Abstract
This longitudinal study of 194 very low birth weight (VLBW) and 184 normal birth weight (NBW) infants, hypothesized that the causal pathway between birth group (VLBW, NBW) and mutans streptococci (MS) acquisition (presence) at 18-20 months is mediated by biological, behavioral, and caregiver MS levels. Biological (number of teeth at 8 and 18-20 months, enamel hypoplasia) and behavioral (brushing/cleaning, sweet snacks, breast feeding, dental access) factors were assessed using dental exams and caregiver questionnaire responses at 8 and 18-20 months. Infant MS acquisition and caregiver levels were assessed from saliva and plaque samples collected at 8 and 18-20 months. Structural equations model evaluated the causal pathway with latent variables for biology and behavior. MS presence was similar between birth groups at 18-20 months (VLBW: 40%; NBW: 49%), but significantly higher for NBW at 8 months. Increased number of teeth at 8 and 18-20 months was associated with biological risk. Infants whose caregivers had a one point higher score on MS had a significantly 1.5 higher odds of MS presence. Caregiver behavior was not associated with MS presence. Early Intervention efforts should focus on delaying initial acquisition and improving caregiver awareness of taking care of erupting primary teeth.
Keywords: Children, Longitudinal, Epidemiology, Mutans streptococci, birth weight
Mutans streptococci (MS) are an important risk factor for Early Childhood Caries (1, 2). The proportion of infants with MS detected ranges from less than 30% in pre-dentate to over 80% in 24 month infants among diverse populations, with disparately higher acquisition among infants of lower socio-economic status (3–10).The MS colonization in preterm and full-term infants was 49% and 68% at 9 and 18 months respectively and the prevalence was significantly higher in pre-term at 24 months, but not at prior ages (11).
In the youngest children MS acquisition takes place primarily by vertical transmission and predominantly by mothers (1, 12). The MS colonization coincides with the so-called window of infectivity, i.e., with increase in number of erupted primary teeth (11, 13) but colonization can also take place after this window (14). However, it is the early MS colonization that increases the caries risk of the child later in life (15, 16). Developmental defects of enamel (DDE), particularly enamel hypoplasia (17, 18) is significantly associated with MS colonization. Preterm infants had 26 times greater risk for hypoplasia compared to full-term and this contributed to increased odds of colonization (11).
Other factors associated with acquisition of MS in infancy although less established include: Caesarean section (C-section) delivered infants acquired Streptococcus mutans earlier (19) but other studies did not find an association (10, 20); and lower S. mutans with antibiotic use (3). Behavioral factors that increase the frequency of exposure by presenting a favorable environment for MS colonization include: frequent sugar/sweet consumption (3, 10, 21); caregiver pre-tasting of food (3, 10); bottle feeding (22); infrequent tooth brushing/cleaning (22); and caregiver unrestored cavities suggesting problematic dental access (22).
The existing studies have utilized standard regression techniques with biological and behavioral factors as individual risk predictors that have limitations in disentangling the causal process underlying MS acquisition in infants. Structural equation modeling (SEM) allows the specification of this direct and indirect relationship underlying the pathway of MS acquisition. Further, only one longitudinal study exists (11) that has followed preterm (not specifically very low birth weight) and full-term infants for MS colonization. Our earlier findings indicated increased enamel defects in the permanent teeth of VLBW adolescents (23) and primary teeth of VLBW infants (24), but we did not report on MS colonization in these longitudinal studies. Thus, the objective of this study was to investigate the extent of the differences in MS presence (8 and 18-20 months) between birth group (VLBW, NBW); and whether the pathway for the effect of birth group on MS presence at 18-20 months would be through the mediating influences of biological and behavioral factors, and caregiver MS levels.
Materials and methods
A longitudinal cohort design was used. Socio-demographic, medical, biological, behavioral, and caregiver MS variables (birth, 8 and 18-20 months) were utilized to study the presence of MS in infants at 8 and 18-20 months of age.
Study setting and participants
The cohort consisted of 468 infants and mothers randomly recruited at birth from 2 hospitals whose neonatal intensive-care units treat the majority of infants with medical complications (24). To coincide with the primary tooth eruption patterns, follow-up visits were conducted at approximately 8 and 18-20 months of corrected age (i.e., actual weeks since date of birth minus weeks premature). Participation rates were 82% (n=386) and 81% (n=378) at 8 and 18-20 months, respectively. The study protocol was approved by The Institutional Review Boards of University Hospitals Case Medical Center and MetroHealth Medical Center. All study procedures were undertaken with the written consent and understanding of each subject's parent/guardian and according to ethical principles, including the World Medical Association Declaration of Helsinki.
Demographic and medical assessments
Caregiver socio-demographic and infant medical data were abstracted at birth from medical records and included: age, race (African-American vs. Caucasian/other), education (<12 years, ≥12 years), marital status (single, other), socioeconomic status (SES: low, high) (25), birth group (VLBW: <1500 g and preterm <37 wk gestation; NBW: ≥ 2500 g and full-term ≥ 37 wk gestation), vaginal or C- section delivery, antibiotic use (no, yes) during postpartum hospitalization, and gender. The caregivers were predominantly (95%) biological mothers and the same caregivers completed 8 and 18-20 month visits.
Microbiological collection and outcomes
At the 8 and 18-20 month visit, MS from saliva and plaque were determined from the caregiver and infant using the Dentocult SM Strip Mutans test (Orion Diagnostica, Espoo, Finland). This test assesses both S. mutans and Streptococcus sobrinus, and has good specificity and sensitivity when validated against conventional culture tests, and good inter-rater reliability (26). The caregivers were asked to refrain from eating, feeding their children, and brush their own or their children's teeth one hour prior to sample collection. Stimulated (paraffin wax chewing for one minute) saliva was collected from the caregiver and unstimulated saliva was collected from the infant. For both the caregiver and child, saliva was collected by pressing the rough surface of the Dentocult saliva strip against the saliva on the participant's tongue and removed through the participants gently closed lips. For 18-20 month infants and caregivers, four samples of plaque from the cervical one thirds of the buccal of maxillary first molars and lingual of mandibular first molars was collected. With a sterile disposable swab (FisherFinest, Fisher Health Care, Houston, TX, USA), the maxillary right buccal surface of the first maxillary molar and the mandibular left lingual surface of the first molar was sampled. With a second sterile swab, the maxillary left buccal surface of the first molar and the mandibular right lingual surface of the first molar were sampled. The second molars were sampled if first molars were missing. For infants with no teeth at 8 months, the sterile swabs sampled the gum in all four quadrants. Each sample was smeared on the plaque site strip containing four areas (for smearing) provided by the manufacturer. The plaque and the saliva strip were incubated back to back in a manufacturer provided vial containing selective medium and bacitracin for 72 h at 35 to 37°C and dried at room temperature. The strip site giving the highest reading for MS was recorded.
The MS colonies were read sideways (to distinguish true colonies from background discoloration) under standardized lighting using magnification and compared to a standard chart with categories `0–1', `2', `3' corresponding to <105, 105 – 106, and >106 CFU/mL of saliva or plaque respectively. The study staff was trained and calibrated by a gold standard examiner (ES) in reading the density of the MS colonies on the strips and comparing it to the standard chart. Three study staff read all the saliva and plaque strips blinded to the birth group status of the infant. Inter-rater Kappa ranged from 0.79 to 0.94. The result for the infant was dichotomized into two categories: absence (0) and presence (1, 2, 3) of MS detected. The MS data was used in the analysis as follows: for the infant, an overall MS score was further calculated if the dichotomized saliva and/or plaque levels were present or absent; for the caregiver, a new 0 to 3 ordinal score was calculated based on the average of the original plaque and saliva ordinal scores.
Biological factors
The three indicators in latent variable Biological factors (FBiol) represented biological risk associated with acquisition of MS and was measured through visual dental examination: (i) number of teeth present at 8 months and (ii) 18-20 months; and (iii) presence of developmental enamel defects according to the modified DDE index (27). The DDE index type (hypoplasia, opacity, combination defects) was utilized to calculate the summary measure for the mean number of teeth affected by hypoplasia for each infant and later dichotomized (0=no, ≥1=yes for hypoplasia). A higher FBiol score indicates a higher number of teeth at 8 and at 18-20 months, and a higher probability of hypoplasia at 18-20 months. Thus, a higher FBiol score indicated higher levels for biological risk factors for MS.
Behavioral factors
The four indicators in latent variable Behavioral factors (FBehav) represented behavioral risk promoting MS acquisition and were measured through the same caregiver questionnaire (at 8 and/or 18-20 months). The following behavioral factors were used for analysis: “How often are the child's teeth usually cleaned or brushed?” with answer choices ranging from “don't clean or brush teeth” to “almost every day” given a score of “0” and answer choices “once a day”, “twice a day”, and “more than twice a day” given a score of “1”; “Do you have a dentist to go to, if you need dental care?” with answer choice “no” given a score of “0” and “yes” given a score of “1”; “Has your child ever been breast fed?” with answer choice “no” given a score of “0” and “yes” given a score of “1”; and “Do you use sweet snacks as a reward for the child?” with “yes” given a score of “0” and “no” given a score of “1”. A fifth indicator, pre-tasting infant's food, “Do you chew the child's food before giving it to the child?” (yes, no) was considered, but was dropped as caregivers reported that few solid foods were given at this age, with most children eating semi-soft/toddler foods and drinking milk, juice or water. A higher FBehav score indicates lower levels for risky behaviors, an inverse relationship with brushing, dentist access, and breast feeding, and positive relationship with sweet snacks which were all considered protective against MS acquisition.
Data analysis
Descriptively, socio-demographic, medical, biological, behavioral and unadjusted MS outcomes were compared between the VLBW and NBW groups utilizing t-test for sample means and chi-square tests for categorical variables.
The pathways for MS presence at 18-20 months were assessed by fitting an SEM model corresponding to the model in Fig. 1. The SEM model assumed Bernoulli/logistic regression models for binary outcomes (infant MS, and the latent variable indicators except number of teeth); multinomial/proportional odds model for caregiver MS; negative binomial/log linear regression models for number of teeth at 8 and 18-20 months; and normality/linear regression models for the two latent variables (FBehav, FBiol). The latent variables are standardized to have variance (or standard deviation) equal to 1, so that their coefficients are interpreted on a scale of standard deviation units. The models were fit using maximum likelihood with Monte Carlo simulation for numerical integration in MPlus Version 6.12. The `MLF' estimator in MPlus obtained approximate standard errors and chi-square test p-values for all the model coefficients. Model goodness of fit was assessed using the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) (28). The root mean squared error of approximation (RMSEA) goodness-of-fit criterion was computed based on a linear version of the above SEM model. Statistical significance was determined based on 0.05 alpha-level criterion.
Figure 1.
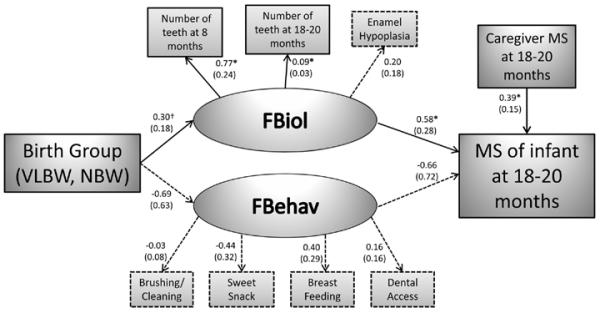
Structural equation model of the pathway between birth group and Infant MS presence at 18-20 months.
The model involves four stages (in causal order): 1) socio-demographics (caregiver age, education, race, marital status) at birth; 2) birth group (VLBW, NBW); 3) latent variables for biology and behavior; caregiver MS levels at 18-20 months; 4) infant MS presence at 18-20 months. Solid arrows with *, denote the presence of a statistically significant (p <0.05) relationship. Dotted arrows denote non-significant (p >0.05) relationship. Solid arrow with † denote trend towards significance with p <0.10. Estimated coefficients with the standard errors for the estimates (in the parentheses) are displayed. The latent variables (FBiol and FBehav) were derived by using confirmatory factor analysis.
As some variables had missing data, we used a complete case analysis as an initial approach. To check robustness of the results, we also conducted multiple imputation using the unrestricted variance covariance model in MPlus to fill in missing data for the model variables. Maternal education, race, marital status, and infant number of teeth at 8 months were imputed to address 2.9%, 3.4%, 0.53%, 14% of missing data respectively. We obtained 20 completed data sets, which were combined for overall inferences using the method of Rubin (29).
Results
Sample characteristics and bivariate analysis
A total of 386 (VLBW: 204, NBW: 182) and 378 (VLBW: 194, NBW: 184) infant-caregiver (95% biological mothers) dyads participated at the 8 and 18-20 month visits respectively
The proportion of 8 and 18-20 month infants showing MS colonization was 22% and 44% respectively. Table 1 indicates that the MS detected at 8 month visits were significantly (p < 0.05) higher in the NBW compared to VLBW infants (Overall: 28% vs. 17%). At 18-20 months, there were no significant differences in MS presence between the two groups. The VLBW and NBW caregivers were similar in MS levels (dichotomized and ordinal) at 8 and 18-20 months. At 18-20 months, the VLBW had significantly lower number of teeth (15.0±3.0) compared to NBW (15.6±2.7) infants.
Table 1.
Mutans streptococci (MS) at 8 and 18-20 months between very low birth weight (VLBW) and normal birth weight (NBW) infants
| 8 months |
18-20 months |
|||||
|---|---|---|---|---|---|---|
| VLBW (n=204)‡ | NBW (n=182)‡ | P-value | VLBW (n=194)‡ | NBW (n=184)‡ | P-value | |
| Infant | ||||||
| Number of teeth | 4.1±2.8 | 4.2±2.6 | 0.75 | 15.0±3.0 | 15.6±2.7 | 0.05* |
| MS Overall | ||||||
| Absent | 169(83.2%) | 127(71.8%) | 0.007* | 116(59.8%) | 94(51.1%) | 0.09 |
| Present | 34(16.8%) | 50(28.2%) | 78(40.2%) | 90(48.9%) | ||
| MS Saliva † | ||||||
| Absent | 179(88.2%) | 139 (78.5%) | 0.01* | 134(69.1%) | 119(64.7%) | 0.36 |
| Present | 24(11.8%) | 38 (21.5%) | 60(30.9%) | 65(35.3%) | ||
| 0 | 179(88.2%) | 139(78.5%) | 0.007* | 134(69.1%) | 119(64.7%) | 0.24 |
| 1 | 23(11.3%) | 28(15.8%) | 30(15.4%) | 41(22.3%) | ||
| 2 | 1(0.5%) | 8(4.5%) | 13(6.7%) | 14(7.6%) | ||
| 3 | 0(0.0%) | 2(1.2%) | 17(8.8%) | 10 (5.4%) | ||
| MS Plaque † | ||||||
| Absent | 178(87.7%) | 140(79.1%) | 0.02* | 123(63.4%) | 101(54.9%) | 0.09 |
| Present | 25(12.3%) | 37(20.9%) | 71(36.6%) | 83(45.1%) | ||
| 0 | 178(87.7%) | 140(79.1%) | 0.08 | 123(63.4%) | 101(54.9%) | 0.28 |
| 1 | 22(10.8%) | 28(15.8%) | 32(16.5%) | 44(23.9%) | ||
| 2 | 2(1.0%) | 4(2.3%) | 10(5.1%) | 10(5.4%) | ||
| 3 | 1(0.5%) | 5(2.8%) | 29(15.0%) | 29(15.8%) | ||
| Mother/Caregiver | ||||||
| MS Overall | ||||||
| Low | 51(25.2%) | 48(27.1%) | 0.68 | 40(20.7%) | 52(28.4%) | 0.08 |
| High | 151(74.8%) | 129(72.9%) | 153(79.3%) | 131(71.6%) | ||
| MS Saliva † | ||||||
| Low | 61(30.2%) | 63(35.6%) | 0.26 | 54(28.0 %) | 64(35.0%) | 0.14 |
| High | 141(69.8) | 114(64.4%) | 139(72.0%) | 119(65.0%) | ||
| 0 | 31(15.4%) | 29(16.4%) | 0.67 | 29(15.0%) | 32(17.5%) | 0.42 |
| 1 | 30(14.8%) | 34(19.2%) | 25(13.0%) | 32(17.5%) | ||
| 2 | 65(32.2%) | 52(29.4%) | 51(26.4%) | 49(26.8%) | ||
| 3 | 76(37.6%) | 62(35.0%) | 88(45.6%) | 70(38.2%) | ||
| MS Plaque † | ||||||
| Low | 78(38.6%) | 70(39.6%) | 0.85 | 72(37.3%) | 76(41.5%) | 0.40 |
| High | 124(61.4%) | 107(60.5%) | 121(62.7%) | 107(58.5%) | ||
| 0 | 42(20.8%) | 45(25.4%) | 0.51 | 34(17.6%) | 44(24.0%) | 0.43 |
| 1 | 36(17.8%) | 25(14.1%) | 38(19.7%) | 32(17.5%) | ||
| 2 | 42(20.8%) | 31(17.5%) | 30(15.5%) | 23(12.6%) | ||
| 3 | 82(40.6%) | 76(43.0%) | 91(47.2%) | 84 (45.9%) | ||
Significantat p<0.05
Ordinal categories are Dentocult scores 0 to 3, dichotomous categories are absent (0) and present (1, 2, 3) for infant. For caregiver low (0,1) and high (2, 3)
Due to a few missing data, the table numbers may not add up to the totals for VLBW and NBW.
Table 2 (bivariate analysis) indicates that presence of MS in the VLBW and NBW infants at 18-20 months were significantly associated with biological factors, such as higher number of teeth present at 8 and 18-20 months. At 8 months, MS presence for both VLBW and NBW infants was associated with higher number of teeth. In the NBW group, infants with MS presence had significantly greater proportion of caregivers with high MS levels, but not in VLBW. Among the behavioral variables at 18-20 months, breast feeding was significantly lower among NBW infants with MS presence.
Table 2.
Relationship between infant mutans streptococci presence and other factors at 18-20 months visit
| Very Low Birth Weight (VLBW) n= 194 |
Normal Birth Weight (NBW) n=184 |
|||||
|---|---|---|---|---|---|---|
| Present | Absent | p-value | Present | Absent | p-value | |
| Socio-demographic | ||||||
| Mom age | 26.1±6.0 | 26.2±5.8 | 0.92 | 26.1±5.0 | 27.5±5.8 | 0.08 |
| Mom race | ||||||
| Other | 16(21.1%) | 35(31.8%) | 0.11 | 18(20.7%) | 39(42.9%) | 0.002* |
| Black | 60(79.0%) | 75(68.2%) | 69(79.3%) | 52(57.1%) | ||
| Mom education | ||||||
| <12 yr | 12(16.2%) | 20(17.7%) | 0.79 | 16(18.4%) | 13(14.1%) | 0.44 |
| >=12 yr | 62(83.8%) | 93(82.3%) | 71(81.6%) | 79(85.9%) | ||
| Mom marital status | ||||||
| Single | 60(76.9%) | 73(63.5%) | 0.047* | 72(80.0%) | 54(58.1%) | 0.001* |
| Other | 18(23.1%) | 42(36.5%) | 18(20.0%) | 39(41.9%) | ||
| Socio-economic status (SES) | ||||||
| Low | 57(79.2%) | 76(67.9%) | 0.09 | 62(71.3%) | 49(53.9%) | 0.02* |
| High | 15(20.8%) | 36(32.1%) | 25(28.7%) | 42(46.1%) | ||
| Medical | ||||||
| Gender | ||||||
| Male | 37(47.4%) | 58(50.0%) | 0.73 | 55(61.1%) | 47(50.0%) | 0.13 |
| Female | 41(52.6%) | 58(50.0%) | 35(38.9%) | 47(50.0%) | ||
| Mode of delivery | ||||||
| C-section | 42(55.3%) | 74(63.8%) | 0.24 | 31(34.8%) | 37(40.2%) | 0.45 |
| Vaginal | 34(44.7%) | 42(36.2%) | 58(65.2%) | 55(59.8%) | ||
| Antibiotic use | ||||||
| Yes | 68(88.3%) | 107(94.7%) | 0.11 | 67(76.1%) | 71(79.8%) | 0.56 |
| No | 9(11.7%) | 6(5.3%) | 21(23.9%) | 18(20.2%) | ||
| Biology | ||||||
| No. of teeth at 8 months | 3.2±3.6 | 2.1±2.5 | 0.02* | 2.7±2.8 | 3.0±3.1 | 0.56 |
| No. of teeth at 18-20 months | 15.9±2.5 | 14.4±3.1 | <0.001* | 16.3±2.5 | 14.9±2.7 | <0.001* |
| Enamel Hypoplasia | ||||||
| Yes | 19(24.4 %) | 41(35.3%) | 0.10 | 10(11.1%) | 5(5.3%) | 0.15 |
| No | 59(75.6%) | 75(64.7%) | 80(88.9%) | 89(94.7%) | ||
| Caregiver mutans streptococci | ||||||
| High | 69(88.5%) | 99(85.3%) | 0.53 | 80(88.9%) | 73(77.7%) | 0.04* |
| Low | 9(11.5%) | 17(14.7%) | 10(11.1%) | 21(22.3%) | ||
| Behavior | ||||||
| Access to dentist | ||||||
| Yes | 65(84.4%) | 94(82.5%) | 0.72 | 74(84.1%) | 84(90.3%) | 0.21 |
| No | 12(15.6%) | 20(17.5%) | 14(15.9%) | 9(9.7%) | ||
| Brushing/Cleaning | ||||||
| No brushing | 35(44.9%) | 50(43.1%) | 0.81 | 36(40.0%) | 43(45.7%) | 0.43 |
| Before 8 mos | 43(55.1%) | 66(56.9%) | 54(60.0%) | 51(54.3%) | ||
| Chew food | ||||||
| Yes | 19(24.7%) | 24(20.9%) | 0.54 | 21(23.3%) | 20(21.3%) | 0.73 |
| No | 58(75.3%) | 91(79.1%) | 69(76.7%) | 74(78.7%) | ||
| Sweet snack | ||||||
| Yes | 36(46.2%) | 46(40.4%) | 0.42 | 45(50.6%) | 35(37.2%) | 0.07 |
| No | 42(53.8%) | 68(59.6%) | 44(49.4%) | 59(62.8%) | ||
| Breastfeeding | ||||||
| Yes | 55(71.4%) | 84(72.4%) | 0.88 | 43(47.8%) | 67(71.3%) | 0.001* |
| No | 22(28.6%) | 32(27.6%) | 47(52.2%) | 27(28.7%) | ||
Significant at p < 0.05
Structural equation modeling analysis
Fig. 1 shows the hypothesized structural model for presence of MS in infants at 18-20 months with imputed data (n=378). All paths were adjusted for caregiver age, race, education, and marital status. Technical details of the structural equation modeling (SEM) are described in the Supporting Appendix S1. The RMSEA was 0.080 indicating reasonable goodness of fit (30). The results for the fit of the SEM without imputation (that is n=325 complete cases) were similar to those using multiple imputation.
The NBW had a non-significant trend (p=0.092) for an estimated 0.30 (standard deviations) greater mean score for biological risk (FBiol) compared to the VLBW infants (Fig. 1). FBiol was significantly indicated by increased number of teeth at 8 and 18-20 months, but not hypoplasia. Infants with a one unit (1 standard deviation) greater FBiol had a significantly, estimated 1.8-fold (e0.58), higher odds of MS presence at 18-20 months (Fig. 1). Birth group did not have an effect on FBehav, and subsequently FBehav was not associated with MS presence. Infants whose caregivers had a one point higher score on MS had an estimated 1.5-fold higher odds of MS presence, and this was significant (P=0.007).
Discussion
Our hypothesis using structural equation modeling (SEM) indicates that the causal pathway for MS presence in infants less than two years is predominantly biological. As VLBW infants had lower number of teeth at 18-20 months and slower tooth development, i.e. greater proportion with unerupted teeth at 8 months, they had lower biological risk during the first two years. But, our results also indicate that at 18-20 months the VLBW have similar MS presence with the emergence of primary teeth indicating future susceptibility to MS. These results are similar to a prior study (11) that indicated 18 month preterm and full-term infants had similar S. mutans prevalence, but at 24 months the preterm group had significantly higher S. mutans prevalence than full- term. We also report that the behavioral pathway has a limited role in MS presence in infants younger than two years. The relative importance of the biological pathway suggests that a non-shredding surface is required for the acquisition and colonization of MS as reported previously (2). Biological variables cannot be modified, therefore caregivers should be informed of the importance of oral hygiene and dietary habits from infancy.
We found an overall MS presence of 22% and 44% at 8 and 18-20 months respectively, lower than previously reported among preterm and full-term infants (11). However, lower prevalence have also been reported for one-year-old children of mothers with high salivary MS counts (14), and in a predominantly African-American sample (19). We speculate that medically compromised infants in our sample may have had limited caregiver contact, received routine antibiotics, and medically well-monitored during their extensive hospital stay (mean 72 days), despite 85% of the caregivers having high MS levels. Although antibiotic use was similar between the VLBW and NBW infants, the duration of use was not easily available from the medical charts and may have yielded different results. Also, we did not find an association between C-section and MS presence contradicting prior findings (19), but similar to other studies (10, 20). The extensive hospital stay may have delayed exposure to the caregiver's microbiota, thus influencing our results.
The SEM results indicate that caregiver's higher levels of MS were significantly associated with MS presence in infants indicating vertical transmission as suggested previously (1, 12). In bivariate analysis, this association was significant for NBW but not for VLBW infants, even though both VLBW and NBW caregivers had similar high levels of MS. Horizontal transmission from other members of the family or child care individuals (2) may be likely with VLBW infants as many individuals provide care during the first year of life.
The majority of VLBW compared to NBW (31% versus 8%) had enamel hypoplasia at 18-20 months (24), but the presence of enamel hypoplasia did not significantly increase the MS acquisition in infants prior to two years of age. Our findings are contradictory to those found in Chinese children 3 to 5 yr of age (17). However, our results are not comparable to this study as our infants were much younger, and other confounding factors were not controlled for in the Chinese sample. Enamel hypoplasia presents a favorable environment for MS colonization, but the VLBW because of slower tooth eruption may not have had the defects for long and it is likely that hypoplasia may be a significant factor at later ages.
Interestingly, none of the behavioral factors were significant mediators for MS presence. Since our latent variable is an underlying construct for behavior, our results are not directly comparable to other studies that have used individual risk factors. However, our findings are similar to a Finnish study (9) that found oral health related habits were not related to MS colonization. Possible explanations are that behavioral risk factors may not have been present for a long time, and these factors could be more related to ECC.
The strengths of this study is the longitudinal follow-up of a large group of VLBW and NBW infants to assess risk factors for MS presence. The limitations include recall and social desirability bias from questionnaire responses that may have influenced our behavioral findings. Additionally, the other biological factors such as salivary composition, saliva buffer capacity, salivary flow rate, pH and oral innate immune factors that may be associated with the acquisition of MS were not investigated in this study. These factors may play a biological role in influencing the colonization and pathogenic activity of MS in saliva and this might offer an explanation regarding the variances in colonization vulnerability (6, 31). It is also possible that other bacteria could have been growing in the medium, but care was taken to train our examiners to read the strips appropriately. Further, when samples are taken from both saliva and plaque as in this study, the role of other bacteria is likely less of an issue.
This study identifies the significant role of certain biological factors in the acquisition of MS in infants. Early intervention efforts should focus on delaying initial acquisition and improving caregiver awareness of taking care of erupting primary teeth.
Supplementary Material
Acknowledgements
We thank all study participants, investigators, staff, dental examiners, clinic coordinators. Study supported by Grants R01DE017947 from the National Institute of Dental and Craniofacial Research, and CTSC UL1 RR024989 and CTSC UL1TR000439 from the National Center for Research Resources.
Footnotes
Conflicts of ionterest The authors declare no potential conflicts of interest.
Supporting Information Additional Supporting Information may be found in the online version of this article:
References
- 1.TANZER JM, LIVINGSTON J, THOMPSON AM. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65:1028–1037. [PubMed] [Google Scholar]
- 2.BERKOWITZ RJ. Mutans Streptococci: Acquisition and Transmission. Pediatr Dent. 2006;28:106–109. [PubMed] [Google Scholar]
- 3.WAN AK, SEOW WK, PURDIE DM, BIRD PS, WALSH LJ, TUDEHOPE DI. Oral colonization of Streptococcus mutans in six-month-old predentate infants. J Dent Res. 2001;80:2060–2065. doi: 10.1177/00220345010800120701. [DOI] [PubMed] [Google Scholar]
- 4.BEIGHTON D, BRAILSFORD S, SAMARANAYAKE LP, BROWN JP, PING FX, GRANTMILLS D, HARRIS R, LO EC, NAIDOO S, RAMOS-GOMEZ F, SOO TC, BURNSIDE G, PINE CM. A multi-country comparison of caries-associated microflora in demographically diverse children. Community Dent Health. 2004;21(Suppl):96S–101S. [PubMed] [Google Scholar]
- 5.ERSIN NK, ERONAT N, COGULU D, UZEL A, AKSIT S. Association of maternal-child characteristics as a factor in early childhood caries and salivary bacterial counts. ASDC J Dent Child. 2006;73:105–111. [PubMed] [Google Scholar]
- 6.LAW V, SEOW WK, TOWNSEND G. Factors influencing oral colonization of mutans streptococci in young children. Aust Dent J. 2007;52:93–100. doi: 10.1111/j.1834-7819.2007.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 7.OLAK J, MÄNDAR R, KARJALAINEN S, SÖDERLING E, SAAG M. Dental health and oral mutans streptococci in 2–4-year-old Estonian children. Int J Paediatr Dent. 2007;17:92–97. doi: 10.1111/j.1365-263X.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 8.ALVES AC, NOGUEIRA RD, STIPP RN, PAMPOLINI F, MORAES AB, GONCALVES RB, HÖFLING JF, LI Y, MATTOS-GRANER RO. Prospective study of potential sources of Streptococcus mutans transmission in nursery school children. J Med Microbiol. 2009;58:476–481. doi: 10.1099/jmm.0.005777-0. [DOI] [PubMed] [Google Scholar]
- 9.MEURMAN P, PIENIHÄKKINEN K, ERIKSSON AL, ALANEN P. Mutans streptococci colonization associates with the occupation of caretaker, a practise-based study. Int J Paediatr Dent. 2010;20:144–150. doi: 10.1111/j.1365-263X.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- 10.THAKUR R, SINGH MG, CHAUDHARY S, MANUJA N. Effect of mode of delivery and feeding practices on acquisition of oral Streptococcus mutans in infants. Int J Paediatr Dent. 2012;22:197–202. doi: 10.1111/j.1365-263X.2011.01176.x. [DOI] [PubMed] [Google Scholar]
- 11.WAN AK, SEOW WK, PURDIE DM, BIRD PS, WALSH LJ, TUDEHOPE DI. A longitudinal study of Streptococcus mutans colonization in infants after tooth eruption. J Dent Res. 2003;82:504–508. doi: 10.1177/154405910308200703. [DOI] [PubMed] [Google Scholar]
- 12.DOUGLASS JM, LI Y, TINANOFF N. Association of mutans streptococci between caregivers and their children. Pediatr Dent. 2008;30:375–387. [PubMed] [Google Scholar]
- 13.CAUFIELD PW, CUTTER GR, DASANAYAKE AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res. 1993;72:37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- 14.SÖDERLING E, ISOKANGAS P, PIENIHÄKKINEN K, TENOVUO J, ALANEN P. Influence of maternal xylitol consumption on mother-child transmission of mutans streptococci: 6-year follow-up. Caries Res. 2001;35:173–177. doi: 10.1159/000047452. [DOI] [PubMed] [Google Scholar]
- 15.KOHLER B, ANDREEN I. Mutans streptococci and caries prevalence in children after early maternal caries prevention: a follow-up at eleven and fifteen years of age. Caries Res. 2010;44:453–458. doi: 10.1159/000320168. [DOI] [PubMed] [Google Scholar]
- 16.LAITALA M, ALANEN P, ISOKANGAS P, SÖDERLING E, PIENIHÄKKINEN K. A cohort study on the association of early mutans streptococci colonisation and dental decay. Caries Res. 2012;46:228–233. doi: 10.1159/000337303. [DOI] [PubMed] [Google Scholar]
- 17.LI Y, NAVIA JM, CAUFIELD PW. Colonization by mutans streptococci in the mouths of 3- and 4-year-old Chinese children with or without enamel hypoplasia. Arch Oral Biol. 1994;39:1057–1062. doi: 10.1016/0003-9969(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 18.CAUFIELD PW, LI Y, BROMAGE TG. Hypoplasia-associated severe early childhood caries--a proposed definition. J Dent Res. 2012;91:544–550. doi: 10.1177/0022034512444929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LI Y, CAUFIELD PW, DASANAYAKE AP, WIENER HW, VERMUND SH. Mode of delivery and other maternal factors influence the acquisition of Streptococcus mutans in infants. J Dent Res. 2005;84:806–811. doi: 10.1177/154405910508400905. [DOI] [PubMed] [Google Scholar]
- 20.TAIPALE T, PIENIHÄKKINEN K, SALMINEN S, JOKELA J, SÖDERLING E. Bifidobacterium animalis subsp. lactis BB-12 administration in early childhood: a randomized clinical trial of effects on oral colonization by mutans streptococci and the probiotic. Caries Res. 2012;46:69–77. doi: 10.1159/000335567. [DOI] [PubMed] [Google Scholar]
- 21.THORILD I, LINDAU-JONSON B, TWETMAN S. Prevalence of salivary Streptococcus mutans in mothers and in their preschool children. Int J Paediatr Dent. 2002;12:2–7. [PubMed] [Google Scholar]
- 22.PLONKA KA, PUKALLUS ML, BARNETT AG, WALSH LJ, HOLCOMBE TF, SEOW WK. A longitudinal study comparing mutans streptococci and lactobacilli colonisation in dentate children aged 6 to 24 months. Caries Res. 2012;46:385–393. doi: 10.1159/000339089. [DOI] [PubMed] [Google Scholar]
- 23.NELSON S, ALBERT JM, LOMBARDI G, WISHNEK S, ASAAD G, KIRCHNER HL, SINGER LT. Dental caries and enamel defects in very low birth weight adolescents. Caries Res. 2010;44:509–518. doi: 10.1159/000320160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NELSON S, ALBERT JM, GENG C, CURTAN S, LANG K, MIADICH S, HEIMA M, MALIK A, FERRETTI G, EGGERTSSON H, SLAYTON RL, MILGROM P. Increased enamel hypoplasia and very low birthweight infants. J Dent Res. 2013;92:788–794. doi: 10.1177/0022034513497751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.HOLLINGSHEAD AB. Two factor index of social position. Yale University Press; New Haven, CT: 1957. [Google Scholar]
- 26.KARJALAINEN S, SÖDERLING E, PIENIHÄKKINEN K. Validation and inter-examiner agreement of mutans streptococci levels in plaque and saliva of 10-year-old children using simple chair-side tests. Acta Odontol Scand. 2004;62:153–157. doi: 10.1080/00016350410001559. [DOI] [PubMed] [Google Scholar]
- 27.FDI COMMISSION ON ORAL HEALTH, RESEARCH AND EPIDEMIOLOGY A review of developmental defects of enamel index (DDE index) Int Dent J. 1992;42:411–426. [PubMed] [Google Scholar]
- 28.BOLLEN KA. Structural equations with latent variables. Wiley; New York: 1989. [Google Scholar]
- 29.LITTLE RJA, RUBIN DB. Statistical Analysis with Missing Data. 2nd ed Wiley; New York: 2002. [Google Scholar]
- 30.KLINE RB. Principles and practices of structural equation modeling. Guilford; New York: 1998. [Google Scholar]
- 31.LENANDER-LUMIKARI M, LOIMARANTA V. Saliva and dental caries. Adv Dent Res. 2000;14:40–47. doi: 10.1177/08959374000140010601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


