapularis (“deer ticks”) in endemic areas. However, B. microti is also readily transmissible by blood transfusion, and transfusion-transmitted babesiosis (TTB) is increasingly recognized as posing a risk to the blood supply.2,3 While B. microti infection results in asymptomatic or mild clinical findings in most immunocompetent hosts, infection in selected patient subsets, notably those who are immunosuppressed, asplenic, and/or at extremes of age, may lead to severe or even fatal disease.3,4 Overrepresentation of transfusion recipients among these high-risk groups accounts for the relatively high mortality ascribed to TTB.4,5
Currently, the only mandated strategy for TTB mitigation in use is a question regarding history of babesiosis posed directly to the potential donor before donation. The failure of this approach is evidenced by more than 150 cases of TTB that have been reported since 1979 with at least 12 fatalities since 2005.3 Despite being acknowledged as the foremost infectious risk to the US blood supply at present,5 there are as yet no validated, US Food and Drug Administration (FDA)-approved and commercially available tests for B. microti screening in blood donors.
We report the development of a high-throughput enzyme immunoassay (EIA) that detects antibodies to B. microti, and B. microti seroreactivity was determined with this EIA in samples from New York Blood Center (NYBC) blood donors collected over a 4-month period in 2012. This pilot study was used to optimize the cutoff of the EIA and to validate the EIA and confirmatory algorithms before an FDA licensure trial launched in 2013.
Materials and Methods
Serum samples from babesiosis patients
Serum samples were obtained at the time of diagnosis from 74 symptomatic patients from endemic areas of the northeastern and midwestern United States that were clinically diagnosed and laboratory confirmed as having babesiosis. Of this group, 58 of 63 were positive by blood smear, 24 of 25 were positive by polymerase chain reaction (PCR), and 72 of 74 were positive by immunofluorescent assay (IFA) with a titer of at least 64, as reported by the physicians who cared for these patients and provided patient serum samples for this study.
Twenty-four of these 74 sera were used exclusively in assay development, 26 were used exclusively in postdevelopment performance validation, and 24 were used in both phases; this distribution was based solely on sample availability. Of the 26 sera used exclusively for postdevelopment performance validation, 12 of 16 were positive by blood smear, 12 of 12 were positive by PCR, and 25 of 26 were positive by IFA with a titer of at least 64.
Blood donor samples used for EIA evaluation and determination of seroprevalence
For assay development and validation, 1003 serum samples from healthy, asymptomatic blood donors living in Arizona, an area considered nonendemic for B. microti transmission, were obtained from Creative Testing Solutions (Tempe, AZ). These donor serum samples were deidentified and represented residual volumes from routine collections.
For a seroprevalence study with the investigational EIA, a total of 15,000 routine blood donor serum samples that were collected between August 6 and November 30, 2012, were deidentified and stored until batch testing. Five-thousand samples each were collected by NYBC from donors residing in an endemic area considered high-risk for Babesia microti transmission (Suffolk County, NY) and from donors in low-risk endemic areas (Manhattan and Brooklyn, NY) and similarly by United Blood Services-Arizona from donors in a nonendemic area (Arizona). Arizona donors with past travel outside the region were not excluded, however.
B. microti EIA
The B. microti EIA is based on four synthetic peptide antigens selected from the BMN1 family of antigens identified as immunodominant and specific for B. microti in previous studies.6–;9 These antigens are distantly related to Plasmodium merozoite surface antigens but are otherwise without known homologs. The peptides are biotinylated to enable immobilization to a streptavidin-coated microplate. The combination of four peptide antigens with differing antigenic specificities resulted in higher overall sensitivity than any single peptide with respect to detection of the well-characterized clinical babesiosis sera.
The assay is configured in a standard indirect enzyme-linked immunosorbent assay (ELISA) format, in which B. microti-specific antibodies in the serum sample are captured by binding to the immobilized peptide antigens. After a washing step, detection of the bound serum antibodies is effected by incubation with monoclonal secondary antibodies targeting immunoglobulin (Ig)G and IgM heavy chains coupled to horseradish peroxidase (HRP). After a second wash step, the soluble substrate tetramethylbenzidine is added, which is converted by HRP to a visible product. A stop reagent is then added to halt further enzyme activity, after which absorbance is read at 450 nm. All reagents along with the coated microplate are provided in a kit configuration. Details of the procedure are provided in Appendix S1 (available as supporting information in the online version of this paper).
Peripheral blood smear examination
Peripheral blood smears were prepared from EDTA-preserved whole blood samples using the method of Houwen10 and allowed to air dry completely before staining. A minimum of two microscope slides were prepared from each sample from donors collected from B. microti–endemic areas.
Smears were stained using Wright’s-Giemsa stain (Easy I, Azer Scientific, Morgantown, PA). Microscopic examination of peripheral blood smears to detect the presence of B. microti or other blood parasites, and quantification of the organisms detected, was performed according to the method of Blevins and coworkers11 utilizing NCCLS-recommended standards (300 fields at 1000× per slide). Any positive or inconclusive results were confirmed by independent review before the final interpretation.
PCR applied to red blood cell preparations
The PCR method developed by Bloch and colleagues12 was used. Details of the procedure are provided in Appendix S1.
IFA
IFAs were carried out according to the method of Chisholm and coworkers13 using B. microti substrate slides obtained from Fuller Laboratories (Fullerton, CA). IgG and IgM antibodies were detected using an FITC-labeled goat anti-human antibody conjugate (Jackson Immunoresearch, West Grove, PA). Slides were analyzed with a microscope (Optiphot-2, Nikon, Melville, NY) equipped with rhodamine and fluorescein filters at 400× magnification. The cutoff for interpretation of an IFA result as positive was 64. Serum samples found to be IFA positive were retested independently at Tufts University using a similar protocol but with substrate slides prepared freshly.
Seroprevalence study
Aliquots of residual serum and/or EDTA plasma and EDTA-packed red blood cells (RBCs) derived from 10,000 NYBC and 5000 United Blood Services-Arizona blood donors were unlinked from any individually identifiable information and were processed and stored frozen after completion of routine donor testing. Samples were tested for evidence of B. microti following the scheme in Fig. 1A. Accordingly, all serum samples were tested by EIA. Samples yielding an initially reactive or gray zone result were retested in duplicate and were classified as repeat reactive, gray zone, or nonreactive (see Appendix S1 and Results section for definitions). Samples with repeat-reactive and gray zone EIA results were further subjected to testing by IFA, peripheral blood smear examination, and quantitative PCR. The latter was used to determine the proportion of seroreactive samples with evidence of persistent parasitemia.
Figure 1.
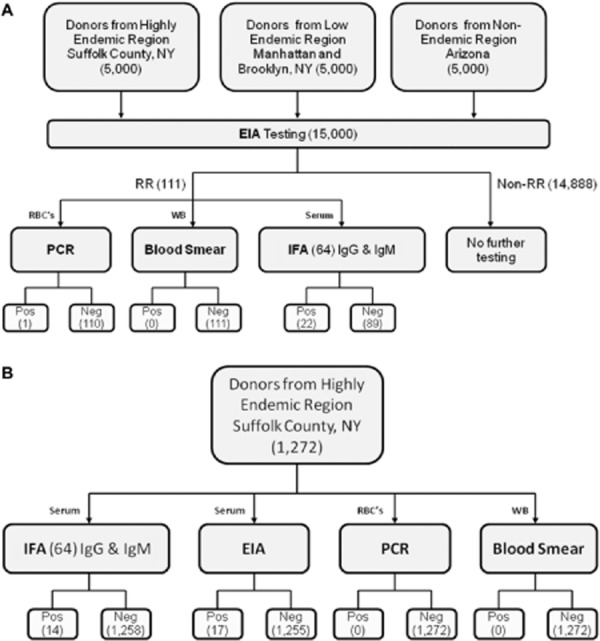
Flow charts showing the two arms of the study. Numbers in parentheses represent numbers of samples at each stage. RR = repeat reactive.
In a second branch of the study (Fig. 1B), a subset of 1272 consecutive, otherwise unselected donor samples from a B. microti–endemic area (Suffolk County, NY) were tested with IFA and PCR to ascertain the frequency of samples which were positive by IFA irrespective of the EIA result and the magnitude of parasitemia as determined by PCR among donors who lack B. microti–specific antibodies detectable by EIA.
For the purposes of the study we included any EDTA whole blood, EDTA plasma and/or serum, and residual EDTA-anticoagulated RBCs from routine blood donation within the defined areas that had a minimum volume of 1.0 mL off-the-clot serum, 3 mL of EDTA plasma, and 4.0 mL of EDTA-packed RBCs and met the minimum sample acceptability requirements for the B. microti EIA assay. All samples were identified by individual sample type and no pooling of plasma with different anticoagulants, pooling of serum, plasma, or RBC occurred. Samples were excluded if they had less than the required minimum volume; had been stored at 2 to 8°C or warmer for more than 5 days postcollection; or were grossly hemolyzed, grossly icteric, or grossly lipemic.
Human subjects
Approval for the study protocol was obtained from the institutional review board at New York Blood Center. No subjects were specifically enrolled into the study and all personal identifiers were removed from the samples before storage. Therefore, no specific informed consent was required beyond the routine consent to donate blood, which included information pertaining to the potential use of data and residual donor blood for research and development. No samples were drawn specifically for this study. All samples were obtained in accordance with “Guidance on Informed Consent for In Vitro Diagnostic Device Studies Using Leftover Human Specimens That Are Not Individually Identifiable,” issued on April 25, 2006, by the US Department of Health and Human Services FDA Center for Devices and Radiological Health Office of In Vitro Diagnostic Device Evaluation.
Statistical analysis
Significance was calculated by two-tailed Fisher’s exact test; p values of less than 0.05 were considered significant. Confidence intervals were determined by the Clopper-Pearson exact method.
Results
EIA performance on clinical babesiosis patients and control donors
The peptide EIA detected 69 (93.2%) of the 74 serum samples from clinically diagnosed, laboratory-confirmed babesiosis patients at the provisional cutoff of 0.3, calculated as described in Appendix S1. Of 72 sera in this group that were positive by IFA at a titer of 64, the peptide EIA detected 69 (95.8%). The sensitivity of EIA detection of clinical babesiosis patient sera was identical at 88% in nonoverlapping subsets of 24 and 26 sera used in assay development and in postdevelopment performance validation, respectively (p = 1.0). Among donors from a nonendemic area, five of 1003 were found to be repeat reactive, yielding an apparent specificity of 99.5%. Risk factors such as travel history for the five EIA-reactive donors were not known. EIA absorbance values for the babesiosis patient sera were distributed over a broad range, with a median absorbance of 1.11, while the median absorbance for nonendemic donor sera was 0.046 (Fig. 2).
Figure 2.
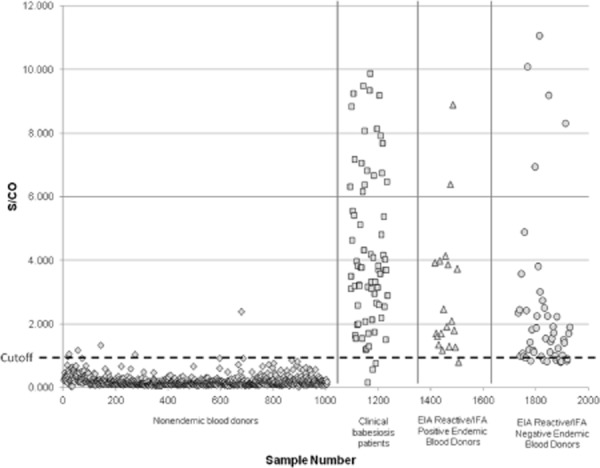
Distribution of S/CO values in the B. microti EIA for healthy blood donors from a non-endemic area (⋄, n = 1003), clinical babesiosis patients that were positive by IFA at 1:64 (□, n = 72), and blood donors from a high-risk endemic area that were EIA repeat reactive or repeat gray zone (n = 69), subdivided between IFA-positive (△, n = 19) and IFA-negative (○, n = 50) groups. The original EIA cutoff is shown as a horizontal dashed line.
Testing of the 1272 randomly selected sera from a highly endemic region by EIA, IFA, PCR, and blood smear yielded samples that were reactive by EIA or IFA, but none that were positive by PCR or blood smear. Comparison of serum reactivities by EIA versus IFA indicated overall concordance of 98.5% (95% confidence interval, 97.7%-99.1%), with 98% of the samples found negative by both methods. Among the 25 seroreactive samples, 17 were repeat reactive by EIA and 14 by IFA, with 19 samples in total exhibiting discrepant results between the two assays (11 EIA repeat reactive/IFA nonreactive and 8 EIA nonreactive/IFA reactive), yielding an unweighted kappa value of 0.38 for agreement between the two tests.
B. microti seroprevalence study
Of 15,000 donations tested with the investigational B. microti EIA, 124 were initially reactive and 111 were repeat reactive (Table 1). The frequency of repeat-reactive samples at the provisional cutoff was 1.08% (54/5000) in the high-risk endemic area (Suffolk County, NY), 0.74% (37/5000) in the lower-risk area (Manhattan and Brooklyn, NY), and 0.40% (20/5000) in the nonendemic area (Arizona). These values represent averages over the roughly 4-month study period, but frequencies of reactivity varied considerably from month to month in the low-risk endemic group (0.25%-1.02%) while remaining more stable in the high-risk endemic and nonendemic groups (0.90%-1.2% and 0%-0.54%, respectively). The overall concordance between EIA and IFA among all donor samples was 99.34%. One-third of the EIA repeat-reactive samples from the high-risk endemic area were also positive by IFA at a 64 cutoff. Of these 18 samples, 10 had an IFA titer of 64, with the remaining 11 reaching higher titers, the highest at 2048. The mean signal-to-cutoff (S/CO) ratio for EIA repeat-reactive samples from the high-risk endemic area was 3.30, with no significant difference between IFA-positive and IFA-negative subsets (Fig. 2). For the low-risk endemic area and nonendemic areas, the mean S/CO values were 2.53 and 1.97, respectively. One seropositive sample from the high-risk endemic area for which the S/CO ratio was 3.3 and IFA IgM titer was 64 was also positive by PCR. All other EIA-reactive samples were negative by PCR and peripheral blood smear examination. The rate of EIA repeat reactivity for the nonendemic group was significantly different from that of the high-risk endemic group (p = 0.0002) and marginally different from that of the low-risk endemic group (p = 0.05). The difference in rates of EIA repeat reactivity between high-risk and low-risk endemic groups was not significant, however (p = 0.09).
Table 1.
B. microti seroprevalence results for donor sera from endemic and nonendemic regions*
| Sample category | B. microti EIA initially reactive, original C/O† | B. microti EIA repeat reactive | IFA positive | PCR positive | Blood smear positive | |
|---|---|---|---|---|---|---|
| Original C/O† | Revised C/O† | |||||
| High-risk endemic donors (Suffolk County, NY) (n = 5000) | 1.14 (57) | 1.08 (54) | 0.92 (46) | 0.36 (18) | 0.02 (1) | 0 |
| Lower-risk endemic donors (Brooklyn and Manhattan, NY) (n = 5000) | 0.86 (43) | 0.74 (37) | 0.54 (27) | 0.06 (3) | 0 | 0 |
| Nonendemic donors (Arizona) (n = 5000) | 0.48 (24) | 0.40 (20) | 0.16 (8) | 0.02 (1) | 0 | 0 |
| Clinical babesiosis cases (n = 74) | 95.9 (71) | 93.2 (69) | 100 (74) | 44.6 (33) | 60.8 (45) | |
Data are reported as percent (number) of samples in each category.
See Materials and Methods and Appendix S1 for description of original cutoff and revised cutoff.
Thirty-nine of 15,000 donor samples yielded S/CO values in the gray zone upon repeat testing; the frequencies of such gray zone samples were not different between high-risk, low-risk, and non-endemic zones (p > 0.84, Table 2). One donor sample among the high-risk endemic group with gray zone S/CO values was reactive by IgG IFA with a titer of 64 but none were positive by PCR or blood smear.
Table 2.
Reactivity of samples initially scored in the gray zone in the B . microti EIA
| Region | EIA initially gray zone | EIA reactive or gray zone upon retest | IFA reactive | PCR positive | Blood smear positive |
|---|---|---|---|---|---|
| High-risk endemic (n = 5000) | 19 | 14 | 1 | 0 | 0 |
| Lower-risk endemic (n = 5000) | 14 | 13 | 0 | 0 | 0 |
| Nonendemic (n = 5000) | 17 | 12 | 0 | 0 | 0 |
Numbers of samples in each category are shown.
The single IFA reactive sample in this table exhibited an estimated IFA titer of 1:64 for IgG, and was scored in the EIA gray zone initially and upon retest. An additional sample not included in Tables1 and 2 was initially EIA reactive but in the gray zone on retesting.
Evaluation of alternative cutoffs for the EIA
A retrospective analysis of EIA data was carried out to examine the cost versus benefit of varying the cutoff, which had been provisionally established based on a more limited sample set. This analysis indicated that a 25% increase in cutoff value (see Appendix S1 for details) would not reduce the frequency of detection of EIA repeat-reactive samples from the high-risk endemic region that were also IFA positive, while it would reduce the frequency of EIA repeat-reactive samples that were IFA negative in the nonendemic group by 12 of 20 or 60% (from 0.40% to 0.16%), a substantial effect. The higher cutoff value would reduce the detection of clinical babesiosis sera by two of 74 or 2.7%. A reanalysis of EIA reactivities at the higher cutoff yielded rates of repeat reactivity of 0.92% (46/5000) in the high-risk endemic group, 0.54% (27/5000) in the low-risk endemic group, and 0.16% (8/5000) in the nonendemic group (Table 1).
Discussion
Seropositivity for B. microti has been reported at more than 1% in blood donations from B. microti–endemic areas in the United States, and the distribution of B. microti seropositivity is expanding beyond areas traditionally regarded as endemic.5,14 This finding is not unique to the United States: B. microti, previously considered rare in Europe,15 has been identified in localized regions of Germany and Switzerland, with human seroprevalence reported to be between 1 and 9% in studies of blood donors and selected at-risk patient populations.16 Most B. microti infections in healthy individuals appear to be undiagnosed and to self-resolve with time. As such, seropositivity is an indication of prior exposure but not necessarily of active infection; however active infection almost invariably leads to seropositivity. This situation is reflected in the various diagnostic tests applied to detection of B. microti infection, which include microscopic examination of Giemsa-stained blood smears, indirect immunofluorescence (IFA), PCR, and hamster inoculation. Each approach has limitations when applied to donor screening.17,18
The IFA method originally described more than 30 years ago13 remains the only currently available serologic method but has never been standardized in a validated kit.17 IFA requires microscopy skills, specific training, and access to a fluorescence microscope, which is practical for some reference laboratories but not a technique amenable to routine high-throughput use and practice by nonspecialists—all, effectively, requirements for a blood screening assay. Examination of thin blood smears for piroplasms similarly requires a microscope and skilled operator and is subject to the same limitations.11 PCR, used as a surrogate assay for infectivity, demands a highly controlled environment to avoid contamination and artifactual results, complex and expensive instrumentation and reagents, and a high degree of training to perform properly. Furthermore, nucleic acid testing in use for transfusion screening has been optimized for the detection of viral infection in plasma and is not amenable to the detection of B. microti DNA, which requires evaluation of RBC preparations. EIA or ELISA methods, on the other hand, present distinct advantages. An EIA formatted in conventional 96-well microplates can be carried out by laboratory personnel without specific training beyond pipetting skills, does not require equipment more sophisticated or costly than pipettors and a microplate reader, and is suitable for either low or higher volume testing using existing microplate-based instrumentation, which is found in many clinical laboratories.
The peptide EIA described in this study brings these advantages to donor screening for B. microti exposure. An earlier study of Connecticut blood donors using a recombinant EIA with antigens also derived from the BMN1 family was the first to suggest the potential of this approach, but different antigen sequences were used, which exhibited a relatively high false-positive rate, reported as approximately 5%.19 However, a prototype EIA developed by Houghton and colleagues8 that employed two tandem BMN1 peptide sequences demonstrated promising sensitivity and specificity in preliminary studies on clinical and blood donor sera. An EIA based on a crude lysate of parasitemic hamster blood and intended for clinical diagnosis has also been described, for which the specificity was 94.1%.20 By comparison, the investigational EIA that was developed and evaluated in this present study demonstrated high sensitivity for clinically defined babesiosis cases and high specificity among healthy blood donors—more than 99.8% with an optimized cutoff. Determination of the intrinsic specificity of the EIA was limited by the fact that the nonendemic donor population that was tested was not subject to exclusion of individuals with a history of travel to endemic areas or other risk factors. Additionally, the possibility that seropositivity in the nonendemic donor population may be due to undocumented cross-reactivity with nonpathogenic Babesia species that are known to be endemic in the US Southwest cannot be excluded.21 The rate of EIA seroreactivity was significantly greater in endemic versus nonendemic areas as predicted, while the subdivision of the endemic area into high-risk and low-risk groups did not appear to contribute additional information.
In this initial study in endemic and nonendemic blood donor populations, the overall rates of EIA seroreactivity were similar to those reported in several previous studies based on IFA methods.22–;24 A comparison of the sample sets identified as reactive by EIA versus IFA suggests that the two methods detect overlapping, but not identical subsets (corroborated by the kappa value of 0.38, interpreted as “fair” agreement). This may be a consequence of differences in the antigen composition of the two assays; IFA is based on fixed, whole-cell antigen, while the peptide EIA is based on a combination of specific peptides. The limitations in validation of methods imposed by the relatively small number of well-characterized B. microti cases that have been available for this purpose, combined with the lack of a standardized IFA method, make it difficult to resolve the observed discrepancies between EIA and IFA results. We are further pursuing this question through analysis of serum reactivities to individual antigens by B. microti immunoblot. Additionally, the relative accuracy and cost/benefit ratio of algorithms involving combinations of serologic assays or serologic assays with PCR to identify blood donors with active B. microti infection merits further investigation.
One objective of this study was the optimization of a cutoff for the EIA. The incorporation of a “gray zone” below the provisional cutoff was intended to provide data that would indicate the cost versus benefit of a still lower cutoff. While 41% of the initially reactive samples in the nonendemic area and 25% in the endemic areas were within this gray zone, only one of the 50 total samples in the gray zone had marginal IFA reactivity and none were positive by PCR or blood smear. Hence a lower cutoff corresponding to the lower limit of the gray zone was not found to offer any obvious benefit in improved sensitivity, but more likely served to decrease assay specificity.
On the other hand, the effect of an increase in the cutoff was evaluated retrospectively on the collected study data. A 25% increase in cutoff value had very little effect on sensitivity with respect to both clinical babesiosis cases and IFA-confirmed blood donors, but a substantial effect in reducing the rate of apparent false positivity in the nonendemic control group. Based on this analysis, the EIA cutoff was raised (as described in Appendix S1) for use in further studies.
The detection of a single PCR-positive sample among the EIA-seropositive subset of 10,000 donors from endemic areas of New York may be a result of two factors—first, the timing of sample collection, which commenced past the peak of the tick transmission season, and second, the application of PCR as a second-tier test carried out on samples found seropositive by EIA. The latter would account for the lack of detection of window period cases, which would not have been tested by PCR in this scheme. However, no such cases were detected among the subset of 1272 donors screened by PCR in parallel with serologic methods. Moreover, as the RBC preparations were made from freshly drawn blood samples within several days of collection, the possibility of sample degradation, although it cannot be excluded, appears minimal. Given the higher, albeit relatively low rate of PCR-positive, potentially infectious donors reported in other studies19,22,23 even in the peak tick transmission season, this result may simply reflect the small population that was tested or the incidence of infection during time of collection.
In summary, the rate of seroreactivity measured by an investigational B. microti EIA in this study in blood donors residing in an area endemic for B. microti is consistent with rates reported in several previous studies of other endemic populations. These donor study results, together with data that validate the performance of the EIA on clinically diagnosed babesiosis patient sera, support the possible utility of this EIA in donor screening procedures that are currently under consideration. A recent cost/benefit analysis suggests antibody-based screening of blood donors in endemic regions as the most cost-effective testing approach to reducing the risk of TTB.25 To this end, we have initiated a more extensive seroprevalence study and pivotal trial under an investigational new device protocol aimed at supporting eventual licensure of the B. microti EIA for blood screening.
Acknowledgments
The authors are grateful to Dr Patricia Wilkins (Centers for Disease Control and Prevention, Atlanta, GA) and Dr Timothy Lepore (Nantucket Cottage Hospital, Nantucket, MA) for providing serum samples from patients diagnosed with babesiosis. The following individuals contributed technical assistance: Tzong-Hae Lee, Leilani Montalvo, Valerie Winkelman, Ardis Decker, Randy Spizman, and Austin Morgan.
Glossary
- IFA(s)
immunofluorescent assay(s)
- NYBC
New York Blood Center
- S/CO
signal to cutoff
- TTB
transfusion-transmitted babesiosis
Conflict of Interest
AEL, JLE, XN, HW, and NXK are employees of Immunetics, the developer of the Babesia microti EIA kit.
Supporting Information
Appendix S1. Supplemental methods.
References
- Vannier E, Krause P. Human babesiosis. N Engl J Med. 2012;366:2397–2407. doi: 10.1056/NEJMra1202018. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24517274. [DOI] [PubMed] [Google Scholar]
- Gubernot DM, Lucey CT, Lee KC. Babesia infection through blood transfusions: reports received by the US Food and Drug Administration, 1997-2007. Clin Infect Dis. 48:25–30. doi: 10.1086/595010. , et al. [DOI] [PubMed] [Google Scholar]
- Herwaldt BL, Linden JV, Bosserman E. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med. 155:509–519. doi: 10.7326/0003-4819-155-8-201110180-00362. , et al. [DOI] [PubMed] [Google Scholar]
- Krause PJ, Gewurz BE, Hill D. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2009;46:370–376. doi: 10.1086/525852. , et al. [DOI] [PubMed] [Google Scholar]
- Leiby DA. Transfusion-transmitted Babesia spp.: bull’s-eye on Babesia microti. Clin Microbiol Rev. 2011;24:14–28. doi: 10.1128/CMR.00022-10. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3021205&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodes MJ, Houghton RL, Bruinsma ES. Serological expression cloning of novel immunoreactive antigens of Babesia microti. Infect Immun. 2000;68:2783–2790. doi: 10.1128/iai.68.5.2783-2790.2000. , et al. . Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=97488&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer MJ, Lodes MJ, Reynolds LD. Identification and characterization of putative secreted antigens from Babesia microti. J Clin Microbiol. 2003;41:723–729. doi: 10.1128/JCM.41.2.723-729.2003. , et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton RL, Homer MJ, Reynolds LD. Identification of Babesia microti-specific immunodominant epitopes and development of a peptide EIA for detection of antibodies in serum. Transfusion. 42:1488–1496. doi: 10.1046/j.1537-2995.2002.00215.x. , et al. [DOI] [PubMed] [Google Scholar]
- Homer MJ, Bruinsma ES, Lodes MJ. A polymorphic multigene family encoding an immunodominant protein from Babesia microti. J Clin Microbiol. 2000;38:362–368. doi: 10.1128/jcm.38.1.362-368.2000. , et al. . Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88725&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houwen B. Blood film preparation and staining procedures. Clin Lab Med. 22:1–14. doi: 10.1016/s0272-2712(03)00064-7. , v. [DOI] [PubMed] [Google Scholar]
- Blevins SM, Greenfield RA, Bronze MS. Blood smear analysis in babesiosis, ehrlichiosis, relapsing fever, malaria, and Chagas disease. Cleve Clin J Med. 75:521–530. doi: 10.3949/ccjm.75.7.521. [DOI] [PubMed] [Google Scholar]
- Bloch EM, Lee TH, Krause PJ. Development of a real-time polymerase chain reaction assay for sensitive detection and quantitation of Babesia microti infection. Transfusion. 2002;53:2299–2306. doi: 10.1111/trf.12098. , et al. [DOI] [PubMed] [Google Scholar]
- Chisholm ES, Ruebush TK, Sulzer AJ. Babesia microti infection in man: evaluation of an indirect immunofluorescent antibody test. Am J Trop Med Hyg. 1978;27(1 Pt 1):14–19. doi: 10.4269/ajtmh.1978.27.14. , et al. . Available from: http://europepmc.org/abstract/MED/343608/reload=0. [DOI] [PubMed] [Google Scholar]
- Weld ED, Eimer KM, Saharia K. Transfusion medicine illustrated. The expanding range and severity of babesiosis. Transfusion. 50:290–291. doi: 10.1111/j.1537-2995.2009.02563.x. , et al. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A, Gray JS, Hunfeld KP. Human babesiosis in Europe: what clinicians need to know. Infection. 41:1057–1072. doi: 10.1007/s15010-013-0526-8. [DOI] [PubMed] [Google Scholar]
- Hunfeld KP, Lambert A, Kampen H. Seroprevalence of Babesia infections in humans exposed to ticks in midwestern Germany. J Clin Microbiol. 2002;40:2431–2436. doi: 10.1128/JCM.40.7.2431-2436.2002. , et al. . Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=120565&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Telford SR, Ryan R. Diagnosis of babesiosis: evaluation of a serologic test for the detection of Babesia microti antibody. J Infect Dis. 169:923–926. doi: 10.1093/infdis/169.4.923. , et al. [DOI] [PubMed] [Google Scholar]
- Krause PJ, Telford S, Spielman A. Comparison of PCR with blood smear and inoculation of small animals for diagnosis of Babesia microti parasitemia. J Clin Microbiol. 1996;34:2791–2794. doi: 10.1128/jcm.34.11.2791-2794.1996. , et al. . Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=229405&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiby DA, Chung AP, Gill JE. Demonstrable parasitemia among Connecticut blood donors with antibodies to Babesia microti. Transfusion. 45:1804–1810. doi: 10.1111/j.1537-2995.2005.00609.x. , et al. [DOI] [PubMed] [Google Scholar]
- Loa CC, Adelson ME, Mordechai E. Serological diagnosis of human babesiosis by IgG enzyme-linked immunosorbent assay. Curr Microbiol. 2004;49:385–389. doi: 10.1007/s00284-004-4373-9. , et al. [DOI] [PubMed] [Google Scholar]
- Burkot TR, Schneider BS, Pieniazek NJ. Babesia microti and Borrelia bissettii transmission by Ixodes spinipalpis ticks among prairie voles, Microtus ochrogaster, in Colorado. Parasitology. 121(Pt 6):595–599. , et al. [PubMed] [Google Scholar]
- Johnson ST, Van Tassell ER, Tonnetti L. Babesia microti real-time polymerase chain reaction testing of Connecticut blood donors: potential implications for screening algorithms. Transfusion. 2013;53:2644–2649. doi: 10.1111/trf.12125. , et al. . Available from: http://www.ncbi.nlm.nih.gov/pubmed/23445322. [DOI] [PubMed] [Google Scholar]
- Tonnetti L, Thorp AM, Deisting B. Babesia microti seroprevalence in Minnesota blood donors. Transfusion. 53:1698–1705. doi: 10.1111/j.1537-2995.2012.03948.x. , et al. [DOI] [PubMed] [Google Scholar]
- Johnson ST, Cable RG, Tonnetti L. Seroprevalence of Babesia microti in blood donors from Babesia-endemic areas of the northeastern United States: 2000 through 2007. Transfusion. 49:2574–2582. doi: 10.1111/j.1537-2995.2009.02430.x. , et al. [DOI] [PubMed] [Google Scholar]
- Simon MS, Leff JA, Pandya A. Cost-effectiveness of blood donor screening for Babesia microti in endemic regions of the United States. Transfusion. 2014;54:889–899. doi: 10.1111/trf.12492. , et al. [Internet] (3 Pt 2): [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplemental methods.


