Abstract
Background
Advances in cancer treatments continue to reduce the incidence of lymphedema. Yet, many breast cancer survivors still face long-term post-operative challenges as a result of developing lymphedema. The purpose of this study was to preliminarily evaluate The-Optimal-Lymph-Flow program, a patient-centered education and behavioral program focusing on self-care strategies to enhance lymphedema risk reduction by promoting lymph flow and optimize body mass index.
Methods
A prospective, longitudinal, quasi-experimental design with repeated-measures was used. The study outcomes included lymph volume changes by infra-red perometer and body mass index by a bioimpedance device at pre-surgery baseline, 2-4 weeks after surgery, 6-month, and 12-month follow-up. A total of 140 patients were recruited and participated in The-Optimal-Lymph-Flow program; 134 patients completed the study with 4% attrition rate.
Results
Fifty-eight percent patients had axillary node dissection and 42% had sentinel lymph node biopsy. The majority (97%) of patients maintained and improved their preoperative limb volume and body mass index at the study endpoint of 12 months following cancer surgery. Cumulatively, 2 patients with sentinel lymph node biopsy and 2 patients with the axillary lymph node dissection had measurable lymphedema (>10% limb volume change). At 12-month follow-up, among the 4 patients with measurable lymphedema, 2 patients' limb volume returned to pre-operative level without compression therapy but by maintaining The-Optimal-Lymph-Flow exercises to promote daily lymph flow.
Conclusions
This educational and behavioral program is effective to enhance lymphedema risk reduction. The study provided initial evidence for emerging change in lymphedema care from treatment-focus to proactive risk reduction.
Keywords: Lymphedema, Breast Cancer, Risk Reduction
Introduction
Lymphedema is one of the unfortunate outcomes of breast cancer treatment since it negatively impacts survivors' overall quality of life [1-3]. Breast cancer-related lymphedema is characterized by an accumulation of lymph fluid in the interstitial spaces of the affected limb, leading to chronic ipsilateral limb swelling coupled with multiple distressing symptoms [3-5]. Breast cancer survivors who undergo breast surgery, dissection of lymph nodes and vessels, and radiation are known to have a compromised lymphatic system, leading to ineffective lymphatic drainage, thus risk for lymphedema [6-7]. In addition to the risk factor of compromised lymphatic drainage from cancer treatment, higher body mass index (BMI) is also an established risk factor for lymphedema [8-11]. Physiologically, a larger body mass creates a disproportion in lymph transport and capacity, resulting in excess extracellular fluid [12]. Women are 1.11 times more at risk for developing lymphedema with every increase of 1kg/m2 in their BMI [8-11].
Patient education focusing on risk reduction strategies holds great promise for reducing the risk of lymphedema [13]. Research evidence demonstrates that patient education remains an important predictor of lymphedema outcome after controlling for confounding cancer treatment-related risk factors [13]. Risk factors, such as compromised lymphatic drainage and higher BMI, may be modified through self-care strategies. Current patient education emphasizes precautionary lifestyle behaviors, such as avoidance of repetitive limb movement, lifting weighted objects, needle punctures, blood draw, and the use of compression garments for air travel in the affected limb [14-15]. To date, there is a paucity of high quality evidence to support these practices that reduce the risk of lymphedema [14-15]. One early study showed a significant decrease in limb volume (LV) using the compression garments for patients with >3% LV increase over a 4-week short duration [16]. Research is lacking to provide evidence to reduce the risk or halt the progression of lymphedema through self-care strategies targeting risk factors of compromised lymphatic drainage and higher BMI. To address this important clinical need, we conducted a pilot study to preliminarily evaluate a patient-centered educational and behavioral lymphedema risk reduction program (The-Optimal-Lymph-Flow) focusing on promoting lymph flow and optimizing BMI over a 12-month period after cancer surgery.
Methods
Study Design
A prospective, longitudinal, quasi-experimental design with repeated-measures was used to: (1) Evaluate the safety, feasibility, and acceptability of The-Optimal-Lymph-Flow program; and (2) Preliminarily evaluate the efficacy of the program, specifically limb volume (LV) change and BMI. The study was approved by the institutional review board at NYU Langone Medical Center.
Study Population
We recruited women who were over age 21, first time diagnosis of breast cancer (Stage I-III), and scheduled for surgical treatment, including lumpectomy or mastectomy, sentinel lymph node biopsy (SLNB) or axillary lymph node dissection ALND). Women with metastatic cancer (Stage IV), prior history of breast cancer and lymphedema, and bilateral breast cancer were excluded. Between April 2010 and June 2012, we prospectively enrolled 140 women and followed the participants for 12 months after surgery. All the participants received the The-Optimal-Lymph-Flow program.
The-Optimal-Lymph-Flow Program
The-Optimal-Lymph-Flow is a patient-centered educational and behavioral program focusing on self-care risk reduction strategies to promote lymph flow and optimize BMI by targeting known lymphedma risk of compromised lymphatic system and high BMI. Easy-to-learn self-care strategies include shoulder mobility exercises to promote shoulder function, muscle-tightening-breathing, muscle-tightening-pumping exercises, and large muscle exercises to promote lymph flow and drainage, as well as general instructions to encourage nutrition-balanced (more vegetables and fruits) and portion-appropriate diet (feeling 75% full for each meal) to strive for maintaining pre-operative BMI. Table 1 describes the self-care strategies and physiological rationales. Trained nurses delivered the intervention during a 30-minute face-to-face meeting with each patient. Patients demonstrated back the shoulder exercises, muscle-tightening-breathing, and muscle-tightening-pumping exercises.
Table 1. The-Optimal-Lymph-Flow Program Strategies and Rationales**.
| **Risk Reduction Strategies | Rationales | Frequency & Situations | |
|---|---|---|---|
| Promoting lymph Flow |
|
|
|
|
|
|
|
|
|
|
|
| Improving Limb Functional Status |
|
|
|
| Optimizing BMI |
|
|
|
|
|
|
For step-by-step instruction for each exercise, please contact the corresponding author.
Outcome Evaluation
The study outcomes were LV change by infra-red perometer and BMI by a bioimpedance device at pre-surgery baseline, 2-4 weeks after surgery, 6-month, and 12-month follow-up after surgery as well as safety, feasibility, and acceptability of The-Optimal-Lymph-Flow program. Lymphedema was defined as a perometer measurement of ≥10% LV increase from baseline in the ipsilateral arm in comparison with the changes in the contralateral arm, using the formula: Lymphedema = (Ipsilateral Frustum LVFollow-up / Ipsilateral Frustum LVbaseline) / (Contralateral Frustum LVFollow-up / Contralateral Frustum LVbaseline) [17-18]. Because even a 5% LV increase enables detectable differences in quality of life and symptom reporting [4, 16-17], participants with a perometer measurement of ≥5%-<10% LV increase during any follow-up assessment were encouraged to increase the frequency of muscle-tightening-breathing and pumping exercises until the LV returned to preoperative level verified by a perometer measurement in 4 weeks. Participants with a perometer measurement ≥10% LV increase during any follow-up assessment were referred for lymphedema therapy, usually consisting of Complete Decongestive Therapy [19].
Data Collection Procedure
We collected data on demographic and clinical information, LV and BMI at pre-surgery baseline, 2-4 weeks, 6-month, and 12-month after surgery. An electrical bioimpedance device (InBody 520, Biospace Co., Ltd) was used to measure the participants' weight, BMI was calculated using the formula: weight (kg) / height (m2).
Infra-red Perometry 350S (Juzo, Cuyahoga Falls, OH) was performed on each arm as it is held horizontally. The perometer maps a 3-dimensional graph of the affected and non-affected limbs using numerous rectilinear light beams, and interfaces with a computer for data analysis and storage. A 3-dimensional limb image was generated and limb volume calculated. This optoelectronic method has a standard deviation of 8.9 ml (arm), less than 0.5% of LV with repeated measuring [16-17].
Safety of The-Optimal-Lymph-Flow program was assessed by asking the participants if the program created any discomfort or injury to them at each follow-up visit. Feasibility of the program was evaluated in terms of intervention delivering time by the trained nurses. Acceptability of the program was assessed by asking the participants the following questions: if the program helped the participants to (1) understand how to reduce the risk of lymphedema; (2) reduce the fear and anxiety of developing lymphedema; and (3) develop a plan to reduce the risk of lymphedema. Acceptability of the program was also assessed by the participants' practice of the risk reduction behaviors during the study period. A structured interview guide (Lymphedema Risk Reduction Behavior Checklist) was used to quantitatively and qualitatively assess patients' practice of risk reduction behaviors at the study endpoint of 12-month after surgery[13].
Data Analysis
Characteristics of the participants were summarized using descriptive statistics (means, standard deviations for continuous variables and frequency distributions and proportions for qualitative variables). Distributions of baseline patient demographic and clinical characteristics were compared for patients with SLNB and ALND using Chi-Squared tests for contingency tables and one-way analysis of variance for continuous variables. All statistical tests were conducted at the 0.05 significance level (2-sided) and 95% confidence intervals (CI) were provided for estimates.
Mixed effects regression models were used with subject specific intercepts (and slopes if appropriate) with fixed effects of time to evaluate the changes from baseline in outcome measures of LV and BMI over time. Participants were included as a random effect to allow for the incorporation within a subject as well as missing data over time [23]. Correlations were examined to identify potential interactions and redundancies among variables. Predictive covariates (such as age, ethnicity, lumpectomy vs. mastectomy, SNLB vs. ALND) were included in the models.
Results
Participants
A total of 197 women responded to the study invitation. Of the 197 patients screened, 178 (90.4%) were eligible for the study and 140 (78.7%) of those eligible consented to participate in the study. Those patients who were eligible but did not enroll in the study provided the following reasons: significant travel distance, difficulty in finding transportation or childcare, or stress from cancer diagnosis.
Among the 140 patients, 134 patients completed the study with a 4% attrition rate. The reasons for those who did not complete the 12-month follow-up were significant travel distance (3 patients), death from cardiac related event (1 patient), and withdrawal of consent due to stress from chemotherapy (2 patients). Since lymph node procedures are major risk factors for lymphedema [3,18], we stratified the participants into axillary node dissection (ALND) group (n=81; 58%) and sentinel lymph node biopsy (SLNB) group (n=59; 42%). Participants in the two groups were comparable with no significant differences in terms of education, marital status, and employment status. The participants in the SLNB group were significantly older than those in the ALND group. Significantly more women in the ALND group had mastectomy, more lymph nodes removed, and chemotherapy. No significant differences were found between ALND and SLNB groups in terms of body weight and BMI and radiotherapy. Cancer surgery was performed on the side ipsilateral to the dominant hand in 67 patients (48%) and on the side of the nondominant hand in 73 patients (52%). Table 2 provides a summary of theses comparisons of demographic and clinical characteristics.
Table 2. Demographic and Clinical Characteristics of Participants by Type of Node Dissection.
| Total N=140 | SLNB n=59 | ALND n= 81 | p Value* | |
|---|---|---|---|---|
| Mean (SD; Range) |
Mean (SD; Range) |
Mean (SD; Range) |
||
| Age at Diagnosis (in years) | 56.0 (11.8; 25-84) |
58.5 (10.7; 42-84) |
54.1 (12.3; 25-81) |
0.03 |
| Body Weight (in pounds) | ||||
| Before Surgery | 155.8 (36.9; 104.7-278.6) |
152.3 (35.9; 104.7-277.3) |
157.7 (37.8; 106-278.6 |
0.48 |
| 2-4 Weeks After Surgery | 154.9 (36.1; 102.9-279.9) |
153.0 (36.3; 102.9-273.4) |
156.3 (36.1; 106.9-279.9) |
0.60 |
| At 6 Months | 153.5 (35.0; 102.7-282.8) |
152.1 (35.8; 102.7-277.5) |
154.6 (34.7; 108.7-282.8) |
0.68 |
| At 12 Months | 154.8 (36.1; 104.9-284.4) |
153.3 (36.6; 106.2-281.8) |
155.8 (35.7; 104.9-284.4) |
0.69 |
| Body Mass Index (BMI) | ||||
| Before Surgery | 26.6 (5.6; 17.7-46.1) |
26.6 (5.7; 17.7-46.1) |
26.6 (5.6; 18.1-41.4) |
0.96 |
| 2-4 Weeks After Surgery | 26.4 (5.5; 17.1-45.5) |
26.5 (5.8; 17.1-45.5) |
26.3 (5.3; 17.9-41.6) |
0.83 |
| At 6 months | 26.2 (5.3; 17.1-46.2) |
26.5 (5.7; 17.1-46.2) |
26.0 (5.0; 18.6-40.1) |
0.60 |
| At 12 months | 26.4 (5.5; 17.1-46.9) |
26.7 (5.9; 17.9-46.9) |
26.2 (5.2; 17.1-39.7) |
0.62 |
| Highest Level of Education | n(%) | n(%) | n(%) | 0.28 |
| High School or Below | 25 (18) | 12 (20) | 13 (16) | |
| Associate's Degree | 22 (16) | 9 (15) | 13 (16) | |
| Bachelor's Degree | 51 (36) | 20 (34) | 31 (38) | |
| Master's Degree | 30 (21) | 12 (20) | 18 (22) | |
| Doctoral Degree | 12 (9) | 6 (10) | 6 (8) | |
| Marital Status | 0.17 | |||
| Married | 77 (55) | 32 (54) | 44 (55) | |
| Partnered | 6 (4) | 0 (0) | 6 (7) | |
| Divorced/Separated | 19 (14) | 8 (14) | 11 (14) | |
| Widowed | 13 (9) | 9 (15) | 4 (5) | |
| Single or Never Partnered | 25 (18) | 10 (17) | 15 (19) | |
| Ethnicity | ||||
| Asian | 18 (13) | 8 (13) | 10 (12) | |
| African American or Black | 13 (9) | 3 (5) | 10 (12) | |
| White | 102 (73) | 46 (78) | 56 (69) | |
| Hispanic/Latino | 7 (5) | 2 (3) | 5 (6) | |
| Employment Status | 0.70 | |||
| Unemployed | 45 (32) | 20 (34) | 25 (31) | |
| Employed | 95 (68) | 39 (66) | 56 (69) | |
| Surgery | n (%) | n (%) | n (%) | 0.02 |
| Mastectomy | 21 (15) | 7 (12) | 14 (17) | |
| Lumpectomy | 61 (44) | 34 (58) | 27 (33) | |
| Immediate Reconstruction | 58 (41) | 18 (30) | 40 (50) | |
| Radiotherapy | 94 (67) | 37 (63) | 57 (70) | 0.35 |
| Neoadjuvant chemotherapy | 17 (12) | 0 (0) | 17 (21) | 0.05 |
| Adjuvant chemotherapy | 55 (39) | 11 (19) | 44 (54) | 0.05 |
| Number of nodes removed |
Median 8 |
Median 2 |
Median 13 |
<0.001 |
One-way analysis of variance was used for continuous variables and Chi-square tests for contingency tables.
Lymphedema and Limb Volume Changes
At the study endpoint of the 12-month following cancer surgery, no patients in the SLNB and ALND group exceeded ≥10% LV increase. Two patients (3%) in the SLNB group had 10% LV increase at the 2-4 weeks post-surgery visit; 2 patients (2%) in the ALND group had 10% LV increase at 6-month follow-up. All of the four women with 10% LV increase at the 2-4 weeks post-surgery and at the 6-month visit decreased their LV to <5% at the 12-month visit. Among the 12 patients (8.5%) with >5%-<10% LV increase at the 6-month follow-up, all the patients decreased their LV to <5% at the 12-month follow-up but one patient sustained 6% LV increase. Two patients with 0-<5% LV at the post-surgery visit progressed to >10% LV increase at the 6-month visit but decreased to <5% at the12-month visit; 7 patients without LV increase in the ALND group at the 6-month visit progressed to >5%-<10% LV increase at the 12-month visit.
For the affected and unaffected limbs, LV decreased monotonically over the time (mixed effects regression analyses: affected p=0.0004, unaffected p<0.0001), adjusted for age, ethnicity, SLNB vs. ALND, and mastectomy vs. lumpectomy. African American participants had the highest LV while Asian American participants had the lowest LV for both affected and unaffected limb at the baseline and at the 12-month visit. Figure 1.
Figure 1. Limb Volume Changes Over 12-Month Time Period.
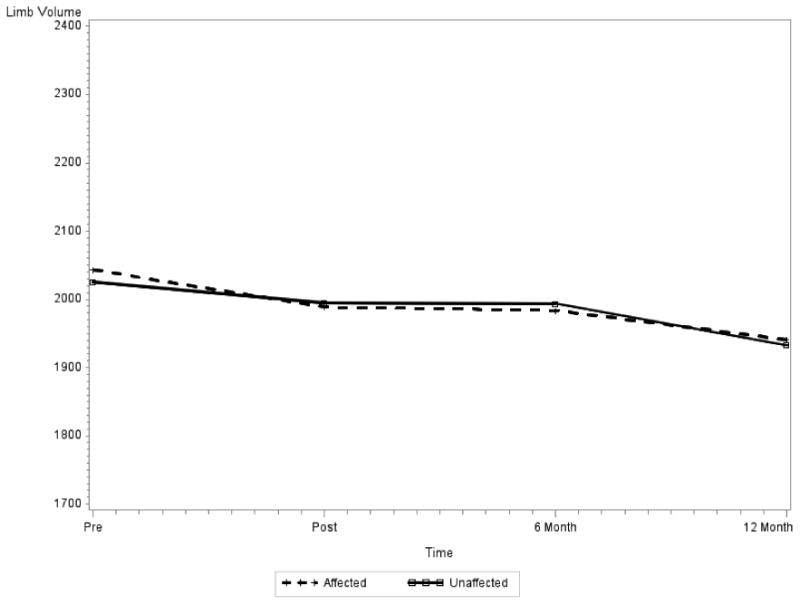
Note: As shown above, the limb volume is decreasing over time, for both affected and unaffected arms (affected p=0.0004, Unaffected p<0.0001)
Among the 8 patients who received lymphedema therapy, two of the 4 patients from the SLNB group had >10% LV increase; two of the 4 patients from the ALND group had 10% LV increase. Only 3 patients wore a compression sleeve daily and 2 patients used bandaging when the swelling was worse. Among 52 participants who had air travel at least once during the 12-month study period, 50 patients did travel without wearing a compression sleeve and glove and they did not develop lymphedema, among whom 30 patients underwent ALND. Among the 2 patients, one patient who wore a compression sleeve and glove during air travel did not develop lymphedema, and the other patient who only wore a compression sleeve did develop lymphedema in the affected hand during the travel. Her hand swelling returned to normal in 4 weeks after daily muscle-tightening-breathing and pumping exercises.
Body Weight and BMI
Participants' body weight did not change significantly over the 12 month study period (p=0.41 after adjustment for age, ethnicity, SLNB vs. ALND, and mastectomy vs. lumpectomy). Ethnicity was significantly related to weight (p<0.001); African American women had higher body weight on average at the baseline. There was no significant change in participants' BMI over the 12 months from the pre-surgery baseline, post-surgery, 6-month and 12-month follow-up visit (adjusted for age, ethnicity, SLNB vs. ALND, and mastectomy vs. lumpectomy; mixed effects regression analysis), participants' BMI were close at four time points (p=0.67). Age and ethnicity were significantly related to BMI at pre-surgery baseline (Age p=0.03, Ethnicity p<0.001). Older patients and African American patients had higher BMI (Age p=0.004; Ethnicity p<0.001). Figure 2.
Figure 2. Body Mass Index and Weight Over 12-Month Time Period.
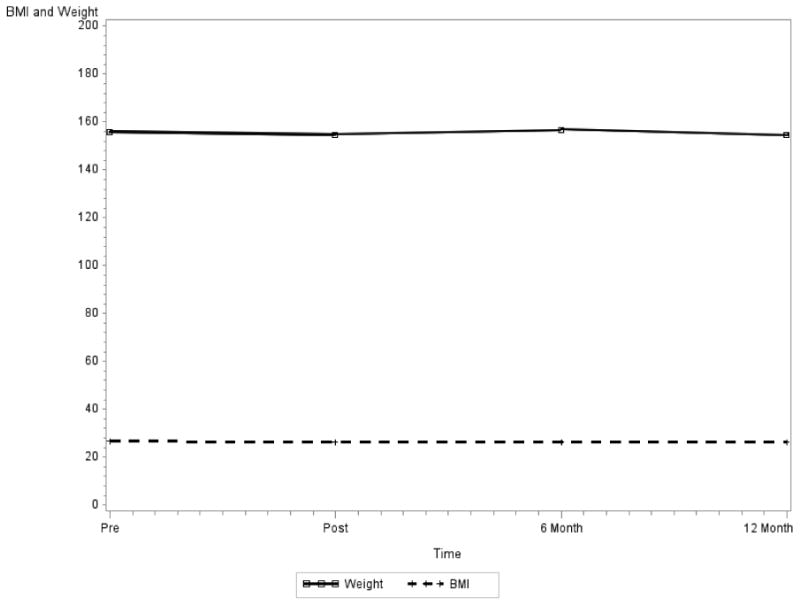
Note: BMI and Weight are not significantly changing over time (BMI p=0.6650, Weight p=0.4086)
Safety, Feasibility, and Acceptability
At each follow-up visit, no participants reported injury or discomfort associated with The-Optimal-Lymph-Flow program. It took about 30 minutes for the trained nurses to deliver the program. Among the 134 patients who completed the study, more than 90% of the participants performed the risk reduction strategies. Quantitative and qualitative evaluation provided positive support for The-Optimal-Lymph-Flow program. See Table 3.
Table 3. Quantitative and Qualitative Evaluation of The-Optimal-Lymph-Flow Program.
| n (%) | n (%) | |||
|---|---|---|---|---|
|
| ||||
| **Risk Reduction Strategies | Yes | No | ||
|
| ||||
| Have you been performing Muscle-Tightening Deep Breathing? | •Daily | 82 (62%) | 7 (5%) | |
| •2-3 Times a Week | 33 (25%) | |||
| •When discomfort occurs | 11 (8%) | |||
|
| ||||
| Have you been performing Muscle-Tightening Pumping? | •Daily | 83 (62%) | 5 (4%) | |
| •2-3 Times a Week | 30 (23%) | |||
| •When discomfort occurs | 15 (11%) | |||
|
| ||||
| Have you been performing Large Muscle Exercises? | •Daily | 66 (49%) | 31 (23%) | |
| •2-3 Times a Week | 37 (28%) | |||
|
| ||||
| Have you been performing Shoulder Exercises? | •After Surgery | 134 (96%) | •After Surgery | 6 (4%) |
| •12-Month after Surgery | 25 (18.7%) | •12-Month after Surgery | 109 (81.3%) | |
|
| ||||
| Have you been eating nutrition-balanced and portion -appropriate diet? | •Daily | 106 (79%) | 5 (4%) | |
| •2-3 Times a Week | 24 (18%) | |||
|
| ||||
| **No significant differences in practicing the risk reduction strategies between patients undergoing SLNB and ALND. | ||||
| *** The-Optimal-Lymph-Flow Program… | Yes | No | ||
|
| ||||
| Helped me to understand how to reduce my risk of lymphedema. | 121 (90.3%) | 13 (9.7%) | ||
|
| ||||
| Helped me to reduce my fear and anxiety of developing lymphedema. | 117 (87.3%) | 17 (12.7%) | ||
|
| ||||
| Helped me to develop a plan to reduce my risk of lymphedema. | 105 (78.4%) | 29 (21.6%) | ||
|
| ||||
| Created injury or discomfort to me. | 0 (0%) | 134 (100%) | ||
|
| ||||
| ***No significant differences in positive responses between patients underwent SLNB and ALND. | ||||
|
| ||||
|
Summary of Themes from Qualitative Data 123 (91.8%) participants Provided Qualitative Evaluation of The-Optimal-Lymph-Flow Program | ||||
|
| ||||
| Themes | Representative Quotes | |||
| Empowerment | ** “Being aware of lymphedema risk and informed about it helped me tremendously. I didn't know what lymphedema was… I felt more in control rather than just hoping I would not get lymphedema. I was doing something to prevent it. It gave me a sense of empowerment.” ** “The best thing I have done for myself was to participate in The-Optimal-Lymph-Flow program. I only had 1 node removed. I thought that I am fine. During the radiation, I had slight swelling in my arm and I started religiously doing the breathing and pumping exercises. It worked and now I am doing the exercises every day because I feel good after doing the exercises. Without the program, I probably would be like my friend who has a huge arm now.” ** “I truly believe that participating in The-Optimal-Lymph-Flow program has been the pillar of strength for me following my mastectomy and lymph node dissection. The program enabled me to feel armed with knowledge and preventive measures to keep me from getting lymphedema.” |
|||
|
| ||||
| Easy to Do | ** “It is a simple program and awareness of the result is also motivating.” ** “I was very pleased that I could reduce my risk with very simple techniques (breathing, pumping, & walking).” ** “The pumping & breathing are something I can do. I can do more and feel better.” |
|||
|
| ||||
| Skills Building | ** “Wonderful to have been aware before surgery of the exercises and get into the habit of doing them. After surgery, I didn't have to review the direction and just began to do what I had been doing already.” ** “I am doing the right exercise (breathing and pumping) on the daily basis. I feel good and I feel that I owe it to myself.” ** “Being aware of factors that contribute to developing lymphedema and specific measures to alleviate symptoms has been instrumental in reducing any swelling. I have experienced and have motivated/aware of making choice to reduce my risk.” |
|||
|
| ||||
| Psychological Benefits: Fear and Anxiety Relief | ** “This program was wonderful for not making me feel like a victim and being able to take charge of my own feeling.” ** “…eased my concerns about lymphedema.” ** “Give me more confidence and motivation to get better.” ** “Helps me to be more aware of having cancer and understanding the way it is affecting my day-to-day living. The process of measurement and follow-ups are essential to maintain good health. I have a whole new outlook on life.” |
|||
|
| ||||
| Forget Doing the Exercises | ** “In the beginning I followed the directions for breathing and pumping and other precautions carefully. After about 4 months I became casual because I feel very good.” ** “I forget doing the exercises a lot because my arm feels good.” |
|||
Discussion
Annually, more than 230,000 women are diagnosed with breast cancer in the US [20]. A large number of women each year still face the life-time risk of developing this progressive and debilitating condition even with the most conservative estimates suggesting that 3% of women with SLNB and 20% of those with ALND developed lymphedema at 12 months following breast cancer surgery [18,21-22]. Provision of patient education and risk reduction strategies is critical to the improvement of patients' quality of life.
Although clinical and personal risk factors have been identified, rational preventive strategies derived from scientific evidence are lacking. Consistent with previous research [16,18], preoperative baseline and consecutive follow-up measurements are vital to successfully detect fluid accumulation. In our study, patients were provided with their measurements at each time point and encouraged to continue The-Optimal-Lymph-Flow strategies. Our study demonstrated safety, acceptance, and feasibility of self-care behaviors to promote lymph flow and optimize BMI. This is an important initial step targeting identified lymphedema risk of compromised lymphatic system and high BMI. Our study provided initial evidence that self-care strategies may be effective, pragmatic, and low-cost for reducing lymphedema risk.
Over 50% of breast cancer survivors were found to be exceedingly worried about their risk of developing lymphedema [18]. Over 90% of our patients reported that The-Optimal-Lymph-Flow program helped them to reduce their fear and anxiety of developing lymphedema. Perhaps, one of the contributions of our study is that the The-Optimal-Lymph-Flow program has increased patients' awareness of lymphedema, demonstrated patients' willingness to perform self-care strategies and feeling empowered and less anxious. Such findings are clinically relevant to support that patients should be educated regarding their lymphedema risk. Furthermore, empowering patients by focusing on what patients can do rather than what patients can avoid perhaps is the key, as our patients stated in the qualitative data. Table 3.
The full range of factors that influence the etiology and progression of lymphedema remains unknown and this knowledge gap has hindered the development of effective risk reduction strategies. Although some LV changes in our study might be due to the natural fluctuation during the first year of cancer surgery, in comparison to the most recent data that 3% of women with SLNB and 20% of those with ALND developed lymphedema with the same definition as in our study, i.e. >10% LV increase at 12 months following breast cancer surgery [18], no patients in our study increased ≥10% LV at the 12-month follow-up. Cumulatively, only 2 patients with SLNB (3%) and 2 patients with ALND (2%) had measurable lymphedema (>10% LV increase) at 2-4 weeks and at 6-month follow-up. Research demonstrated that women with mild lymphedema are more prone to progress to severe lymphedema [3]. In our study, among the 12 patients with >5%-<10% LV increase at the 6-month follow-up, all the patients decreased their LV to <5% at the 12-month visit but one patient from ALND group sustained 6% LV increase. Our patients demonstrated that self-care strategies helped to maintain pre-operative LV and halt the progression of fluid accumulation. It should be noted that general instructions on having nutrition-balanced and portion-appropriate diet and physical activities daily or weekly was effective for our patients to maintain their pre-operative BMI.
We recognize that this pilot study was limited because it did not have a randomized-controlled design. The strengths of our study included well-designed intervention targeting known lymphedema risk of compromised lymphatic system and high BMI, well-defined outcomes, adequate sample size for a feasibility study, prospective and consecutive repeated measurement at meaningful time points, as well as quantitative and qualitative evaluation of the program. It should be noted that the majority of patients discontinued shoulder exercises at 12-month follow-up. Our patients perceived that either there was no need or forgot doing the shoulder exercises because of their shoulder and limb function returned to the pre-surgical level. One acceptance testing was to discern which behaviors was initiated, continued, or discontinued by the participants. Our data showed that perhaps shoulder exercises might be discontinued once the function of the affected shoulder and limb has returned to pre-surgical level. Nevertheless, future research requires a larger study with a randomized-controlled design to evaluate the overall effectiveness of The-Optimal-Lymph-Flow program and to determine the dosage and contribution of individual component of the program. Research should continue providing evidence on the need to avoid air travel or wear compression garments during air travel and the ethnic differences in limb volume.
Acknowledgments
This study was supported by the Avon Foundation, National Institute of Health (NIMHD Project # P60 MD000538-03), Judges and Lawyers for Breast Cancer Alert, and the Vital Fund. Dr. Judith D. Goldberg was partially supported by NYU School of Medicine CCSG NCI 5 P30 CA16087-32. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Partial findings were presented at the 2012 Breast Cancer Symposium (September 13-15, 2012) in San Francisco, CA.
Footnotes
Statement of Financial Interest: All the authors have no financial interest or commercial association with information submitted in manuscript.
List of Products Used: No products, devices, or drugs were used or identified in the manuscript.
References
- 1.Fu MR, Ridner SH, Hu SH, et al. Psychosocial Impact of Lymphedema: A systematic review of literature from 2004 to 2011. Psycho-Oncol. 2013;22(7):1466–84. doi: 10.1002/pon.3201. Epub 2012 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chachaj A, Malyszczak K, Pyszel K, et al. Physical and psychological impairments of women with upper lymphedema following breast cancer treatment. Psycho-Oncol. 2009;19:299–305. doi: 10.1002/pon.1573. [DOI] [PubMed] [Google Scholar]
- 3.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(6):1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Armer JM, Stewart BR, Shook RP. 30-Month Post-Breast Cancer Treatment Lymphoedema. J Lymphoedema. 2009;4(1):14–18. [PMC free article] [PubMed] [Google Scholar]
- 5.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. Consensus document of the International Society of Lymphology Lymphology. 2003;36:84–91. [PubMed] [Google Scholar]
- 6.Stanton AW, Modi S, Mellor RH, et al. Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphatic Res & Biol. 2009;7(1):29–45. doi: 10.1089/lrb.2008.1026. [DOI] [PubMed] [Google Scholar]
- 7.Brorson H, Aberg M, Svensson H. Chronic lymphedema and adipocyte proliferation: Clinical therapeutic implications. The Lymphatic Continuum. National Institutes of Health, Bethesda, USA, 2002. Lymphat Res Biol. 2003;1:88. [Google Scholar]
- 8.Mak SS, Yeo W, Lee YM, et al. Predictors of lymphedema in patients with breast cancer undergoing axillary lymph node dissection in Hong Kong. Nurs Res. 2008;57:416–425. doi: 10.1097/NNR.0b013e31818c3de2. [DOI] [PubMed] [Google Scholar]
- 9.Kwan ML, Darbinian J, Schmitz KH, et al. Risk factors for lymphedema in a prospective breast cancer survivorship study: the Pathways Study. Arch Surg. 2010;145:1055–1063. doi: 10.1001/archsurg.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed RL, Schmitz KH, Prizment AE, et al. Risk factors for lymphedema in breast cancer survivors, the Iowa Women's Health Study. Breast Cancer Res Treat. 2001;130:981–991. doi: 10.1007/s10549-011-1667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paskett ED, Naughton MJ, McCoy TP, et al. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16:775–782. doi: 10.1158/1055-9965.EPI-06-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruocco V, Schwartz RA, Ruocco E. Lymphedema: An immunologically vulnerable site for development of neoplasms. J Am Acad Dermat. 2002;47:124–127. doi: 10.1067/mjd.2002.120909. [DOI] [PubMed] [Google Scholar]
- 13.Fu MR, Chen C, Haber J, et al. The effect of providing information about lymphedema on the cognitive and symptom outcomes of breast cancer survivors. Ann Surg Oncol. 2010;17(7):1847–1853. doi: 10.1245/s10434-010-0941-3. Epub 2010 Feb 6. [DOI] [PubMed] [Google Scholar]
- 14.Cemal Y, Pusic A, Mehrara BJ. Preventative measures for lymphedema: separating fact from fiction. J Am Coll Surg Oct. 2011;213(4):543–51. doi: 10.1016/j.jamcollsurg.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen I, Gordon S, Selby A. Breast cancer-related lymphoedema risk reduction advice: a challenge for health professionals. Cancer Treat Rev. 2008;34(7):621–8. doi: 10.1016/j.ctrv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Stout NL, Binkley JM, Schmitz KH, Andrews K, Hayes SC, Campbell KL, et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer. 2012;118(8 Suppl):2191–200. doi: 10.1002/cncr.27476. [DOI] [PubMed] [Google Scholar]
- 17.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–217. doi: 10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin SA, Bagaria S, Gibson T, et al. Trends in risk reduction practices for the prevention of lymphedema in the first 12 months after breast cancer surgery. J Am Coll Surg. 2013;216:380–389. doi: 10.1016/j.jamcollsurg.2012.11.004. 216 (3) [DOI] [PubMed] [Google Scholar]
- 19.Lasinski BB, Thrift KM, Squire D, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. Physic Med & Rehab. 2012;4(8):580–601. doi: 10.1016/j.pmrj.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 20.American Cancer Society. Breast Cancer Facts & Figures 2013-2014. Atlanta: American Cancer Society, Inc.; 2013. [Google Scholar]
- 21.Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27(12):2007–14. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Data Analysis. New Jersey: John Wiley & Sons; 2004. [Google Scholar]


