Abstract
Background
We analyzed the effect of peri-transplant prophylaxis on the epidemiology of bacteremia in a 12-year contemporary cohort of allogeneic HSCT recipients at our center.
Methods
This was an observational study of 1,052 consecutive adult HSCT from 2000 to 2011. Formal prophylaxis with vancomycin only, fluoroquinolone (FQ) only, or vancomycin+FQ was implemented in 2006. The cumulative incidence of day 100 bacteremia was compared between the Early Period (2000-2005) and the Recent Period (2006-2011). Predictors for pre-engraftment bacteremia were analyzed with Cox-proportional hazard models in a subcohort of 821 HSCT who received myeloablative or reduced intensity conditioning (MA/RIC).
Results
The incidence of bacteremia decreased in the Recent Period (32% vs 27%; P=0.002), whereas the rates of resistance in gram-negative rods (GNR) and vancomycin-resistant enterococci (VRE) were similar between the two Periods (P values are not statistically significant.) In multivariate analyses, prophylaxis with vancomycin only or vancomycin+FQ was protective (HR=0.5; CI=0.30-0.72) and (HR=0.3; CI=0.12-0.52, P<0.01). Vancomycin or vancomycin+FQ eliminated viridans streptococcal bacteremia (VSB); vancomycin+FQ decreased GNR bacteremia (HR=0.35; CI=0.15-0.85).
Conclusions
Vancomycin-based prophylaxis peri-transplant in MA/RIC HSCT was associated with elimination of VSB and may be considered at centers with high incidence of this infection.
Keywords: allogeneic transplant, bacteremia, vancomycin prophylaxis, fluoroquinolone prophylaxis, viridans streptococci
INTRODUCTION
Bacteremia is a recognized cause of morbidity and mortality in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) (1-3). Neutropenia, indwelling intravenous catheters, and mucositis of the gastrointestinal tract are major risk factors for bacteremia in the peri-transplant period (4, 5).
Various antibiotic prophylaxis strategies have been studied in cancer patients to reduce bacterial infection during chemotherapy. While fluoroquinolone (FQ) prophylaxis is currently recommended in neutropenic patients with hematologic malignancies due to a demonstrated reduction in mortality (6), its use has been associated with an increase in infections due to FQ-resistant bacteria, Clostridium difficile enterocolitis, and vancomycin-resistant enterococci (VRE) (7-9). Importantly, FQ prophylaxis has been reported as a risk factor for viridans streptococcal bacteremia (VSB) in neutropenic patients with cancer (10, 11). At present, the choice of prophylaxis at each transplant center is based on local epidemiology and physician preference.
At Memorial Sloan Kettering Cancer Center (MSKCC), peri-transplant antibiotic prophylaxis was not routinely administered prior to 2006. The incidence of pre-engraftment VSB was 7.4% with an attributable morality of 21% (12). Receipt of two or more doses of intravenous (IV) vancomycin from day -7 through day +7 was associated with protection from VSB (12). In contrast, FQ or beta-lactam antibiotics pre-transplant were not shown to be protective (12). Prevention of VSB was therefore a high priority in our center. In February 2004, we started a single center, randomized, open label study to evaluate the effectiveness of prophylactic vancomycin compared to empiric vancomycin given at first neutropenic fever for prevention of VSB. Twenty-eight patients were enrolled in the study (14 patients in each arm). Two patients in the empiric vancomycin arm developed VSB and required intensive care unit admission and one subsequently died due to VSB. Because of a trend for lower incidence of VSB in the prophylaxis arm (0% vs 14%, P=0.14; data not published), the study was terminated in November 2005. Since 2006, we implemented IV vancomycin prophylaxis, alone or with FQ, during the pre-engraftment period to prevent VSB.
The primary objective of this study was to describe the overall incidence of bacteremia and rates of antimicrobial resistance in blood culture isolates during the first 100 days post transplant in a 12-year contemporary cohort of adult allogeneic HSCT recipients. In addition, we sought to assess the effect of the implementation of routine peri-transplant antibacterial prophylaxis with IV vancomycin by comparing the incidence of bacteremia and antimicrobial resistance rates before (Early Period, 2000 -2005) and after (Recent Period, 2006-2011) this intervention. Finally, we examined the impact of antibiotic prophylaxis on pre-engraftment bacteremia in patients who received myeloablative or reduced intensity conditioning (MA/RIC) regimens.
METHODS
Patients and standards of care
The study was reviewed and approved by the MSKCC Institutional Review Board. MSKCC is a 432-bed tertiary care facility in New York City. The study cohort is comprised of 1,052 consecutive adult patients who underwent allogeneic HSCT at MSKCC between January 1, 2000 and December 31, 2011.
Antibiotic primary prophylaxis strategies
Patients received prophylaxis for Pneumocystis jiroveci, herpes viruses, and fungi as per standard guidelines [4]. From January 2000 through December 2005 (Early Period), antibiotic prophylaxis in the peri-transplant period was not routinely given. Since January 2006 (Recent Period), recipients of peripheral blood or marrow allografts undergoing MA/RIC regimens received IV vancomycin 1 gram every 12 hours as prophylaxis for VSB. Recipients of umbilical cord blood (UCB) allografts received vancomycin and FQ (ciprofloxacin 500 mg orally every 12 hours or 400 mg IV every 12 hours). Starting July 2011, all patients undergoing MA/RIC received IV vancomycin and ciprofloxacin regardless of stem cell source.
Antibiotic prophylaxis was started two days prior to stem cell infusion (day -2). FQ prophylaxis was continued until engraftment or onset of febrile neutropenia (FN), whichever occurred first. Vancomycin prophylaxis was continued until day+7, after which continuation of vancomycin was at the discretion of the treating physician as needed for management of FN or treatment of a documented infection.
Management of Febrile Neutropenia
The first-line empiric regimen for FN was ticarcillin/clavulanate and amikacin until June 2002 when the regimen was switched to piperacillin/tazobactam plus amikacin. The hospital converted to piperacillin/tazobactam monotherapy in the summer of 2010. Empiric therapy for FN was discontinued when patients were afebrile and had an absolute neutrophil count (ANC) at least 500/mm3 with anticipated rising trend. Antibiotic regimen and duration were adjusted for allergies, documented infections, or other clinical indications (13).
Transplant Protocols
MA and RIC transplant protocols have been previously described (14-16). In brief, in recipients of adult donor T-cell depleted (TCD) allografts, conditioning regimens containing hyperfractionated total body irradiation (HFTBI) consisted mainly of either thiotepa and fludarabine or thiotepa and cyclophosphamide. All non-HFTBI regimens included mainly busulfan and melphalan with or without fludarabine. Peripheral blood grafts underwent CD34+ cell selection using the ISOLEX 300i magnetic cell selection system (Baxter, Deerfield, IL), followed by sheep red blood cell–rosette depletion until 2010 when CD34+ cell selection was performed by the CliniMACS cell selection system™ (Miltenyi Biotech, Gladbach, Germany). Bone marrow (BM) grafts were used upon donor preference and were depleted of T cells by sequential soybean lectin agglutination and sheep red blood cell–rosette depletion. All adult donor allograft recipients undergoing T-cell depletion received anti-thymocyte globulin (ATG) to prevent graft rejection, except for those patients receiving a transplant from a HLA-matched related donor and conditioned with HFTBI+thiotepa+fludarabine (14). Patients who received ex vivo TCD allografts did not receive exogenous graft-versus-host disease (GVHD) prophylaxis post-transplantation. Unmodified adult donor grafts were given with high dose or RI conditioning as clinically appropriate with tacrolimus-based immunosuppression. Conditioning in UCB transplant recipients has been previously described, and immunosuppression was with a calcineurin inhibitor (predominantly cyclosporine-A) and mycophenolate mofetil.
Laboratory methods
Blood cultures were obtained at the discretion of the treating physician for workup of fever or other clinical signs of infection. Follow-up blood cultures were obtained routinely in patients with prior positive cultures. No routine surveillance blood cultures were drawn during the study period. Blood cultures were processed by the Clinical Microbiology Laboratory at MSKCC using the BACTEC™ 9240 systems (BD Diagnostics, Sparks, MD). Antimicrobial susceptibility testing was performed on either the MicroScan autoScan or Walkaway instruments (Siemens Healthcare Diagnostics, Inc., Tarrytown, NY) and data interpreted based on published guidelines (17, 18). Extended broad spectrum β-lactamase (ESBL) detection was based on resistance to ceftazidime and/or cefotaxime with confirmation (3-fold decrease in MIC when ceftazidime and/or cefotaxime were tested with clavulanic acid) by the MicroScan instrument (17, 18). Isolates were defined as multidrug-resistant (MDR) if they were resistant to at least 2 of the following: cefepime, piperacillin/tazobactam, or carbapenems. Pathogens with intermediate susceptibility were considered resistant.
Definitions
Antibiotic prophylaxis was defined as administration of one or more doses of vancomycin intravenously and/or FQ orally or intravenously from day-2 through day+2, regardless of routine prophylaxis or for clinical indication.
Bacteremia was defined as the isolation of a bacterial pathogen from one or more blood cultures from day -7 to day 100 post-transplant. For common skin contaminants such as coagulase negative staphylococci (CoNS), corynebacteria, Bacillus spp. and diphtheroids recovery from two or more consecutive blood cultures was required (19). If more than one pathogen was isolated from the same blood culture, each pathogen was counted to calculate pathogen-specific rates.
Pre-engraftment bacteremia was defined as bacteremia occurring from day -2 to the day of neutrophil engraftment. Date of neutrophil engraftment was defined by the first day of three consecutive days with an ANC >500/mm3. Incidence of pre-engraftment bacteremia for MA/RIC is expressed as number of cases per 1,000 patient-days at risk.
Data collection
An institutional Clinical Research Data Base (CRDB) was utilized to prospectively collect patient's demographics, transplant characteristics, survival, laboratory, and microbiology data. Administration of antibiotics in the peri-transplant period was extracted from pharmacy records.
Statistical analysis
The primary objective of the study was to assess the effect of peri-transplant antibacterial prophylaxis on the epidemiology of bacteremia from day-7 to day 100 in a contemporary cohort of 1,052 HSCT during a 12-year period by comparing overall incidence types of bacteria and rates of antibacterial resistance between early and recent periods as defined by prophylactic antibiotic administration.
Chi-square test, Fisher's exact test, and Kruskal-Wallis rank sum test were used to compare baseline transplant characteristics between the two periods (Early Period and Recent Period). The log rank test was used to compare cumulative incidence between Early Period and Recent Period. Death, relapse, or second HSCT were competing risks for bacteremia.
Our secondary objective was to define predictors for pre-engraftment bacteremia in a subcohort of 821 MA/RIC HSCT. Patients were considered at risk for pre-engraftment bacteremia from day -2 until engraftment, death prior to engraftment, or development of bacteremia, whichever occurred first. The incidence of pre-engraftment bacteremia per 1,000 patient-days was estimated by dividing the total number of pre-engraftment bacteremia cases by the sum of patient-days at risk.
Time-dependent Cox-proportional hazard model was used to estimate the Hazard ratios (HRs) and 95% confidence intervals (CIs) for each risk factor. To establish a multivariate model for risk factors, a stepwise forward selection approach was used with significance level of entry as 0.3 and significance level of stay of 0.1 in multivariate analysis. Antibiotic prophylaxis and transplant periods were entered as a priori variables. We considered two-sided P value ≤ 0.05 to be statistically significant. Statistical analyses were performed using Statistical Analysis Software (SAS) version 9.2 (Cary, NC, USA).
RESULTS
Epidemiology of bacteremia
Table 1 shows the baseline characteristics of the 1,052 HSCT. Three hundred fifty -six (34%) patients received HSCT in the Early Period and 696 (66%) patients in the Recent Period. Baseline and transplant characteristics differed substantially between the two Periods.
Table 1.
Characteristics of the transplant cohort (n=1,052), by transplant period.
| Characteristics | Early Period (2000-2005) N (%) | Recent Period (2006-2011) N (%) | P value |
|---|---|---|---|
| Total N = 356 | Total N = 696 | ||
| Age | <0.01 | ||
| Median age, years (range) | 46 (19-71) | 52(18-73) | |
| Sex | 0.48 | ||
| Male | 154 (43) | 284 (41) | |
| Female | 202 (57) | 412 (59) | |
| Diagnosis | <0.01 | ||
| Acute leukemia/MDS | 211 (59) | 397 (57) | |
| CML | 27 (8) | 18 (3) | |
| Lymphoma/CLL | 87 (24) | 217 (31) | |
| Multiple myeloma | 11 (3) | 52 (7) | |
| Other1 | 20 (6) | 12 (2) | |
| Donor Type | <0.01 | ||
| Matched related | 198 (56) | 245 (35) | |
| Matched unrelated | 89 (25) | 228 (33) | |
| Mismatched 2 | 69 (19) | 223 (32) | |
| Stem cell source | <0.01 | ||
| Bone marrow | 105 (29) | 26 (4) | |
| Peripheral blood | 251 (71) | 565 (81) | |
| Umbilical cord blood | 0 | 105 (15) | |
| T-cell Depletion | <0.01 | ||
| No | 116 (33) | 356 (51) | |
| Yes | 240 (67) | 340 (49) | |
| Conditioning | <0.01 | ||
| Myeloablative | 222 (62) | 325 (47) | |
| Reduced intensity | 67 (19) | 216 (31) | |
| Non-myeloablative | 67 (19) | 155 (22) |
Continuous variables are displayed as mean (range), categorical variables are displayed as N (%).
Other: Myeloproliferative disorder, solid tumor, non-malignant hematologic disorder.
related or unrelated.
Abbreviations: MDS: myelodysplastic syndrome; CML: chronic myeloid leukemia; CLL: chronic lymphocytic leukemia.
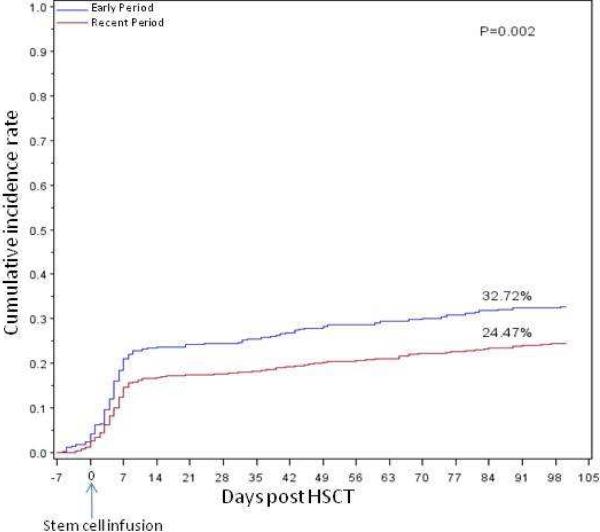
Two hundred eighty-nine (27.5%) patients had 341 episodes of bacteremia from day -7 to 100 with a total 362 bacterial isolates. The cumulative incidence of bacteremia decreased significantly (Early Period 33% vs Recent Period 24%; P=0.002) (Figure 1). However, for those patients who became bacteremic the median time to onset from stem cell infusion was 5 days during both periods (P=0.23).
Figure 1. Cumulative incidence of bacteremia in 1,052 HSCT recipients by transplantperiod.
Cumulative incidence rate of bacteremia by transplant period, starting from day -7. On Day 100, 33% of patients in Early Period 2000-2005 developed bacteremia, as compared to 24% of patients in Recent Period 2006-2011. The difference is statistically significant (P=0.002)
Table 2 shows the bacterial isolates by period. In the Early Period, Gram-positive bacteria comprised 63% of all isolates compared to 49% in the Recent Period. Notably, viridans streptococci comprised 18% of bacteria in the Early Period and only 2% in the Recent Period. VRE comprised 22 % and 29% of bacteria in the Early Period and the Recent Period, respectively (P=0.19). While the relative proportion of VRE in the Recent Period was numerically higher in the Recent Period, the percentage of patients with VRE bacteremia was similar in the two periods. Thirty- five of 356 (9.8%) patients had VRE bacteremia in the Early Period vs 58 of 696 (8.3%) in the Recent Period.
Table 2.
Bacteria isolated from blood cultures from 1,052 HSCT recipients according to transplant period .
| Organism | Early Period (2000-2005) N (%) | Recent Period (2006-2011) N (%) |
|---|---|---|
| Total N=159 | Total N=203 | |
| Gram-positive | 100 (63) | 100 (49) |
| Mucositis related | ||
| Viridans group streptococci | 29 (18) | 4 (2) |
| Enterococci | 44 (28) | 62 (30) |
| Vancomycin sensitive | 9 (6) | 4 (2) |
| Vancomycin resistant (VRE) 1 | 35 (22) | 58 (29) |
| Skin-related | ||
| Staphylococci | 23 (15) | 34 (17) |
| Coagulase-negative staphylococci | 20 (13) | 28 (14) |
| Staphylococcus aureus | 3 (2) | 6 (3) |
| Corynebacterium JK | 3 (2) | 0 |
| Bacillus spp. | 1 (0) | 0 |
| Gram-negative | 59 (37) | 103 (51) |
| Enterobacteriaceae | 35 (22) | 76 (36) |
| Escherichia coli | 14 (9) | 29 (14) |
| Klebsiella spp. | 13 (8) | 29 (14) |
| Enterobacter spp. | 6 (4) | 13 (6) |
| Serratia spp. | 2 (1) | 5 (2) |
| Non-fermenting GNR | 21 (13) | 23 (11) |
| Ps. aeruginosa | 5 (3) | 9 (4) |
| Ps. fluorescens | 0 | 2 (1) |
| S. maltophilia | 0 | 6 (3) |
| Acinetobacter spp. | 10 (6) | 4 (2) |
| Fusobacterium spp. | 6 (4) | 2 (1) |
| Other2 | 3 (2) | 4 (2) |
| Total number of allogeneic HSCT | 356 | 696 |
Includes all isolates from day-7 to day 100.
E. faecium comprised 34/35 (97%) of VRE in period A and 57/58 (98%) of VRE in Period B.
Includes: 1 Neisseria spp, 1 Bacteroides fragilis, 1 Achromobacter xylosoxidans, 1 Hemophilus parainfluenzae, 1 Roseomonas spp., 1 Proteus mirabilis, 1 Clostridium spp.
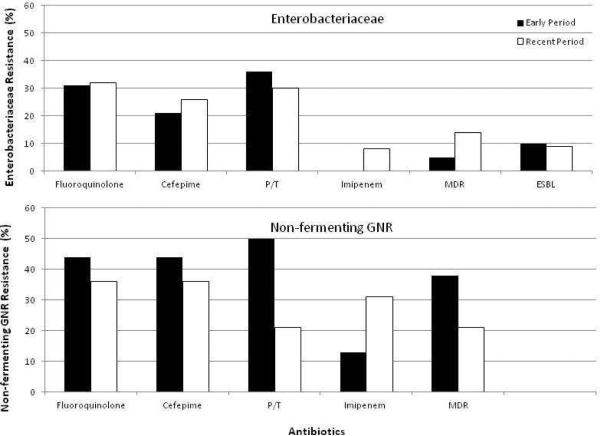
Figure 2 shows rates of antimicrobial resistance in Gram-negative rods (GNR) in the Early and Recent periods. ESBL-producing and MDR organisms were infrequent in both periods. There were no significant differences in rates of resistance in Enterobacteriaceae or non-fermenting GNR between the two periods.
Figure 2. Rates of antimicrobial resistance in Enterobacteriaceae and non-fermenting aerobic GNR.
Y- axis: percentage of resistant isolates. Black bar: Early Period 2000-2005; Blank bar: Recent Period 2006-2011. Pairwise comparison was performed between Early and Recent period for each antibiotic, P values are not significant for all.
1Fluoroquinolone resistance denotes resistance to ciprofloxacin or/and levofloxacin
2P/T:piperacillin/tazobactam
34 ESBL in Early Period and 7 ESBL in Recent Period contributed to the resistance of Cefepime and P/T.
4Non-fermenting GNR includes only Ps. aeruginosa and Acinetobacter spp.
5MDR (Multidrug resistance) was defined as resistant to at least 2 of the following: fluoroquinolone, cefepime, piperacillin/tazobactam, or carbapenems.
Pre-engraftment bacteremia
Vancomycin only or vancomycin+FQ prophylaxis was only administered to MA/RIC HSCT for prevention of pre-engraftment bacteremia. We next sought to examine the effect of vancomycin prophylaxis on the incidence of pre-engraftment bacteremia in MA/RIC HSCT. Ten patients who had bacteremia prior to antibiotic prophylaxis (day-2) and 220 patients who received non-myeloablative conditioning regimens were excluded from the analysis. This resulted in a cohort of 821 patients who received MA/RIC (Table 3).
Table 3.
Characteristics of the MA/RIC transplant cohort (n=821), by antibiotic prophylaxis
| Characteristic | None N (%) | Vancomycin Only N (%) | Vancomycin+FQ N (%) | FQ Only N (%) |
|---|---|---|---|---|
| Total N=140 | Total N=405 | Total N=240 | Total N=36 | |
| Age | ||||
| Median age, years (Range) | 47.2 (21.6 - 71.1) | 49 .8 (18.5 - 73) | 52.9 (21.4 - 71.3) | 46 .8 (21.6 - 68.4) |
| Sex | ||||
| Male | 67 (48) | 246 (61) | 128 (53) | 22 (61) |
| Female | 73 (52) | 159 (39) | 112 (47) | 14 (39) |
| Diagnosis | ||||
| Acute Leukemia/MDS | 89 (63) | 290 (72) | 189 (79) | 25 (69) |
| CML | 19 (14) | 19 (5) | 6 (3) | 1 (3) |
| Lymphoma/CLL | 24 (17) | 66 (16) | 30 (12) | 8 (22) |
| Multiple myeloma | 5 (4) | 26 (6) | 12 (5) | 1 (3) |
| Other1 | 3 (2) | 2 (1) | 3 (1) | 1 (3) |
| Donor Type | ||||
| Matched related | 89 (63) | 158 (39) | 75 (31) | 5 (14) |
| Matched unrelated | 32 (23) | 138 (34) | 71 (30) | 15 (42) |
| Mismatched2 | 19 (14) | 109 (27) | 94 (39) | 16 (44) |
| Stem Cell Source | ||||
| Bone marrow | 55 (39) | 34 (8) | 16 (7) | 5 (14) |
| Peripheral blood | 85 (61) | 342 (85) | 188 (78) | 22 (61) |
| Umbilical cord blood | 0 (0) | 29 (7) | 36 (15) | 9 (25) |
| T-Cell Depletion | ||||
| No | 56 (40) | 139 (34) | 81 (34) | 21 (58) |
| Yes | 84 (60) | 266 (66) | 159 (66) | 15 (42) |
| Conditioning | ||||
| Myeloablative | 112 (80) | 272 (67) | 140 (58) | 19 (53) |
| Reduced intensity | 28 (20) | 133 (33) | 100 (42) | 17 (47) |
| Transplant Period | ||||
| Early Period (2000-2005) | 131 (94) | 101 (25) | 43 (18) | 11 (31) |
| Recent Period (2006-2011) | 9 (6) | 304 (75) | 197 (82) | 25 (69) |
Continuous variables are displayed as mean (range), categorical variables are displayed as N (%).
Other: Myeloproliferative disorder, solid tumor, non-malignant hematologic disorder.
related or unrelated.
Abbreviations: MDS: myelodysplastic syndrome; CML: chronic myeloid leukemia; CLL: chronic lymphocytic leukemia.
Table 3 shows the characteristics of MA/RIC HSCT by types of peri-transplant prophylaxis. Of the 821 patients, 140 patients received no prophylaxis, 405 patients received vancomycin only, 240 patients received vancomycin+FQ and 36 patients received FQ only.
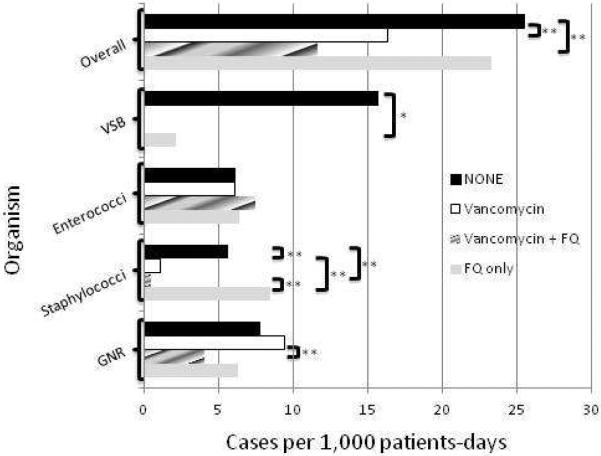
One hundred and eighty-eight of 821(22.9%) patients developed pre-engraftment bacteremia. Figure 3 shows the incidence of pre-engraftment bacteremia by prophylactic regimen. The overall incidence of bacteremia was lower in patients who received vancomycin only or vancomycin+FQ prophylaxis compared to no prophylaxis.
Figure 3. Incidence of pre-engraftment bacteremia in MA/RIC by prophylaxis.
X- axis: Cases of bacteremias per 1,000 patient-days at risk. Black bar: No prophylaxis (none); White bar: Vancomycin only; Striped bar: Vancomycin+FQ; Grey bar: FQ only.
Abbreviations: VSB: viridans streptococci; GNR: gram-negative rods; FQ: fluoroquinolone. Brackets represent significant differences between prophylaxis strategies. The asterisks mean statistically significant: *P<0.05 and **P<0.01.
Specifically, VSB was absent in patients who received vancomycin prophylaxis. The incidence of enterococcal bacteremia was similar across the four groups. Seventy-two of 73 (99%) episodes of enterococcal bacteremia were caused by VRE. Compared to “no antibiotic prophylaxis”, the incidence of staphylococcal bacteremia was decreased for vancomycin only (P<0.01) and vancomycin+FQ (P<0.01). The incidence of GNR bacteremia was lower in patients who received vancomycin+FQ compared to patients who received vancomycin only prophylaxis (P<0.01).
Risk factor analysis for pre-engraftment bacteremia
Patient and transplant characteristics, antibiotic prophylaxis, and period of transplant were examined as potential risk factors for pre-engraftment bacteremia in univariate and multivariate models (Table 4). In univariate analysis, risk factors for bacteremia were T-cell depletion, myeloablative conditioning with HFTBI and fludarabine, and early transplant period. Prophylaxis with vancomycin (vancomycin only or vancomycin+FQ) was protective (P<0.01 for vancomycin only and P<0.001 for vancomycin+FQ). Prophylaxis with FQ only did not reach statistical significance, but the number of patients receiving FQ only was low. In multivariate analysis, T-cell depletion and UCB transplantation were associated with increased risk for bacteremia. The period of transplant and conditioning with HFTBI+fludarabine were not significant in multivariate analysis (Table 4).
Table 4.
Risk factors for pre-engraftment bacteremia.
| Variable | Incidence3 | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Overall | 17.0 | ||||
| Age | 0.24 | ||||
| 10 years increase | 1.10 (0.96 - 1.20) | ||||
| Sex | 0.72 | ||||
| Male | 16.5 | REF | |||
| Female | 17.6 | 1.05 (0.79 - 1.4) | |||
| Diagnosis | 0.27 | ||||
| Acute leukemia/MDS | 18.9 | REF | |||
| CML | 16.8 | 0.89 (0.47 - 1.68) | |||
| Lymphoma/CLL | 11.0 | 0.62 (0.39 - 0.97) | |||
| Multiple myeloma | 11.4 | 0.53 (0.23 - 1.19) | |||
| Other1 | 12.7 | 0.86 (0.21 - 3.46) | |||
| Donor Type | 0.35 | ||||
| Matched related | 18.9 | REF | |||
| Matched unrelated | 15.2 | 0.79 (0.55 - 1.12) | |||
| Mismatched | 16.4 | 0.99 (0.71 - 1.39) | |||
| Stem Cell Source | 0.46 | <0.01 | |||
| Bone marrow | 15.4 | REF | REF | ||
| Peripheral blood | 17.8 | 0.91 (0.60 - 1.39) | 0.97 (0.59 - 1.61) | ||
| Cord blood | 14.5 | 1.21 (0.69 - 2.13) | 3.43 (1.49 - 7.89) | ||
| T-Cell Depletion | <0.01 | <0.01 | |||
| No | 10.2 | REF | REF | ||
| Yes | 22.5 | 1.66 (1.21 - 2.29) | 2.86 (1.83 - 4.47) | ||
| Conditioning | 0.02 | 0.07 | |||
| Reduced intensity | 16.2 | REF | REF | ||
| Myeloablative | 17.4 | ||||
| HFTBI with F | 23.0 | 1.54 (1.07 - 2.22) | 1.14 (0.77 - 1.68) | ||
| HFTBI other | 12.6 | 0.76 (0.48 - 1.21) | 0.68 (0.41 - 1.14) | ||
| Non-HFTBI | 16.1 | 1.03 (0.70 - 1.52) | 1.35 (0.89 - 2.03) | ||
| Transplant Period | 0.04 | 0.99 | |||
| Early Period (2000-2005) | 20.2 | REF | REF | ||
| Recent Period (2006-2011) | 15.3 | 0.73 (0.55 - 0.98) | 1.00 (0.66-1.2) | ||
| Antibiotic Prophylaxis2 | <0.01 | <0.01 | |||
| None | 27.6 | REF | REF | ||
| Vancomycin only | 16.3 | 0.57 (0.40 - 0.81) | 0.46 (0.30 - 0.72) | ||
| Vancomycin+FQ | 11.6 | 0.42 (0.28 - 0.64) | 0.31 (0.12 - 0.52) | ||
| FQ only | 23.3 | 0.83 (0.43 - 1.59) | 0.67 (0.33-1.367) | ||
Cox-proportional hazard model was used for univariate and multivariate analysis of risk factors for pre-engraftment bacteremia in the MA/RIC transplant cohort. Stepwise model selection with 0.3 as significance level to enter and 0.1 as significant level to be retained. Antibiotic prophylaxis and period of transplant were entered as a priori variables.
Other: Myeloproliferative disorder, solid tumor, non-malignant hematologic disorder.
Antibiotic prophylaxis is defined as exposure to ≥ 1 dose or more of vancomycin or fluoroquinolone (ciprofloxacin or levofloxacin) starting between Day −2 to Day +2 of stem cell infusion.
number of cases per 1,000 patient-days.
Abbreviations: HR: hazard ratio; CI: confidence interval; REF: reference group; NA: not available; MDS: myelodysplastic syndrome; CML: chronic myeloid leukemia; CLL: chronic lymphocytic leukemia; HFTBI: hyperfractionated total body irradiation.
Abbreviations: HR: hazard ratio; CI: confidence interval; REF: reference group; NA: not available; MDS: myelodysplastic syndrome; CML: chronic myeloid leukemia; CLL: chronic lymphocytic leukemia; HFTBI: hyperfractionated total body irradiation.
Pathogen-specific risk factors
Our two prophylactic approaches (vancomycin only and vancomycin+FQ) differed in antimicrobial spectrum. We therefore sought to assess the impact of prophylaxis on bacteremia by specific pathogens. Patient and transplant characteristics, antibiotic prophylaxis, and period of transplant were examined as potential risk factors for bacteremia by each pathogen category (viridans streptococci, enterococci, staphylococci, and GNR) in stepwise multivariate regression analysis (Table 5).
Table 5.
Incidence of pre-engraftment bacteremia and Hazard Ratio (95% CI) of antibiotic prophylaxis for specific pathogens.
| Antibiotic Prophylaxis | Overall | Viridans Streptococci1 | Enterococci2 | Staphylococci1 | GNR3 | |
|---|---|---|---|---|---|---|
| None | Incidence | 27.6 | 15.8 | 6.2 | 5.6 | 7.9 |
| Univariate | Reference | Reference | Reference | Reference | Reference | |
| Multivariate | Reference | Reference | Reference | Reference | Reference | |
| Vancomycin only | Incidence | 16.3 | 0 | 6.1 | 1.1 | 9.5 |
| Univariate | 0.57 (0.40 - 0.81)* | NA | 0.93 (0.47 - 1.83) | 0.19 (0.07 - 0.53)* | 1.19 (0.66 - 2.14) | |
| Multivariate | 0.46 (0.30 - 0.72)* | NA | 0.92 (043 - 200) | 0.14 (0.04 - 0.52)* | 0.89 (0.43 - 1.85) | |
| Vancomycin+FQ | Incidence | 11.6 | 0 | 7.5 | 0.6 | 4.1 |
| Univariate | 0.42 (0.28 - 0.64)* | NA | 1.17 (0.58 - 2.37) | 0.10 (0.02 - 0.48)* | 0.54 (0.26 - 1.12) | |
| Multivariate | 0.31 (0.19 - 0.53)* | NA | 1.07 (0.47 - 2.44) | 0.07 (0.01 - 0.43)* | 0.35 (0.15 - 0.85)* | |
| FQ only | Incidence | 23.3 | 2.1 | 6.4 | 8.5 | 6.4 |
| Univariate | 0.83 (0.43 - 1.59) | 0.13 (0.02-0.98) | 0.98 (0.27 - 3.52) | 1.47 (0.46 - 4.70) | 0.80 (0.23 - 2.79) | |
| Multivariate | 0.67 (0.33 - 1.37) | 0.17 (0.02-1.50) | 1.01 (0.27 - 3.80) | 1.07 (0.26 - 4.46) | 0.60 (0.16 - 2.24) | |
Hazard ratios from univariate and multivariate analysis are displayed as Hazard Ratio and 95% confidence interval (95% CI) in the table. Antibiotic prophylaxis: ≥ 1 dose of antibiotic starting between day −2 to +2 of stem cell infusion. No patient who received vancomycin or vancomycin with fluoroquinolone prophylaxis developed bacteremia from viridans streptococci; hence, no hazard ratio could be calculated. Incidence was per 1,000 patient-days at risk. No prophylaxis (None) was considered the reference group; NA: not available; GNR: gram-negative rods.
Notes: Stepwise model selection with 0.3 as significance level to enter and 0.1 as significant level to be retained in the model.
multivariate model adjusted for transplant periods.
multivariate model adjusted for age, stem cell source, T-cell depletion, and, transplant periods.
multivariate model adjusted for stem cell source, T-cell depletion, and, transplant period.
*one asterisk means statistically significant (P<0.01).
Viridans streptococci
The incidence of VSB with vancomycin (vancomycin only and vancomycin+FQ) was 0 cases per 1,000 patient-days compared to 15 with no antibiotic prophylaxis. Patients who received FQ prophylaxis only had a VSB incidence of 2.1 cases per 1,000 patient-days. Since there were no cases of bacteremia in patients who received vancomycin, no regression analysis of vancomycin prophylaxis was performed for VSB.
Enterococci
The incidence of enterococcal bacteremia was 6.2 cases per 1,000 patient-days without prophylaxis compared to 6.4 with FQ only prophylaxis and 7.5 with vancomycin+FQ prophylaxis. In univariate analysis, antibiotic prophylaxis was not a significant risk factor for enterococcal bacteremia (Table 5). Age, stem cell source, T-cell depletion, and the a priori variables remained in the stepwise multivariate analysis. Antibiotic prophylaxis and period of transplant were not significant (Table 5). Whereas increased age (HR=1.29; CI=1.05-1.59, P=0.02 for each 10 year increase), UCB allograft (HR=4.18; CI=1.12 - 14.62, P=0.04), and T-cell depletion (HR=2.79; CI=1.36 - 5.72, P<0.01) were associated with increased risk.
Staphylococci
Vancomycin prophylaxis reduced the incidence of staphylococcal bacteremia from 5.6 cases per 1,000 patient-days without any prophylaxis to 1.1 with vancomycin only and 0.6 with vancomycin+FQ. In univariate analysis, vancomycin-containing prophylaxis was significant (Table 5). After stepwise selection, only the a priori variables remained in the multivariate analysis. Vancomycin prophylaxis (with or without FQ) was protective (Table 5). Transplant period was not associated with staphylococcal bacteremia (HR=1.61; CI= 0.48 – 5.33, P=0.44).
GNR
The incidence of GNR bacteremia was 7.9 cases per 1,000 patient-days without prophylaxis and 4.1 with vancomycin+FQ prophylaxis. In univariate analysis, vancomycin+FQ prophylaxis was significant. After stepwise selection, stem cell source and T-cell depletion as well as the a priori variables remained in the multivariate analysis. Vancomycin+FQ was protective (HR=0.35; CI=0.15 - 0.85, P=0.02). FQ only was associated with decreased risk but did not reach statistical significance (HR=0.60; CI=0.16 - 2.24, P=0.44) (Table 5). T-cell depletion was a risk factor for GNR bacteremia (HR=2.36; CI=1.25 - 4.44, P<0.01).
DISCUSSION
Antibiotic prophylaxis is effective in reducing febrile episodes and bacterial infections in chemotherapy-induced FN, but only a recent meta-analysis showed a survival benefit (6). Several national guidelines now make a qualified recommendation that FQ prophylaxis could be considered in high-risk patients anticipated to be neutropenic for more than 7 days, including those undergoing allogeneic HSCT, and yet vancomycin, either used as prophylaxis or first-line treatment for FN, is discouraged (4) (13) (20). This is problematic as viridans streptococci isolated from neutropenic patients are often resistant to FQs (10). Furthermore, FQ use is a recognized risk factor for VSB, which carries high mortality in neutropenic patients (11, 12). Since 2006, we routinely use vancomycin prophylaxis peri-transplant for MA and RIC HSCT at MSKCC. In the present study, we show that implementation of routine vancomycin prophylaxis was associated with a significant decrease in overall incidence of bacteremia from day -7 to 100 in the entire HSCT cohort. The major benefit of formal prophylaxis was observed during the first 10 days posttransplant. Beyond day +10 there was no significant difference in the incidence of bacteremia between the Early Period (13.1%) and Recent Period (9.6%) (P=0.1306). We next conducted an analysis of MA/RIC HSCT to assess the impact of prophylaxis on pre-engraftment bacteremia. Vancomycin only prophylaxis was associated with complete elimination of VSB. Vancomycin+FQ was associated with an even further reduction in overall incidence of bacteremia due to a decrease in GNR bacteremia.
Several gradual changes in transplant practice over the 12-year study period could potentially impact the incidence of bacteremia, including increased recipient age, different underlying diseases, increased use of mismatched donors and UCB, and greater use of reduced intensity conditioning regimens. To adjust for potential additional confounders we forced the transplant period as a variable into our model. In multivariate analysis, we show that vancomycin only was associated with a 54% reduction and vancomycin+FQ with a 69% reduction in pre-engraftment bacteremia while the transplant period was not significant.
Prior published studies of vancomycin prophylaxis in the 1990s showed significant reductions in bacteremia caused by Gram-positive organisms although none demonstrated a reduction in mortality due to their limited sample size. VSB however was relatively uncommon in those studies (21, 22). In our large observational cohort, vancomycin-containing prophylaxis was associated with elimination of VSB (from 15 cases per 1,000 patient-days without prophylaxis to 0 cases with vancomycin only). Elimination of VSB was particularly important given a 21% case fatality rate for VSB in our HSCT patients (12). The present study did not examine the impact of vancomycin prophylaxis on early mortality. Vancomycin- containing prophylaxis also decreased the incidence of pre-engraftment staphylococcal bacteremia albeit to a much lesser extent than VSB.
Vancomycin+FQ prophylaxis was associated with a greater reduction in overall incidence of bacteremia compared to vancomycin alone due to a reduction in GNR bacteremia. When we adjusted for transplant characteristics and antibiotic prophylaxis, T-cell depletion was a risk factor for GNR bacteremia. Vancomycin+FQ prophylaxis however reduced the risk in TCD allograft recipients.
A valid concern of FQ prophylaxis is the potential for selection of resistant organisms. Breakthrough infections due to FQ -resistant Enterobacteriaceae, P. aeruginosa, and other bacteria have been described in cancer patients receiving FQ prophylaxis (8) (23) (24). There are also increasing reports of infections due to ESBL-producing and MDR pathogens tied to FQ prophylaxis (25, 26). At our center, rates of ESBL and multidrug resistance in GNR isolates were in general low and stable for the duration of the study. Rates of resistance to imipenem albeit low, were higher in the Recent Period, consistent with an increase in carbapenemase-producing Klebsiella pneumoniae (KPC) within our center and hospitals in the USA (27). However, continued surveillance is needed as emergence of FQ -resistant GNR over time has been reported with levofloxacin prophylaxis in HSCT (28). Whether FQ prophylaxis will be able to maintain a protective effect in the long-term is in question if the environmental prevalence of FQ-resistant bacteria continues to rise in the United States (29) (30) (8).
VRE has emerged as an important pathogen in HSCT patients over the last 10 years. UCB, VRE colonization, and increased co-morbidities are recognized risk factors for VRE bacteremia (21, 22, 31, 32). While concerns have been raised about the emergence of VRE with exposure to vancomycin among other antibiotics (25, 33), the incidence of enterococcal bacteremia at our center was similar before and after implementation of antibiotic prophylaxis (7.1 cases vs 6.6 cases per1,000 patient-days in Early and Recent Periods, respectively). Rather, increased age, UCB transplant, and TCD HSCT were risk factors for VRE bacteremia in multivariate analysis.
We found that T-cell depletion was a risk factor for pre-engraftment bacteremia and specifically for enterococcal and GNR bacteremia. Compared to conventional HSCT, recipients of TCD allografts were more likely to receive HFTBI-containing conditioning regimens (48.5% in TCD arm vs 22.4% in conventional arm, P<0.0001). Specifically, HFTBI+fludarabine (27.5% in TCD arm vs 2.2% in conventional arm, P<0.0001) was associated with increased risk for pre-engraftment bacteremia in univariate analysis. We have previously reported an association of HFTBI+ fludarabine with VSB (12). HFTBI- containing regimens are typically associated with more severe mucositis compared to all chemotherapy conditioning regimens. The interaction effect between T-cell depletion and HFTBI could partially account for the association between TCD and pre-engraftment bacteremia in HSCT.
The main strengths of our study are: 1) our large and diverse cohort of HSCT including cord blood and TCD allografts; 2) the reporting of trends in the epidemiology of bacteremia in HSCT over a 12-year period; and 3) the analysis of risk factors for bacteremia, focusing specifically on the impact of antibacterial prophylaxis.
The limitations of our study are inherent to its single-center, observational, non-randomized nature examining two time periods with differing patient and transplant characteristics. In addition, infection control practices and an active antibiotic stewardship program at our institution or changes in epidemiology of microbes in the community are likely to have contributed to our findings. Furthermore, perturbation of individual patients’ microbiota by peri-transplant antibiotics may have an impact on broader transplant outcomes which was beyond the scope of our analysis. Studies to dissect the impact of antibiotics on microbiota and transplant outcomes are actively being pursued at our center (34).
In summary, implementation of antibacterial prophylaxis with IV vancomycin in allogeneic HSCT with MA/RIC was associated with in overall reduction of bacteremia in the first 100 days and a significant decrease in pre-engraftment bacteremia at our center without changes in rates of VRE or resistant GNR. Specifically, vancomycin prophylaxis was associated with elimination of VSB and reduced staphylococcal bacteremia. The addition of a FQ was associated with reduction in GNR bacteremia. Our findings are especially relevant as T-cell depletion and the use of alternative graft sources such as cord bloods are expected to have broader applicability. Nevertheless, we recognize that antibiotic exposure is associated with the emergence of bacterial resistance and that continued surveillance is warranted to weigh the risks and benefits of prophylaxis and adjust prophylactic regimens according to local epidemiology and transplant practices.
Highlights.
We analyzed the effect of prophylaxis on the epidemiology of bacteremia in HSCT.
Prophylaxis was associated with decreased incidence of bacteremia up to Day 100.
Vancomycin was protective against streptococcal and staphylococcal bacteremia.
Fluoroquinolone was protective against Gram-negative rod (GNR) bacteremia.
Prophylaxis did not impact incidence of VRE and resistant GNR.
Acknowledgments
The study was supported in part by NIH grant P01CA023766 Immunobiology of marrow allografts for Leukemia (AAJ, SG and JNB). The authors thank Dr Dennis Neofytos for critical review of the manuscript and Ms Candice Cooper for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Almyroudis NG, Fuller A, Jakubowski A, Sepkowitz K, Jaffe D, Small TN, et al. Pre-and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transplant infectious disease : an official journal of the Transplantation Society. 2005;7(1):11–7. doi: 10.1111/j.1399-3062.2005.00088.x. doi: 10.1111/j.1399-3062.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 2.Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone marrow transplantation. 2005;36(9):757–69. doi: 10.1038/sj.bmt.1705140. doi: 10.1038/sj.bmt.1705140. [DOI] [PubMed] [Google Scholar]
- 3.Liu CY, Lai YC, Huang LJ, Yang YW, Chen TL, Hsiao LT, et al. Impact of bloodstream infections on outcome and the influence of prophylactic oral antibiotic regimens in allogeneic hematopoietic SCT recipients. Bone marrow transplantation. 2011;46(9):1231–9. doi: 10.1038/bmt.2010.286. doi: 10.1038/bmt.2010.286. [DOI] [PubMed] [Google Scholar]
- 4.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–238. doi: 10.1016/j.bbmt.2009.06.019. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wingard JR. Advances in the management of infectious complications after bone marrow transplantation. Bone marrow transplantation. 1990;6(6):371–83. [PubMed] [Google Scholar]
- 6.Gafter-Gvili A, Fraser A, Paul M, Leibovici L. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med. 2005;142(12 Pt 1):979–95. doi: 10.7326/0003-4819-142-12_part_1-200506210-00008. [DOI] [PubMed] [Google Scholar]
- 7.Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerging infectious diseases. 2002;8(8):802–7. doi: 10.3201/eid0808.010418. doi: 10.3201/eid0805.010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kern WV, Klose K, Jellen-Ritter AS, Oethinger M, Bohnert J, Kern P, et al. Fluoroquinolone resistance of Escherichia coli at a cancer center: epidemiologic evolution and effects of discontinuing prophylactic fluoroquinolone use in neutropenic patients with leukemia. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2005;24(2):111–8. doi: 10.1007/s10096-005-1278-x. doi: 10.1007/s10096-005-1278-x. [DOI] [PubMed] [Google Scholar]
- 9.Muto CA, Pokrywka M, Shutt K, Mendelsohn AB, Nouri K, Posey K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2005;26(3):273–80. doi: 10.1086/502539. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 10.Han XY, Kamana M, Rolston KV. Viridans streptococci isolated by culture from blood of cancer patients: clinical and microbiologic analysis of 50 cases. Journal of clinical microbiology. 2006;44(1):160–5. doi: 10.1128/JCM.44.1.160-165.2006. doi: 10.1128/JCM.44.1.160-165.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tunkel AR, Sepkowitz KA. Infections caused by viridans streptococci in patients with neutropenia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;34(11):1524–9. doi: 10.1086/340402. doi: 10.1086/340402. [DOI] [PubMed] [Google Scholar]
- 12.Jaffe D, Jakubowski A, Sepkowitz K, Sebti R, Kiehn TE, Pamer E, et al. Prevention of peritransplantation viridans streptococcal bacteremia with early vancomycin administration: a single-center observational cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(11):1625–32. doi: 10.1086/425612. doi: 10.1086/425612. [DOI] [PubMed] [Google Scholar]
- 13.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(4):427–31. doi: 10.1093/cid/ciq147. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 14.Jakubowski AA, Small TN, Young JW, Kernan NA, Castro-Malaspina H, Hsu KC, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110(13):4552–9. doi: 10.1182/blood-2007-06-093880. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, Childs BH, Mackinnon S, Boulad F, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91(3):1083–90. [PubMed] [Google Scholar]
- 16.Ponce DM, Sauter C, Devlin S, Lubin M, Gonzales AM, Kernan NA, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(5):799–803. doi: 10.1016/j.bbmt.2013.02.007. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. Supplement M100-S1. Wayne: p. PA2009. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing. Wayne: p. PA2013. [Google Scholar]
- 19.Prevention CfDCa Centers for Disease Control and Prevention, editor. CDC/NHSN Surveillance Definitions for Specific Types of Infections [Google Scholar]
- 20.Baden LR, Bensinger W, Angarone M, Casper C, Dubberke ER, Freifeld AG, et al. Prevention and treatment of cancer-related infections. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10(11):1412–45. doi: 10.6004/jnccn.2012.0146. [DOI] [PubMed] [Google Scholar]
- 21.Arns da Cunha C, Weisdorf D, Shu XO, DeFor T, Pastor JD, 3rd, Johnson JR. Early gram-positive bacteremia in BMT recipients: impact of three different approaches to antimicrobial prophylaxis. Bone marrow transplantation. 1998;21(2):173–80. doi: 10.1038/sj.bmt.1701057. doi: 10.1038/sj.bmt.1701057. [DOI] [PubMed] [Google Scholar]
- 22.Schots R, Trullemans F, Van Riet I, Kaufman L, Hafsia A, Meddeb B, et al. The clinical impact of early gram-positive bacteremia and the use of vancomycin after allogeneic bone marrow transplantation. Transplantation. 2000;69(7):1511–4. doi: 10.1097/00007890-200004150-00053. [DOI] [PubMed] [Google Scholar]
- 23.Carratala J, Fernandez-Sevilla A, Tubau F, Callis M, Gudiol F. Emergence of quinolone-resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1995;20(3):557–60. doi: 10.1093/clinids/20.3.557. discussion 61-3. [DOI] [PubMed] [Google Scholar]
- 24.Bucaneve G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. The New England journal of medicine. 2005;353(10):977–87. doi: 10.1056/NEJMoa044097. doi: 10.1056/NEJMoa044097. [DOI] [PubMed] [Google Scholar]
- 25.Rangaraj G, Granwehr BP, Jiang Y, Hachem R, Raad I. Perils of quinolone exposure in cancer patients: breakthrough bacteremia with multidrug-resistant organisms. Cancer. 2010;116(4):967–73. doi: 10.1002/cncr.24812. doi: 10.1002/cncr.24812. [DOI] [PubMed] [Google Scholar]
- 26.Garnica M, Nouer SA, Pellegrino FL, Moreira BM, Maiolino A, Nucci M. Ciprofloxacin prophylaxis in high risk neutropenic patients: effects on outcomes, antimicrobial therapy and resistance. BMC infectious diseases. 2013;13(1):356. doi: 10.1186/1471-2334-13-356. doi: 10.1186/1471-2334-13-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landman D, Babu E, Shah N, Kelly P, Olawole O, Backer M, et al. Transmission of carbapenem-resistant pathogens in New York City hospitals: progress and frustration. The Journal of antimicrobial chemotherapy. 2012;67(6):1427–31. doi: 10.1093/jac/dks063. doi: 10.1093/jac/dks063. [DOI] [PubMed] [Google Scholar]
- 28.Therriault BL, Wilson JW, Barreto JN, Estes LL. Characterization of bacterial infections in allogeneic hematopoietic stem cell transplant recipients who received prophylactic levofloxacin with either penicillin or doxycycline. Mayo Clinic proceedings. 2010;85(8):711–8. doi: 10.4065/mcp.2010.0006. doi: 10.4065/mcp.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez L, Garau J, Estrada C, Marquez M, Dalmau D, Xercavins M, et al. Ciprofloxacin prophylaxis in patients with acute leukemia and granulocytopenia in an area with a high prevalence of ciprofloxacin-resistant Escherichia coli. Cancer. 2003;97(2):419–24. doi: 10.1002/cncr.11044. doi: 10.1002/cncr.11044. [DOI] [PubMed] [Google Scholar]
- 30.Ng ES, Liew Y, Earnest A, Koh LP, Lim SW, Hsu LY. Audit of fluoroquinolone prophylaxis against chemotherapy-induced febrile neutropenia in a hospital with highly prevalent fluoroquinolone resistance. Leukemia & lymphoma. 2011;52(1):131–3. doi: 10.3109/10428194.2010.518655. doi: 10.3109/10428194.2010.518655. [DOI] [PubMed] [Google Scholar]
- 31.Kamboj M, Chung D, Seo SK, Pamer EG, Sepkowitz KA, Jakubowski AA, et al. The changing epidemiology of vancomycin-resistant Enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol Blood Marrow Transplant. 2010;16(11):1576–81. doi: 10.1016/j.bbmt.2010.05.008. doi: 10.1016/j.bbmt.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vydra J, Shanley RM, George I, Ustun C, Smith AR, Weisdorf DJ, et al. Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(6):764–70. doi: 10.1093/cid/cis550. doi: 10.1093/cid/cis550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford CD, Reilly W, Wood J, Classen DC, Burke JP. Oral antimicrobial prophylaxis in bone marrow transplant recipients: randomized trial of ciprofloxacin versus ciprofloxacin vancomycin. Antimicrobial agents and chemotherapy. 1998;42(6):1402–5. doi: 10.1128/aac.42.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(7):905–14. doi: 10.1093/cid/cis580. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]