Abstract
OBJECTIVES
To examine whether day-to-day variations in sleep behaviors, ongoing sleep disturbance and fatigue predict the cortisol diurnal rhythm in women recently diagnosed with early stage breast cancer.
Methods
Women (N=130, age=55. 6±9.4 years) collected saliva 5×/day/2 days for cortisol. Diaries were used to assess prior-day nap duration, nocturnal awakenings, sleep latency, and morning restfulness. Ongoing fatigue and sleep disturbance were measured using the Multidimensional Fatigue Symptom Inventory and the Pittsburg Sleep Quality Inventory. Data were analyzed using multilevel growth curve modeling.
Results
Greater ongoing fatigue (b=0.035, p = .032), or sleep disturbance (b=0.026, p = .006) predicted a slower cortisol decline. Greater ongoing fatigue also predicted higher awakening cortisol (b=0.154, p = .030) and lower cortisol awakening response (CAR) (b=−0.146, p = .005). Longer prior-day naps predicted higher CAR (b=0.042, p=.050), and a steeper cortisol decline (b=−0.035, p = .003). Longer sleep latency predicted both a greater cortisol linear decline (b=−0.013, p < .001), and a greater quadratic slope curvature (b=0.0007, p < .001). Feeling less rested in the morning predicted lower awakening cortisol (b=−0.187, p= .004), higher CAR (b=0.124, p=.016) and a slower cortisol decline (b=0.023, p=.042).
CONCLUSIONS
Both daily variations in sleep behaviors and ongoing sleep disturbance and fatigue associated with a disrupted cortisol rhythm. In contrast, prior-day napping associated with a more robust cortisol rhythm. These findings are particularly relevant to women with breast cancer who often experience sleep disturbance and fatigue. Additional research is needed to determine causal pathways between sleep disturbance and dysregulation of the hypothalamic-pituitary-adrenal axis in patients with breast cancer.
Keywords: breast cancer, diurnal cortisol rhythm, cortisol awakening response, sleep disturbance, fatigue, napping
Introduction
Healthy individuals exhibit a diurnal pattern of cortisol secretion, with high levels of cortisol at awakening that further increase 50–60% at 30–45 minutes post-awakening (i.e., the cortisol awakening response or CAR) (1). Subsequently, cortisol levels rapidly decline in the first few hours after awakening, and then more gradually decline throughout the day to reach a nadir during sleep (2). The cortisol diurnal rhythm is under neural regulation, largely mediated through the suprachiasmatic nucleus and a light-sensitive extra-pituitary pathway that modulates adrenal sensitivity to ACTH. It is also subject to hormonal negative feedback, whereby cortisol inhibits hypothalamic-pituitary-adrenocortical (HPA) activity by interacting with glucocorticoid and mineralocorticoid receptors (3). Superimposed upon this regulation, the cortisol diurnal rhythm is also responsive to emotional, social, and physical experiences (4). Recent evidence demonstrates that cortisol levels, especially the cortisol awakening level and the CAR, are sensitive to day-to-day experiences (1, 5, 6). Disruption of the cortisol rhythm can adversely affect health, and for women with breast cancer, such disruption may impair cancer control (7).
Women with breast cancer show substantial variation in their cortisol diurnal rhythm, with some exhibiting a flattened rhythm (lower morning rise or higher level at bedtime) (7–9). Disruption of the cortisol rhythm in women with breast cancer is associated not only with a reduced quality of life (7, 10), but also increased cancer severity and poorer prognosis (8, 9). Further, in women with advanced metastatic breast cancer, a flatter cortisol slope, independent of other prognostic factors, was found to predict earlier mortality (7). These findings emphasize the importance of clarifying intra-individual differences in behaviors that associate with a disrupted cortisol rhythm in cancer patients. Fatigue and sleep disturbance are common behavioral symptoms experienced by cancer patients, and each have been linked to altered dynamics of the cortisol diurnal rhythm (7, 11). However, the relationships between sleep disturbances/fatigue and HPA axis regulation are complex and reciprocal. For example, disruption of the cortisol diurnal rhythm may contribute to and/or exacerbate sleep disturbance and fatigue in cancer patients, as the HPA axis plays a role in maintaining alertness and modulating sleep (12). Conversely, sleep onset, nocturnal awakenings, and sleep duration are also known to influence the profile of the next-day cortisol diurnal rhythm (13).
We previously demonstrated women with early stage breast cancer to report high levels of fatigue after surgery and during cancer treatment (14). Such fatigue may be attributed to sleep disturbance, which is more prevalent and intense prior to treatment onset and during the early weeks of treatment (15). Others demonstrated sleep disruption in advanced cancer patients to be linked to a flattened diurnal cortisol rhythm (7, 11). Likewise in a small preliminary study, a flatter cortisol diurnal rhythm was observed in long-term breast cancer survivors with persistent fatigue (10). However, little is known about the relationship among sleep quality, fatigue and the dynamics of the cortisol rhythm in women newly diagnosed with early stage breast cancer. Furthermore, there is no understanding of whether prior-day variations in sleep quality and napping alter the next-day cortisol rhythm pattern. Others show short daytime napping provides neurobehavioral benefit for those with sleep loss (16, 17), and daytime napping in healthy individuals reverses the effects of sleep loss on cortisol and IL-6 levels (18).
The purpose of this study, therefore, was to examine individual differences in the cortisol diurnal rhythm in relation to day-to-day fluctuations in sleep behavior, and napping. Women newly diagnosed with early stage breast cancer were enrolled and completed measures of prior-day sleep behaviors, including napping, in conjunction with measures of more ongoing sleep quality and fatigue, to determine associations with the day-to-day variations in the cortisol diurnal rhythm. Prior-day sleep behaviors were measured by daily diary, which assessed prior-day nap frequency and duration, latency to fall asleep, frequency and duration of nocturnal awakenings, and next day morning report of restfulness. Multilevel growth modeling was used to estimate diurnal cortisol profiles for each individual. Three-level hierarchical growth curve models were tested to consider the nesting of moments within days and days nested within persons (19). It was hypothesized that the cortisol diurnal rhythm profile would be associated not only with more long-term sleep disturbance and fatigue, but also with prior-day variations in sleep quality and napping.
Methods
Participants
The sample was drawn from a larger ongoing longitudinal study evaluating the effect of mindfulness-based stress reduction on psychosocial stress and immune dysregulation in women with breast cancer. For the present study, only data from the first assessment period (prior to the intervention) were evaluated. Women were recruited from three breast oncology clinics located in west-suburban Chicago. Eligible women were identified after their breast surgery when their surgical pathology report was available. Women were excluded if diagnosed with recurrent breast cancer or other cancers, immune-based or inflammatory disease, major psychiatric disorder or cognitive dysfunction. They were also excluded if they were substance abusers, used tobacco products, were taking corticosteroids, or if prescribed systemic cancer chemotherapy. Women were enrolled at least 2-weeks post-surgery after effects of anesthesia and surgical stress dissipated (20). A total of 146 women were enrolled on average 7±5 weeks after their breast cancer surgery. Sixteen women reported a recent respiratory condition or used sleep aid medication the night before saliva sampling and were subsequently excluded from the final analysis. Thus, the final sample included 130 participants with data collected from September 2008 to October 2012.
Procedure
Women completed sleep diaries for 3 consecutive days, to capture day-to-day variation in sleep behavior. On day 2 and 3, women collected saliva: at awakening, 30-minutes post-awakening, 1200h, 1700h, and at bedtime; saliva was used to determine the cortisol diurnal rhythm profile (described below). Women recorded the exact time of day for each saliva collection. Also, women completed the Pittsburgh Sleep Quality Index and the Multidimensional Fatigue Inventory, described below, to assess more prolonged or “ongoing” fatigue and sleep disturbance. This study was approved by the Institutional Review Board for the Protection of Human Subjects of all participating sites and informed consent was obtained from all participants.
Assessment
Ongoing fatigue and sleep disturbance
To capture the ongoing experience of fatigue and sleep disturbance during the weeks prior to cortisol sampling, the Multidimensional Fatigue Symptom Inventory Short Form (MFSI-SF; (21)) and Pittsburgh Sleep Quality Inventory (PSQI; (22)) were administered. The MFSI-SF measures overall fatigue and fatigue experienced in five domains (general, emotional, physical, mental, vigor). Respondents indicated their level of fatigue during the preceding one-week period (0= not at all to 4= extremely), with a higher score indicating greater fatigue. The fatigue total score represents total fatigue across all domains (subtracting vigor scale score) with a range of −24 to 96. Factor analysis demonstrated good fit for the 5 factors. Previously reported internal consistency ranges from .87−.96 (21). Cronbach alpha for the total fatigue scale in the present sample was 0.94. The PSQI measures quality and patterns of sleep over the preceding one-month period differentiating "poor” from "good" sleep on 7 subscales: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. A global sum of "5" or greater indicates "poor" sleep (range = 0 to 21). Cronbach alpha in the present sample was 0.83 for the Global Sleep Quality scale, when each of the seven components was treated as an “item” in the analysis. PSQI has high validity and reliability with cancer patients (22, 23).
Daily measures of sleep and fatigue
Day-to-day variation in quality of sleep and morning restfulness was assessed using a modified version of the sleep diary from the Washington Women's Health Diary (24). Women completed diaries every morning and every night prior to bedtime. At wakeup they answered questions about the previous night, including: time they went to bed, time to fall asleep (i.e., sleep latency), and number and duration of nocturnal awakenings. Women also recorded the time of awakening and rated how rested they felt that morning (0 = extremely rested to 4 = not at all rested), with higher scores indicating feeling less rested. At bedtime, women reported whether they napped during the day, and if so, for how long (in minutes). They also recorded any acute health issues, exercise behavior, and use of over the counter or prescription medications, including sleep aids.
Salivary cortisol diurnal measurement
To evaluate the cortisol diurnal profile, participants collected saliva using salivettes (Sarstedt, Inc, Newton, NC) at 5 times over two consecutive days, as noted in Procedure (25). The parameters defining the cortisol profile were: wake-up cortisol, size of the cortisol awakening response (CAR), and linear and quadratic slope from wake-up to bedtime. Salivary cortisol was measured in duplicate by immunoassay (Salimetrics, LLC, State College, PA). Intra-assay precision was 3.35–3.65% and inter-assay precision was 3.75–6.41%. Sensitivity is < 0.003 µg/dL (26).
Control variables
Demographic information, including age, race, marital status, education, and employment status, was obtained by self-report. Depressive symptoms and perceived stress were assessed as covariates, using the Center for Epidemiologic Studies Depression scale (CES-D; (27) and the Perceived Stress Scale (PSS; (28), respectively. Cancer pathology, staging, and treatment were obtained from medical records. Health behaviors (i.e., exercise, tobacco and medication use, and co-morbidities) were collected by self-report. The Charlson Co-morbidity Index-CCI was calculated and used to statistically control for pre-existing medical conditions. This index factors chronological age with comorbidities to create a sum score for each participant (29).
Statistical analysis
Preliminary analyses were performed using IBM SPSS 20.0 (Chicago, IL). Summary descriptive statistics for all variables were calculated and normality of distribution examined. Cortisol values were natural log-transformed to adjust for a positively skewed distribution. A modest skew was also detected for the latency to fall asleep (skewness = 1.16, SD = 0.19), however transformations offered no advantages to approximate normal distribution (30). Subsequently, raw scores were used in the final analysis.
Hierarchical linear modeling (HLM) was performed using HLM 7.0 software for computing multilevel model for change (19). HLM is based on full maximum likelihood estimation and was used to examine the associations among cortisol and the day-to-day variation in sleep behaviors, in conjunction with ongoing fatigue and sleep disturbance. Hierarchical growth modeling allows for examination of moment-varying, day-varying and person-varying factors within the same model (5, 19). HLMs also estimate variance components associated with the initial level and the time trend, which is indicative of the sample’s heterogeneity.
Three-level HLMs were computed. Level of cortisol for each person at each moment was the dependent variable. Predictor variables included moment-level predictors (Level 1), day-level (Level 2), and ongoing individual differences (Level 3). In order to fit the data to model the shape of each individual’s diurnal rhythm and the size of their CAR, time since awakening and CAR variables were included at Level 1. The time since awakening variable was computed by subtracting the wakeup time from the exact time of each cortisol sample, such that time upon awakening was zero. A quadratic term (hours since waking squared) was included to capture the curvilinear nature of the diurnal cortisol profile. To model the size of CAR, a dummy-coded variable CAR was also a part of the Level 1 model. To predict changes in the diurnal cortisol rhythm from day-to-day, sleep diary variables the day before (i.e., minutes to fall asleep, nocturnal awakenings, duration of nap time) and ratings of morning fatigue on the day of each cortisol sampling were entered at Level 2. Level 3 included predictors of ongoing fatigue (as assessed by MFSI) and sleep disturbances (as assessed by PSQI).
The HLM analysis was performed in three stages. First, to investigate the distribution of variation of cortisol across moments, days and persons, an unconditional model (i.e., model with no covariates) was fit to the data. The variance components were estimated to evaluate individual variation around the sample-wide model estimates. The second stage of HLM analysis examined the potential effects of the following variables: demographic (age, race, education, marital status and BMI), prior-day exercise, co-morbidities, medication usage (including anti-depressants), and cancer treatment variables (cancer stage, type of surgery, radiation therapy, time since start of radiation therapy, and anti-estrogen cancer therapy). Effects of depressive symptoms (CES-D) and women’s perception of stress (PSS) were also tested. To achieve the most parsimonious models, only variables that had a significant effect (t-value above 2.00) and retained their significance in combination with other predictor variables were left in the final model. Note, no effect of cancer treatment variables including radiation therapy, medication usage, depressive symptoms and perception of stress were found to affect the cortisol diurnal rhythm. During the third and final stage, potential moderating effects of daily sleep behaviors (assessed by sleep diary) and ongoing fatigue and sleep disturbance (assessed by MFSI, PSQI) were examined. These variables were standardized to aid in the interpretation and were entered simultaneously.
In the models, the intercept represents average cortisol level upon awakening, linear and quadratic slopes reflect change across the day from time of awakening to bedtime (excluding CAR), and CAR coefficient reflects each woman’s CAR. The slope coefficients are interpreted as a percent change per one standard deviation change in the predictor variable, after applying the following transformation : B%change=[exp[Braw]−1]. To return the cortisol intercept value to the original measurement scale (µg/dl), the inverse of natural-log was applied (see Table 3). All Level 2 and Level 3 predictor variables were standardized and co-variables were grand mean centered.
Table 3.
Final Hierarchical Linear Model of Association between Sleep Behavior Variables, Fatigue and the Diurnal Cortisol Rhythm (N = 130).
| Fixed Effects | Coefficient | SE | Interpretation |
|---|---|---|---|
| Cortisol Intercept (Wakeupa) | |||
| Intercept | −1.216*** | 0.066 | Awakening level = 0.296b |
| MFSI | 0.154* | 0.081 | 14% increase per +1SD |
| PSQI | −0.067 | 0.497 | --- |
| Nap duration | −0.184** | 0.062 | 17% decrease per +1SD |
| Nocturnal awakenings | 0.088* | 0.044 | 9% increase per +1SD |
| Latency to fall asleep | 0.009 | 0.008 | --- |
| Morning restfulnessc | −0.187** | 0.062 | 17% decrease per +1SD |
| Linear Time Slope | |||
| Intercept | −0.191*** | 0.013 | 17% decrease per hr at wakeupd |
| MFSI | 0.035* | 0.016 | 3.4% flatter |
| PSQI | 0.026** | 0.009 | 2.7% flatter |
| Nap duration | −0.035*** | 0.009 | 3.4% steeper |
| Nocturnal awakenings | −0.021 | 0.012 | --- |
| Latency to fall asleep | −0.013*** | 0.002 | 1.3% steeper |
| Morning restfulness | 0.023* | 0.011 | 2.3% flatter |
| Co-morbidity Index | 0.004** | 0.002 | 0.4% flatter |
| Quadratic Time Slope | |||
| Intercept | 0.004*** | 0.0007 | 0.4% Increase per hr2 |
| MFSI | 0.0009 | 0.0011 | --- |
| PSQI | −0.0015 | 0.0011 | --- |
| Nap duration | −0.0017** | 0.0005 | 0.1% less curvature |
| Nocturnal awakenings | 0.0009 | 0.0009 | --- |
| Latency to fall asleep | 0.0007** | 0.0001 | 0.07% more curvature |
| Morning restfulness | 0.0005 | 0.0008 | --- |
| Cortisol Awakening Response (CAR) | |||
| Intercept | 0.394*** | 0.039 | 48% increase 30-min post wakeup |
| MFSI | −0.146** | 0.050 | 13.5% decrease per +1SD |
| PSQI | −0.002 | 0.051 | --- |
| Nap duration | 0.042* | 0.021 | 4.3% increase per +1SD |
| Nocturnal awakenings | −0.029 | 0.043 | --- |
| Latency to fall asleep | 0.009 | 0.008 | --- |
| Morning restfulness | 0.124** | 0.049 | 12% increase per +1SD |
Notes. aTime was coded 0 at the wakeup time.
Because cortisol values were natural-log transformed, the inverse of that transformation (the exponential function) was applied to return the intercept to the original scale of measurement.
Higher Morning restfulness score indicates lower levels of feeling rested on the morning of saliva collection.
Linear time slope intercept represents instantaneous rate of change at awakening. All Level 1 predictors are uncentered; Level 2 and Level 3 predictors are either standardized or grand-mean centered. MFSI= Multidimensional Fatigue Symptom Inventory, PSQI= Pittsburgh Sleep Quality Inventory.
p < .001,
p < .01,
p ≤ .05.
Results
Descriptive characteristics of participants
As summarized in Table 1, women were predominantly Caucasian (77%), married (72%), and well educated. The majority had Stage 0 or Stage I breast cancer (78%). All women underwent surgical removal of their cancer (101 had breast conserving surgery and 29 had a mastectomy). Most women (n=110) received adjuvant radiation therapy; 25% of the women had begun their radiation treatment and on average were 3 ±2.5 weeks into their treatment at assessment. The effects of radiation therapy and time since the start of radiation therapy were examined as covariates during stage 2 of the HLM analysis and were not significant predictors of the cortisol diurnal rhythm. Descriptive data for the fatigue and sleep measures are provided in Table 2. Data from the sleep diaries reported in Table 2 were averaged across 2 days. As indicated by the PSQI and MFSI measures, women reported poor sleep (M = 8.1, SD = 3.6) and increased fatigue (M = 16.4, SD = 11.8) (22, 31). Intercorrelations (assessed by Pearson or Spearman rank correlation coefficients as appropriate) between sleep/fatigue variables are reported in Table 4. Greater sleep disturbance (PSQI) was associated with greater fatigue (MFSI) (r = .49, p < .001) and prolonged sleep latency (ρ =.21, p = .016). Greater levels of morning restfulness were related to less fatigue (r = −.37, p < .001), less sleep disturbance (r = −.37, p < .001), shorter duration of nocturnal awakenings (r = −.26, p = .003) and shorter sleep latency (ρ = −.29, p < .001). Raw cortisol values for the 2-days of saliva collection are provided in Table S1, Supplemental Digital Content 1.
Table 1.
Demographic Characteristics (N = 130).
| Age (yrs) Mean ± SD | 55. 6 ± 9.4 |
| Education (yrs) Mean ± SD | 15.4 ± 2.8 |
| Race | n (% Yes) |
| White | 100 (76.9%) |
| African American | 17 (13.1%) |
| Hispanic | 7 (5.4%) |
| PI/Asian | 6 (4.6%) |
| Marital Status | |
| Married | 93 (71.5%) |
| Divorced/Separated | 20 (15.4%) |
| Single | 17 (13.1%) |
| Income | |
| $10,000–29,000 | 12 (9.2%) |
| $30,000–59,000 | 23 (17.7%) |
| $60,000 and higher | 95 (73.1%) |
| Stage of Cancer | |
| Stage 0 | 28 (21.5%) |
| Stage I | 73 (56.2%) |
| Stage II | 29 (22.3%) |
| Treatmenta | |
| Radiation therapyb | 32 (24.6%) |
| Time since start a (Mean ±SD) | 3.1±2.5 |
| Surgery Type | |
| Breast Conserving | 101 (77.7%) |
| Mastectomy | 29 (22.3%) |
Notes. a Treatment that has started before T1 assessment.
SD= standard deviation
Most women (n = 110) received radiation therapy as part of their cancer treatment; 25% of the women had begun their radiation treatment and on average were 3 ± 2.5 weeks into their treatment at assessment.
Table 2.
Descriptive Data for Sleep Behaviors and Fatigue (N = 130).
| Variables | Mean ± SD | Range |
|---|---|---|
| Time of awakening (hrs) | 7:00 a.m. ± 1.3 | 4:00 a.m. – 11:50 a.m. |
| Latency to fall asleep (min) | 20.3 ± 23 | 1 – 150 |
| Quality of sleep | 5.7 ± 1.9 | 1 – 9 |
| Morning restfulness | 2.1 ± 0.9 | 0 – 4 |
| Nap (% Yes) | 42 % | |
| Nap duration (min) | 18.7 ± 20.9 | 0 – 110 |
| Nocturnal awakenings (% Yes) | 92 % | |
| Nocturnal awakenings duration (min) | 21.5 ± 28.2 | 0 – 180 |
| MFSI total score | 16.4 ± 11.8 | −21 – 87 |
| PSQI total score | 8.1 ± 3.6 | 2 – 18 |
Note. Sleep diary variables were averaged across 2 assessment days. SD = standard deviation.
MFSI=Multidimensional Fatigue Symptom Inventory; PSQI= Pittsburgh Sleep Quality Index.
Table 4.
Intercorrelations Between Dependent Variables (N = 130).
| MFSI | PSQI | Nap duration |
Nocturnal awakenings |
Latency to fall asleep |
Morning restfulness |
|
|---|---|---|---|---|---|---|
| MFSI | .498*** | −.078 | .158 | .092 | −.372** | |
| PSQI | .033 | .192 | .212** | −.374** | ||
| Nap duration | −.035 | .048 | .127 | |||
| Nocturnal awakenings | −.002 | −.256** | ||||
| Latency to fall asleep | −.291** | |||||
| Morning restfulness |
Notes. *p <.05,
p <.01,
p <.001.
MFSI = Multidimensional Fatigue Symptom Inventory; PSQI = Pittsburgh Sleep Quality Index.
The effects of daily sleep factors, ongoing sleep disturbance and fatigue
Results of the final HLM analysis are shown in Table 3. On average, across two days, women showed a typical diurnal pattern of cortisol: high values upon awakening (b = −1.216, = 0.296 µg/dl), a 48% increase in CAR (b = 0.394) and an initial decline at awakening (b = −0.191 = 17% decrease per hour at wakeup). The quadratic term was significant, indicating that there was a curvature to the diurnal rhythm in addition to the linear decline over the day. For example, the rate of the decline was 14% decrease per hour at 1200hr, was reduced to 11% by 1700hr and finally tapered off to 5% at bedtime. The rate of the decline was most rapid within the first 8 hours and tapered off by the end of the day (16 hours later). Of the covariates tested (i.e., demographic and cancer treatment variables), only the co-morbidity index significantly associated with the linear rate of change in cortisol level, in that women with a greater co-morbidity index exhibited a flatter linear slope (b = 0.004, p = .051). Significant associations are demonstrated in Figures 1–3. Note these figures are graphical representations of the predicted estimates computed by HLM, and not sample mean values.
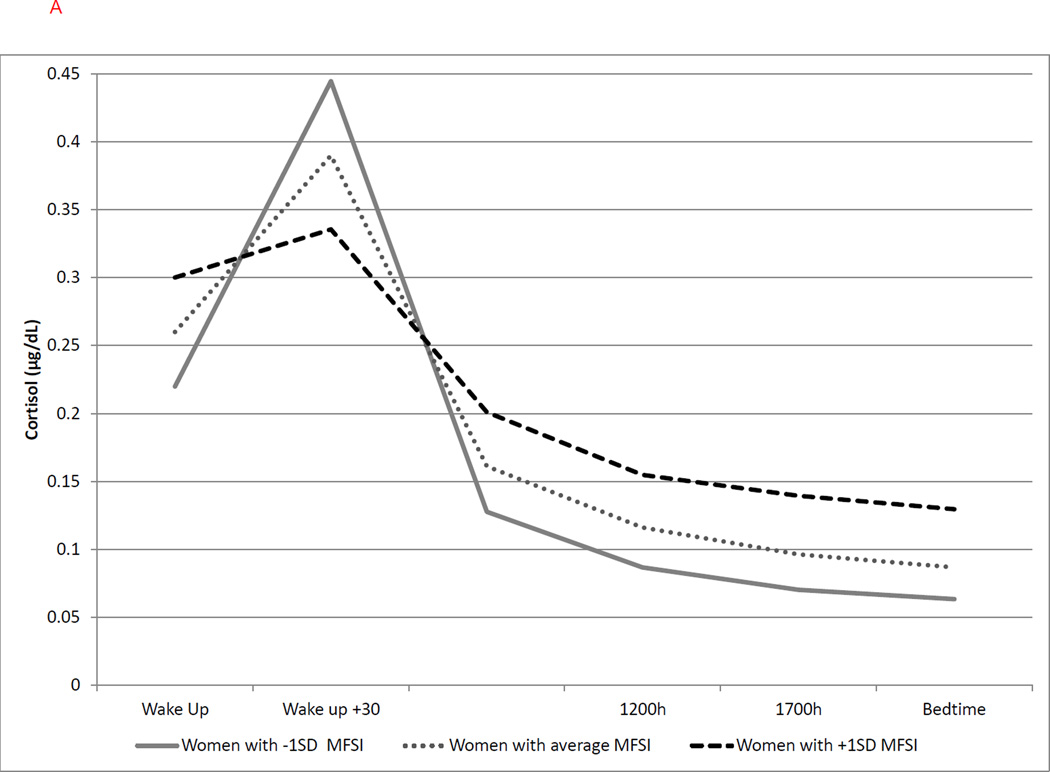
Figure 1.
(a–b). Graphical representation of the effects of ongoing fatigue (MFSI score) and sleep disturbance (PSQI score) on cortisol diurnal rhythm. These effects are modeled either as the average value as well as ±1SD of the sample mean values. Graphs are estimated by the hierarchical linear models from the time of awakening (Wake Up) through bedtime.
(a) Greater ongoing fatigue (MFSI) was associated with higher cortisol upon awakening (b = 0.154, p = .030), smaller CAR (b = −0.146, p = .005), and flatter linear slope (b = 0.035, p = .032), but not quadratic slope. MFSI = Multidimensional Fatigue Inventory; Solid line represents MFSI scores at −1 SD; dotted line represents average MFSI scores; dashed line represents MFSI scores at +1 SD.
b) Greater reduction in quality of sleep (PSQI) was associated with a flatter linear slope (b = 0.026, p = .006), but not cortisol upon awakening, CAR or quadratic slope.
PSQI = Pittsburgh Sleep Quality Index; Solid line represents PSQI scores at −1 SD; dotted line represents average PSQI scores; dashed line represents PSQI scores at +1 SD.
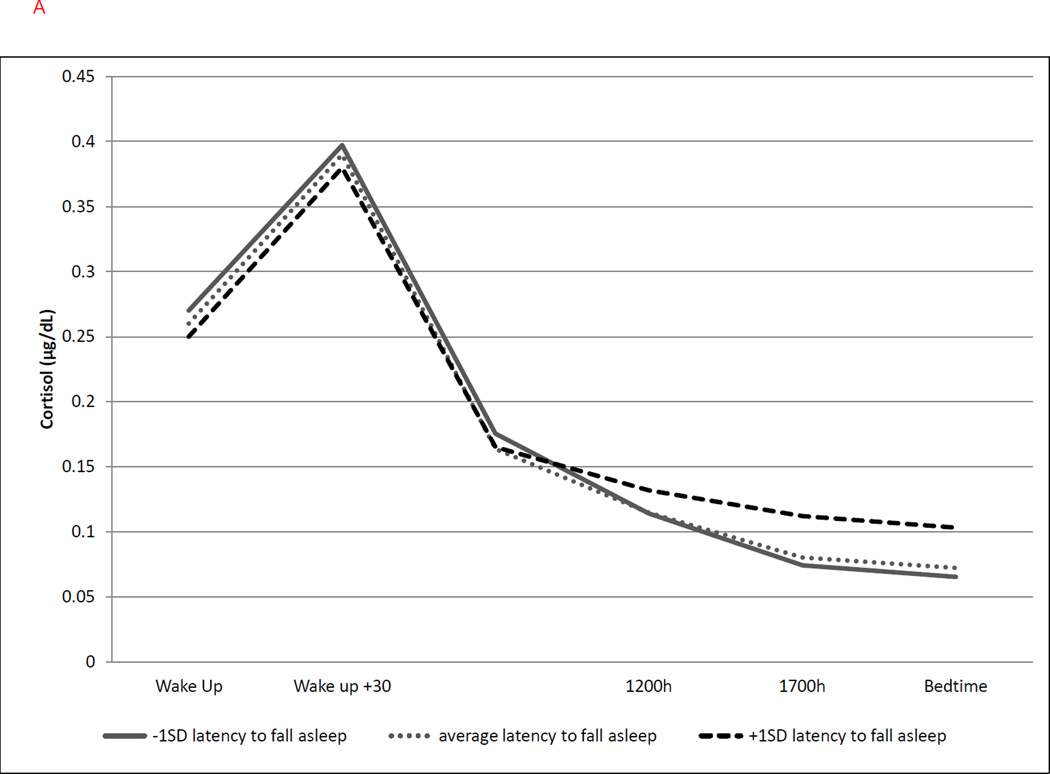
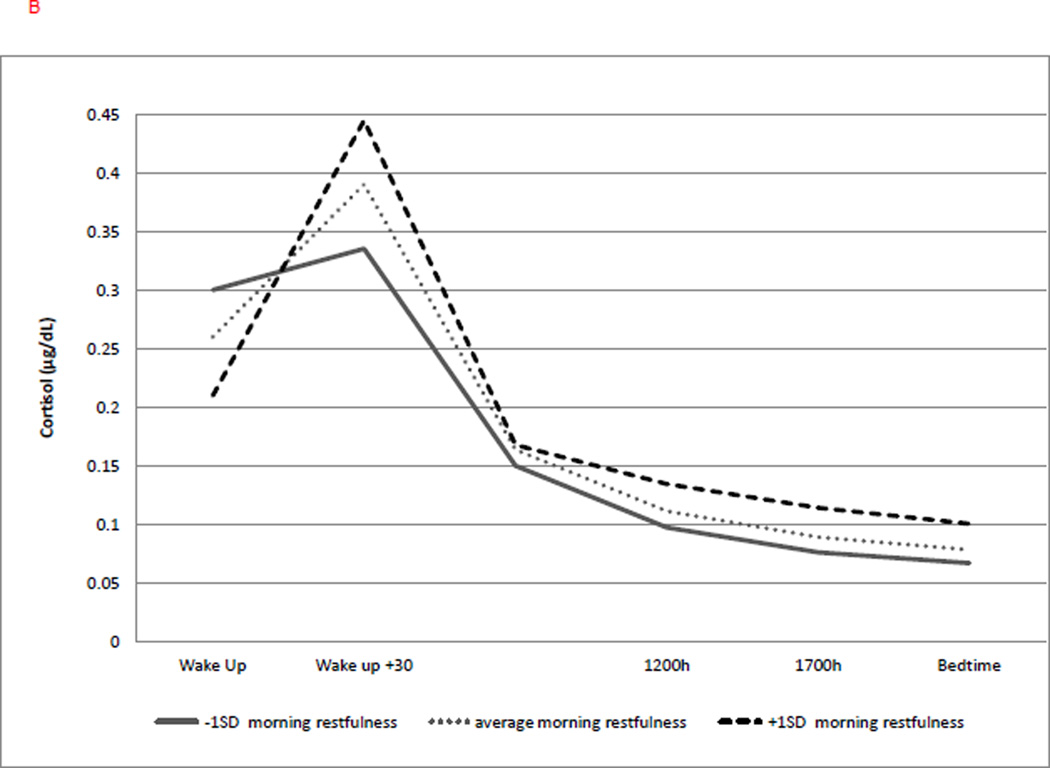
Figure 3.
(a–b). Graphical representation of the effects of prior-night latency to fall asleep and same-day morning restfulness on the cortisol diurnal rhythm. These effects are modeled either as the average value as well as ±1SD of the sample mean values. Graphs are estimated by the hierarchical linear models from the time of the awakening (Wake Up) through bedtime.
(a) Longer prior night sleep latency was associated with steeper linear slope (b = −0.013, p < .001) and a greater quadratic slope (b = 0.0007, p < .001), but not cortisol at awakening or CAR. Solid line represents duration of sleep latency at −1SD; dotted line represents average duration of sleep latency; dashed line represents duration of sleep latency at +1 SD.
(b) Lower morning restfulness was associated with lower cortisol at awakening (b = −0.187, p = .004), a greater CAR (b = 0.124, p = .016), and a flatter linear slope (b = 0.023, p = .042), but not quadratic slope. Solid line represents level of morning restfulness at −1SD; dotted line represents average level of morning restfulness; dashed line represents level of morning restfulness at +1 SD.
Ongoing fatigue and quality of sleep
Significant associations between ongoing fatigue and quality of sleep and cortisol parameters were found (see Table 3). Women reporting greater levels of ongoing fatigue (MFSI) had higher cortisol levels upon awakening, with a 14% increase per 1SD increase in fatigue (b = 0.154, p = .030). See Figure 1a. An elevation in ongoing fatigue was also associated with a less pronounced CAR (b = −0.146, p = .005; with a 13.5% decrease per 1SD increase in fatigue), as well as a slower decline in cortisol over the day (b = 0.035, p = .032; with a 3.4% flatter linear slope per 1SD increase in fatigue). Poor sleep quality (PSQI) was significantly associated with linear slope (b = 0.026, p = .006), with a 2.7% flatter slope for every 1SD increase in poor quality of sleep, but was not associated with cortisol upon awakening, CAR, or quadratic change. See Figure 1b. Similar to ongoing fatigue, reduction in sleep quality predicted a slower cortisol decline in linear slope (b = 0.026, p = .006).
Daily sleep behaviors
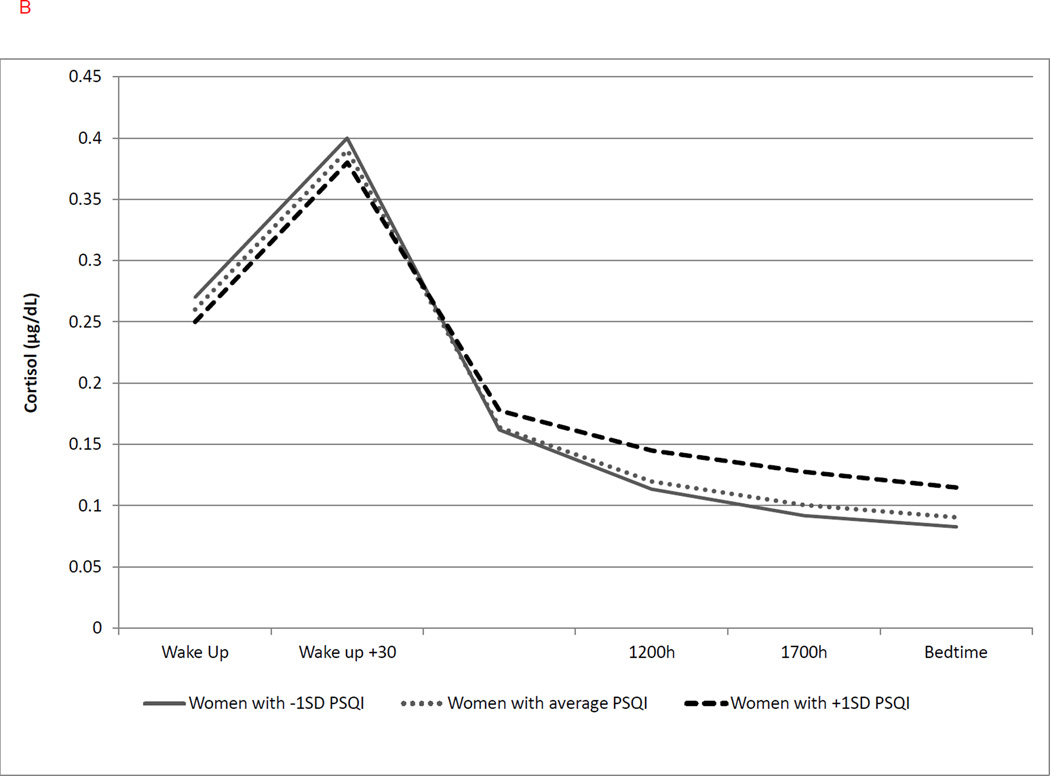
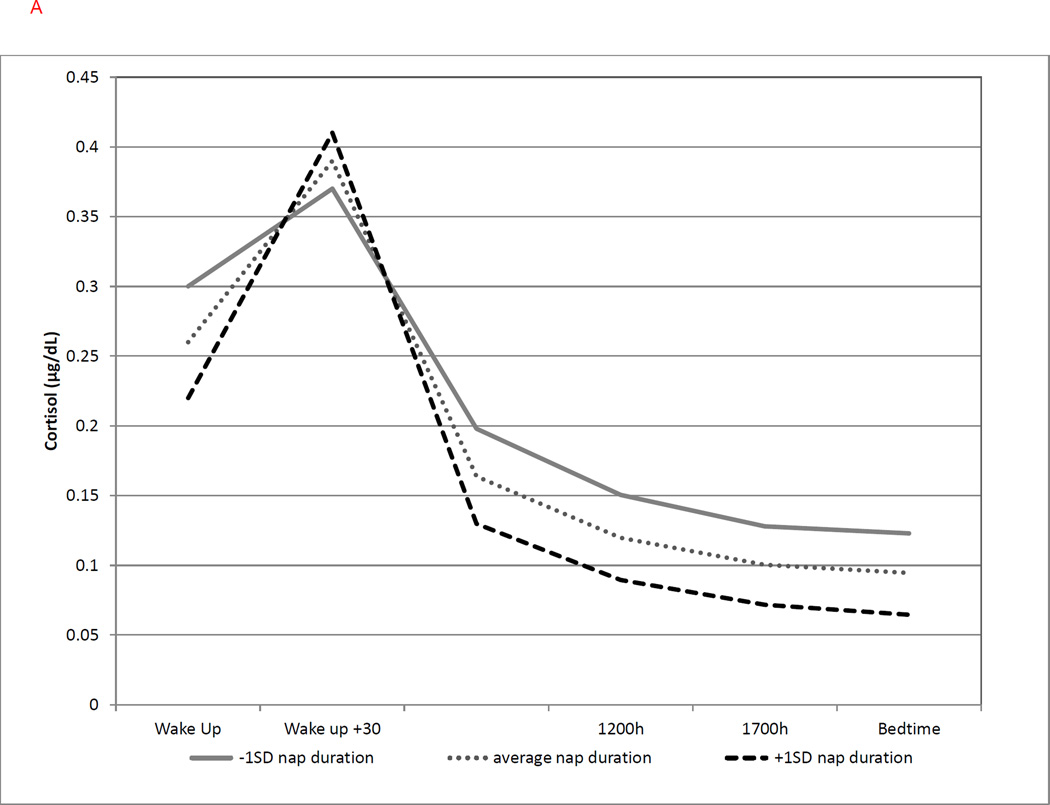
The following sleep diary variables, as measured across each day, were found to be significantly associated with cortisol parameters: nap duration, duration of nocturnal awakening, and morning restfulness were associated with cortisol levels at awakening; nap duration and morning restfulness were associated with linear slope and with the CAR; and sleep latency was associated with the linear and quadratic slopes. As shown in Table 3 and Figure 2a, greater duration of prior-day naps was associated with lower cortisol at awakening (b = −0.184, p = .004; with a 17% decrease for every 1SD increase in nap time) and a greater CAR (b = 0.042, p = .050; with a 4.3% increase for every 1SD) on the following day. A greater duration of prior-day naps was also associated with a steeper cortisol decline (b= −0.035, p = .003; with a 3.4% steeper decline in linear slope per 1SD) and less quadratic slope curvature (b = −0.0017, p < .001) over the following day.
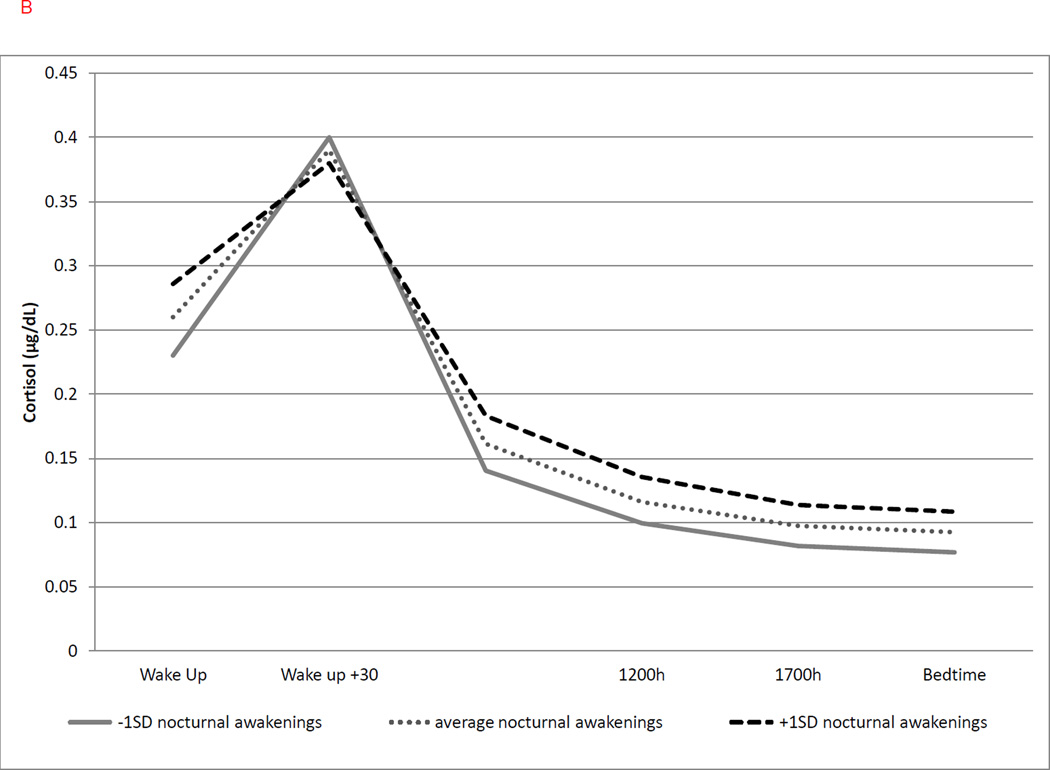
Figure 2.
(a–b). Graphical representation of the effects of duration of prior-day nap duration and prior-night nocturnal awakenings on cortisol diurnal rhythm. These effects are modeled either as the average value as well as ±1SD of the sample mean values. Graphs are estimated by the hierarchical linear models from the time of the awakening (Wake Up) through bedtime.
(a) Longer prior-day nap duration was associated with lower cortisol at awakening (b = −0.184, p = .004), greater CAR (b = 0.042, p = .050), steeper linear (b = −0.035, p = .003) and flatter quadratic slopes (b= −0.0017, p < .001). Solid line represents nap duration at −1SD; dotted line represents average nap duration; dashed line represents nap duration at +1SD.
(b) Greater duration of nocturnal awakenings was associated with a greater cortisol at awakening only (b = 0.088, p = .043) and not CAR or linear/quadratic slope. Solid line represents duration of nocturnal awakenings at −1SD; dotted line represents average duration of nocturnal awakenings; duration; dashed line represents duration of nocturnal awakenings at +1 SD.
A longer prior-night duration of nocturnal awakenings was significantly associated with greater average cortisol at wakeup (b = 0.088, p = .043; with a 9% increase in cortisol for 1 SD increase in duration of nocturnal awakenings). Yet, the duration of nocturnal awakenings was not a significant predictor of CAR or linear or quadratic change over time (Figure 2b). Latency to fall asleep the night before was significantly associated with both linear and quadratic rate of change in cortisol but not with cortisol level at awakening or CAR. Longer sleep latency was associated with a steeper decline in the linear slope of cortisol throughout the following day (b = −0.013, p < .001; with a 1.3% steeper decline for every 1 SD) but greater curvature in the slope as indexed by the quadratic slope (b = 0.0007, p < .001, Figure 3a).
Lastly, a decreased feeling of restfulness on the day of saliva collection was associated with a lower cortisol level upon awakening (b = −0.187, p = .004; with a 17% decrease in cortisol for every1 SD decrease in morning restfulness) and a greater CAR (b = 0.124, p = .016), with a 12% increase in CAR for every 1 SD increase in morning restfulness. Also, a decreased feeling of morning restfulness was associated with a slower decline of cortisol over the day (b = 0.023, p = .042; with a 2.3 % flatter slope for every 1 SD increase in morning restfulness). However, morning restfulness was not a significant predictor of the quadratic trajectory (Figure 3b).
Discussion
Few studies have modeled the day-to-day dynamics of associations between sleep behaviors and the diurnal cortisol rhythm, together with an individual’s ongoing experience of sleep and fatigue, as accomplished in this study. Key findings revealed that prior-day variations in sleep behaviors, as well as ongoing or cumulative experience of sleep disturbance and fatigue, independently influenced the day-to-day pattern of the cortisol diurnal rhythm in women with early stage breast cancer. Specifically, women reporting either ongoing fatigue or poor sleep quality exhibited a slower decline of cortisol during the day (i.e., flatter slope) but in addition, ongoing fatigue was associated with a higher cortisol level at awakening and a lower CAR. In contrast, prior-day nap duration associated with a more dynamic cortisol diurnal rhythm marked by a lower cortisol level at awakening, a higher CAR, and a steeper decline of cortisol throughout the day. Our evaluation of prior-day sleep behaviors revealed that longer nocturnal awakenings were associated with a higher cortisol at awakening, while longer latency to fall asleep was associated with an increased linear decline in slope, as well as a greater quadratic curvature of the cortisol slope. Moreover, women who did not feel rested on the morning of cortisol sampling exhibited lower levels of cortisol at awakening, an increased CAR, and a slower decline in cortisol over the day.
Our finding that ongoing sleep disturbance and fatigue associated with a flatter cortisol slope in women newly diagnosed with early stage breast cancer is consistent with prior work evaluating women with advanced metastatic breast cancer (11), as well as preliminary findings observed in a small sample of breast cancer survivors 1–2 years post-treatment (32). Yet, there is limited research on the relationship among fatigue, poor sleep, and the pattern of the cortisol rhythm in women recently diagnosed with early stage breast cancer. One investigation examined women awaiting their breast cancer surgery, with the majority of that sample (86%) having a diagnosis of Stage 0 thru Stage IIIA. Findings from that study revealed that women with poor circadian consistency of rest/activity patterns (assessed by actigraphy) exhibited flatter cortisol slopes (33). Another study of women without metastasis, who were on average 22 months post diagnosis, found those women reporting poor sleep quality and shorter sleep duration to have a flatter diurnal slope (34). Our results, in conjunction with these prior studies, demonstrate that associations between ongoing fatigue and sleep disturbance and disruption of the cortisol diurnal rhythm are present in women no matter what the stage of their cancer and such disruption manifests early on during diagnosis and treatment, as well as much later during survivorship.
In our sample, 42% of women reported taking a nap, which varied from 5 to 110 minutes. A novel finding from the present study was that longer prior-day nap duration was associated with a more dynamic cortisol rhythm (i.e., greater CAR and steeper decline throughout the day). Little is known about the effects of prior-day napping on cortisol levels. Some evidence, however, shows that cortisol secretion is sensitive to daytime sleep in healthy adults. For example, in individuals who took a nap on the day following a night of total sleep deprivation, cortisol levels were found to be lower during the nap, but to increase during the post-nap period (18). Yet others report a decrease in cortisol after a daytime nap (35). To our knowledge the present report is the first to describe the effects of prior-day nap duration on the cortisol diurnal rhythm in women with breast cancer.
The potential health benefits of napping remain under investigation; however, sleep hygiene protocols commonly recommend avoidance of daytime naps to prevent disturbance of nighttime sleep. Yet, contrary to this thinking, others demonstrated in healthy adults that afternoon naps produced little effect on the duration or quality of subsequent nighttime sleep; moreover, 24-hour sleep time was increased and those who napped had better cognitive and psychomotor performance after the nap and over the day (36). Daytime napping can also improve emotional state (37). Contrasting data, however, shows that habitual and prolonged daytime napping can be associated with poor health outcomes (38, 39). Whether daytime naps are beneficial to health depends upon many factors, including the duration of the nap, time of day that napping occurs, if the nap is planned or reactive, and the person’s age and existing health condition (17). Our observation that daytime napping associates with a steeper pattern of the cortisol diurnal rhythm is impetus to further examine the influence of napping on the dynamics of the cortisol diurnal rhythm in cancer populations, as some evidence shows cortisol and disruption of the cortisol diurnal rhythm influence cancer control (7, 40–42). Although the precise mechanism is poorly understood, cortisol can inhibit apoptosis of cancer cells (43, 44) and down-regulate expression of the human tumor suppressor gene, BRCA1 (45). Prior work also demonstrated that social isolation in rodents increased the corticosterone response to acute stress and such increase was associated with greater mammary tumor progression (46). Further evaluation of approaches that lower the cortisol response to stress and/or promote a more dynamic cortisol rhythm is needed as such approaches may benefit cancer control.
A person’s sleep-wake cycle can modulate immune function (47) in that prolonged sleep disturbance can promote a proinflammatory state by altering neuroendocrine pathways, including cortisol secretion (48). Napping may reverse such effects. For example, in adults who napped after a night of sleep deprivation, indices of inflammation were reduced and the reduction was associated with a decrease in cortisol levels immediately after the nap (35). This is pertinent to cancer patients, as inflammation contributes to tumor development and progression, including breast tumors (49, 50); thus the reduction of inflammation may serve as another pathway whereby napping might benefit those with cancer-related sleep disturbance and fatigue.
Our findings demonstrate that ongoing sleep disturbance and fatigue, as well as the day-to-day experience of sleep disturbance and restfulness disrupts the cortisol rhythm in a manner that results in more prolonged exposure to higher cortisol levels during the late afternoon and evening hours. We observed that women who reported feeling less rested in the morning, as well as women who reported greater ongoing fatigue and sleep disturbance, exhibited a slower decline of cortisol over the day. Also, women reporting longer latency to fall asleep the night prior to saliva sampling had more prolonged exposure to higher cortisol levels. But in this case, longer sleep latency was associated with greater increase in the curvature of the quadratic slope of the cortisol rhythm, instead of a flatter linear slope, as observed for ongoing fatigue and sleep disturbance. This indicates that the decline in cortisol level tapered off earlier in the day and was greater at bedtime for women with longer sleep latency, compared to women with shorter sleep latency. These results demonstrate the importance of accounting for the quadratic change in cortisol, which is not typically evaluated.
We also observed that both day-to-day variations in sleep behaviors and ongoing fatigue influenced the magnitude of the CAR. The CAR is a distinct phenomenon of the cortisol diurnal rhythm and is under discrete neural and hormonal regulation (3). The precise biological function of CAR remains unclear (3, 11), however, it is theorized that CAR serves as an adaptive mechanism to prepare individuals for the demands of the upcoming day (i.e., the stress anticipation hypothesis) (5, 6). Although largely untested, the stress anticipation hypothesis is supported by research demonstrating higher CAR during week-days versus week-ends (51–53) and during athletic competition days versus non-competition days (54). Further support for the stress anticipation hypothesis is provided by a study of healthy adults that demonstrated an association between a higher CAR and an attenuated stress response to stressors encountered later that same day (55). Our results show that women newly diagnosed with breast cancer exhibit a higher CAR when they report feeling less rested in the morning. Although speculative, this may represent an adaptive response of the HPA axis to the anticipation of a more taxing day. In contrast, we observed that greater ongoing fatigue associated with a lower CAR. This finding is consistent with meta-analysis of 147 studies, which found robust associations between fatigue, burnout, and a reduced CAR (56). It should be emphasized, however, that neither heightened nor blunted CAR can be interpreted as maladaptive. In fact, it is theorized that it is not the size of the CAR that characterizes a "healthy" cortisol pattern, but the day-to-day flexibility and responsivity to changes in daily circumstances. That is, the CAR is dysfunctional when it fails to adjust the organism energetic supply to situational demands of a particular day (53).
Several limitations of this study are acknowledged. First, the design precluded a determination of causal directions. It is possible that the disruption of the cortisol rhythm led to the altered sleep behaviors and experiences of fatigue and sleep disturbance, and not the reverse. For instance, in healthy elderly, Adam et al (2006) found that cortisol levels at awakening predicted fatigue, but fatigue the day before did not predict wakeup cortisol (5). Ample evidence demonstrates an important role for the HPA axis in maintaining alertness and modulating sleep (12). Additional research is therefore needed to determine the causal pathways because HPA axis dysregulation and sleep disturbances/fatigue may be bi-directional. Second, we were unable to partial out the contribution of cancer-related biological factors to the dysregulation of the cortisol rhythm. Others demonstrate in ovarian cancer patients that tumor reduction/elimination through chemotherapy associates with normalization of the cortisol rhythm and tumor-derived inflammation (57). Women in our sample, however, had early stage cancer and were evaluated after surgical removal of their cancer; thus, reducing the possibility that tumor-related inflammation contributed to the observed disruption of the cortisol diurnal rhythm. Third, although the magnitude of the variation in cortisol rhythm dynamics observed in our data are comparable to previous effect sizes for change in cortisol levels observed in response to day-to-day variations in emotional experiences (i.e., tension, anger) in healthy adults (5), the health implications of such changes in cortisol rhythm remains to be determined. Fourth, our investigation used a short sampling time frame (three days). A longer sampling period for both daily experiences and cortisol would enhance understanding of the dynamics of associations between day-to-day variations in sleep behaviors and the diurnal cortisol rhythm. Also, our study relied upon subjective assessments of sleep quality. More objective measures of sleep quality, such as actigraphy or polysomnography, are needed to more carefully evaluate the relationship between sleep architecture and the cortisol rhythm in women with breast cancer. Lastly, women were not asked to provide the time at which naps were taken. Although the evidence is equivocal, in healthy adults a short afternoon nap can be restorative, providing short-term increases in alertness and cognitive performance, as well as more long-term health benefits (58). Yet, putative benefits of nap-timing depend on many factors, such as sleep quality during the nap, quality of sleep during preceding night(s), and stability of the sleep/wake cycle (16, 17). Despite limitations, our findings demonstrate that napping is associated with a more dynamic cortisol diurnal rhythm, suggesting a potential biological mechanism whereby napping might confer health benefits for women with breast cancer.
In summary, to our knowledge this is the first study to examine daily changes in sleep behavior, in relation to day-to-day variation in cortisol rhythm in women newly diagnosed with early stage breast cancer. Our findings demonstrate that the dynamics of the cortisol diurnal rhythm is not only associated with the experience of more prolonged fatigue and poor sleep quality, but is also sensitive to day-to-day variations in sleep behaviors, nap duration, and morning feeling of restfulness. Thus, although typically treated as a random error, day-to-day variability in the cortisol diurnal rhythm systematically associates with day-to-day changes in sleep behaviors. These findings emphasize the importance of continuing to investigate the dynamic interplay between one’s experiences and the nature of the cortisol rhythm. It is possible that similar alterations in the cortisol diurnal rhythm may occur in other populations, both healthy and ill, who experience fatigue and sleep disturbance. For individuals with cancer, understanding factors that disrupt the cortisol diurnal rhythm is significant, as a more dynamic cortisol rhythm supports cancer control mechanisms and improves outcomes (7, 40–42).
Supplementary Material
Acknowledgements
The authors thank the women who participated in the study. Gratitude is also extended to Karen Fishe, RN, MSN for assistance in subject recruitment and Thomas Schenone for laboratory analysis.
Funding: NIH R01 CA125455 to LWJ and HLM and American Cancer Society (ACS PF-12-261-01-CPPB) to DT.
Glossary
- CAR
Cortisol Awakening Response
- CCI
Charlson Co-morbidity Index
- PSQI
Pittsburg Sleep Quality Inventory
- MFSI
Multidimensional Fatigue Symptom Inventory
- CESD
Center for Epidemiologic Studies Depression
- PSS
Perceived Stress Scale
- HLM
Hierarchical linear modeling
Footnotes
Conflicts of Interest: None
Contributor Information
Dina Tell, Department of Health Promotion, Loyola University Chicago, Marcella Niehoff School of Nursing.
Herbert L. Mathews, Department of Microbiology and Immunology, Loyola University Stritch School of Medicine.
Linda Witek Janusek, Department of Health Promotion, Loyola University Chicago, Marcella Niehoff School of Nursing.
References
- 1.Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- 2.Posener JA, Schildkraut JJ, Samson JA, Schatzberg AF. Diurnal variation of plasma cortisol and homovanillic acid in healthy subjects. Psychoneuroendocrinology. 1996;21:33–38. doi: 10.1016/0306-4530(95)00033-x. [DOI] [PubMed] [Google Scholar]
- 3.Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neuroscience & Biobehavioral Reviews. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: An Overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- 5.Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U S A. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 8.Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Touitou Y, Levi F, Bogdan A, Benavides M, Bailleul F, Misset JL. Rhythm alteration in patients with metastatic breast cancer and poor prognostic factors. J Cancer Res Clin Oncol. 1995;121:181–188. doi: 10.1007/BF01198101. [DOI] [PubMed] [Google Scholar]
- 10.Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Palesh O, Zeitzer JM, Conrad A, Giese-Davis J, Mustian KM, Popek V, Nga K, Spiegel D. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. J Clin Sleep Med. 2008;4:441–449. [PMC free article] [PubMed] [Google Scholar]
- 12.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- 13.Balbo M, Leproult R, Van Cauter E. Impact of Sleep and Its Disturbances on Hypothalamo-Pituitary-Adrenal Axis Activity. International Journal of Endocrinology. 2010 doi: 10.1155/2010/759234. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witek Janusek L, Tell D, Albuquerque K, Mathews HL. Childhood adversity increases vulnerability for behavioral symptoms and immune dysregulation in women with breast cancer. Brain Behav Immun. 2013;30:S149–S162. doi: 10.1016/j.bbi.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas KS, Bower J, Hoyt MA, Sepah S. Disrupted sleep in breast and prostate cancer patients undergoing radiation therapy: the role of coping processes. Psychooncology. 2010;19:767–776. doi: 10.1002/pon.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers NL, Dorrian J, Dinges DF. Sleep, waking and neurobehavioural performance. Front Biosci. 2003;8:s1056–s1067. doi: 10.2741/1174. [DOI] [PubMed] [Google Scholar]
- 17.Dhand R, Sohal H. Good sleep, bad sleep! The role of daytime naps in healthy adults. Curr Opin Pulm Med. 2006;12:379–382. doi: 10.1097/01.mcp.0000245703.92311.d0. [DOI] [PubMed] [Google Scholar]
- 18.Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, Fang J, Sarrigiannidis A, Chrousos GP. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–E261. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- 19.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2 ed. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 20.Whelan RL, Franklin M, Holubar SD, Donahue J, Fowler R, Munger C, Doorman J, Balli JE, Glass J, Gonzalez JJ, Bessler M, Xie H, Treat M. Postoperative cell mediated immune response is better preserved after laparoscopic vs open colorectal resection in humans. Surg Endosc. 2003;17:972–978. doi: 10.1007/s00464-001-8263-y. [DOI] [PubMed] [Google Scholar]
- 21.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27:140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Landis CA, Frey CA, Lentz MJ, Rothermel J, Buchwald D, Shaver JL. Self-reported sleep quality and fatigue correlates with actigraphy in midlife women with fibromyalgia. Nurs Res. 2003;52:140–147. doi: 10.1097/00006199-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 26.Saban, Mathews HL, Bryant FB, O'Brian TE, Witek Janusek L. Depressive symptoms and diurnal cortisol patterns among female caregivers of stroke survivors. (manuscript under review) Biological Research For Nursing. 2011;14:369–404. doi: 10.1177/1099800412439458. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 28.Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. The Social Psychology of Health. 1988:31–67. [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Tabachick BG, Fidell LS. Using Multivariate Statistics. 6 ed. Boston: Allyn and Bacon; 2013. [Google Scholar]
- 31.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 32.Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med. 2005;67:277–280. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- 33.Dedert E, Lush E, Chagpar A, Dhabhar FS, Segerstrom SC, Spiegel D, Dayyat E, Daup M, McMasters K, Sephton SE. Stress, Coping, and Circadian Disruption Among Women Awaiting Breast Cancer Surgery. Ann Behav Med. 2012;44:10–20. doi: 10.1007/s12160-012-9352-y. [DOI] [PubMed] [Google Scholar]
- 34.Ho RT, Fong TC, Chan CK, Chan CL. The associations between diurnal cortisol patterns, self-perceived social support, and sleep behavior in Chinese breast cancer patients. Psychoneuroendocrinology. 2013;38:2337–2342. doi: 10.1016/j.psyneuen.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Faraut B, Boudjeltia KZ, Dyzma M, Rousseau A, David E, Stenuit P, Franck T, Antwerpen PV, Vanhaeverbeek M, Kerkhofs M. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction⋆. Brain Behav Immun. 2011;25:16–24. doi: 10.1016/j.bbi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Campbell SS, Stanchina MD, Schlang JR, Murphy PJ. Effects of a month-long napping regimen in older individuals. J Am Geriatr Soc. [Comparative Study Research Support, N.I.H., Extramural] 2011;59:224–232. doi: 10.1111/j.1532-5415.2010.03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo Z, Inoue S. A short daytime nap modulates levels of emotions objectively evaluated by the emotion spectrum analysis method. Psychiatry Clin Neurosci. 2000;54:207–212. doi: 10.1046/j.1440-1819.2000.00660.x. [DOI] [PubMed] [Google Scholar]
- 38.Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, Robbins J. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 39.Asada T, Motonaga T, Yamagata Z, Uno M, Takahashi K. Associations between retrospectively recalled napping behavior and later development of Alzheimer's disease: association with APOE genotypes. Sleep. 2000;23:629–634. [PubMed] [Google Scholar]
- 40.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nature reviews Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 42.Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, Rainwater K, Ritchie JM, Yang M, Sood AK. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–4521. [PubMed] [Google Scholar]
- 43.Volden PA, Conzen SD. The influence of glucocorticoid signaling on tumor progression. Brain Behav Immun. 2013;30(Supplement):S26–S31. doi: 10.1016/j.bbi.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moran TJ, Gray S, Mikosz CA, Conzen SD. The Glucocorticoid Receptor Mediates a Survival Signal in Human Mammary Epithelial Cells. Cancer Res. 2000;60:867–872. [PubMed] [Google Scholar]
- 45.Antonova L, Mueller CR. Hydrocortisone down-regulates the tumor suppressor gene BRCA1 in mammary cells: A possible molecular link between stress and breast cancer. Genes Chromosomes Cancer. 2008;47:341–352. doi: 10.1002/gcc.20538. [DOI] [PubMed] [Google Scholar]
- 46.Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD, McClintock MK. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proceedings of the National Academy of Sciences. 2009;106:22393–22398. doi: 10.1073/pnas.0910753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zielinski MR, Krueger JM. Sleep and innate immunity. Front Biosci (Schol Ed) 2011;3:632–642. doi: 10.2741/s176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vgontzas AN, Zoumakis E, Bixler EO, Lin H-M, Follett H, Kales A, Chrousos GP. Adverse Effects of Modest Sleep Restriction on Sleepiness, Performance, and Inflammatory Cytokines. Journal of Clinical Endocrinology & Metabolism. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 49.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 50.Goldberg J, Schwertfeger K. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Current Drug Targets. 2010;11:1133–1146. doi: 10.2174/138945010792006799. [DOI] [PubMed] [Google Scholar]
- 51.Schlotz W, Hellhammer J, Schulz P, Stone AA. Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom Med. 2004;66:207–214. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- 52.Thorn L, Hucklebridge F, Evans P, Clow A. Suspected non-adherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology. 2006;31:1009–1018. doi: 10.1016/j.psyneuen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Mikolajczak M, Quoidbach J, Vanootighem V, Lambert F, Lahaye M, Fillée C, de Timary P. Cortisol awakening response (CAR)’s flexibility leads to larger and more consistent associations with psychological factors than CAR magnitude. Psychoneuroendocrinology. 2010;35:752–757. doi: 10.1016/j.psyneuen.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Rohleder N, Beulen SE, Chen E, Wolf JM, Kirschbaum C. Stress on the dance floor: the cortisol stress response to social-evaluative threat in competitive ballroom dancers. Pers Soc Psychol Bull. 2007;33:69–84. doi: 10.1177/0146167206293986. [DOI] [PubMed] [Google Scholar]
- 55.Powell DJ, Schlotz W. Daily Life Stress and the Cortisol Awakening Response: Testing the Anticipation Hypothesis. PLoS ONE. 2012;7:e52067. doi: 10.1371/journal.pone.0052067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Penedo F, Lucci JA, Iii, Ganjei-Azar P, Mendez L, Markon K, Lubaroff DM, Thaker PH, Slavich GM, Sood AK, Lutgendorf SK. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: Relationships with depression, fatigue, and disability. Brain Behav Immun. 2013;30(Supplement):S126–S134. doi: 10.1016/j.bbi.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18:272–281. doi: 10.1111/j.1365-2869.2008.00718.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.