Abstract
A novel synthesis of the translocator protein (TSPO) ligand 7-chloro-N,N,5-trimethyl-4-oxo-3-phenyl-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide (SSR180575, 3) was achieved in four steps from commercially available starting materials. Focused structure−activity relationship development about the pyridazinoindole ring at the N3 position led to the discovery of 7-chloro-N,N,5-trimethyl-4-oxo-3(6-fluoropyridin-2-yl)-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide (14), a novel ligand of comparable affinity. Radiolabeling with fluorine-18 (18F) yielded 7-chloro-N,N,5-trimethyl-4-oxo-3(6-[18F]fluoropyridin-2-yl)-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide (18F-14) in high radiochemical yield and specific activity. In vivo studies of [18F]-14 revealed this agent as a promising probe for molecular imaging of glioma.
Keywords: TSPO, Glioma, PET, Fluorine-18, SSR180575
There exists a continuing demand for the development and validation of improved imaging biomarkers for cancer. Such biomarkers may aid in cancer diagnosis, predict clinical outcome, and quantify response to therapeutic intervention. Imaging biomarkers of glioma are limited and routinely include magnetic resonance imaging (MRI), computed tomography (CT), and less frequently, positron emission tomography (PET). Of these, PET may be the most suitable given its exquisite sensitivity, quantitative nature, and ability to analyze the molecular basis of the tumor. For most oncology studies, 2-deoxy-2-[18F]fluoroglucose ([18F]FDG) is commonly utilized, as this tracer reflects glucose metabolism, which is usually elevated in cancer cells.1 However, relatively high glucose uptake in normal brain results in poor tumor-to-background ratios that can confound glioma detection. Moreover, [18F]FDG uptake can be affected by a myriad of tangential metabolic processes. This has led many groups to evaluate other PET tracers of metabolism in this setting, including amino acid–based PET probes such as [11C]MET,2 [18F]FET,3 and [18F]FDOPA.4 While these probes can accumulate in tissues that exhibit enhanced amino acid transport,5-7 they can be hampered by nonspecific accumulation.6 Thus, there remains a need to develop and validate additional PET imaging biomarkers suitable for glioma detection and characterization.
We have explored translocator protein (TSPO) expression as a target for molecular imaging of cancer with PET.8-13 TSPO, formerly the peripheral benzodiazepine receptor (PBR), is an 18 kDa protein that takes part in a wide breadth of cellular processes, including cell proliferation, apoptosis, steroid biosynthesis, and cholesterol metabolism.14 Though TSPO has historically been leveraged as a PET biomarker in neuroinflammation,15-17 its elevated expression has also been reported in many types of cancer.18 This overexpression in tumors has been reported to correlate with disease progression and diminished survival and can be indicative of aggressive and potentially metastatic tumors.18 Within this context, our laboratory has investigated the use of TSPO PET ligands to image colon cancer,8 breast cancer,9 and glioma,12,13,19 as these agents could potentially serve as useful cancer imaging biomarkers. Moreover, on a mechanistic level, we have utilized TSPO molecular imaging probes to visualize colon cancers arising in genetically engineered mice with aberrant Tgfβsignaling,8 as well as tumors arising in mice following loss of Apc function.20 These studies suggest the potential importance TSPO PET agents to detect key molecular events in oncology and to possibly serve as companion diagnostics alongside targeted inhibitors of these pathways.
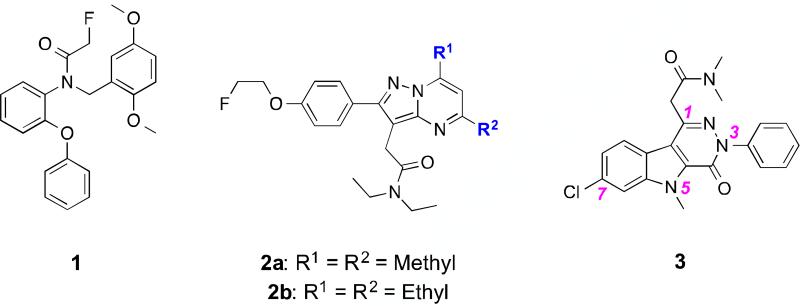
We recently reported the first utilizations of two TSPO-specific PET ligands for quantitative assessment of TSPO expression in preclinical glioma, the aryloxyanilide N-[18F]fluoroacetyl-N-(2,5-dimethoxybenzyl)-2-phenoxyaniline ([18F]PBR06, [18F]-1),11 and the pyrazolopyrimidine N,N-diethyl-2-(2-(4-(2-[18F]fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide ([18F]DPA-714, [18F]-2a)12 (Figure 1). Through focused library synthesis and structure−activity relationship (SAR) development of the 5,6,7-substituted pyrazolopyrimidine scaffold of [18F]-2a, we developed 2-(5,7-diethyl-2-(4-(2-[18F]fluoroethoxy)phenyl)pyrazolo[1,5-a]pyrimidin-3-yl)-N,N– diethylacetamide ([18F]-2b, [18F]VUIIS1008), a novel and highly potent TSPO PET ligand exhibiting a 36-fold enhancement in affinity compared to [18F]-2a and accessible in high radiochemical yield and specific activity.13 Subsequent in vivo studies of [18F]-2b demonstrated this agent to possess several properties for molecular imaging of TSPO-expressing cancers.13,21
Figure 1.
Representative TSPO ligand scaffolds: Aryloxyanilide (1, PBR06); Pyrazolopyrimidine (2a, DPA-714; 2b, VUIIS1008); Pyridazinoindole (3, SSR180575).
In addition to the aryloxyanilides and pyrazolopyrimidines, pyridazinoindoles are another series of potent TSPO ligands, as represented by 7-chloro-N,N,5-trimethyl-4-oxo-3-phenyl-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide (SSR180575, 3).22,23 Developed originally by Sanofi-Aventis, the specificity and high-affinity of 3 for TSPO has prompted research in cardiovascular24,25 and renal pathologies,26 as well as neurodegenerative indications,23 inflammatory disorders,22,27-29 and HIV pathogenesis.30
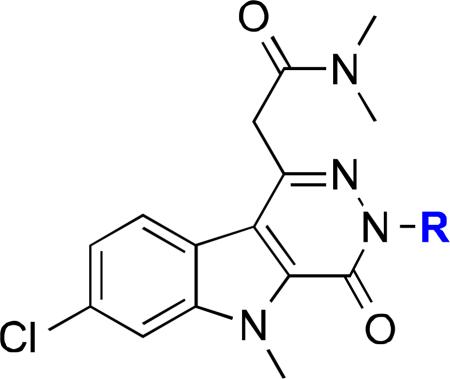
The goal of this study was to determine whether optimization of 3, specifically at the N3 position, would yield TSPO ligands with comparable affinity and potential to serve as PET imaging ligands. These experiments led to the synthesis of 3 in only four steps and the subsequent development of 7-chloro-N,N,5-trimethyl-4-oxo-3(6-fluoropyridin-2-yl)-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide (14), a novel pyridazinoindole TSPO ligand exhibiting binding comparable to 3 and structural features suitable for radiolabeling with fluorine-18 (18F). Radiofluorination of either the 2-chloro (15) or 2-bromo (16) precursor gave 7-chloro-N,N,5-trimethyl-4-oxo-3(6-[18F]fluoropyridin-2-yl)-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide ([18F]-14), which was subsequently evaluated in vivo in a preclinical model of glioma (C6). [18F]-14 exhibited modest accumulation in normal brain, yet robust accumulation in tumor tissue, which facilitated excellent imaging contrast. [18F]-14 was fully displaceable by administration of non-radioactive 14 halfway through the PET scan. Overall, these studies illuminate [18F]-14 as a promising, novel PET ligand for evaluating TSPO expression in gliomas and potentially other solid tumors and diseases.
The original synthesis of SSR180575 (3) remains a patented process at seven total steps.31 Moreover, the patented SAR studies of 3 targeted four areas on the scaffold, the acetamide functionality at C1 and the N3, N5, and C7 positions of the tricyclic pyridazinoindole ring (Figure 1).31,32 The synthesis developed and reported herein requires only four steps from a commercially available starting material (Scheme 1). A small library of pyridazinoindoles (Table 1) was assembled, with the point of divergence at the final condensation step with strategic arylhydrazines (8) (Scheme 1). The design of the library focused primarily on incorporation of fluorine onto an aromatic ring and potential substituents that would facilitate radiolabeling with 18F.
Scheme 1.
Synthetic methodology developed to generate SSR180575 (3) and a focused library of N3-substituted pyridazinoindoles (9-16).
Table 1.
Affinity of Pyridazinoindoles
| ||||
|---|---|---|---|---|
| Compd | R | C6 Glioma Ki (pM)b | Heart Ki (pM)b | Kidney Ki (pM)b |
| 3a | Ph | 1.23 ± 0.08 | 0.762 ± 0.15 | 0.596 ± 0.04 |
| 9 | 2-fluorophenyl | 0.422 ± 0.06 | 0.591 ± 0.10 | 1.18 ± 0.04 |
| 10 | 3-fluorophenyl | 0.280 ± 0.07 | 0.180 ± 0.02 | 0.212 ± 0.02 |
| 11 | 3-nitrophenyl | 0.889 ± 0.2 | 1.18 ± 0.09 | 0.678 ± 0.2 |
| 12 | 4-fluorophenyl | 0.671 ± 0.2; 3020 ± 1007d | 0.877 ± 0.03 | 0.459 ± 0.03; 969 ± 752d |
| 13 | 2-pyridyl | 2409 ± 795.4 | 4936 ± 662.3 | 6796 ± 830.9 |
| 14c | 3-fluoro-2-pyridyl | |1.19 ± 0.05; 1770 ± 232.6d | 3.21 ± 0.4 | 2.21 ± 0.4 |
| 15 | 3-chloro-2-pyridyl | 2.34 ± 0.4 | 0.676 ± 0.08 | 0.495 ± 0.03 |
| 16 | 3-bromo-2-pyridyl | 1.48 ± 0.2 | 0.281 ± 0.01 | 0.965 ± 0.02 |
SSR180575.
Ki ± S.E.M. versus [3H]PK11195.
Ki versus [3H]flunitrazepam in rat brain lysate >10 μM.
Mixed affinity binding. All lysates procured from athymic nude rats. All experiments performed in triplicate.
The overall synthetic methodology is presented in Scheme 1. Starting from a commercially available indole (4), deprotonation with sodium hydride in DMF, followed by treatment with methyl iodide, gave the desired N-methylated intermediate (5) (85%). Acylation of (5) at C3 was achieved using TiCl4 and methyl chloro-3-oxopropanoate in dichloroethane at 40 °C for 15 h, which gave keto diester 6 (46%). The necessary N,N-dimethylamide moiety at C1 (7) was achieved through displacement of the methoxy group of (6) with dimethylamine in toluene and THF in a sealed tube at 110 °C for 15 h. Final condensation with phenylhydrazine (8, R=phenyl) gave the lead compound, SSR1805875 (3), in 32% yield. To date, this stands as the shortest reported synthesis of this particular TSPO ligand.
Diversification at the N3 position of 3 was achieved through condensation of key intermediate 7 with a series of monosubstituted aryl hydrazines (8) (31-50%). The hydrazines chosen focused upon variation of the endogenous N3-phenyl ring, with particular attention towards preliminary SAR and functional groups amenable to PET ligand development, namely the presence of a fluorine atom and groups that would facilitate radiolabeling with 18F through an ipso-type substitution. The groups explored included: a substituted phenyl ring (2-, 3-, 4-positions) (9-12); a 2-pyridyl ring (13); a substituted 2-pyridyl ring (14-16).
To evaluate binding to TSPO, radiometric binding assays were carried out with the compounds in a variety of athymic nude rat cell lysates (C6, heart, kidney) using N-(sec-butyl)-1-(2-chlorophenyl)-N-methyl-[3H]-isoquinoline-3-carboxamide ([3H]PK11195).11,12 For central benzodiazepine receptor (CBR) binding, radioligand displacement was carried out against [3H]flunitrazepam in healthy, athymic nude rat brain lysate. Affinities are expressed as Ki ± S.E.M. (pM) in Table 1. All experiments were performed in triplicate.
Of the eight analogs synthesized, seven proved extremely potent in all three lysates, with Ki values comparable to those of the parent SSR180575 (3): in C6 lysate, 0.280-2.34 pM versus 1.23 pM for 3; in heart 0.180-3.21 pM versus 0.762 pM for 3; in kidney, 0.212-2.21 pM versus 0.596 pM for 3. Only one analog, compound 13, bearing a 2-pyridyl ring at N3, did not appreciably bind TSPO in any of the lysates. Interestingly, a portion of the compounds synthesized showed evidence of mixed (low, high) binding affinities. In C6 lysate, compounds 12 and 14 demonstrated high-affinity binding at 0.671 and 1.19 pM, and low-affinity binding at 1770 and 3020 pM, respectively. Compound 12 demonstrated similar mixed binding in kidney lysate (0.459 and 969 pM), but not the heart. While multiple PK11195 TSPO binding sites have been reported and merit consideration,33 recent research has also implicated a genetic polymorphism for such binding variations in the human brain.34,35 However, genetic sequencing of the C6 cell line from the specific clonal population used for this study confirmed its wild-type status. The mixed binding we observed could potentially be a result of overlapping points of interaction that the analogs and PK11195 possess for TSPO.
Overall, the modifications made to lead compound 3, with the exception of 13, were well tolerated. While substitution of the N3-phenyl ring with 2-pyridine did adversely affect binding across all three lysates, comparable biological activity was regained with halogen substitution at the 3-position of the ring (14-16). This influence on potency could be attributed to an electronic effect of the halogen atom and merits further consideration in future studies. Similarly, 2-, 3-, and 4-fluorosubstitution on the endogenous phenyl ring with fluorine (9, 10, 12, respectively) proved to minimally affect TSPO affinity, as did 4-nitro-substitution (11). This series of compounds are among the most potent ligands reported for TSPO in C6 glioma (1 Ki = 6170 pM;11 2a Ki = 9730 pM;12 2b Ki = 270 pM13). Moreover, in our lab, compound 14 represents a 1000-fold increase in potency over 2b.
Of the synthesized series, the 3-fluoro analog 10 was initially considered the candidate PET tracer, particularly given its activity and ready availability of its 3-nitro precursor (11). However, effective substitution of a nitro group with 18F- on a benzene ring requires a highly deactivated ring system, preferably with electron-withdrawing groups either ortho or para to the nitro moiety. Neither was the case for 11 as a precursor. Similar arguments could also be made for consideration of 9 or 12 as PET tracer candidates. Compound 14 was ultimately chosen as the candidate PET tracer, with 15 and 16 as possible precursors. The selectivity of 14 for TSPO over CBR was evaluated through displacement of [3H]flunitrazepam in rat cerebral cortex membranes (Table 1). The selectivity of 14 for TSPO over CBR was verified with [3H]flunitrazepam, which gave a Ki >10 μM.
Isotopic labeling of 3 with both 11C and 18F has been previously reported in the patent literature.36 In the research literature, Thominiaux et al. reported 11C labeling at the 5-methylpyridazino[4,5-b]indole ([indole-N-methyl-11C]SSR180575) and N,N-dimethylacetamide ([acetamide-N-methyl-11C]SSR180575)37 moieties. Within the context of radiochemical yield and purity, and in vivo pharmacological properties, [indole-N-methyl-11C]SSR180575 was advanced as a candidate for imaging neuroinflammation37 and afforded higher image contrast when compared to the traditional isoquinoline [11C]PK11195 in a model of acute neuroinflammation (rat).38 Moreover, competition studies demonstrated a high specific binding of [11C]SSR180575 for TSPO.38 However, as far as the authors are aware, no studies to date have been reported for the use of isotopically labeled SSR180575 for PET imaging in oncology. Since our goal was to explore 3 as a potential radiopharmaceutical lead for translation into preclinical cancer imaging studies, radiofluorination was deemed to be the most effective means to achieve this goal. Though useful in the research setting, the 20.4 min half-life of 11C limits broader utility of the tracer (shipment to satellite locations, dynamic PET studies), thus making 18F (109.4 min half-life) an attractive alternative.
We initially applied microfluidic-radiolabeling approaches to elucidate stability and ascertain labeling feasibility of precursors 15 and 16. Using a commercial microfluidic module (NanoTek®) that enabled carefully controlled stoichiometry between [18F]- and precursor at set temperatures (°C) with controllable reaction times (μL/min flow rates), we carried out multiple, small-scale, sequential radiolabelings in a relatively short amount of time, allowing rapid optimization of labeling conditions (Table 2). The information gathered with the NanoTek® module was used to better inform the transition to the GE TRACERlab™ FXF-N module for larger, preclinical-scale productions. Using cyclotron-generated 18F- and K+-K2.2.2/K2CO3 in anhydrous DMSO, a series of reaction temperatures (80-180 °C) at a transfer rate of 40 μL/min was investigated, with product formation monitored by radio-TLC for % 18F-incorporation (Table 2). For the chloro precursor (15), product formation was observed at 180 °C (1.41%) at a transfer rate of 40 μL/min. Maintaining a reaction temperature of 180 °C and decreasing the transfer rate to 14 μL/min gave modest incorporation (5.2%, Table 2, entry 3). Similar results were obtained using precursor 16 (Table 2, entry 6). Despite modest yields, the data generated from these experiments also highlighted the thermal stability (up to 180 °C) of both 15 and 16, verified by HPLC of crude reaction mixtures. Bromo-precursor 16 was ultimately chosen going forward given its optimal chromatographic resolution from the final product ([18F]-14). These observations provided a confident basis to pursue the radiolabeling reaction in a sealed system at 180 °C for a longer period of time.
Table 2.
Initial radiofluorination of precursor 15 & 16 using microfluidics.
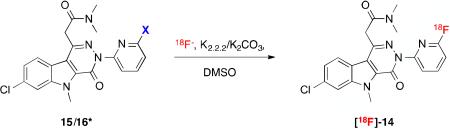
| ||||
|---|---|---|---|---|
| Run | X | Temp (°C) | Transfer Rate (μl) | RCY (%) |
| 1 | Cl | 140 | 40 | 0 |
| 2 | Cl | 160 | 40 | 0 |
| 3 | Cl | 180 | 14 | 5.2 |
| 4 | Br | 140 | 40 | 0 |
| 5 | Br | 160 | 40 | 1 |
| 6 | Br | 180 | 60 | 7 |
*Precursors 15 & 16 stable up to 180 °C.
Adaptation of these reaction conditions to large-scale box productions enabled preclinical production of [18F]-14 in the GE TRACERlab™ FXF-N module with labeling conditions of 170 °C for 15 min. A lowering of the reaction temperature from 180 to 170 °C was deemed necessary due to the limitations of heating reliability at such an extreme temperature using the TRACERlab™ platform. Purification of [18F]-14 was carried out with preparative HPLC in 45% ethanol and 55% water. The retention time of [18F]-14 was 23-28 min according to gamma detection and corresponded to the UV retention time of nonradioactive 14. Radiochemical purity was consistently greater than 99% (n=9), with decay-corrected yields ranging from 9.3-19.3% and specific activities as high as 5559 Ci/mmol (206 TBq/mmol) (n=9). However, the apparent specific activity was diminished using 42.5% ethanol and 57.5% water, despite the longer retention time.
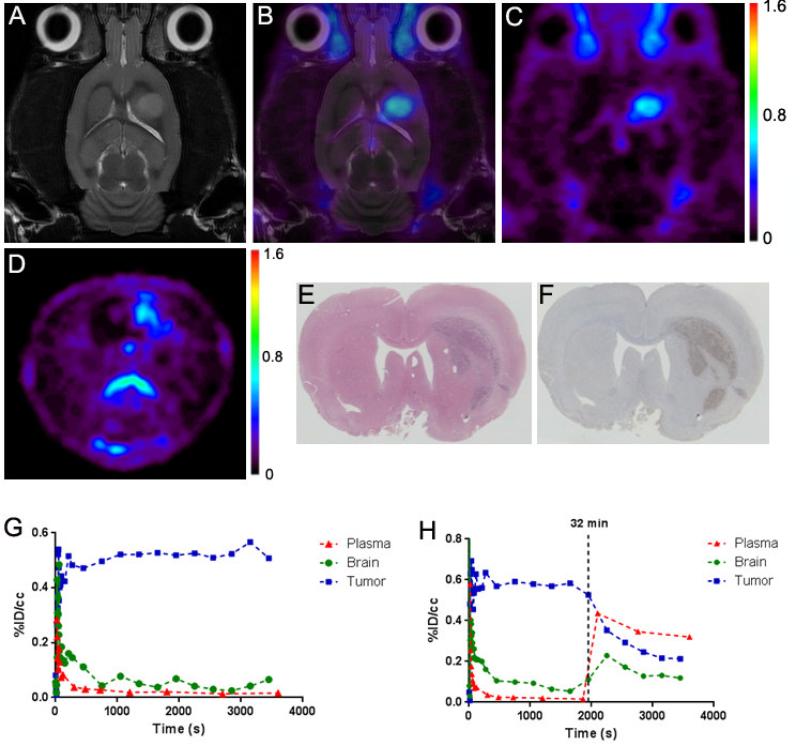
The in vivo performance of [18F]-14 was evaluated in glioma-bearing (C6), male Wistar rats using microPET imaging, with a typical study shown in Figure 2. MRI (T2-weighted) was used to localize tumors and for registration of anatomical features with PET (Fig. 2A).11,12,18 Dynamic PET imaging with [18F]-14 illustrated that the majority of tracer accumulation in the brain was localized to the tumor, with modest accumulation that exceeded plasma in contralateral, non-tumor brain (Fig. 2B & 2C). The tumor-selective characteristics of [18F]-14 afforded excellent imaging contrast between tumor and contralateral tissue. Figure 2G illustrates time−activity curves (TACs) typical of representative studies for tumor (blue), normal brain (green), and plasma (red) over a 90-min dynamic acquisition. After an initial spike in radioactivity consistent with tracer injection and rapid distribution, [18F]-14 quickly cleared the plasma (red). We found that [18F]-14 accumulation in the tumor (blue), relative to normal brain (green), reached a tumor-to-normal brain ratio greater than 10:1. Preliminary radio-TLC analysis of plasma samples (2, 12, 30, 60 min) taken during the scan (n=2) suggested minimal tracer metabolism over the 60 minutes (% parent at 60 min > 90%).
Figure 2.
Imaging of preclinical glioma using [18F]-14. (A) T2–weighted MRI (coronal). (B) Fused MRI-PET (coronal). (C) Dynamic PET (coronal). (D) Dynamic PET (axial). (E) H&E histology. (F) TSPO immunohistochemistry. (G) Typical dynamic PET TAC. (H) Displacement study PET TAC.
To validate the PET, imaging-matched brains were processed for post-mortem staining and immunohistochemistry for TSPO. Ex vivo histological analysis correlated well with PET imaging data, with close agreement between tumor tissue (H&E, Fig. 2E), elevated TSPO expression (immunohistochemistry, Fig. 2F), and tumor accumulation of [18F]-14 (Fig. 2D).
To evaluate the in vivo TSPO specificity of [18F]-14, we performed displacement studies in C6-bearing rats using the non-radioactive analog (14). As shown in the TAC in Figure 2H, during the dynamic PET study, a bolus (IV) infusion of nonradioactive 14 (10 mg/kg) was administered approximately 30 minutes after injection of [18F]-14. Summation of the first 30 minutes of the PET scan prior to injection of cold 14 (0–30 min) demonstrated typical accumulation characteristics of [18F]-14. However, summation of the final 30 minutes of the PET scan (30– 60 min) demonstrated significant displacement of [18F]-14 from tumor tissue (blue). TAC analysis demonstrated that after injection of 14, tumor binding was reduced to approximately 40% of the peak tumor uptake. During tumor displacement, we observed a minor influx of tracer into normal brain (green). The displaced [18F]-14 then rapidly cleared the normal brain and entered the plasma, subsequently elevating plasma (red) radioactivity. These studies suggest a level of displaceable binding indicative of a high degree of specific binding and reversibility of [18F]-14 to TSPO in tumor tissue.
In this work, we report the shortest published synthesis of the pyridazinoindole TSPO ligand SSR180575 (3) to date. Initial SAR exploration at the N3 position of 3 provided a series of analogs of comparable potency, one of which (14) could be adapted into a potential PET imaging tracer ([18F]-14). Subsequent preclinical studies illuminated [18F]-14 as a promising, novel TSPO PET ligand for imaging glioma. Compared to previous TSPO PET ligands in oncology, the first generation ligand [11C]PK1119539 and the second generation ligands [18F]PBR06,19 [18F]DPA-714,12 and [18F]VUIIS1008,13,21 [18F]-14 has demonstrated improved binding affinity and in vivo imaging characteristics. These advantages, in addition to the in vivo stability and high signal-to-noise achieved between tumor and surrounding normal brain, suggest the potential of this PET ligand for detection of TSPO-expressing tumors in the brain and neuroinflammation. Future head-to-head comparisons between this tracer and emerging, potent TSPO ligands13,21 are underway, as well as exploration of other promising applications in oncology, neuroinflammation, and other diseases.
Supplementary Material
Acknowledgments
We acknowledge funding from the National Institutes of Health (K25 CA127349, P50 CA128323, S10 RR17858, U24 CA126588, 1R01 CA163806, P50 CA095103, P30 DK058404), The Kleberg Foundation, and The Lustgarten Foundation. Dr. M. Noor Tantawy, George H. Wilson, and Dr. Daniel Colvin are acknowledged for support of the imaging studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Synthetic and analytical results for all compounds; methods; supplemental figures. This material is available free of charge via the Internet at:
References
- 1.Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Lancet Neurol. 2010;9:906. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 2.Rheims S, Rubi S, Bouvard S, Bernard E, Streichenberger N, Guenot M, Le Bars D, Hammers A, Ryvlin P. Neurooncology. 2014 doi: 10.1093/neuonc/nou022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen NL, Suchorska B, Wenter V, Eigenbrod S, Schmid-Tannwald C, Zwergal A, Niyazi M, Drexler M, Bartenstein P, Schnell O, Tonn JC, Thon N, Kreth FW, la Fougere C. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:198. doi: 10.2967/jnumed.113.122333. [DOI] [PubMed] [Google Scholar]
- 4.Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Grogan T, Elashoff D, Geist C, Silverman DH, Phelps ME, Chen W. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:3550. doi: 10.1158/1078-0432.CCR-13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman S, Pirotte BJ. Methods Mol Biol. 2011;727:291. doi: 10.1007/978-1-61779-062-1_16. [DOI] [PubMed] [Google Scholar]
- 6.la Fougere C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Neuro Oncol. 2011;13:806. doi: 10.1093/neuonc/nor054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirotte B, Goldman S, Massager N, David P, Wikler D, Vandesteene A, Salmon I, Brotchi J, Levivier M. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2004;45:1293. [PubMed] [Google Scholar]
- 8.Deane NG, Manning HC, Foutch AC, Washington MK, Aronow BJ, Bornhop DJ, Coffey RJ. Mol Cancer Res. 2007;5:341. doi: 10.1158/1541-7786.MCR-06-0225. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt SK, Manning HC, Bai M, Bailey SN, Gallant P, Ma G, McIntosh L, Bornhop DJ. Mol Imaging Biol. 2010;12:349. doi: 10.1007/s11307-009-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manning HC, Goebel T, Thompson RC, Price RR, Lee H, Bornhop DJ. Bioconjug Chem. 2004;15:1488. doi: 10.1021/bc049904q. [DOI] [PubMed] [Google Scholar]
- 11.Buck JR, McKinley ET, Hight MR, Fu A, Tang D, Smith RA, Tantawy MN, Peterson TE, Colvin D, Ansari MS, Baldwin RM, Zhao P, Guleryuz S, Manning HC. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52:107. doi: 10.2967/jnumed.110.081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang D, Hight MR, McKinley ET, Fu A, Buck JR, Smith RA, Tantawy MN, Peterson TE, Colvin DC, Ansari MS, Nickels M, Manning HC. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:287. doi: 10.2967/jnumed.111.095653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang D, McKinley ET, Hight MR, Uddin MI, Harp JM, Fu A, Nickels ML, Buck JR, Manning HC. Journal of medicinal chemistry. 2013;56:3429. doi: 10.1021/jm4001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Trends Pharmacol Sci. 2006;27:402. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Varrone A, Mattsson P, Forsberg A, Takano A, Nag S, Gulyas B, Borg J, Boellaard R, Al-Tawil N, Eriksdotter M, Zimmermann T, Schultze-Mosgau M, Thiele A, Hoffmann A, Lammertsma AA, Halldin C. Eur J Nucl Med Mol Imaging. 2013;40:921. doi: 10.1007/s00259-013-2359-1. [DOI] [PubMed] [Google Scholar]
- 16.Suridjan I, Rusjan PM, Voineskos AN, Selvanathan T, Setiawan E, Strafella AP, Wilson AA, Meyer JH, Houle S, Mizrahi R. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takano A, Piehl F, Hillert J, Varrone A, Nag S, Gulyas B, Stenkrona P, Villemagne VL, Rowe CC, Macdonell R, Tawil NA, Kucinski T, Zimmermann T, Schultze-Mosgau M, Thiele A, Hoffmann A, Halldin C. EJNMMI research. 2013;3:30. doi: 10.1186/2191-219X-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batarseh A, Papadopoulos V. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buck JR, Saleh S, Uddin MI, Manning HC. Tetrahedron letters. 2012;53:4161. doi: 10.1016/j.tetlet.2012.05.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell AE, Vlacich G, Zhao ZY, McKinley ET, Washington MK, Manning HC, Coffey RJ. American journal of physiology. Gastrointestinal and liver physiology. 2014;307:G16. doi: 10.1152/ajpgi.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang D, Nickels ML, Tantawy MN, Buck JR, Manning HC. Mol Imaging Biol. 2014 doi: 10.1007/s11307-014-0743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bribes E, Bourrie B, Esclangon M, Galiegue S, Vidal H, Casellas P. Eur J Pharmacol. 2002;452:111. doi: 10.1016/s0014-2999(02)02231-8. [DOI] [PubMed] [Google Scholar]
- 23.Ferzaz B, Brault E, Bourliaud G, Robert JP, Poughon G, Claustre Y, Marguet F, Liere P, Schumacher M, Nowicki JP, Fournier J, Marabout B, Sevrin M, George P, Soubrie P, Benavides J, Scatton B. J Pharmacol Exp Ther. 2002;301:1067. doi: 10.1124/jpet.301.3.1067. [DOI] [PubMed] [Google Scholar]
- 24.Leducq N, Bono F, Sulpice T, Vin V, Janiak P, Fur GL, O'Connor SE, Herbert JM. J Pharmacol Exp Ther. 2003;306:828. doi: 10.1124/jpet.103.052068. [DOI] [PubMed] [Google Scholar]
- 25.Vin V, Leducq N, Bono F, Herbert JM. Biochemical and biophysical research communications. 2003;310:785. doi: 10.1016/j.bbrc.2003.09.079. [DOI] [PubMed] [Google Scholar]
- 26.Kunduzova OR, Escourrou G, De La Farge F, Salvayre R, Seguelas MH, Leducq N, Bono F, Herbert JM, Parini A. Journal of the American Society of Nephrology : JASN. 2004;15:2152. doi: 10.1097/01.ASN.0000133563.41148.74. [DOI] [PubMed] [Google Scholar]
- 27.Galiegue S, Tinel N, Casellas P. Curr Med Chem. 2003;10:1563. doi: 10.2174/0929867033457223. [DOI] [PubMed] [Google Scholar]
- 28.Bribes E, Galiegue S, Bourrie B, Casellas P. Immunology Letters. 2003;85:13. doi: 10.1016/s0165-2478(02)00177-3. [DOI] [PubMed] [Google Scholar]
- 29.Bribes E, Bourrie B, Casellas P. Immunology Letters. 2003;88:241. doi: 10.1016/s0165-2478(03)00083-x. [DOI] [PubMed] [Google Scholar]
- 30.Leducq-Alet N, Vin V, Savi P, Bono F. Biochemical and biophysical research communications. 2010;399:475. doi: 10.1016/j.bbrc.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Ferzaz B, Benavides J, Marguet F, Froissant J, Marabout B, Evanno Y, Sevrin M, Janiak P, Organization WIP. Sanofi-Synthelabo: France. 2000:32. [Google Scholar]
- 32.Evanno Y, Dubois L, Sevrin M, Marguet F, Froissant J, Bartsch R, Gille C, Organization WIP. Synthelabo S. A.: France. 1999 [Google Scholar]
- 33.Scarf AM, Kassiou M. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52:677. doi: 10.2967/jnumed.110.086629. [DOI] [PubMed] [Google Scholar]
- 34.Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:1. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoder KK, Nho K, Risacher SL, Kim S, Shen L, Saykin AJ. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:1320. doi: 10.2967/jnumed.112.118885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benavides J, Boutin H, Castel M-N, Damont A, Dolle F, Hantraye P, Marguet F, Rooney T, Rivron L, Tavitian B, Thominiaux C, Sanofi-Aventis Fr. Commissariat a l'Energie Atomique C.E.A.; France: 2010. p. 40. [Google Scholar]
- 37.Thominiaux C, Damont A, Kuhnast B, Demphel S, Helleix SL, Boisnard S, Rivron L, Chauveau F, Boutin H, Camp NV, Boisgard R, Roy S, Allen J, Rooney T, Benavides J, Hantraye P, Tavitian B, Dolle F. Journal of Labelled Compounds & Radiopharmaceuticals. 2010;53:767. [Google Scholar]
- 38.Chauveau F, Boutin H, Van Camp N, Thominiaux C, Hantraye P, Rivron L, Marguet F, Castel MN, Rooney T, Benavides J, Dolle F, Tavitian B. Eur J Nucl Med Mol Imaging. 2011;38:509. doi: 10.1007/s00259-010-1628-5. [DOI] [PubMed] [Google Scholar]
- 39.Starostarubinstein S, Ciliax BJ, Penney JB, Mckeever P, Young AB. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:891. doi: 10.1073/pnas.84.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.