Abstract
The goal of this study was to investigate Achilles tendon tissue displacement patterns under passive and eccentric loading conditions. Nine healthy young adults were positioned prone on an examination table with their foot secured to a rotating footplate aligned with the ankle. Subjects cyclically rotated their ankle over a 25 deg range of motion at 0.5 Hz. An inertial load geared to the footplate induced eccentric plantarflexor contractions with dorsiflexion. Passive cyclic ankle motion was also performed over the same angular range of motion. An ultrasound transducer positioned over the distal Achilles tendon was used to collect radiofrequency (RF) images at 70 frames/sec. Two-dimensional ultrasound elastographic analysis of the RF data was used to track tendon tissue displacements throughout the cyclic motion. Non-uniform tissue displacement patterns were observed in all trials, with the deeper portions of the Achilles tendon consistently exhibiting larger displacements than the superficial tendon (average of 0.9–2.6 mm larger). Relative to the passive condition, eccentric loading consistently induced smaller tissue displacements in all tendon regions, except for the superficial tendon in a flexed knee posture. Significantly greater overall tissue displacement was observed in a more extended knee posture (30 deg) relative to a flexed knee posture (90 deg). These spatial- and posture-dependent displacement patterns suggest that the tendon undergoes nonuniform deformation under in vivo loading conditions. Such behavior could reflect relative sliding between the distinct tendon fascicles that arise from the gastrocnemius and soleus muscles.
Keywords: Achilles Tendon, Non-uniform Motion, Ultrasound Elastography
Introduction
The frequency and type of tendon loading is an important determinant of injury potential and mechanobiological responses. For example, Achilles tendinopathies are most commonly observed in individuals involved in activities that subject the tendon to repetitive loading (e.g. running) (Maffulli et al., 2003). It has been hypothesized that this repetitive loading gives rise to the localized fibrillar damage and tissue degeneration that develops in tendinopathies (Riley, 2008). However, somewhat paradoxically, exercises involving repeat eccentric loading of the injured tendon have shown effectiveness in treating some individuals with mid-substance tendinopathies (Alfredson, 2003; Fahlstrom et al., 2003; Maffulli et al., 2008). Although the underlying mechanism of this conservative treatment is not well understood, it is believed that the shear loading induced via eccentric exercises may stimulate the tenocytes to induce anabolic responses (Fong et al., 2005; Maeda et al., 2011). These observations highlight the importance of understanding tissue deformation patterns that arise from different in vivo loading paradigms.
There is increasing recognition that architectural features can give rise to complex deformation patterns within tendons. For example, micromechanical studies have demonstrated that tendon tissue stretch likely involves a combination of fascicle stretch and inter-fascicle sliding (Thorpe et al., 2013; Thorpe et al., 2012). These observations are highly relevant to the Achilles tendon, which consists of distinct fascicles arising from the soleus, medial gastrocnemius and lateral gastrocnemius muscles (Szaro et al., 2009). Distally, the architecture of the Achilles tendon is characterized by a helical twist which causes the relative positioning of these fascicles to vary along the Achilles length. In the mid-substance of the free Achilles tendon, the fascicles from the medial gastrocnemius are located in the superficial portion of the tendon, and fascicles from the soleus are primarily in the mid and deep portions of the tendon (Szaro et al., 2009). This complex architecture, paired with independent loading from the soleus and gastrocnemius muscles (Arndt et al., 1999; Ivanenko et al., 2004; Winter and Yack, 1987), contribute to the potential for development of non-uniform deformation in the free Achilles tendon.
Quantitative ultrasound techniques have recently emerged that allow for the assessment of in vivo behavior of the Achilles tendon. For example, prior studies have used ultrasound-based manual tracking of anatomical landmarks to show that different amounts of strain are taken up by the free tendon and aponeurosis (Arampatzis et al., 2005; Magnusson et al., 2001; Magnusson et al., 2003; Muramatsu et al., 2001). Along-tendon non-uniform deformation could arise from variations in loading along the aponeurosis (Finni et al., 2003) as well as spatially varying tendinous tissue stiffness (DeWall et al., 2014). However, much less is known about spatial variations in tendon deformation across the tendon thickness. A prior study using ultrasound speckle tracking discovered variations in tissue displacement between the superficial, mid and deep layers of the Achilles tendon during passive stretch (Arndt et al., 2012), which suggested the existence of differential stretch in tendon fascicles that originate in the soleus and gastrocnemius muscles. It is likely that such behavior could vary with active muscle loading and limb posture, given that knee flexion shortens gastrocnemius muscle-tendon operating lengths. Indeed, prior studies have shown that gastrocnemius contributions to ankle plantarflexor strength are greatly reduced in flexed knee postures (Arampatzis et al., 2006; Cresswell et al., 1995).
The goal of this study was to use ultrasound elastography (Chernak and Thelen, 2012; Slane and Thelen, 2014) to measure Achilles tendon displacement patterns under both passive and eccentric loading conditions in two knee postures. We hypothesized that eccentric loading would induce more non-uniform tendon motion than observed under passive stretch. We also hypothesized that knee posture would alter relative tendon tissue motion between the soleus and gastrocnemius portions of the Achilles tendon, due to a posture-induced change in loading sharing between gastrocnemius and soleus fascicles. Specifically, we hypothesized that when the knee is extended, distal tendon tissue motion would increase in the more taut gastrocnemius muscle-tendon unit.
Methods
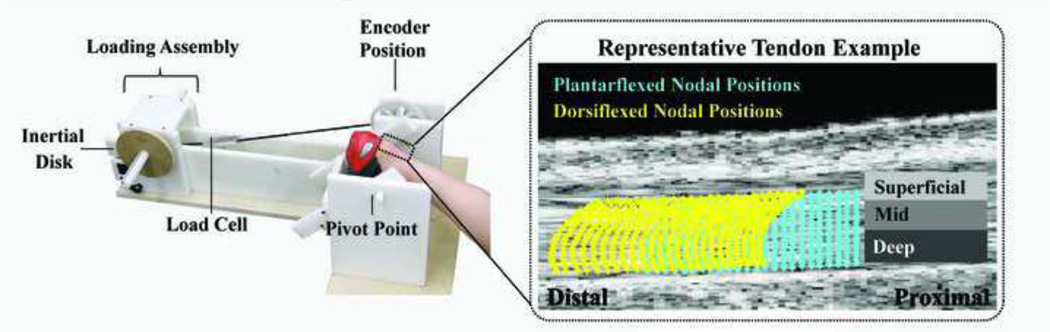
Nine healthy young adults (5F/4M; 24 ± 1 yrs) with no self-reported history of Achilles tendon injury were recruited for this study. Written consent was obtained from each subject as per our Institutional Review Board requirements prior to testing. Subjects were first asked to walk at a comfortable pace for six minutes to pre-condition the plantarflexor muscle-tendon units (Hawkins et al., 2009). Subjects were then positioned prone on an examination table with their foot secured in a rigid shoe, which was then attached to a rotating footplate (Fig. 1). Care was taken to adjust the axis of the footplate to ensure that it aligned well with the sagittal ankle axis of rotation. The footplate was coupled via a stiff belt and gear-train to rotating inertial disks. Eccentric plantarflexor activity was used to decelerate the rotating disks as the ankle moved toward peak ankle dorsiflexion. Loads were recorded using two load cells (LCM300 Futek, Irvine, CA) mounted on the belts of the loading assembly and recorded at 1000 Hz. Ankle angle was simultaneously recorded using an encoder mounted on the footplate rotation shaft. Load cell and encoder data were subsequently used in post-hoc inverse dynamic analyses to compute the internal sagittal ankle moment.
Figure 1.
An overview of the experimental setup and image analysis. Inertial disks were used to induce eccentric plantarflexor contractions when moving from a plantarflexed to dorsiflexed position. An ultrasound transducer placed over the distal Achilles tendon was used to collect RF data throughout the cyclic trials. In post-hoc analysis, initial nodal positions were defined within the tendon from an image collected in the most plantarflexed position. Two-dimensional elastography was then used to track the subsequent motion of nodes located in superficial, mid and deep portions of the tendon.
Subjects performed eccentric and passive ankle trials at fixed knee flexion angles of 30 deg and 90 deg, referred to as extended and flexed knee postures, respectively. In eccentric trials, subjects were asked to cyclically dorsi- and plantarflex their ankle between 0 and 30 deg of plantarflexion at a rate of 0.5 Hz. Assuming the subject performs the task sinusoidally, the task induces a peak angular speed of ~90 deg/s in the middle of the range of motion. A metronome was used to guide the cyclic rate, and subjects were provided real-time angular feedback to maintain the desired range of motion. Inertial disks on the loading device induced eccentric ankle plantarflexor moments, with the peak load of ~30 Nm occurring when the subject was near their most dorsiflexed position. For both knee angles, inactive passive trials were also conducted in which a researcher guided the ankle through the same range of motion at the same cyclic rate as in the eccentric loading trials. Subjects were instructed to remain relaxed in the passive trials and the researcher monitored the footplate loading to ensure compliance. Trial order was randomized, and subjects were given one minute of practice with each loading condition prior to data collection.
Rectangular ultrasound standoff pads (178 × 127 mm, 16 mm thick) were created by heating a commercial pad (Aquaflex, Parker Laboratories, Fairfield, NJ) and letting it settle in a mold. The standoff pad was placed over the Achilles tendon and secured with an elastic ankle brace. A 10 MHz linear array transducer was then manually positioned to image the Achilles tendon, with the inferior edge of the transducer positioned just superior to the superior edge of the calcaneus (Fig. 1b). For each test condition, three eight-second trials of cine ultrasound radiofrequency (RF) data were collected of the Achilles tendon at 70 frames/sec. All trials were then visually reviewed to ensure that out-of-plane motion was small and that tendon fascicles remained in view throughout loading. At least one trial for each of the subjects and for each of the loading conditions met the required criteria and was included in the analysis.
Tissue displacements were computed retrospectively from the RF data. Tissue tracking was performed using a 2D cross-correlation based elastography technique that has been described previously (Chernak and Thelen, 2012), and validated in phantom and ex vivo experiments (Chernak and Thelen, 2012; Slane and Thelen, 2014). Briefly, ankle angle data were used to define each cycle of motion, with the most plantarflexed position designated as the cycle start. To ensure that only tendinous tissue was included, a region of interest (ROI) was manually defined within an image of the Achilles tendon from the most plantarflexed position. A grid of nodes was then positioned (spaced: 0.5 × 0.2 mm) within the ROI (8.1 × 2.3 mm). Frame-to-frame nodal motion was assessed by finding the displacements that maximized the cross-correlation of rectangular kernels (2 mm × 1 mm) centered at the nodes in each pair of successive frames. Nodal displacements were regularized using a 2nd order surface fit (Slane and Thelen, 2014). Nodal tracking was repeated in the reverse direction and a weighted average of the nodal displacement trajectories from the forward and backward tracking results was computed (Pelc, 1995).
Displacement profiles for each trial were computed by averaging the along-fiber nodal displacements into twelve bins across the thickness of the tendon. For each bin, we computed the distal displacement when moving from the most plantarflexed to the most dorsiflexed angle. For statistical comparison, we then further averaged the nodal displacements into three equally sized depths of the tendon: superficial, mid and deep. A repeated measures ANOVA was used to evaluate the effects of loading condition (passive, eccentric) and depth (superficial, mid, deep) on the absolute distal tendon displacements. To evaluate loading effects on non-uniformity, we computed the relative displacements between specific region (superficial, mid, deep) and the displacement of the whole ROI. A repeated measures ANOVA was used to evaluate the effects of loading condition and depth on these relative displacements. Tukey post-hoc analyses (p < 0.05) were used to test the primary hypotheses.
Results
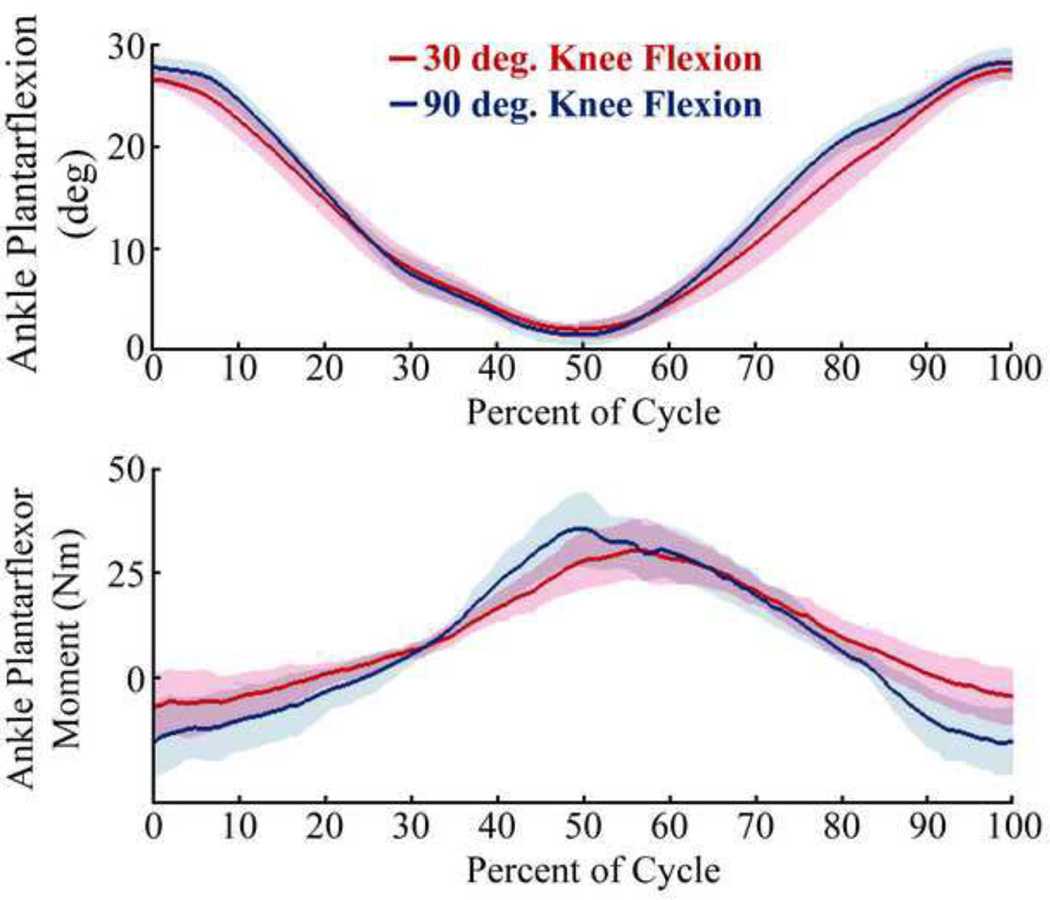
Achilles tendon thickness averaged 4.5 (± 0.5) mm for the 9 subjects tested. There were no significant differences in ankle angular excursion (25.6 ± 1.7 deg) between the passive motion and eccentric loading conditions (Fig. 2a). In the eccentric condition, the device induced a peak ankle plantarflexor moment (33.6 ± 8.3 Nm) in a nearly fully dorsiflexed posture (Fig. 2b). No significant difference in peak ankle moment was observed between eccentric loading tasks performed in extended and flexed knee postures. However, peak ankle moment did occur slightly and significantly earlier in the loading cycle for the flexed knee case (Extended: 57 ± 3% of cycle, Flexed: 52 ± 4% of cycle, p = 0.007).
Figure 2.
Average (±1 s.d.) temporal patterns of the plantarflexion angle and internal ankle moment over an eccentric task motion cycle, with 0% corresponding to the most plantarflexed position. Ankle range of motion and peak ankle moment were not significantly different when performed with 30 and 90 deg of knee flexion.
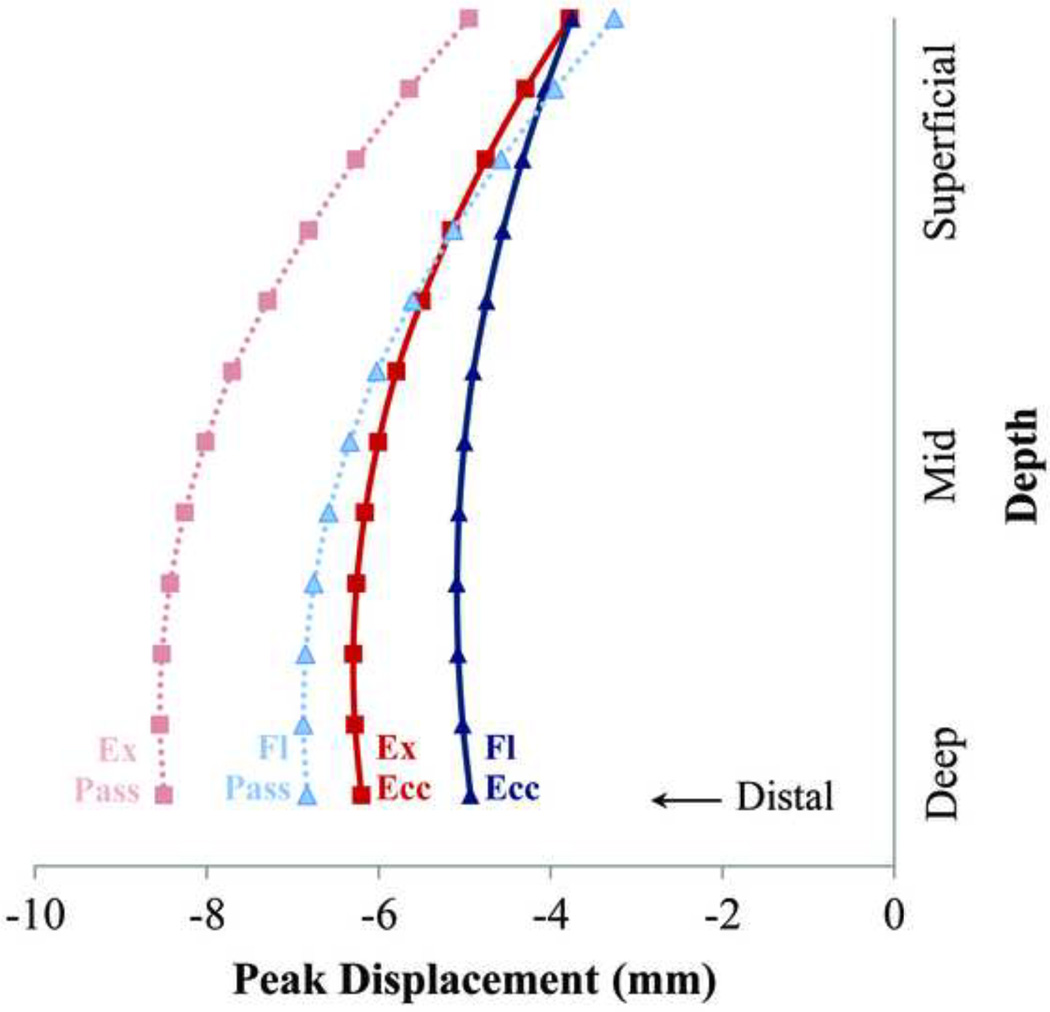
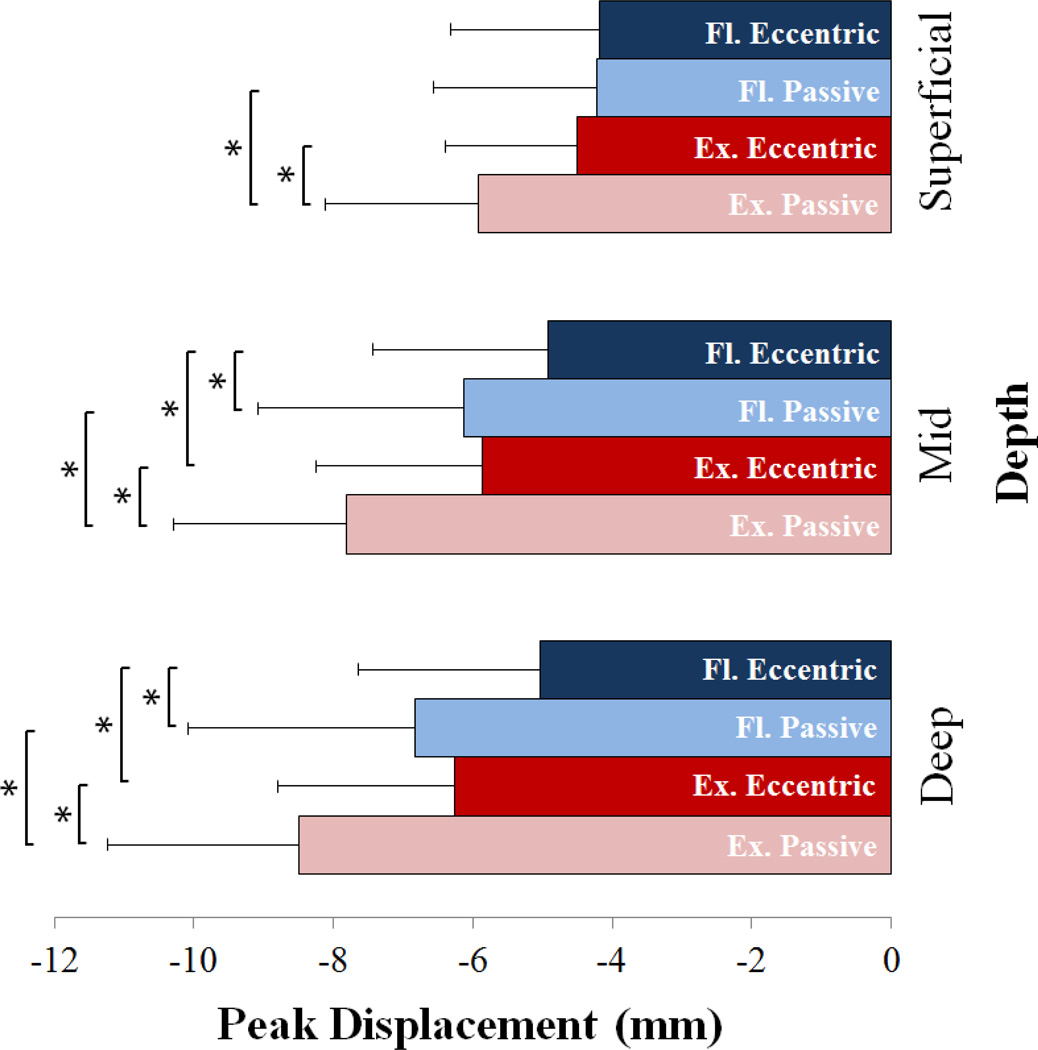
Non-uniform tissue displacement patterns were observed across the tendon thickness, with the greatest displacement observed in the deeper tissue for all four test conditions (Fig. 3). Knee angle had a significant effect on the magnitude of tissue displacements observed, with greater mid and deep tissue displacement (p < 0.002) observed in the extended knee posture relative to the flexed knee posture for both loading conditions in the mid and deep portions of the tendon (Fig. 4). Superficial tissue displacements were greater in the extended posture for the passive loading condition but not the eccentric loading condition. In the extended knee posture, significantly greater displacements were measured in the passive loading condition than in eccentric loading condition at all depths (p < 0.0002). In the flexed knee posture, significantly greater passive displacements were only observed in the mid and deep portions of the tendon (p < 0.0002).
Figure 3.
The average (across 9 subjects) peak nodal displacements (negative corresponds with distal) at different tissue depths for passive (Pass) and eccentric (Ecc) loading tasks. Larger tissue displacements were observed when the knee was extended (Ex; 30 deg) compared with when the knee was flexed (Fl; 90 deg).
Figure 4.
Average (+1 s.d.) peak tissue displacements (negative corresponds with distal) when moving from plantarflexed to dorsiflexed positions. Knee posture (Fl: flexed, Ex: extended) and loading conditions significantly affected tissue displacements in all layers, except for the superficial tendon which exhibited similar displacements under both loads when the knee was flexed. *p < 0.05.
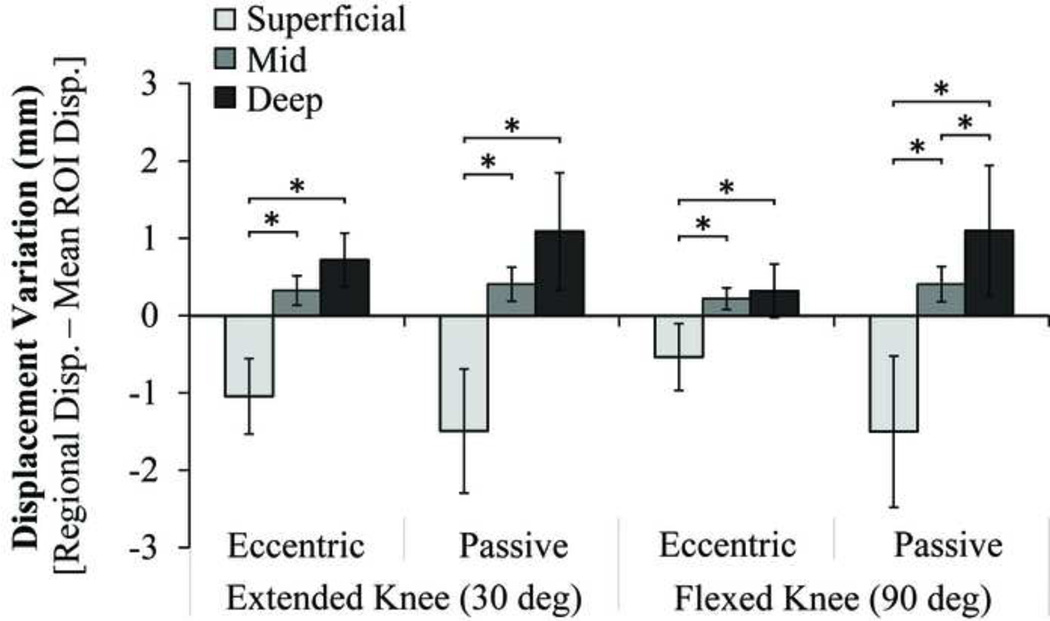
There were depth-dependent significant (p < 0.03) differences in the relative tissue displacements between the superficial and mid layers for all four tasks (Fig. 5). Differences between the mid and deep tissue displacements were only significant under the passive flexed condition (p < 0.05).
Figure 5.
Superficial, mid and deep tissue displacements, relative to the average ROI displacements for each condition. Depth-dependent differences in relative tissue displacements are evident for each of the tasks, though differences between the mid and deep layers are only significant in the passive flexed knee trial. *p < 0.05.
Discussion
Our data suggest that the Achilles tendon undergoes non-uniform displacement during both passive and eccentric plantarflexor loading. Specifically, we consistently observed greater displacement in the mid and deep tendon relative to the more superficial tendon when dorsiflexing the ankle. Similar displacement patterns were previously observed with passive dorsiflexion (Arndt et al., 2012) and may reflect the tendon undergoing shear deformation and variable stretch over the tendon cross-section. Such behavior may be relevant to consider when assessing both injury factors and mechanobiological stimuli.
Contrary to our first hypothesis, we did not consistently observe an increase in the non-uniformity of tendon motion with eccentric loading relative to passive loading. This hypothesis was based on the idea that eccentric loading may generate greater tissue shear stress and fluid flow that stimulates tenocytes to induce anabolic responses (Grigg et al., 2009). However, it should be noted that the eccentric exercises commonly performed for Achilles tendinopathy involve considerably larger loading than used in this study. Further, heavy slow resistance training has been suggested as an alternative to eccentric loading (Kongsgaard et al., 2009) in treating patellar tendinopathy, which we did not evaluate in this study. Thus, although this study suggests non-uniform deformation arises with eccentric loading on the tendon, further study is needed to determine how load magnitude and type may affect tissue displacement patterns. Such data are needed to translate mechanobiological studies that demonstrate the importance of tissue loading for promoting tissue homeostasis and repair responses (Fong et al., 2005; Maeda et al., 2011) into efficacious rehabilitation exercises that may best alleviate symptoms in patients with Achilles tendinopathy (Alfredson, 2003; Fahlstrom et al., 2003; Maffulli et al., 2008).
We did observe a significant effect of knee angle on tendon tissue motion (Fig. 4). As hypothesized, we generally observed greater tendon displacements in the extended knee posture relative to the flexed knee posture under both passive and eccentric loading conditions. The only exception to this was in the superficial Achilles, which exhibited similar motion in extended and flexed knee postures under eccentric loading. Greater distal displacement would imply that the portion of the tendon distal to the ROI is undergoing less stretch with dorsiflexion when the knee is extended. This posture effect could result from the more stretched gastrocnemius inducing a preload that stiffens the Achilles tendon, thereby reducing the additional stretch that arises with dorsiflexion. We observed knee flexion to affect motion in all three tissue layers. Although biomechanically, knee flexion only affects the muscle-tendon length of the biarticular gastrocnemius, a change in gastrocnemius muscle length can alter the relative load sharing across the triceps surae (Cresswell et al., 1995). Indeed, there is evidence of a posture-dependent change in load sharing in our data. When the knee was flexed, eccentric loading significantly altered motion in the mid and deep portions of the tendon but not in the superficial tendon (Fig. 4). This may reflect preferential loading of the soleus in a flexed knee posture, since the shortened gastrocnemius is at a length poorly tuned for force generation (Arampatzis et al., 2006; Cresswell et al., 1995). Greater soleus loading would potentially increase the distal stretch of the mid and deep fascicles of the Achilles tendon, thereby contributing to the observed reduction in displacement with eccentric loading.
There is ample anatomical evidence of the intricate hierarchical structure of the tendon that likely affects internal deformations. Distinct bundles of fascicles arise from the medial gastrocnemius, lateral gastrocnemius and soleus. These fascicle bundles intertwine and undergo approximately a 90 deg helical twist distally (White, 1943). We imaged the free Achilles tendon, with the distal edge of our image located slightly proximal to the superior calcaneus. The ROI was centered on the tendon and positioned proximally to allow for the distal translation to remain within the image during dorsiflexion. Anatomically at this position, we believe that superficial displacements of the ROI would primarily reflect the portions of the tendon arising from the medial gastrocnemius, with the mid and deeper displacements of the ROI most likely reflecting fascicles arising from the soleus (Szaro et al., 2009). Lateral gastrocnemius tendon fascicles do twist and appear in deeper layers in the most distal portion of the Achilles tendon, but likely were not a major component of the ROI at the level of the tendon we tracked. Therefore, the non-uniform motion that we observed may reflect relative sliding between the tendon fascicles originating in the medial gastrocnemius and soleus. This explanation would be consistent with recent in vitro tendon studies that have suggested that inter-fascicle sliding is an important component of tendon micromechanical behavior (Thorpe et al., 2013; Thorpe et al., 2012).
It was somewhat surprising that we observed less motion in the superficial Achilles tendon relative to the mid and deep tendon. The moment arm about the ankle would be larger for the superficially positioned fascicles of the medial gastrocnemius and thus could be expected to undergo greater displacement with dorsiflexion. However, there are three potential explanations for our observations. First, it is possible that the tendon fascicles originating in the medial gastrocnemius are more compliant, resulting in greater stretch distal to the ROI and thus less displacement within the image window. Indeed, the longer length of the distal gastrocnemius tendon relative to the soleus distal tendon (Cummins et al., 1946) may result in it having lower compliance. Alternatively, it is possible that there is a component of twist to the motion of the gastrocnemius fascicles during dorsiflexion that is not captured in our two-dimensional tracking technique. In this case, the motion we measured would not be purely a function of dorsiflexion and tendon stretch. A third explanation is that a portion of the superficial tendon we imaged could originate from lateral gastrocnemius muscle fascicles, which rotate distally and insert onto the anterior aspect of the calcaneus. This anterior insertion would diminish the tendon moment arm about the ankle. It should be noted that anatomical studies have reported a fairly wide variation in Achilles tendon architecture, such that it is challenging to ascertain the specific fascicles being imaged based on location alone.
There are a few limitations in this study to consider. An important component of this study was the evaluation of non-uniform tendon motion under distinct loading conditions and limb postures. To maintain a consistent ankle excursion across different tasks, visual biofeedback of ankle angle was used to guide task performance. Thus, the repeatability of this approach was dependent on the subjects’ or researchers’ ability to maintain the desired range of motion at the desired rate. This method was effective at controlling ankle angle excursions, with no significant differences measured between conditions. However, we did measure a slight change in the timing of eccentric loading patterns (Fig. 2). Our analyses were defined based on displacements between peak plantarflexion and peak dorsiflexion, such that the small change in load timing could have contributed to some of the posture effects observed in the eccentric loading paradigm. The fact that we observed similar posture effects under both passive and eccentric loading conditions (Fig. 3) would suggest this was not a major factor.
A second challenge is the use of 2D ultrasound imaging to capture motion from a 3D structure. This is a limitation that is present in many studies that have used 2D ultrasound to characterize Achilles tendon motion and deformation with loading (Arampatzis et al., 2005; Arndt et al., 2012; Maganaris, 2003; Magnusson et al., 2003; Muramatsu et al., 2001). We have previously performed extensive validation studies of our elastographic tracking technique on ex vivo tendon loading paradigms and found that displacement estimates are highly correlated (R2 > 0.92) with displacements obtained by tracking markers on the tissue surface (Slane and Thelen, 2014). We also used a qualitative evaluation of the data to reduce the number of trials that contained significant out-of-plane motion. Future studies will involve further validation of the ability to measure localized tissue strains to provide additional insight into tissue deformations. Thus, although we are unable to capture the out-of-plane motion that can arise from tendon twist, we are fairly confident in the capacity of this method to track motion within the image plane.
In summary, this study provides evidence of depth-dependent tissue displacements across the Achilles tendon cross-section under both passive and eccentric loading conditions. We observed knee angle to play a significant role in altering tendon tissue motion, with greater tendon tissue displacements measured when the knee was extended. Eccentric loading was also observed to alter Achilles tendon tissue displacements in both knee postures, but in the flexed posture only the mid and deep portions of the tendon were affected. These spatial and posture dependent motion patterns suggest that the Achilles tendon undergoes non-uniform deformation under in vivo loading conditions. Such behavior could reflect relative sliding between the distinct tendon fascicles that arise from the gastrocnemius and soleus muscles.
Acknowledgments
We gratefully acknowledge the support of NIH F31AG043216, the American Society of Biomechanics (ASB) Grant-in-Aid, the International Society of Biomechanics Matching Dissertation Program, and the contributions of Dave Bunger, Dan Volk, Nathan Kleinhans, Tanner Marshall, Alex Ehlers, Dr. Silvia Blemker and Geoffrey Handsfield to this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflict of interest.
References
- Alfredson H. Chronic midportion Achilles tendinopathy: an update on research and treatment. Clin. Sports Med. 2003;22:727–741. doi: 10.1016/s0278-5919(03)00010-3. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Karamanidis K, Stafilidis S, Morey-Klapsing G, DeMonte G, Bruggemann GP. Effect of different ankle- and knee-joint positions on gastrocnemius medialis fascicle length and EMG activity during isometric plantar flexion. J. Biomech. 2006;39:1891–1902. doi: 10.1016/j.jbiomech.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Stafilidis S, DeMonte G, Karamanidis K, Morey-Klapsing G, Bruggenmann GP. Strain and elongation of the human gastrocnemius tendon and aponeurosis during maximal plantarflexion effort. J. Biomech. 2005;38:833–841. doi: 10.1016/j.jbiomech.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Arndt A, Bengtsson AS, Peolsson M, Thorstensson A, Movin T. Non-uniform displacement within the Achilles tendon during passive ankle joint motion. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2012;20:1868–1874. doi: 10.1007/s00167-011-1801-9. [DOI] [PubMed] [Google Scholar]
- Arndt A, Bruggemann GP, Koebke J, Segesser B. Asymmetrical loading of the human triceps surae: I. Mediolateral force differences in the Achilles tendon. Foot Ankle Int. 1999;20:444–449. doi: 10.1177/107110079902000709. [DOI] [PubMed] [Google Scholar]
- Chernak LA, Thelen DG. Tendon motion and strain patterns evaluated with two-dimensional ultrasound elastography. J. Biomech. 2012;45:2618–2623. doi: 10.1016/j.jbiomech.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell AG, Loscher WN, Thorstensson A. Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Exp Brain Res. 1995;105:283–290. doi: 10.1007/BF00240964. [DOI] [PubMed] [Google Scholar]
- Cummins EJ, Anson BJ, et al. The structure of the calcaneal tendon (of Achilles) in relation to orthopedic surgery, with additional observations on the plantaris muscle. Surg Gynecol Obstet. 1946;83:107–116. [PubMed] [Google Scholar]
- DeWall RJ, Slane LC, Lee KS, Thelen DG. Spatial variations in Achilles tendon shear wave speed. J. Biomech. 2014 doi: 10.1016/j.jbiomech.2014.05.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlstrom M, Jonsson P, Lorentzon R, Alfredson H. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg. Sports Traumatol. Arthrosc. 2003;11:327–333. doi: 10.1007/s00167-003-0418-z. [DOI] [PubMed] [Google Scholar]
- Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Mapping of movement in the isometrically contracting human soleus muscle reveals details of its structural and functional complexity. J. Appl. Physiol. 2003;95:2128–2133. doi: 10.1152/japplphysiol.00596.2003. [DOI] [PubMed] [Google Scholar]
- Fong KD, Trindade MC, Wang Z, Nacamuli RP, Pham H, Fang TD, Song HM, Smith RL, Longaker MT, Chang J. Microarray analysis of mechanical shear effects on flexor tendon cells. Plast. Reconstr. Surg. 2005;116:1393–1404. doi: 10.1097/01.prs.0000182345.86453.4f. discussion 1405-1396. [DOI] [PubMed] [Google Scholar]
- Grigg NL, Wearing SC, Smeathers JE. Eccentric calf muscle exercise produces a greater acute reduction in Achilles tendon thickness than concentric exercise. Brit. J. Sport Med. 2009;43:280–283. doi: 10.1136/bjsm.2008.053165. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. The Journal of Physiology. 2004;556:267–282. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsgaard M, Kovanen V, Aagaard P, Doessing S, Hansen P, Laursen AH, Kaldau NC, Kjaer M, Magnusson SP. Corticosteroid injections, eccentric decline squat training and heavy slow resistance training in patellar tendinopathy. Scandinavian journal of medicine & science in sports. 2009;19:790–802. doi: 10.1111/j.1600-0838.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, Ilic D, Baskaran H, Sakai T. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Current Biology. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffulli N, Walley G, Sayana MK, Longo UG, Denaro V. Eccentric calf muscle training in athletic patients with Achilles tendinopathy. Disabil. Rehabil. 2008;30:1677–1684. doi: 10.1080/09638280701786427. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin. Sports Med. 2003;22:675–692. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- Maganaris CN. Tendon conditioning: artefact or property? Proceedings of the Royal Society - Biological Sciences. 2003;270(Suppl. 1):S39–S42. doi: 10.1098/rsbl.2003.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. J. Physiol. 2001;531:277–288. doi: 10.1111/j.1469-7793.2001.0277j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiologica Scandinavica. 2003;177:185–195. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Muraoka T, Takeshita D, Kawakami Y, Hirano Y, Fukunaga T. Mechanical properties of tendon and aponeurosis of human gastrocnemius muscle in vivo. J. Appl. Physiol. 2001;90:1671–1678. doi: 10.1152/jappl.2001.90.5.1671. [DOI] [PubMed] [Google Scholar]
- Riley G. Tendinopathy--from basic science to treatment. Nat Clin Pract Rheumatol. 2008;4:82–89. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- Slane L, Thelen D. The Use of 2D Ultrasound Elastography for Measuring Tendon Motion and Strain. J. Biomech. 2014;47:750–754. doi: 10.1016/j.jbiomech.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaro P, Witkowski G, Smigielski R, Krajewski P, Ciszek B. Fascicles of the adult human Achilles tendon - an anatomical study. Ann. Anat. 2009;191:586–593. doi: 10.1016/j.aanat.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Klemt C, Riley GP, Birch HL, Clegg PD, Screen HR. Helical sub-structures in energy-storing tendons provide a possible mechanism for efficient energy storage and return. Acta. Biomater. 2013;9:7948–7956. doi: 10.1016/j.actbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HR. Specialization of tendon mechanical properties results from interfascicular differences. J. R. Soc. Interface. 2012;9:3108–3117. doi: 10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JW. Torsion of the Achilles tendon: its surgical significance. Archives of Surgery. 1943;46:784–787. [Google Scholar]
- Winter DA, Yack HJ. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroen. Clin. Neuro. 1987;67:402–411. doi: 10.1016/0013-4694(87)90003-4. [DOI] [PubMed] [Google Scholar]