Summary
The formation of cartilage is restricted to the core of the limb bud mesenchyme by ectodermal Wnts, which can irreversibly silence expression of the prochondrogenic transcription factor Sox9. In contrast, fibroblast growth factor (FGF) signals from the apical ectodermal ridge maintain the competence of chondrogenic precursors to undergo chondrogenesis once these cells go out of range of ectodermal Wnt signals. We have found that Wnt signals induce both a repressive chromatin mark (H3K27me3) and DNA methylation over the Sox9 promoter and that Wnt-induced irreversible silencing of the Sox9 gene requires DNA methylation of this locus, which is specifically countered by FGF signals. FGF blocks the recruitment of the de novo DNA methyltransferase, DNMT3A, to the Sox9 promoter by inducing the interaction and phosphorylation of DNMT3A by ERK1/2, and thereby controls whether expression of Sox9 is either irreversibly or reversibly silenced by Wnt signals in limb bud mesenchymal cells.
Introduction
Vertebrate long bones are formed through a process of endochondral ossification. During this process, bone formation begins with the establishment of mesenchymal condensations, which serve as a template for the adult skeletal elements. Chondrocytes then differentiate within the aggregated mesenchyme. Sox9, Sox5 and Sox6 play an essential role in the regulation of chondrogenesis (reviewed in (Lefebvre and Bhattaram, 2010)). Indeed, Sox9 directly activates cartilage differentiation markers, as this transcription factor has been shown to bind to the regulatory elements that drive expression of these genes (reviewed in (Lefebvre and Bhattaram, 2010)). The formation of cartilage in the limb bud is restricted to the core of the limb bud mesenchyme by signals from the ectoderm that block cartilage formation in the periphery of this tissue (Solursh, 1984). While ectopic expression of Wnts that signal via beta-catenin/LEF1/TCF block cartilage formation in the limb bud, conditional loss of beta-catenin expression in the limb bud mesenchyme increases the expression of Sox9 in this tissue (Hill et al., 2005). Together these findings suggest that Wnts secreted by the ectoderm act via a beta-catenin-dependent pathway to block Sox9 expression and cartilage formation in limb bud mesenchymal cells that lie in the peripheral regions of the limb bud. In addition to Wnts secreted by limb bud ectoderm, FGFs secreted by the apical ectodermal ridge (AER) are necessary to maintain: (1) limb bud outgrowth, (2) the viability of chondrogenic precursors that give rise to the cartilage templates of the limb, and (3) the competence for limb bud mesenchymal cells to undergo chondrogenesis once the Wnt signals are removed (ten Berge et al., 2008).
Results
Transient Wnt signals irreversibly block induction of Sox9 expression and chondrogenesis only in the absence of FGF signals
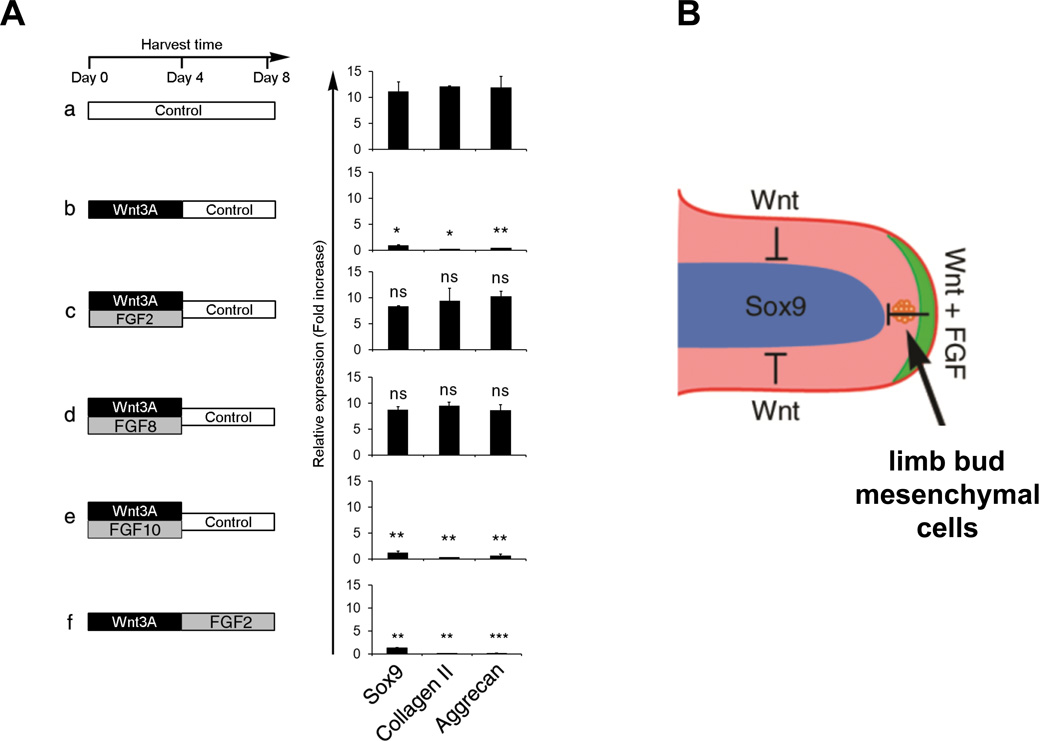
To further elucidate how Wnt and FGF signaling modulates the competence of limb bud mesenchymal cells (LBMCs) to undergo chondrogenesis, we evaluated the expression of Sox9, collagen II and aggrecan in micromass cultures of chicken LBMCs in response to these signals. We observed that Sox9, collagen II and aggrecan were all robustly expressed in LBMCs after 8 days culture in control medium (Figure 1Aa). If however soluble Wnt3A was transiently administered to the explants during only the first 4 days of culture, the expression of Sox9, collagen II and aggrecan was extinguished at day 4 (Figure 2, compare Aa and Ab), and expression of these genes continued to be silenced 4 days after Wnt3A was removed from the culture medium (i.e., at day 8; Figure 1Ab). In concert with prior observations (ten Berge et al., 2008), we observed that transient administration of Wnt3A in the presence of FGF8 for 4 days allowed the subsequent expression of Sox9, collagen II and aggrecan in cultures harvested at day 8 (Figure 1Ad). FGF2, which can substitute for the AER to sustain relatively normal limb development, could similarly restore subsequent chondrogenesis when administered together with Wnt3A (Figure 1Ac). In contrast, FGF10 (which is expressed in limb bud mesenchyme) when administered together with Wnt3A, was unable to restore chondrogenic gene expression in these cultures (Figure 1Ae). Thus, FGF family members (i.e., FGF2 and FGF8) that can substitute for the AER to maintain limb bud outgrowth, can also maintain the chondrogenic potential of limb bud mesenchymal cells transiently exposed to Wnt signals, which are secreted by the overlying ectoderm (displayed schematically in Figure 1B). Interestingly, FGF2 could only restore chondrogenesis when administered simultaneously with Wnt3A, but failed to do so when administered subsequent to Wnt exposure (Figure 1A, compare c and f), indicating that FGF signals must occur concomitant with Wnt signals to maintain chondrogenic competence. Importantly, simultaneous administration of FGF did not disrupt Wnt signaling, as inclusion of FGF2 for the first 4 days of culture did not block the ability of Wnt3A to either induce target genes such as Twist2 (Supplemental Figure 1A), or repress expression of Sox9, collagen II and aggrecan in cultures harvested at day 4 (Figure 2Ac). To begin to explore how FGF maintains the competence for LBMCs to express Sox9 following exposure to transient Wnt signals, we simultaneously treated micromass cultures of LBMCs with Wnt3A and FGF2 in the presence of the MEK1/2 antagonist, U0126. Inhibition of MEK1/2 activity severely blocked the ability of FGF2 to restore chondrogenic gene expression following removal of Wnt3A (Figure 2, compare Ag and Ah; Supplemental Figure 1B). Thus, FGF signaling blocks the ability of transient Wnt signals to stably repress the expression of Sox9, collagen II and aggrecan via a MEK1/2-dependent pathway, and can do so even in the absence of DNA replication (Supplemental Figure 2, A–C).
Figure 1. Transient Wnt signals irreversibly block induction of Sox9 expression and chondrogenesis only in the absence of FGF signals.
(A) LBMCs were isolated from day 4 chicken embryos and cultured in either control medium or medium containing either Wnt3A or FGF2/8/10, for the days indicated. Cells were harvested after 8 days of culture and gene expression was assayed by RT-qPCR. In this and subsequent figures, significance was calculated using Student’s t-test, *P<0.05, **P<0.01, ***P<0.001 and ns (non-significant difference) are indicated and error bar indicates standard error of the mean. The relative expression in b–f was compared with that in a. (B) Wnt signals from the ectoderm block Sox9 expression (and chondrogenesis) in the peripheral regions of the limb bud; FGF signals from the AER maintain both the viability and chondrogenic competence of limb bud mesenchymal cells.
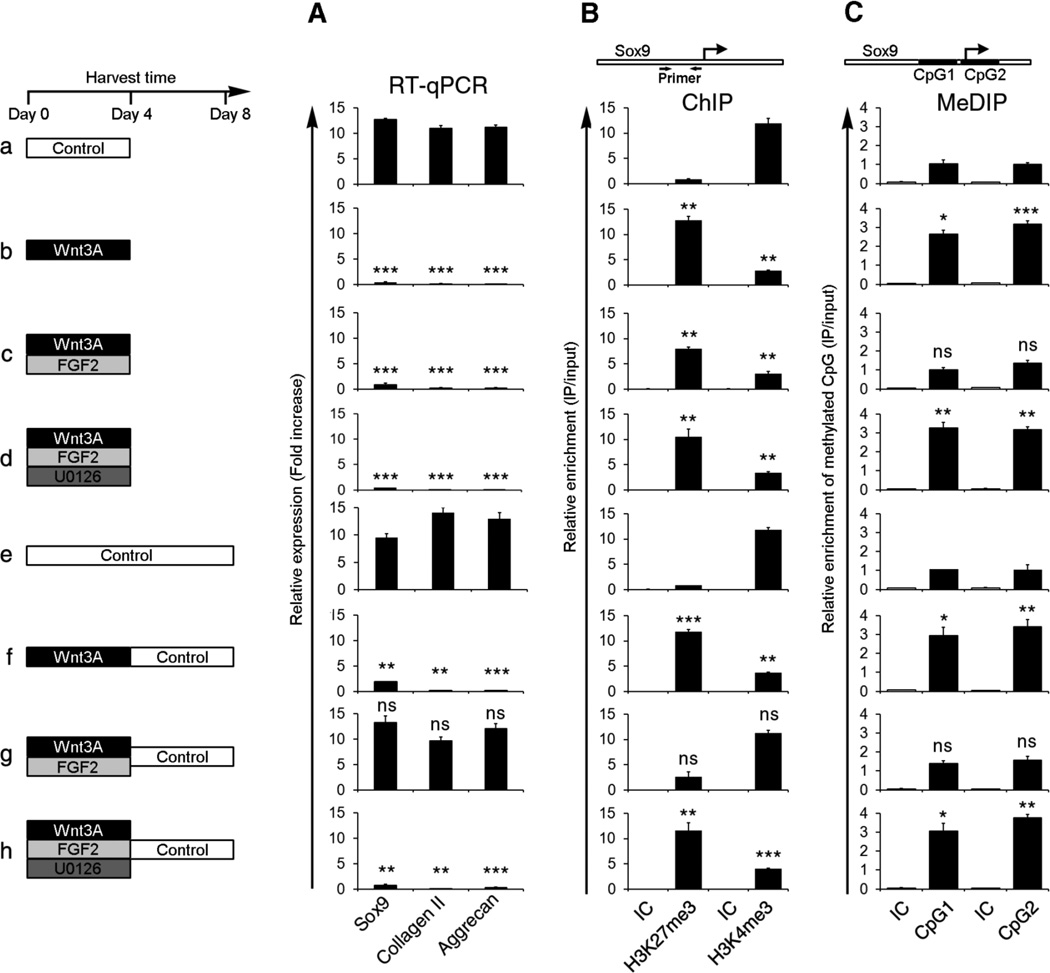
Figure 2. Transient Wnt signals induce stable H3K27me3 modification and CpG methylation over the Sox9 promoter only in the absence of FGF signals.
LBMCs were cultured in medium containing either Wnt3A, FGF2, and the MEK1/2 antagonist, U0126 for the days indicated. Cells were harvested after 4 or 8 days of culture and either (A) gene expression was assayed by RT-qPCR, (B) chromatin IP (ChIP) was performed to assay H3K27me3 and H3K4me3 modifications over the Sox9 promoter or (C) methyl-DNA IP (MeDIP) was performed to assay methyl CpG modification in two CpG islands (CpG1 and CpG2) that encompass the Sox9 promoter. In B and C, Isotype control (IC) IgG IPs are also displayed. In each section (A, B, C) of this Figure, b–d was compared with a; f–h was compared with e.
Transient Wnt signals induce stable H3K27me3 modification over the Sox9 promoter only in the absence of FGF signals
Because Sox9 is the earliest marker of chondrogenic differentiation, we evaluated whether Wnt and FGF signals may modulate chondrogenesis by inducing epigenetic modification of this locus. We first evaluated whether these signals alter histone methylation over the Sox9 promoter. The Sox9 gene in LBMCs cultured in control medium for 4 days is expressed (Figure 2Aa) and its promoter is accordingly marked by both H3K4me3 (Figure 2Ba) and H3K9Ac (Supplemental Figure 2Da) modifications, which are both indicative of active transcription. Correspondingly, this promoter lacks H3K27me3 modification (Figure 2Ba), which is associated with transcriptional silencing. In contrast, the Sox9 gene in LBMCs cultured in the presence of Wnt3A for 4 days (in either the absence or presence of FGF2) is transcriptionally silent (Figure 2, Ab and Ac), and its promoter is accordingly marked by significantly increased levels of H3K27me3, and decreased levels of both H3K4me3 and H3K9Ac modifications (Figure 2, Bb and Bc; Supplemental Figure 2D, b and c). Thus, concomitant administration of FGF2 does not block the ability of WntA3 to initially establish a repressive chromatin structure over the Sox9 promoter.
As discussed above, a transient 4 day exposure of LBMCs to Wnt3A (in the absence of exogenous FGF) resulted in stable repression of Sox9 gene expression in cells harvested at day 8 (Figure 2Af). Consistent with this stable repression of Sox9 gene expression, the Sox9 promoter displayed a relatively high level of H3K27me3 modification and a relatively low level of both H3K4me3 and H3K9Ac modifications (at day 8) in LBMCs that had been cultured for the initial 4 days in Wnt3A-containing medium (Figure 2Bf; Supplemental Figure 2De). In contrast, a transient 4 day exposure of LBMCs to Wnt3A in the presence of FGF2 resulted in the subsequent expression of Sox9 (at day 8) (Figure 2Ag); coupled with a significant decrease in H3K27me3 modification, and corresponding increases in both H3K4me3 and H3K9Ac modifications of the Sox9 promoter (at day 8) (Figure 2Bg; Supplemental Figure 2Df). Taken together, these findings suggest that while concurrent FGF2 administration does not block the initiation of Wnt3A-induced H3K27me3 modification of the Sox9 promoter (at day 4), it disrupts the stability of this histone modification, such that it is lost in the absence of continued Wnt signaling (by day 8 of culture).
Administration of the MEK1/2 antagonist, U0126, blocked the ability of FGF2 to restore Sox9 gene expression (at 8 days) in cells exposed to both Wnt3A and FGF2 for the first four days of culture (Figure 2, compare Ag and Ah). Thus, MEK1/2 activity is necessary for FGF signals to maintain the chondrogenic competence of LBMCs exposed transiently to Wnt signals. Interestingly however, U0126 failed to significantly alter either H3K4me3 or H3K27me3 modification of the Sox9 promoter after culture of LBMCs in Wnt3A plus FGF2 for 4 days (Figure 2, compare Bc and Bd). Thus, the potential to express the Sox9 gene (after 8 days culture) in LBMCs transiently exposed to either Wnt3A, Wnt3A/FGF2, or Wnt3A/FGF2/U0126 (for 4 days) did not correlate with the relative levels of either H3K4me3 or H3K27me3 modification of this locus (after 4 days culture).
Transient Wnt signals induce CpG methylation of the Sox9 promoter only in the absence of FGF signals
We have found that FGF/MEK1/2 signaling does not significantly affect the ability of Wnt3A to induce H3K27me3 modification over the Sox9 promoter but specifically blocks the maintenance of this histone modification after withdrawal of Wnt signaling. Thus, maintenance of the H3K27me3 modification (after transient Wnt signaling) requires another event that is inhibited by FGF signaling. Because DNA methylation has been noted to in some cases reinforce repressive histone modifications, we investigated whether FGF might alter the ability of Wnt signals to induce DNA methylation of two CpG islands that encompass the Sox9 promoter. By employing a methylated DNA-IP (methyl-DIP) assay, we found that a 4 day exposure of LBMCs to soluble Wnt3A increased DNA methylation of both CpG islands surrounding the Sox9 promoter (Figure 2, compare Ca and Cb). Interestingly, the relative level of DNA methylation that was initially established on the Sox9 promoter in the presence of Wnt3A (at 4 days) was stably maintained after withdrawal of Wnt3A for an additional 4 days (Figure 2, compare Ce and Cf). In marked contrast, in the presence of FGF2, Wnt3A administration failed to increase CpG methylation of the Sox9 promoter (Figure 2Cc), and this relative deficit of CpG methylation was maintained after withdrawal of both Wnt3A and FGF2 for an additional 4 days (Figure 2Cg). We examined whether MEK signaling was necessary for FGF2 to inhibit Wnt3A-induced DNA methylation of the Sox9 promoter in LBMCs. Indeed, administration of the MEK1/2 antagonist, U0126, blocked the ability of FGF2 to inhibit Wnt3A-induced DNA methylation of the Sox9 promoter, resulting in increased CpG methylation of this sequence (Figure 2, compare Cc and Cd; Cg and Ch). The increased DNA methylation of the Sox9 promoter in LBMCs treated with Wnt3A/FGF2/U0126 (for 4 days) correlated with the subsequent repression of Sox9 expression in cells harvested 4 days following the removal of Wnt3A/FGF2/U0126 (Figure 2Ah). Thus, MEK signaling is essential for FGF signals to block Wnt-induced DNA methylation of the Sox9 promoter.
To confirm these methyl-DNA IP results, we performed bisulfite sequencing of the second Sox9 CpG island (CpG2) in genomic DNA isolated from LBMCs that had been cultured for 4 days in either control medium, medium containing soluble Wnt3A, or medium containing both Wnt3A and FGF2. Consistent with our methyl-DNA IP results, we found that Wnt3A administration increased cytosine methylation of CpG residues located throughout the Sox9 CpG2, with the greatest effect occurring in the 3’ region of this CpG island (Supplemental Figure 3A; Supplemental Table 1). Most notably, concurrent administration of FGF2 (together with Wnt3A) decreased cytosine methylation of CpG residues throughout the Sox9 CpG2 (Supplemental Figure 3A; Supplemental Table 1). To further evaluate whether the competence for subsequent Sox9 gene expression in LBMCs transiently exposed to both Wnt and FGF signals inversely correlates with DNA methylation of the Sox9 promoter, we treated LBMCs with Wnt3A plus differing levels of FGF2. We found that levels of FGF2 that were sufficient to block Wnt3A-induced DNA methylation of the Sox9 promoter (Supplemental Figure 3B) correlated with FGF2 levels necessary to maintain the competence to express both Sox9 and other chondrogenic differentiation markers, following transient exposure to Wnt3A plus FGF2 (Supplemental Figure 3C). Taken together, our findings indicate that the relative level of DNA methylation that is established over the Sox9 promoter by culture of LBMCs in either control medium, medium containing soluble Wnt3A, or medium containing both Wnt3A and FGF2, inversely correlates with the subsequent expression of this gene four days after these growth factors are removed.
FGF signals block recruitment of DNMT3A to the Sox9 promoter
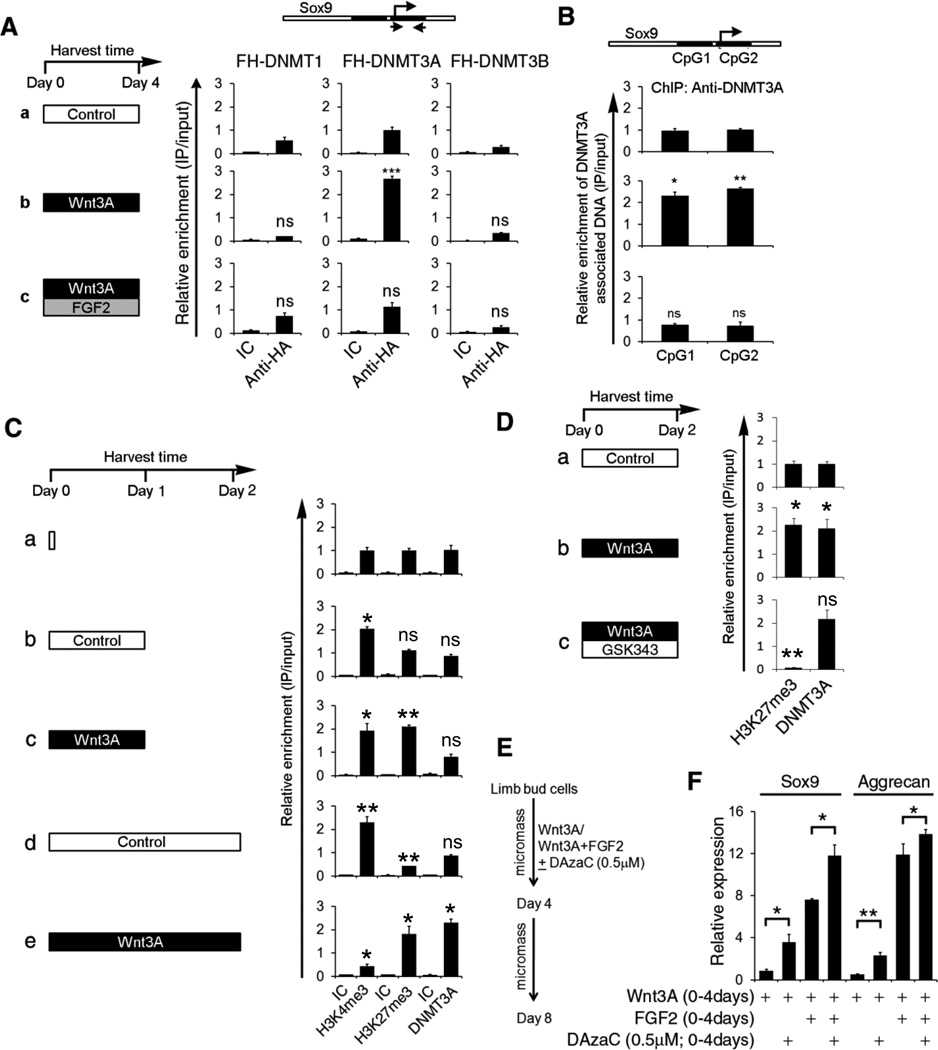
Because transient Wnt signals induce DNA methylation of the Sox9 promoter and FGF signals block this effect, we investigated both whether Wnt signals recruit a DNA methyltransferase to the Sox9 promoter, and whether FGF signals might block this recruitment. LBMCs were infected with retrovirus encoding either Flag/HA (FH)-tagged hDNMT1, hDNMT3A, or hDNMT3B and cultured in either control medium, medium containing soluble Wnt3A, or medium containing both Wnt3A and FGF2. After 4 days of culture, the cells were harvested and the association of exogenous FH-tagged DNMT with the Sox9 promoter was assayed by chromatin IP with anti-HA antibody. We found that Wnt3A specifically induced the association of FH-DNMT3A with the Sox9 promoter, and that Wnt-induced recruitment of FH-DNMT3A did not occur in the presence of FGF2 (Figure 3A). Importantly, neither Wnt nor FGF administration altered expression of the exogenous DNMTs (Supplemental Figure 4A and B), indicating that the effects of these ligands on HA-DNMT3A:Sox9 promoter interaction were post-transcriptional. In addition, we observed that Wnt3A administration to LBMCs similarly induced recruitment of endogenous DNMT3A to both Sox9 CpG islands (Figure 3B), without significantly altering the steady state levels of endogenous DNMT3A (Supplemental Figure 4C). Consistent with the ability of FGF2 and FGF8 (but not FGF10) to maintain the chondrogenic competence of LBMCs transiently cultured in Wnt3A (Figure 1), FGF2 and FGF8 (but not FGF10) similarly blocked Wnt3A-induced recruitment of DNMT3A to the Sox9 promoter (Supplemental Figure 5A). Importantly, Wnt3A-induced recruitment of DNMT3A to chromatin did not occur genome-wide, as Wnt3A administration failed to recruit DNMT3A to either sequences far up-stream of the Sox9 coding region (i.e., at −350 kb) or to the promoter of the TATA Binding Protein (TBP) gene (Supplemental Figure 5B). Taken together, these findings indicate that Wnt3A signaling promotes the recruitment of either exogenous or endogenous DNMT3A to the Sox9 promoter and thereby induces subsequent DNA methylation of this sequence, but only in the absence of concurrent FGF signals.
Figure 3. Wnt3A specifically recruits DNMT3A to the Sox9 promoter; FGF signals block this recruitment.
(A) LBMCs were infected with a retrovirus encoding either Flag/HA(FH)-tagged hDNMT1, FH-hDNMT3A, or FH-hDNMT3B and cultured in either control medium, or medium containing Wnt3A (in either the absence or presence of FGF2) as indicated. Cells were harvested after 4 days of culture and chromatin IP performed to assay FH-hDNMT association with the Sox9 promoter. (B) Uninfected LBMCs were cultured as in (A). Cells were harvested after 4 days of culture and chromatin IP performed to assay endogenous DNMT3A association with the Sox9 promoter. In (A) and (B), Isotype control (IC) IgG IPs are also displayed; b–c was compared to a. (C and D) LBMCs were cultured in either control medium or medium containing Wnt3A (in either the absence or presence of the EZH2 inhibitor, GSK343). Cells were harvested at the indicated time, and chromatin IP performed to assay either H3K4me3 and H3K27me3 modifications, or DNMT3A association with the Sox9 promoter. In (C), Isotype control (IC) IgG IPs are also displayed; values in b–e were compared to a. In (D) for each antibody, values in b and c were compared to a. (E) Diagram of experimental protocol for (F). (F) LBMCs were cultured for days 0–4 in medium containing either Wnt3A or Wnt3A plus FGF2 in either the absence or presence of 5’deoxy-azacytidine (DAzaC) as indicated. Cells were cultured an additional 4 days in control medium (and harvested at day 8). Gene expression was assayed by RT-qPCR and normalized to GAPDH levels.
Wnt signaling increases H3K27me3 modification of the Sox9 promoter prior to inducing the recruitment of DNMT3A
To begin to explore whether Wnt3A-induced chromatin modifications and DNA methylation of the Sox9 promoter are coupled, we analyzed the time course of both these modifications in LBMC cultures. We found that H3K4me3 modification of the Sox9 promoter increased after only 1 day micromass culture of LBMCs, in either the absence or presence of Wnt3A (Figure 3C, compare a–c). In contrast, H3K27me3 was enhanced after 1 day micromass culture of LBMCs specifically in the presence of Wnt3A (Figure 3C, compare a–c). In LBMCs cultured for 2 days in control medium, the H3K27me3 modification was lost over the Sox9 promoter, leaving only the H3K4me3 mark in this position (Figure 3Cd). The recruitment of DNMT3A to the Sox9 promoter was first detectable after 2 days culture of LBMCs in medium containing Wnt3A, and correlated with loss of the H3K4me3 modification from this promoter (Figure 3C, compare d and e). These findings indicate that Wnt3A-induced DNMT3A recruitment to the Sox9 promoter is a relatively late event, suggesting that de novo synthesis of a Wnt3A-induced RNA and/or protein may be required to promote this association.
H3K27me3 modification of the Sox9 promoter is not necessary for Wnt3A-induced association of DNMT3A with this locus
Because increased Wnt3A-induced H3K27me3 modification of the Sox9 promoter preceded recruitment of DNMT3A to this locus, we wondered whether this chromatin modification was necessary for the subsequent association of DNMT3A. To explore this possibility, we cultured LBMCs in medium containing Wnt3A for 2 days, in either the absence or presence of GSK343 (a potent inhibitor of the histone methyltransferase EZH2, which catalyzes the H3K27me3 modification). Interestingly, we found that while treatment of LBMCs with GSK343 completely blocked H3K27me3 modification of the Sox9 promoter, it failed to dampen Wnt3A-induced recruitment of DNMT3A to this locus (Figure 3D). Thus, Wnt-induced H3K27me3 modification of the Sox9 promoter is not necessary to promote the association of DNMT3A with this locus.
DNA methylation is necessary to induce irreversible silencing of Sox9 following transient Wnt signaling
To address whether DNA methylation is necessary for transient Wnt signals to irreversibly silence Sox9 expression, we cultured LBMCs in the presence of Wnt3A in either the absence or presence of 5-Aza-2’-deoxycytidine (outlined in Figure 3E), a drug that blocks DNA methylation. Administration of 5-Aza-2’-deoxycytidine together with Wnt3A for 4 days blocked Wnt3A-induced DNA methylation of the Sox9 promoter (Supplemental Figure 5C). In addition, treatment with 5-Aza-2’-deoxycytidine promoted increased expression of Sox9 in Wnt3A-treated LBMCs (that were subsequently cultured in the absence of Wnt3A for an additional 4 days) to approximately 50% the level observed in LBMCs that had been initially cultured in Wnt3A plus FGF2; and boosted the expression of Sox9 to even higher levels in LBMCs that had been cultured for the initial 4 days in both Wnt3A and FGF2 (Figure 3F). Administration of 5-Aza-2’-deoxycytidine together with Wnt3A also restored the subsequent expression of aggrecan, but to a considerably lesser extent than did initial culture of LBMCs in Wnt3A plus FGF2 (Figure 3F). These findings indicate that DNA methylation is critical for transient Wnt signals to irreversibly silence Sox9 expression in LBMCs, and suggest that Wnt signaling may in addition induce a parallel pathway(s) to silence subsequent expression of cartilage structural markers such as aggrecan. The fact that FGF2 was more efficient than 5-Aza-2’-deoxycytidine administration in promoting subsequent expression of Sox9 in LBMCs transiently cultured in Wnt3A suggest that FGF signals not only block Wnt-induced recruitment of DNMT3A to this locus (and consequent DNA methylation of the Sox9 CpG islands), but must also attenuate additional Wnt-induced repressive epigenetic marks over this locus. Indeed, we had previously observed that concomitant FGF2 administration attenuated the stability of both Wnt3A-induced H3K27me3 modification and inhibition of H3K9 acetylation over the Sox9 promoter (Figure 2B, compare lanes f with g; Supplemental Figure 2D, compare lanes e and f). Thus, FGF signals both block Wnt-induced recruitment of DNMT3A to the Sox9 promoter and in addition destabilize Wnt-induced repressive chromatin modifications over this locus.
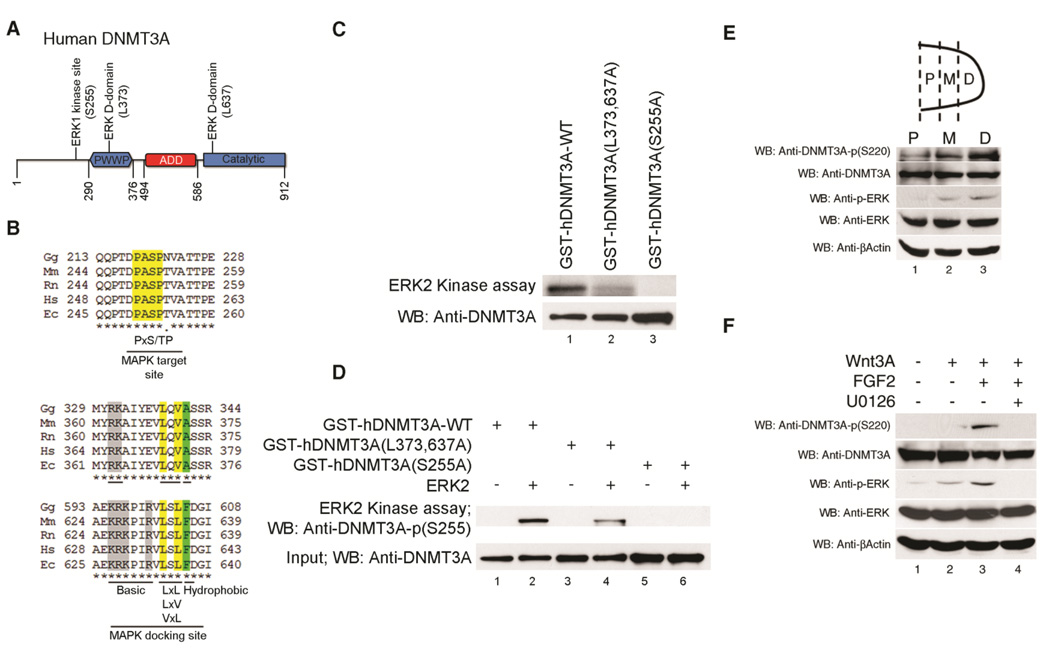
DNMT3A contains two conserved ERK1/2 docking sites and is directly phosphorylated by activated ERK
Because MEK1/2 activity is necessary for FGF signals to block Wnt3A-induced DNA methylation of the Sox9 promoter, we explored whether DNMT3A might be directly phosphorylated by the MEK1/2-activated kinases ERK1/2. Consistent with this hypothesis, we found that DNMT3A contains both a conserved ERK1/2 phosphorylation site in the amino-terminal region as well as two conserved ERK1/2 docking sites located in either the PWWP domain (which can bind to H3K36me3 (Dhayalan et al., 2010) and is required for targeting DNMT3A to heterochromatin (Chen et al., 2004; Ge et al., 2004)) or in the amino-terminal region of the methyltransferase domain (Figure 4A and B). Indeed, bacterially produced and purified mouse DNMT3A (residues 220–908) which contains both the putative ERK1/2 phosphorylation site and docking sites was efficiently phosphorylated by activated ERK2 in vitro (Supplemental Figure 6A and B). In contrast, a bacterially produced sub-fragment of mouse DNMT3A (residues 607–908) which lacks both the putative ERK1/2 phosphorylation site and one of the ERK docking sites was not phosphorylated by activated ERK2 (Supplemental Figure 6B). To better map the ERK2 phosphorylation site in DNMT3A we made GST-fusion proteins with either human DNMT3A-WT or human DNMT3A-(S255A), which contains a serine to alanine mutation in the putative ERK1/2 phosphorylation site. While activated ERK2 efficiently phosphorylated GST-hDNMT3A-WT, this kinase failed to significantly phosphorylate GST-hDNMT3A-(S255A) (Figure 4C, compare lanes 1 and 3), suggesting that S255 in hDNMT3A is a key residue necessary for ERK2 phosphorylation. We generated affinity-purified polyclonal antibodies in rabbits that specifically recognize phosphorylated-S255 in human DNMT3A. This affinity purified antibody, that was made against a 17 amino acid peptide (246a.a.-AVQQPTDPA-phospho-S255PTVATTC-262a.a.) including phosphorylated-S255 in human DNMT3A, only recognized GST-hDNMT3A-WT after incubation with ATP and activated ERK2 (Figure 4D, compare lanes 1 and 2) and specifically did not recognize GST-hDNMT3A-(S255A) even after incubation with ATP and activated ERK2 (Figure 4D, lanes 5 and 6). Importantly, interaction of anti-phospho-S255 with GST-hDNMT3A-WT that had been phosphorylated in vitro by activated ERK2 was specifically competed by a DNMT3A peptide (246a.a.-AVQQPTDPASPTVATTC-262a.a.) containing phospho-S255 but not by a peptide containing non-phosphorylated S255 (Supplemental Figure 6C). Together, these findings indicate that the principal ERK2 phosphorylation site in hDNMT3A is S255.
Figure 4. DNMT3A contains conserved ERK1/2 phosphorylation and docking sites and is directly phosphorylated by activated ERK.
(A) Schematic diagram of human DNMT3A depicting the ERK phosphorylation and docking sites, and the PWWP and ADD (ATRX-DNMT3-DNMT3L) domains. (B) ERK1/2 phosphorylation and docking sites are conserved in vertebrate DNMT3A (Gg: Gallus gallus; Mm: Mus musculus; Rn: Rattus Norvegicus; Hs: Homo sapiens; Ec: Equus caballus). (C) ERK2 kinase assay (employing γ32P-ATP) and either WT or mutant forms of GST-hDNMT3A, as indicated. (D) Western analysis (employing either anti-hDNMT3A-(pS255) or anti-DNMT3A antibodies) of in vitro ERK2-phosphorylated WT or mutant forms of GST-hDNMT3A. (E) Limb buds (isolated from day 4 chicken embryos) were separated into proximal (P), middle (M) and distal (D) regions. Equal protein amounts of each limb bud region were loaded onto SDS-PAGE, and Western analysis was performed with either anti-DNMT3A-(pS220), anti-DNMT3A, anti-pERK, anti-ERK, or anti–beta actin. (F) Limb buds (isolated from day 4 chicken embryos) were cultured for 2 hours in either control medium, or medium containing either soluble Wnt3A, Wnt3A/FGF2, or Wnt3A/FGF2/U0126 as indicated. Equal protein amounts of each explant culture were loaded onto SDS-PAGE, and Western analysis was performed with either anti-DNMT3A-(pS220) (in the presence of competitor non-phosphorylated hDNMT3A peptide), anti-DNMT3A, anti-pERK, anti-ERK, or anti–beta actin.
ERK1/2 docking sites identified in substrates for these kinases have previously been noted to be necessary for efficient phosphorylation of these substrates by ERK1/2. To assay whether the ERK1/2 docking sites located in the PWWP domain and in the methyltransferase domain of DNMT3A are required for efficient phosphorylation of this protein by activated ERK2, we assayed the relative ability of activated ERK2 to phosphorylate either GST-hDNMT3A-WT versus GST-hDNMT3A-(L373A, L637A), which contains point mutations in both ERK1/2 docking sites. Notably, in vitro phosphorylation of GST-hDNMT3A-(L373A, L637A) by activated ERK2 (as assayed either by labeling with γ-32P-ATP or by Western blotting with anti-phospho-S255 antibody) was markedly attenuated relative to GST-hDNMT3A-WT phosphorylation (Figure 4C, compare lanes 1 and 2; Figure 4D, compare lanes 2 and 4). These findings suggest that ERK2 interaction with either L373 and/or L637 in DNMT3A is critical to support efficient phosphorylation of S255A in hDNMT3A by this kinase.
DNMT3A is phosphorylated at the ERK1/2 phosphorylation site in response to FGF signals in limb buds
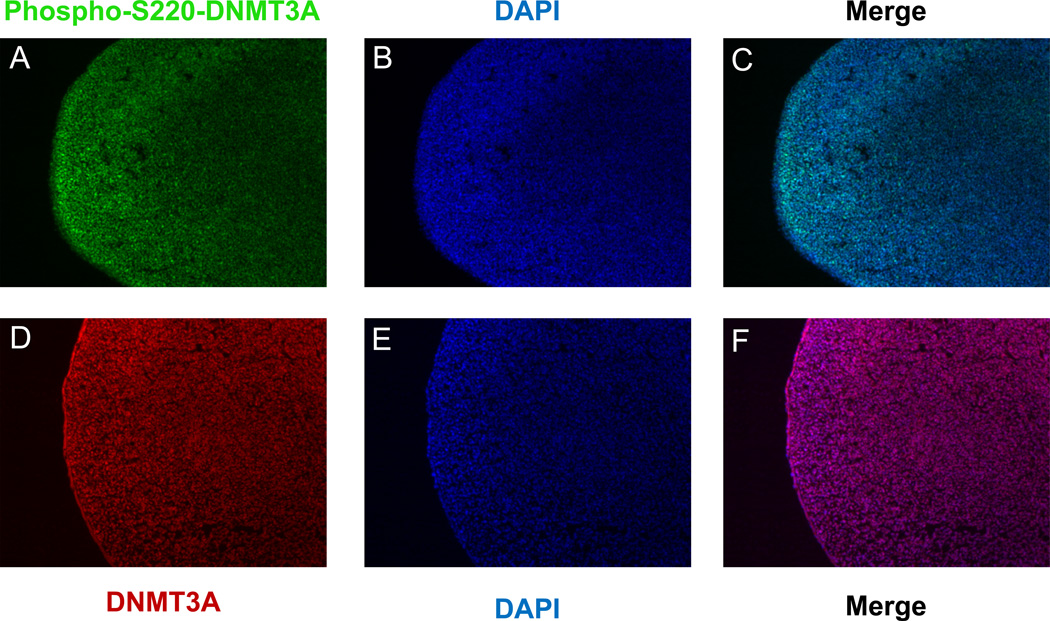
To investigate whether endogenous DNMT3A is phosphorylated at the ERK1/2 phosphorylation site, we dissected limb buds from H. H. stage 20–22 chicken embryos and isolated either the proximal (P), middle (M) or distal (D) regions of this structure. Western analysis revealed that these three regions of the limb bud express equivalent levels of DNMT3A, but that this protein is preferentially phosphorylated at the ERK1/2 phosphorylation site (S220 in chicken DNMT3A) specifically in distal limb bud cells, which also express relatively higher levels of activated phospho-ERK1/2 (Figure 4E; Supplemental Figure 6D). To determine whether FGF signals specifically induce phosphorylation of DNMT3A at the ERK1/2 phosphorylation site via a MEK1/2-dependent pathway, we cultured limb bud explants for two hours in either control medium, or medium containing either soluble Wnt3A, Wnt3A/FGF2, or Wnt3A/FGF2/U0126. While culture of the intact limb buds in Wnt3A alone only slightly boosted phosphorylation of DNMT3A at the ERK1/2 phosphorylation site, culture of limb bud explants in both Wnt3A and FGF2 significantly increased phosphorylation of DNMT3A at this site (Figure 4F, compare lanes 1–3). Inclusion of the MEK1/2 inhibitor U0126 abrogated the increased phosphorylation of both ERK and DNMT3A (at the ERK1/2 phosphorylation site) induced by FGF2 administration. (Figure 4F, lane 4). To assay the spatial localization of ERK-phosphorylated DNMT3A in the limb bud, we assayed its expression by immunofluorescence in cryosections of limb buds. Interestingly, we found that while anti-total DNMT3A recognized approximately equal levels of this protein in proximal and distal regions of the limb bud (Figure 5D), anti-phospho-S220-DNMT3A recognized this protein most predominantly in the distal region of the limb bud mesenchyme adjacent to the AER (Figure 5A). Most importantly, we found that the immunoreactivity of ERK-phosphorylated DNMT3A in limb buds was specifically competed by the phosphorylated DNMT3A peptide (Supplemental Figure 6E), but not by the peptide lacking serine phosphorylation (at the ERK phosphorylation site; Figure 5A).
Figure 5. DNMT3A is phosphorylated at the ERK1/2 phosphorylation site in a proximal-distal gradient in the limb bud.
Cryosections of limb buds (isolated from day 4 chicken embryos) were immunostained with either anti-phospho-S220-DNMT3A (A–C) or anti-total-DNMT3A (D–F); nuclei were visualized by DAPI staining. In (A–C) a DNMT3A peptide lacking serine phosphorylation (at the ERK phosphorylation site) was added along with anti-phospho-S220-DNMT3A. The distal region of the limb bud (containing the AER) is shown on the left.
ERK-DNMT3A interaction and phosphorylation are both necessary for FGF signals to block DNMT3A recruitment to the Sox9 promoter
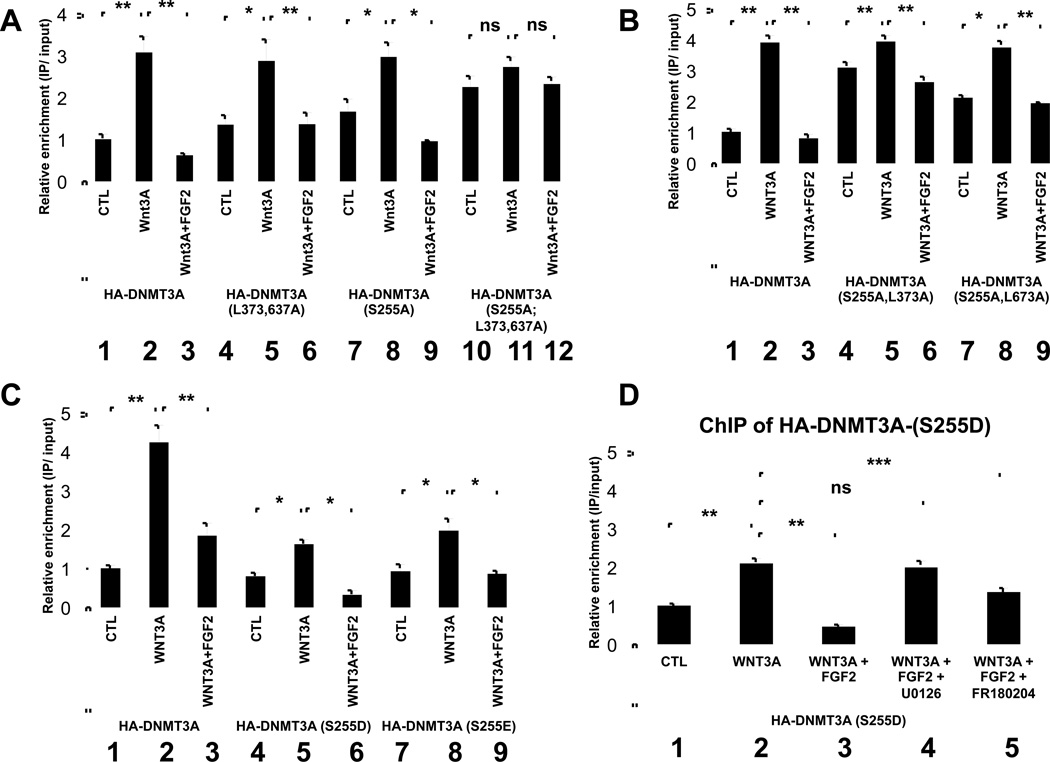
To investigate whether mutation of either the ERK1/2 phosphorylation site and/or the ERK1/2 docking sites in DNMT3A would alter the ability of FGF2 to modulate Wnt-induced recruitment of DNMT3A to the Sox9 promoter, we engineered retroviruses encoding either hDNMT3A-WT, hDNMT3A-(S255A) or hDNMT3A-(L373A, L637A). We infected micromass cultures of LBMCs with these retroviruses and cultured the cells in either control medium or in medium containing Wnt3A alone, or Wnt3A plus FGF2. Like hDNMT3A-WT, we found that Wnt3A administration increased binding of either hDNMT3A-(S255A) or hDNMT3A-(L373A, L637A) to the Sox9 promoter (Figure 6A, lanes 2, 5, and 8). Interestingly however, both mutant forms of DNMT3A showed higher occupancy (than the WT protein) on the Sox9 promoter in LBMCs cultured in control medium (Figure 6A, compare lanes 1, 4, and 7); and less eviction from this promoter (than the WT protein) by FGF2 administration (Figure 6A, compare lanes 3, 6, 9). Consistent with these results, we observed that a mutant form of hDNMT3A that lacked both the ERK1/2 phosphorylation site and ERK1/2 docking sites (hDNMT3A-(S255A, L373A, L637A) was constitutively bound to the Sox9 promoter in LBMCs cultured in control medium, and that administration of Wnt3A only slightly augmented the association of this triple mutant form of hDNMT3A with the Sox9 promoter (Figure 6A, lanes 10–11). In addition, the binding of hDNMT3A-(S255A, L373A, L637A) to the Sox9 promoter was only marginally attenuated in LBMCs cultured in the combination of Wnt3A and FGF2 (Figure 6A, lane 12). Importantly, WT and mutant forms of DNMT3A were all expressed at approximately equal levels in LBMCs cultured in either control medium or in medium supplemented with Wnt3A, or Wnt3A/FGF2 (Supplemental Figure 7A).
Figure 6. Phosphorylation/interaction of DNMT3A with activated ERK attenuates binding of DNMT3A to the Sox9 promoter.
(A–D) LBMCs were infected with retrovirus encoding either WT or mutant forms of hDNMT3A, as indicated, and cultured in either control medium or medium containing Wnt3A in either the absence or presence of FGF2. In (D) either a MEK1/2 inhibitor (U0126) or an ERK inhibitor (FR180204) were also added, where indicated. Cells were harvested after 4 days of culture and chromatin IP (ChIP) was performed (and normalized to input DNA) to assay HA-hDNMT3A association with the Sox9 promoter.
To discern whether one or both of the ERK docking sites was necessary for FGF signaling to evict DNMT3A from the Sox9 promoter, we engineered retroviruses encoding DNMT3A with mutations in both the ERK1/2 phosphorylation site and only one of the two ERK1/2 docking sites (i.e., hDNMT3A-(S255A, L373A) and hDNMT3A-(S255A, L637A)). In contrast to hDNMT3A-WT, we found that hDNMT3A-(S255A, L373A) was significantly associated with the Sox9 promoter in LBMCs cultured in control medium, and that administration of Wnt3A only slightly promoted further recruitment of this mutant form of DNMT3A to this locus (Figure 6B, lanes 4–5). Addition of FGF2 was able to counter the effect of Wnt3A, but was considerably attenuated in its ability to evict hDNMT3A-(S255A, L373A) from the Sox9 promoter (Figure 6B, lane 6). These findings suggest that interaction of activated ERK1/2 with the ERK1/2 docking site located in the PWWP domain of DNMT3A (which is mutated by L373A) is crucial for FGF signals to fully inhibit Wnt3A-induced recruitment of DNMT3A to the Sox9 promoter.
Phosphomimetic mutations at the ERK phosphorylation site in DNMT3A are poorly recruited by Wnt3A, and very efficiently evicted by FGF2, from the Sox9 promoter
To further investigate the role of the ERK phosphorylation site in DNMT3A, we generated phosphomimetic mutations converting S255 of hDNMT3A to either aspartic (D) or glutamic (E) acid. Relative to DNMT3A-WT, we found that both DNMT3A-(S255D) and DNMT3A-(S255E) were poorly recruited to the Sox9 promoter following Wnt3A administration (Figure 6C, compare lane 2 with lanes 5, 8 ). Conversely, we also noted that either phosphomimetic mutation promoted more efficient eviction of the mutant form of DNMT3A from the Sox9 promoter, upon concurrent administration of FGF2 together with Wnt3A (Figure 6C, compare lane 3 with lanes 6, 9). Importantly, WT and mutant forms of DNMT3A containing the phosphomimetic mutations were all expressed at approximately equal levels in LBMCs (Supplemental Figure 7B, C). Together these findings suggest that a negative charge at S255 (as would occur after ERK1/2 phosphorylation) significantly destabilizes Wnt-induced recruitment of DNMT3A to the Sox9 promoter.
Activated ERK1/2 blocks recruitment of DNMT3A to the Sox9 promoter by both catalytic and stoichiometric means
Our findings suggest that FGF disrupts DNMT3A association with the Sox9 promoter via two parallel pathways; one involving the ERK1/2 phosphorylation site and the other dependent upon the ERK1/2 docking site(s). To investigate whether ERK1/2 works in a stoichiometric fashion (in addition to a catalytic one) to block the association of DNMT3A with the Sox9 promoter, we investigated whether ERK1/2 catalytic activity was necessary for FGF signals to evict hDNMT3A-(S255D) from the Sox9 promoter. hDNMT3A-(S255D) contains a phosphomimetic mutation at the ERK1/2 phosphorylation site and is only weakly recruited by Wnt signals, and very efficiently evicted by FGF signals, from the Sox9 promoter (Figure 6D, lanes 1–3). Interestingly, while inclusion of a MEK1/2 inhibitor (U0126) completely blocked FGF-induced eviction of this mutant form of hDNMT3A from the Sox9 promoter (Figure 6D, lane 4), inclusion of an ERK1/2 inhibitor (FR180204) only partially attenuated the ability of FGF2 to evict this mutant form of hDNMT3A from the Sox9 promoter (Figure 6D, lane 5). Consistent with these findings, we found that inclusion of the MEK1/2 inhibitor (U0126) completely blocked expression of Sox9, collagen II, and aggrecan in LBMCs (programmed to express hDNMT3A-(S255D)) that had been cultured in Wnt3A plus FGF2 for the first 4 days of an 8 day culture period (Supplemental Figure 7D, lane 4). In contrast, inclusion of the ERK1/2 inhibitor (FR180204) allowed the expression of these chondrogenic markers in LBMCs (programmed to express hDNMT3A-(S255D)) that had been cultured in Wnt3A plus FGF2 for the first 4 days of an 8 day culture period (Supplemental Figure 7D, lane 5). Importantly, U0126 and FR180204 equally blocked FGF2-induced phosphorylation of DNMT3A in chicken limb bud explants (Supplemental Figure 7E, lanes 3–5), indicating that both reagents can efficiently inhibit ERK1/2-mediated phosphorylation of DNMT3A. While the MEK1/2 inhibitor (U0126) blocks both MEK1/2-mediated phosphorylation of ERK1/2 and accumulation of nuclear-localized ERK1/2; the ERK1/2 inhibitor (FR180204) allows MEK1/2-mediated phosphorylation of ERK1/2 and consequent nuclear localization of ERK1/2 (albeit at a reduced level, due to diminished ERK1/2 auto-phosphorylation; see (Plotnikov et al., 2011)). Thus, we suspect that the ability of FGF2 to promote reversible silencing of Sox9 by Wnt3A in the presence of the ERK1/2 inhibitor FR180204 (and not in the presence of the MEK1/2 inhibitor, U0126), may reflect the accumulation of catalytically inactive ERK1/2 in the nucleus (specifically in the presence of FR180204), which acts to stoichiometrically block the association of both endogenous cDNMT3A and exogenous hDNMT3A-(S255D) with the Sox9 promoter.
Wnt signals modulate the catalytic activity of a DNMT3A complex which contains a mutant form of this protein, that is constitutively bound to the Sox9 promoter
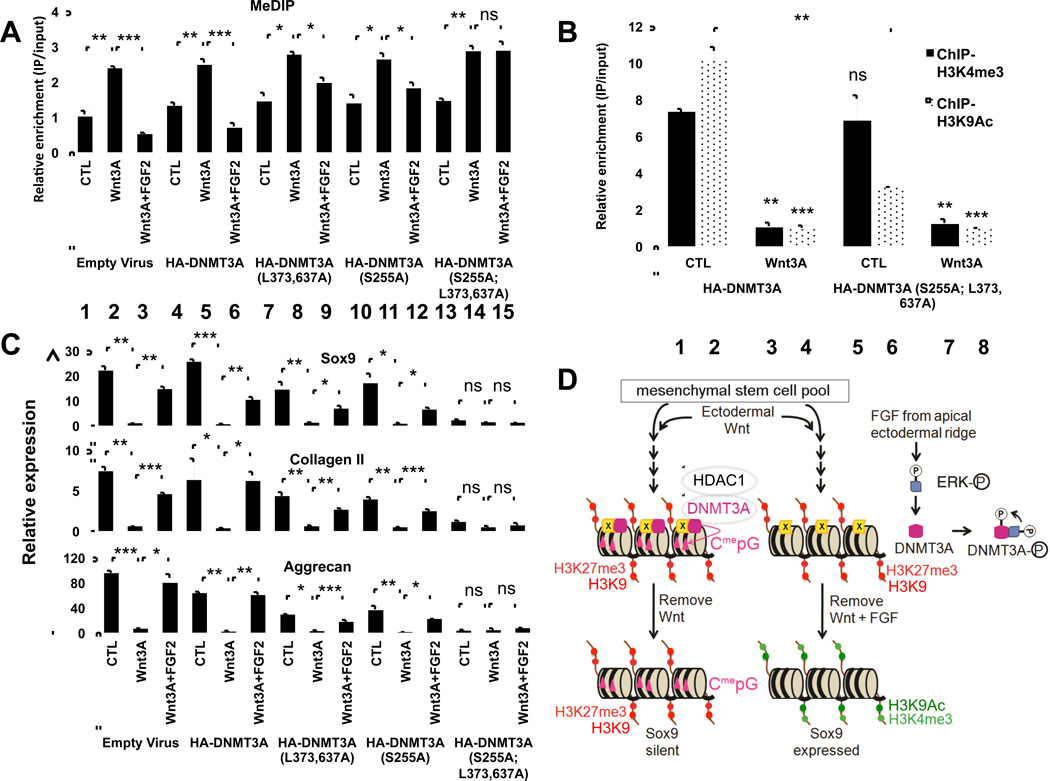
Expression of exogenous hDNMT3A-WT did not alter the relative levels of DNA methylation (as assayed by methyl-DNA IP) of the Sox9 CpG islands in LBMCs cultured in either control medium, medium supplemented with Wnt3A, or medium supplemented with both Wnt3A plus FGF2 (Figure 7, lanes 1–6). Thus, CpG methylation of the Sox9 promoter in LBMCs is primarily controlled by the relative levels of Wnt3A and FGF signaling, rather than by the absolute level of DNMT3A in the cells. Consistent with this idea, Wnt3A administration boosted DNA methylation of the Sox9 CpG islands to approximately the same level in LBMCs programmed to express either hDNMT3A-WT, hDNMT3A-(S255A), hDNMT3A-(L373A, L637A) or hDNMT3A-(S255A, L373A, L637A) (Figure 7A, lanes 5, 8, 11, and 14). In contrast, the ability of FGF2 administration to reverse Wnt3A-induced DNA methylation of the Sox9 CpG islands, was either significantly attenuated or completely abrogated in LBMCs programmed to express mutant forms of DNMT3A which were poor substrates for ERK interaction/phosphorylation (Figure 7A, compare lanes 6, 9, 12, and 15). Thus, mutation of either the ERK phosphorylation site and/or ERK docking sites in DNMT3A either attenuate (in the single mutants) or block (in the combined mutant) the ability of FGF signals to counter Wnt3A-induced DNA methylation of the Sox9 promoter. The inability of FGF signals to alter DNA methylation of the Sox9 promoter in LBMCs programmed to express hDNMT3A-(S255A, L373A, L637A) strongly support the notion that this mutant form of hDNMT3A is either itself catalytically active or serves as a tether to constitutively recruit endogenous cDNMT3A to this locus (as an oligomer with endogenous cDNMT3A). Thus, while FGF/ERK signaling can disrupt Wnt3A-induced interaction of DNMT3A-WT with the Sox9 promoter, this signaling pathway is unable to disrupt Wnt3A-induced interaction of a catalytically active complex containing hDNMT3A-(S255A, L373A, L637A) from the Sox9 promoter.
Figure 7. FGF signals promote DNMT3A phosphorylation by ERK1/2 in distal limb bud mesenchyme and thereby maintain competence for eventual Sox9 gene expression by blocking Wnt signals from inducing stable CpG methylation of the Sox9 locus.
(A–C) LBMCs were infected with retrovirus encoding either WT or mutant forms of DNMT3A and cultured in either control medium or medium containing Wnt3A in either the absence or presence of FGF2. Cells were harvested after 4 days of culture and either methyl-DNA IP (MeDIP) was performed to assay methyl CpG modification (A) or chromatin IP was performed to assay H3K4me3 and H3K9Ac modification of the Sox9 promoter (B). (C) Cells were cultured for another 4 days (in control medium) and harvested at day 8. Gene expression was assayed by RT-qPCR. In (B): lanes 3 and 4 were compared to lanes 1 and 2; lanes 7 and 8 were compared to lanes 5 and 6; and lanes 5 and 6 were compared to lanes 1 and 2, respectively. (D) Wnt signals recruit DNMT3A to the Sox9 promoter; FGF signals block recruitment of DNMT3A to the Sox9 promoter by promoting activated ERK1/2-mediated phosphorylation of (and interaction with) DNMT3A (see text for details).
Interestingly, while association of hDNMT3A-(S255A, L373A, L637A) with the Sox9 promoter was only slightly boosted by Wnt signals (Figure 6A, lanes 10–11), Wnt-induced CpG methylation of this promoter was significantly enhanced in LBMCs programmed to express this mutant form of DNMT3A (Figure 7A, lanes 13–14). Thus, Wnt signals both induce the recruitment of hDNMT3A-WT to the Sox9 promoter and also promote the catalytic activity of an oligomeric complex containing hDNMT3A-(S255A, L373A, L637A) (which is constitutively bound to the Sox9 promoter) to induce CpG methylation of this locus. Prior work has indicated that H3K4me0 enhances (and that H3K4me3 modification inhibits) DNMT3A–mediated CpG methylation (Li et al., 2011). Thus, we think it is relevant in this regards that Wnt3A administration robustly inhibits H3K4me3 modification of the Sox9 promoter in LBMCs programmed to express either DNMT3A-WT or hDNMT3A-(S255A, L373A, L637A) (Figure 7B, compare lanes 1 and 3; lanes 5 and 7). Taken together, our findings suggest that Wnt3A signaling promotes DNA methylation of the Sox9 CpG islands by both recruiting DNMT3A to this locus and inducing DNMT3A catalytic activity by repressing H3K4me3 modification of this promoter.
Expression of a mutant form of DNMT3A that lacks ERK kinase and docking sites can mediate irreversible silencing of Sox9 by Wnt signals even in the presence of FGF2
Because recruitment of hDNMT3A-(S255A, L373A, L637A) to the Sox9 promoter was not blocked by FGF2 administration, we investigated whether expression of this mutant form of DNMT3A in LBMCs would also inhibit the subsequent induction of Sox9 expression following transient exposure to both Wnt3A and FGF2. Following a 4 day exposure to both Wnt3A and FGF2, and a subsequent 4 day culture in the absence of these signals; Sox9, collagen II and aggrecan expression were induced in micromass cultures of either uninfected LBMCs or in cultures infected with virus encoding either hDNMT3A-WT, hDNMT3A-(S255A), or hDNMT3A-(L373A, L637A) (Figure 7C). In contrast, Sox9, collagen II and aggrecan failed to be expressed in similar cultures infected with virus encoding hDNMT3A-(S255A, L373A, L637A) (Figure 7C). Thus, because FGF signals cannot block the association of hDNMT3A-(S255A, L373A, L637A) with the Sox9 promoter (Figure 6A, lane 12), in LBMCs expressing this mutant form of DNMT3A, Sox9 expression is irreversibly silenced by transient Wnt signals in both the absence or presence of FGF2 (Figure 7C).
Interestingly, the expression of Sox9 was also extinguished in LBMCs programmed to express hDNMT3A-(S255A, L373A, L637A) when cultured in control medium (Figure 7C), consistent with the constitutive recruitment of this mutant form of DNMT3A to the Sox9 promoter even in the absence of Wnt signals (Figure 6A, lane 10). In this instance, transcriptional silencing of Sox9 expression occurred in the absence of increased DNA methylation of its promoter (Figure 7A, lane 13) and may reflect the ability of DNMT3A to recruit histone deacetylases (HDACs) to target genes and thereby repress their expression (Fuks et al., 2001). Indeed, we observed that expression of hDNMT3A-(S255A, L373A, L637A) significantly decreased H3K9Ac modification of the Sox9 promoter (by approximately 60%) in LBMCs cultured in control medium (Figure 7B, compare lanes 2 and 6). These findings suggest that recruitment of DNMT3A to the Sox9 promoter blocks expression of this gene by both increasing DNA methylation and attenuating histone acetylation of this locus (in the latter case, via interaction with HDAC1 (Fuks et al., 2001)).
Discussion
FGF signals maintain a competence for eventual Sox9 gene expression by blocking Wnt signals from inducing stable CpG methylation of the Sox9 locus
In the absence of FGF signaling, transient Wnt signals sent from the ectoderm induce both H3K27me3 modification over the Sox9 promoter and simultaneously recruit DNMT3A association to this gene, which promotes subsequent CpG methylation of this promoter in limb bud mesenchymal cells (LBMCs). Interestingly, both the H3K27me3 modification and DNA methylation of the Sox9 promoter is stably maintained after the withdrawal of Wnt signals and correlates with irreversible silencing of Sox9 gene expression in LBMCs (outlined in Figure 7D, left). In the presence of both Wnt and FGF signals (as occurs beneath the AER), transient Wnt signals induce H3K27me3 modification over the Sox9 promoter but neither recruit DNMT3A to the Sox9 promoter nor induce CpG methylation of this sequence (Figure 7D, right). Our findings indicate that MEK1/2 activity is necessary for FGF signals to block de novo DNA methylation of the Sox9 CpG islands. In addition, it seems likely that this effect is mediated via direct ERK1/2-mediated interaction with and phosphorylation of DNMT3A based on four lines of evidence: (1) DNMT3A is a substrate for activated ERK2 phosphorylation in vitro, (2) FGF promotes phosphorylation of endogenous DNMT3A (at the ERK phosphorylation site) in a MEK1/2-dependent fashion in vivo, (3) FGF signals fail to block DNA methylation of the Sox9 CpG islands in LBMCs programmed to express a mutant form of DNMT3A with mutations in both the ERK phosphorylation and docking sites (which is constitutively bound to the Sox9 promoter), and (4) phosphomimetic mutations at the ERK phosphorylation site in DNMT3A strongly impede Wnt3A-induced recruitment of DNMT3A to the Sox9 promoter. In addition, our findings suggest that activated ERK1/2 (which translocates into the nucleus) blocks recruitment of DNMT3A to the Sox9 promoter by both phosphorylating DNMT3A and by binding to the ERK1/2 docking sites in DNMT3A. Thus, nuclear-localized ERK1/2 acts in both a catalytic and stoichiometric capacity to block Wnt-induced recruitment of DNMT3A to the Sox9 promoter.
We speculate that by uncoupling H3K27me3 modification from simultaneous CpG methylation, FGF signals render the H3K27me3 modification unstable, allowing the loss of this chromatin modification from the Sox9 promoter and consequent activation of this gene, when cells go out of range of Wnt signaling (outlined in Figure 7D, right). In this model, FGF signals maintain the competence of limb bud mesenchymal cells to undergo chondrogenesis (once the cells have moved away from the ectodermal Wnt signal) by blocking CpG methylation of the Sox9 promoter, and thereby maintain the reversibility of a Wnt-induced H3K27me3 modification over this sequence. Interestingly, we observed that FGF2 was more efficient than 5-Aza-2’-deoxycytidine administration in promoting subsequent expression of Sox9 in LBMCs transiently cultured in Wnt3A, suggesting that FGF signals not only block Wnt3A-induced recruitment of DNMT3A to the Sox9 promoter, but may also block the stabilization of repressive chromatin marks on this locus. Consistent with this notion, we found that FGF signals attenuate the stability of both Wnt3A-induced H3K27me3 modification and inhibition of H3K9 acetylation over the Sox9 promoter.
Wnt signals both recruit DNMT3A to the Sox9 promoter and induce its DNA methyltransferase activity at this locus
We observed that Wnt signals specifically recruited DNMT3A, but not DNMT3B nor DNMT1 to the Sox9 promoter in chicken limb bud mesenchymal cells, suggesting that DNMT3A plays a unique role in stably silencing Sox9 expression in this tissue. On the other hand, DNMT3A-null mice appear normal at birth, but become runted and die at about 4 weeks of age (Okano et al., 1999). Thus, tissue-specific differentiation and limb pattering can take place (in mice) in the absence of DNMT3A function, and a requirement for DNMT3A activity is manifest after birth. Perhaps chromatin-based transcriptional repressive mechanisms serve to temporarily silence inappropriate gene expression during tissue patterning prior to birth, but these eventually degrade in the absence of appropriate DNMT3A-mediated DNA methylation.
We have found that a mutant form of hDNMT3A that lacks both the ERK1/2 phosphorylation and docking sites (hDNMT3A-(S255A, L373A, L637A)) is constitutively bound to the Sox9 promoter in LBMCs programmed to express this protein. Interestingly however, the ability of hDNMT3A-(S255A, L373A, L637A) (either alone or in complex with endogenous cDNMT3A) to induce CpG methylation of the Sox9 promoter is restrained in the absence of Wnt signals; and is specifically induced by administration of Wnt3A (in either the absence or presence of FGF). We have found that Wnt3A administration to LBMCs inhibits H3K4me3 modification of the Sox9 promoter, which in turn has been shown to inhibit the DNA methyltransferase activity of DNMT3A (Li et al., 2011). Thus, we speculate that the presence of the H3K4me3 chromatin modification at the Sox9 promoter in cells cultured in control medium may act to block the enzymatic activity of the DNMT3A complex containing hDNMT3A-(S255A, L373A, L637A) (which is constitutively recruited to the Sox9 locus, but does not increase DNA methylation of this locus in the absence of Wnt signals). Taken together, our findings suggest that Wnt signals induce both the recruitment of hDNMT3A-WT to the Sox9 promoter and in addition promote the catalytic activity of either WT or mutant forms of hDNMT3A to induce CpG methylation of this locus, by erasing the H3K4me3 modification over the Sox9 promoter.
Our current working hypothesis is that Wnt signals recruit DNMT3A to the Sox9 promoter by promoting the expression of a DNMT3A docking partner (termed “X” in Figure 7D) that is targeted to the Sox9 promoter. In contrast, FGF signals block recruitment of DNMT3A to the Sox9 promoter by promoting activated ERK1/2-mediated phosphorylation of (and interaction with) DNMT3A, which in turn blocks the interaction of DNMT3A with this docking partner. Interestingly, even in the absence of exogenous Wnt3A a basal level of DNMT3A is present at the promoter of the Sox9 gene in LBMCs cultured in control medium, and additional DNMT3A is recruited to this locus in LBMCs cultured in Wnt3A (Supplemental Figure 5B). Indeed, hDNMT3A-(S255A, L373A, L637A) (which is immune to ERK signaling) is constitutively recruited to the Sox9 promoter in LBMCs, where it represses both H3K9Ac modification of the Sox9 promoter and expression of this gene in LBMCs cultured in control medium, presumably by recruiting class I HDACs to this locus (Fuks et al., 2001). Taken together, these findings suggest that basal levels of the DNMT3A docking partner “X” are present at the Sox9 promoter even in the absence of exogenous Wnt3A administration, and that Wnt signaling increases the expression of this docking partner.
Experimental Procedures
Cell culture
Chicken limb bud mesenchymal cells (LBMCs) were harvested from H. H. stage 22–24 chicken embryos. Micromass cultures were generated by plating 2 × 105 LBMCs (pooled from 30–60 chicken embryos) into a 10 microliter spot (i.e., 2 × 107 cells/ml) as described in (ten Berge et al., 2008). Either micromass cultures or intact limb buds (isolated from H. H. stage 20–22 chicken embryos) were maintained in a 50:50 ratio of DMEM:F12 (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 50 µg/ml streptomycin & 50U penicillin and either Wnt3A-conditioned media or control conditioned medium. Culture medium was changed every 24 hr. In some explants either 50 ng/ml FGF2 (Sigma), 150 ng/ml FGF8b (R&D), 50 ng/ml FGF10 (R&D), 500 ng/ml 5-Aza-2’-deoxycytidine (Sigma), 500nM aphidicolin (Sigma), 20nM U0126 (a MEK1/2 inhibitor; Calbiochem), 100 nM FR180204 (an ERK inhibitor; R&D), or 500 ng/ml GSK343 (an EZH2 inhibitor; Glaxo-Smith Kline) were added to the culture medium. Wnt3A-conditioned medium was made as described in (Willert et al., 2003).
Plasmids and viruses
MSCV-FH-hDNMT1, MSCV-FH-hDNMT3A, and MSCV-FH-hDNMT3B plasmids encoding human DNMT1, DNMT3A, and DNMT3B cDNAs were obtained from Dr. Yang Shi (Children’s Hospital, Boston, MA). For FH-hDNMT3A, Site-directed mutagenesis was carried out to obtain mutations in either the ERK2 phosphorylation site (S255) and/or in the ERK1/2 docking sites (L373, L637) to generate MSCV-FH-hDNMT3A-(S255A), MSCV-FH-hDNMT3A-(L373A, L637A), MSCV-FH-hDNMT3A-(S255A, L373A, L637A), MSCV-FH-hDNMT3A-(S255A, L373A), MSCV-FH-hDNMT3A-(S255A, L637A), MSCV-FH-hDNMT3A-(S255D), or MSCV-FH-hDNMT3A-(S255E) using a Quikchange multi site-directed mutagenesis kit (Stratagene). Retroviruses expressing either wild type hDNMT3A or its mutant forms were prepared by co-transfecting 293T cells with expression vehicles encoding GAG-POL and the VSV G protein. For bacterial expression of either wild type or mutant hDNMT3A, the respective cDNAs were cloned into the pGEX-KG vector and GST-tagged proteins were purified using a B-Per GST fusion protein purification kit (Thermo Scientific).
Generation of anti-phospho-S255 hDNMT3A antibody
Anti-phospho-S255 hDNMT3A antibody was produced in rabbits (Covance Research Products). Briefly, a 17 amino acid peptide (246a.a.-AVQQPTDPApSPTVATTC-262a.a.) of hDNMT3A containing phosphorylated serine was conjugated with KLH and injected subcutaneously as an emulsion with Freund’s Complete Adjuvant followed by Freund’s Incomplete Adjuvant every three weeks. Animals were bled 10 days after the final booster. The serum was affinity purified using both negative and positive selection columns containing either the non-phosphorylated or phosphorylated peptide, respectively.
Western analysis
Western blotting was done as previously described (Kumar and Lassar, 2009). The following primary antibodies were used: Anti-beta actin (50 ng/ml; ab6276; Abcam), anti-Flag-HRP (1 µg/ml; A8592, Sigma), anti-HA-HRP (1 µg/ml; sc-805; Santacruz), anti-DNMT3A (1 µg/ml; ab13888, Abcam), and anti-phospho-S255 hDNMT3A (1 µ/ml, described above). For anti-phospho-S255 hDNMT3A Western blots, in some cases we added competitor non-phosphorylated or phosphorylated DNMT3A peptide (246a.a.-AVQQPTDPApSPTVATTC-262a.a.) at 5µg/ml during primary antibody incubation. Horseradish Peroxidase-conjugated either Donkey anti-mouse or anti-rabbit secondary antibodies (Jackson Immunoresearch laboratories) were used. The interactions were detected using enhanced chemiluminescence (Pierce).
Supplementary Material
Acknowledgements
The authors thank Yang Shi (Children’s Hospital, Boston, MA) for generously supplying us with the DNMT retroviral expression vehicles, Xing Zhang and Xiaodong Cheng (Emory University School of Medicine, Atlanta, GA) for generously providing us with bacterially purified fragments of DNMT3A, and Steve Buratowski for his advise during the course of this work. In addition, we thank the Nikon Imaging Facility at Harvard Medical School for the use of their microscopes, and Jennifer Waters for her assistance with photomicroscopy. This work was supported by grants to A.B.L. from the Harvard Stem Cell Institute and the National Institutes of Health (AR055552, AR060735).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
A.B.L. and D. K. were together responsible for the conception and design of the study, analysis and interpretation of data, and the drafting the article. D. K. was responsible for acquisition of data.
References
- Chen T, Tsujimoto N, Li E. The PWWP domain of Dnmt3a and Dnmt3b is required for directing DNA methylation to the major satellite repeats at pericentric heterochromatin. Mol Cell Biol. 2004;24:9048–9058. doi: 10.1128/MCB.24.20.9048-9058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhayalan A, Rajavelu A, Rathert P, Tamas R, Jurkowska RZ, Ragozin S, Jeltsch A. The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. J Biol Chem. 2010;285:26114–26120. doi: 10.1074/jbc.M109.089433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks F, Burgers WA, Godin N, Kasai M, Kouzarides T. Dnmt3a binds deacetylases and is recruited by a sequence-specific repressor to silence transcription. EMBO J. 2001;20:2536–2544. doi: 10.1093/emboj/20.10.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge YZ, Pu MT, Gowher H, Wu HP, Ding JP, Jeltsch A, Xu GL. Chromatin targeting of de novo DNA methyltransferases by the PWWP domain. J Biol Chem. 2004;279:25447–25454. doi: 10.1074/jbc.M312296200. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kumar D, Lassar AB. The transcriptional activity of Sox9 in chondrocytes is regulated by RhoA signaling and actin polymerization. Mol Cell Biol. 2009;29:4262–4273. doi: 10.1128/MCB.01779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Bhattaram P. Vertebrate skeletogenesis. Curr Top Dev Biol. 2010;90:291–317. doi: 10.1016/S0070-2153(10)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BZ, Huang Z, Cui QY, Song XH, Du L, Jeltsch A, Chen P, Li G, Li E, Xu GL. Histone tails regulate DNA methylation by allosterically activating de novo methyltransferase. Cell Res. 2011;21:1172–1181. doi: 10.1038/cr.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Plotnikov A, Chuderland D, Karamansha Y, Livnah O, Seger R. Nuclear extracellular signal-regulated kinase 1 and 2 translocation is mediated by casein kinase 2 and accelerated by autophosphorylation. Mol Cell Biol. 2011;31:3515–3530. doi: 10.1128/MCB.05424-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Solursh M. Ectoderm as a determinant of early tissue pattern in the limb bud. Cell Differ. 1984;15:17–24. doi: 10.1016/0045-6039(84)90025-3. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Brugmann SA, Helms JA, Nusse R. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development. 2008;135:3247–3257. doi: 10.1242/dev.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.