Abstract
Injury to the preterm brain has a particular predilection for cerebral white matter. White matter injury (WMI) is the most common cause of brain injury in preterm infants and a major cause of chronic neurological morbidity including cerebral palsy. Factors that predispose to WMI include cerebral oxygenation disturbances and maternal-fetal infection. During the acute phase of WMI, pronounced oxidative damage occurs that targets late oligodendrocyte progenitors (preOLs). The developmental predilection for WMI to occur during prematurity appears to be related to both the timing of appearance and regional distribution of susceptible preOLs that are vulnerable to a variety of chemical mediators including reactive oxygen species, glutamate, cytokines, and adenosine. During the chronic phase of WMI, the white matter displays abberant regeneration and repair responses. Early OL progenitors responds to WMI with a rapid robust proliferative response that results in a several fold regeneration of preOLs that fail to terminally differentiate along their normal developmental time course. PreOL maturation arrest appears to be related in part to inhibitory factors that derive from reactive astrocytes in chronic lesions. Recent high field MRI data support that three distinct forms of chronic WMI exist, each of which displays unique MRI and histopathological features. These findings suggest the possibility that therapies directed at myelin regeneration and repair could be initiated early after WMI and monitored over time. These new mechanisms of acute and chronic WMI provide access to a variety of new strategies to prevent or promote repair of WMI in premature infants.
Impact of Perinatal White Matter Injury on Preterm Survivors
Although major advances in the care of premature infants have resulted in striking improvements in the survival of very low birth weight (VLBW) infants (< 1.5 kg), improved survival has been accompanied by a significant increase in the number of pre-term survivors with long-term neurological deficits (Wilson-Costello et al. 2005). In multiple parts of the world, ~10-15% of preterm survivors sustain permanent motor impairment (i.e., cerebral palsy; CP) that ranges from mild to profound spastic motor deficits (Beaino et al. 2010; Hack et al. 2005; Liu et al. 2008; Mercier et al. 2010; Miller et al. 2005a). By school age, 25-50% also manifest a broad spectrum of cognitive, visual, social-behavioral, attention and learning disabilities (Anderson et al. 2011; Glass et al. 2008b; Jacobson and Dutton 2000; Litt et al. 2005; Soria-Pastor et al. 2008).
Cerebral white matter injury (WMI) is the major form of brain injury recognized in survivors of premature birth (Volpe 2009). The period of highest risk for WMI is ~23-32 weeks post-conceptional age. In preterm survivors, MRI-defined WMI manifests in the first months of life as abnormal movements that are predictive of CP (Constantinou et al. 2007; Spittle et al. 2009; Spittle et al. 2008). The impact of WMI can be appreciated from a recent large population based study of children with CP. Perinatal WMI, including the necrotic lesions of periventricular leukomalacia (PVL), was the most common finding, seen in almost half (42.5%) of affected children (Bax et al. 2006). Moreover, premature birth alone is associated with a greater risk for reduction in both cerebral white and gray matter volume, which is associated with poorer cognitive development (Aarnoudse-Moens et al. 2009; Anderson and Doyle 2008; Delobel-Ayoub et al. 2009; Kesler et al. 2008; Loeliger et al. 2006; Peterson et al. 2000; Scafidi et al. 2009; Soria-Pastor et al. 2009). Since VLBW infants comprise about 1.5% of the 4 million live births in the U.S. alone each year, the world-wide social and economic burden is considerable. The average lifetime costs per person with CP is estimated to be ~1 million dollars in the U.S. ((CDC) 2004).
Premature infants with WMI are at markedly increased risk for several others forms of brain injury, notably intraventricular hemorrhage (IVH) and intraparenchymal hemorrhage (Volpe 2008a). Whereas medical interventions have resulted in a pronounced decrease in the incidence of IVH (Fowlie and Davis 2003; Volpe 2001), the associated incidence of WMI is not decreasing (Ballabh 2010). Thus, WMI is one of the major neurological problems that affect VLBW infants.
WMI is not exclusively associated with prematurity and is increasingly appreciated in term infants (Lasry et al. 2010; Li et al. 2009; Martinez-Biarge et al. 2012; Pagliano et al. 2007). Infants with complex congenital heart disease (CHD) are at particular risk for WMI and delayed brain maturation (Clouchoux et al. 2012; Licht et al. 2009; Limperopoulos et al. 2010; Miller et al. 2007; Wernovsky et al. 2005). These infants show an increased predilection for a pressure passive circulation (Bassan et al. 2005). Although the risk for WMI would be expected to be lower, since these infants are often full term at birth, WMI is now the major neurological lesion associated with CHD (Galli et al. 2004; Kinney et al. 2005). The basis for this propensity for WMI is unknown, but recent studies support that white matter pathology can precede surgical repair of heart lesions (McQuillen and Miller 2010). This suggests that CHD itself may be a risk factor for WMI.
CELLULAR-MOLECULAR MECHANISMS OF ACUTE WMI
Maturation-dependent Vulnerability of the OL lineage to Oxidative Stress
Since the major period of vulnerability for WMI occurs prior to the onset of myelination, it was initially proposed that the myelination disturbances of WMI might arise from targeted death of OL progenitors that are the source of mature OLs (Back and Volpe 1997). This hypothesis stated that the predilection for WMI is related to a developmentally-regulated susceptibility of more immature stages of the OL lineage to oxidative stress, a well-established sequela of both hypoxia-ischemia and systemic hypotension arising from maternal-fetal infection (Ferriero and Miller 2010; Hagberg et al. 2002). This hypothesis motivated the testing of the relative susceptibility of succesive stages of the OL lineage to clincally relevant insults ranging from oxidative stress to excitotoxicity (Rosenberg et al. 2003).
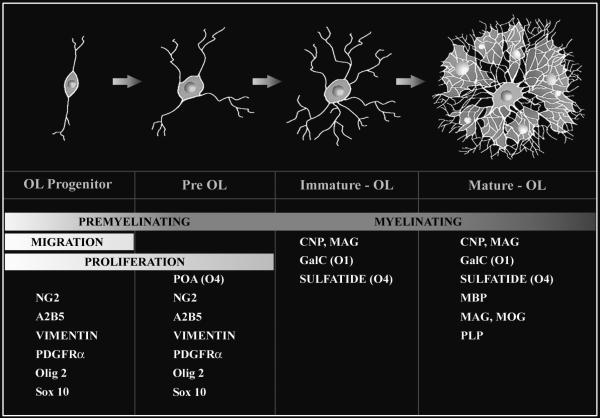
Human fetal OLs develop according to a well-established lineage, defined by stage-specific antibodies specific for sequentially expressed OL cell-surface and myelin-specific epitopes (Back et al. 2001; Back et al. 2002b; Jakovcevski et al. 2009; Jakovcevski and Zecevic 2005). It is feasible to precisely define the timing and features of OL lineage progression both in vitro and in vivo. The successive OL stages are distinguished by a progressively more complex morphology (Figure 1). The OL progenitor (OPC) is the earliest stage committed to the OL lineage. The preOL is a simple multipolar, mitotically active late OL progenitor immunoreactive with the O4 but not the O1 monoclonal antibodies. The immature OL is a post-mitotic complex multipolar cell identified by the O1 antibody that binds to galactocerebroside. The mature OL is identified by myelin-associated markers that include myelin basic protein (MBP).
Figure 1.
Maturation of the oligodendrocyte (OL) lineage. Four principal stages of OL lineage progression are depicted together with their corresponding morphological features and capacity for myelination, migration and proliferation. Each stage is uniquely defined by a combination of marker genes or antibodies. A2B5, O4, O1 refer to mouse monoclonal antibodies. Olig 2 and Sox10 are genes that are highly enriched in premyelinating OLs. Olig 2 is also expressed at later stage in the OL lineage. Abbreviations: CNP (CNPase), 2’:3’-cyclic nucleotide-3’-phosphodiesterase; ; GalC, galactocerebroside; MAG, myelin associated glycoprotein; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein; NG2, chondroitin sulfate proteoglycan 4; PDGFRα, platelet-derived growth factor-alpha; PLP, proteolipid protein.
The developmental window of highest risk for WMI (i.e., ~23-32 weeks postconceptional age) corresponds to a period in human white matter development that precedes the onset of myelination (Back et al. 2001; Rakic and Zecevic 2003). This period coincides with the presence of one major population of preOLs in cerebral white matter and identified the preOL as a potential target for cell death in WMI. The decline in risk for WMI coincides with the onset of a wave of differentiation of preOLs to immature OLs that initiate myelination of cerebral white matter (Back et al. 2002b).
The concept that OL progenitors are selectively targeted by oxidative stress derives from several studies that identified maturation-dependent mechanisms of free radical-mediated injury to the OL lineage both in vitro and in vivo (Back et al. 1998; Back et al. 2002a; Baud et al. 2004a; Fern and Moller 2000; Fragoso et al. 2004; Lin et al. 2004). Many of these studies compared the susceptibility of successive stages in the OL lineage to oxidative stress. PreOLs are markedly more susceptible than mature OLs to intrinsic and extrinsic sources of oxidative stress. Intracellular depletion of glutathione triggered a downstream rise in reactive oxygen species (ROS) that lead to preOL death (Back et al. 1998). Several in vitro studies found that caspase-mediated death of mixed populations of OL progenitors and preOLs occurs after oxidative stress in vitro (Druzhyna et al. 2003; Fragoso et al. 2004; Mronga et al. 2004; Sanchez-Gomez et al. 2003). However, preOLs degenerate from a spectrum of necrotic and apoptotic death in vivo (Back et al. 2002a; Segovia et al. 2008; Alix et al. 2012). Interestingly, the E2-isoprostanes, a lipid peroxidation product, are particularly toxic to OL progenitors in vitro, but not to mature OLs, which suggests that specific compounds generated endogenously from oxidative stress might be a potential mechanism for OL degeneration in WMI (Brault et al. 2004a).
Response of the OL Lineage to Oxidative Stress in vitro
Disturbances in cerebral blood flow, oxygenation and metabolism commonly cause dysregulation of key neurotransmitter systems that are already functional in the preterm brain. In vitro studies have identified glutamate-mediated toxicity to the OL lineage that is both receptor-independent and receptor-mediated. A receptor-independent mechanism is mediated by glutathione depletion with consequent free radical generation (Back et al. 1998; Oka et al. 1993; Yonezawa et al. 1996) and is similar to the phenomenon of oxidative glutamate toxicity that was discovered using immature neurons and neuronal cell lines in culture (Coyle and Puttfarcken 1993). Oxidative glutamate toxicity involves a glutamate-cystine exchanger (system xc− transporter) that is an important source of cellular cystine and, by intracellular reduction, cysteine (Bannai and Kitamura 1980; Murphy et al. 1989a; Sato et al. 1999). This transporter also has been increasingly recognized as an important contributor to extracellular glutamate homeostasis (Kalivas 2009; Lewerenz et al. 2013). Intracellular cystine depletion leads to reduced glutathione synthesis, enhanced free radical generation and disruption of glutathione-dependent antioxidant mechanisms.
The mechanisms of cell death triggered by glutathione depletion are of interest as they may reflect how oxidative injury actually kills cells and may lead to ways to intervene to prevent cell death. Early work provided evidence that this process was dependent upon the metabolism of arachidonic acid (Murphy et al. 1989b). Subsequent work by the Schubert group showed that this form of toxicity required the activation of 12-lipoxygenase (Li et al. 1997). Arachidonic acid metabolism is highly important in cells, and involves three major pathways for the transformation of arachidonic acid: the cyclooxygenase (COX) pathway of prostaglandin synthesis, the lipoxygenase (LOX) pathway of leukotriene synthesis (Katsuki and Okuda 1995), and a third pathway of arachidonic acid metabolism by cytochrome P 450 arachidonic acid monoxygenase (Capdevila and Falck 2002). Lipoxygenases are important not only for the lipid mediators that they produce, but also as a catalytic source of lipid peroxides (Brash 1999). LOX appears to be negatively regulated by glutathione, and positively regulated or activated by lipid peroxides. In fact, the lipid peroxide tone of the cell sets the activity of lipoxygenases. When glutathione levels fall, lipid peroxides rise because their accumulation is controlled by glutathione peroxidases, especially GPx4 (Seiler et al. 2008). LOX is activated by this elevation in peroxide tone and becomes a catalytic source for reactive oxygen species, specifically lipid peroxides. Why there should be such a positive feedback loop, whereby elevation in lipid peroxide tone activates LOX generating more lipid peroxides is unclear, especially given the deleterious effects of 12-LOX activation. Products of lipid peroxidation accumulate in perinatal WMI (Back et al. 2005b; Inder et al. 2002; Welin et al. 2007); and are toxic to developing OLs (Brault et al. 2004b). Relevance to human WMI is suggested by the recent demonstration of 12/15-LOX expression in premyelinating OLs and microglia in the diffuse (non-necrotic) component of WMI (Haynes and van Leyen 2013).
As previously discussed, developing OLs are highly sensitive to death induced by glutathione depletion, and this cell death is also dependent upon 12-LOX activity. Arachidonic acid itself is toxic to OLs, and this toxicity can be blocked by 12-LOX inhibitors (Wang et al. 2004). GPx4 specifically regulates the activity of 12-LOX, and in neurons, the death pathway activated by GPx4 down-regulation is dependent upon the translocation of AIF (Seiler et al. 2008). Studies by DeFranco and coworkers of the effects of GSH depletion in neurons and cell lines (Ho et al. 2008) and our own studies of the mechanism of peroxynitrite toxicity to OLs as well as neurons have shown that MAP kinases are involved in the toxicity of reactive nitrogen species (RNS) (Zhang et al. 2007; Zhang et al. 2006; Zhang et al. 2004). Together, these data indicate that oxidative injury may occur by activation of a regulated pathway that involves 12-LOX, MAP kinases, and AIF (Seiler et al. 2008; Zhang et al. 2007). Inhibiting 12-LOX may be beneficial in the prevention of injury following ischemia to the mature brain (Jin et al. 2008; Pallast et al. 2010; Pallast et al. 2009; Pekcec et al. 2012; van Leyen et al. 2008; van Leyen et al. 2006; Yigitkanli et al. 2012).
OL death induced by glutathione depletion is necrotic (Wang et al. 2004). The idea that necrosis can be a regulated form of cell death has been vigorously pursued (Degterev et al. 2005). A screen of inhibitors that blocked TNFα induced non-apoptotic cell death led to the discovery of necrostatin, which targets RIP1 kinase (Degterev et al. 2008; Hitomi et al. 2008). RIP1 kinase activity is considered to be required for the upstream signaling events inducing regulated necrosis, and there are multiple pathways that may take part in the destruction phase, including lipoxygenase.
Given the evidence that oxidative stress induced cell death in the OL lineage involves a program of cell death mediated by 12-LOX activation, the question arose whether this programmed cell death is a RIP1 kinase dependent form of cell death. Necrostatin (NEC-1) was found to be an effective inhibitor of OL death in vitro caused by cystine deprivation, inhibition of glutathione synthesis by buthionine sulfoximine, or exposure to arachidonic acid (Kim et al. 2010). In addition, necrostatin was very effective at blocking the production of reactive oxygen species, although it is not a free radical scavenger (Degterev et al. 2005). These results suggest that the 12-LOX pathway of injury in OLs is a necroptotic pathway, in the broadest sense that necroptosis is a regulated form of necrotic cell death. Whether RIP 1 kinase is in fact involved would require more rigorous studies to demonstrate that the effects of NEC-1 are due to on-target rather than off-target effects (Degterev et al. 2013; Takahashi et al. 2012; Vandenabeele et al. 2013). The relevance of necroptosis to some forms of neonatal cerebral gray matter injury is supported by studies in which NEC-1 was protective in a model of neonatal hypoxia-ischemia (Chavez-Valdez et al. 2012; Northington et al. 2011). The relative resistance of mature OLs to oxidative stress suggests that mature OLs may have improved mechanisms to maintain cellular redox homeostasis, most notably the maturation of anti-oxidant enzyme systems (Baud et al. 2004a; Baud et al. 2004b; Folkerth et al. 2004a).
Role of Glutamate Receptor-Mediated Toxicity in Acute WMI
Increased expression of the GluR4 subunit occurs between 23-32 weeks gestation in human parietal white matter and P7 rat corpus callosum (Follet et al. 2004). O4 antibody-labeled cells also express calcium permeable GluR2 lacking AMPA receptors (Fern and Moller 2000; Follet et al. 2004; Ong et al. 1996; Sanchez-Gomez et al. 2003; Sanchez-Gomez and Matute 2000). Focal expression of NMDA receptors on the processes of OL progenitors and mature OLs in vivo mediates a rapid, Ca2+-dependent, disintegration of OL processes under in vitro conditions that mimic ischemia, and NMDA receptors have been found on O4+ cells in developing human white matter (de Jesus Domingues et al. 2011; Jantzie et al. 2013; Karadottir et al. 2005; Micu et al. 2006; Salter and Fern 2005). The timing of calcium permeable AMPA type glutamate receptor expression and NMDA receptor expression during human and rat white matter development has been said to coincide with the window of heightened susceptibility to WMI (Jantzie et al. 2013; Talos et al. 2006a; Talos et al. 2006b). However, in these studies the intent was to identify glutamate receptors on O4+ cells, assuming that the O4 marker alone could be used to identify preOLs. It is well-known that immature OLs, which are identified by the expression of the O1 marker, also express the O4 antigen. The significance of these in vivo studies for understanding the pathogenesis of WMI, which appears to target the O4+ O1- preOL, therefore, remains uncertain.
Glutamate toxicity to preOLs in vitro is mediated by ionotropic glutamate receptors (iGluRs) of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate type (Borges et al. 1994; Fern and Moller 2000; Itoh et al. 2002; McDonald et al. 1998; Rosenberg et al. 2003; Sanchez-Gomez et al. 2011; Sanchez-Gomez and Matute 2000; Yoshioka et al. 1995). Although these in vitro studies suggest that calcium permeable AMPA receptors on preOLs might be important in WMI, there remains uncertainty about whether preOLs in vivo express this type of receptor. This issue is especially important because we know from studies of NMDA receptors in vivo (Karadottir et al. 2005; Salter and Fern, 2005; Micu et al. 2006) that anomalies in glutamate receptor expression may occur in vitro. Thus, although calcium permeable AMPA receptors may be expressed on preOLs in vitro, they may be absent from preOLs in vivo.
Excessive glutamate receptor activation during ischemia requires an elevation in extra-cellular glutamate and a concomitant glutamate loss from at least one cellular compartment. The potential sources of glutamate release to cerebral white matter include astrocytes, OLs, axons and cells of the choroid plexus. Mechanisms of glutamate release from axons (Kriegler and Chiu 1993; Stirling and Stys 2010) and astrocytes (Anderson and RA 2000; Bezzi et al. 2004; Domingues et al. 2010; Ye et al. 2003) have been defined, while oligodendroglia express Na-dependent glutamate transporters and a Xc− transporter, which are all potential sources of glutamate release during hypoxia-ischemia (Deng et al. 2003; DeSilva et al. 2009; DeSilva et al. 2007; Domercq et al. 1999; Fern and Moller 2000; Oka et al. 1993). There is evidence for significant release of astrocytic glutamate in isolated immature rat optic nerve during in vitro modeled ischemia (Wilke et al. 2004) as well as glutamate transport mechanisms in immature axons (Arranz et al. 2008). During perinatal hypoxia-ischemia, depletion of glutamate was most pronounced in axons and pre-myelinating stages of the OL lineage (Back et al. 2007). By contrast, astrocytes showed only a small loss of glutamate, which suggested that these cells were neither a significant source nor sink for glutamate in ischemic neonatal white matter. The pronounced depletion of glutamate observed in OLs and axons suggests that re-uptake mechanisms may be immature in the perinatal brain or dysfunctional during hypoxia-ischemia. Glutamate transport inhibition in the adult optic nerve in vivo resulted in excitotoxic degeneration of both OLs and axons mediated by AMPA and kainate receptor over-activation (Domercq et al. 2005).
Role of Glutamate Transporters in Glutamate-mediated Toxicity in Acute WMI
Glutamate transporters constitute a family of 5 genes, known in the human as EAAT1-5 (Danbolt 2001). The important transporters in the forebrain are EAAT1-3, known in the rat as GLAST, GLT-1, and EAAC1. GLAST and GLT-1 are primarily expressed in astrocytes. GLT-1 is the major transporter of the brain, and is expressed in axon terminals as well (Chen et al. 2004). EAAC1 is often called the neuronal transporter. All three transporters are expressed in OLs (DeSilva et al. 2009). The concept of a suicide loop in developing OLs has been suggested, whereby, in the setting of oxygen-glucose deprivation, cells release glutamate by reversal of glutamate transporters, which then acts upon glutamate receptors on the same cells to cause cell death. Evidence suggesting the existence of such a loop is that cell death could be blocked by glutamate receptor antagonists, and, importantly, also by an inhibitor of one particular glutamate transporter, GLT-1 (Fern and Möller 2000). Since all three glutamate transporters expressed in the forebrain are expressed in OLs, it is not clear why GLT-1 in particular is important for the release of glutamate in oxygen-glucose deprivation (OGD). Now that a specific inhibitor of GLAST is available (Jensen et al. 2009), it would be of interest to test whether blocking GLAST also blocks OGD induced OL death, in which case there would be nothing unique about GLT-1, or, whether only GLT-1 can fulfill this pathophysiological role.
Given these experimental data implicating glutamate transporters and specifically GLT-1 in hypoxic-ischemic injury to developing OLs, the expression of the human homolog EAAT2 and other glutamate transporters in developing white matter were investigated using in situ RNA hybridization. EAAT2 expression is developmentally regulated and was increased in immature white matter. Although strongly expressed in developing OLs in 32-week gestational age human brain, there was no detectable EAAT2 expression in OLs in 7 month old human brain. In contrast, EAAT1 and EAAT3 expression either remained constant or, in the case of EAAT3, increased in the more mature brain (DeSilva et al. 2007). A similar pattern was found in the rat, in which GLT-1 was expressed in developing OLs at P7, but no labeling was found in mature OLs (DeSilva et al. 2009). These data support the concept that a fatal feedback loop exists in developing OLs consisting of high expression of the glutamate transporter GLT-1/EAAT2 that provides a source of extracellular glutamate in the setting of energy failure. When activated, the GluR2 lacking AMPA receptors expressed on the same cells mediate excessive influx of calcium, which triggers excitotoxic injury and cell death. This model of excitotoxic injury in the immature brain therefore contrasts with that in the mature brain, and may be a cell-autonomous process in which certain populations of cells provide both the source and the target for pathological accumulations of glutamate. In the mature brain, the glutamate that is thought to kill neurons is derived from other cells—from excitatory terminals, or from neighboring astrocytes (Lipton and Rosenberg 1994). The existence of these cell-autonomous feedback loops may distinguish highly vulnerable targets in the developing brain and account for the particular patterns of cell death that characterize neonatal WMI. Another example of a cell-autonomous feedback loop may be layer V pyramidal cells in the developing cortex. In the last 5 years attention has been focused on the collateral neuronal damage that accompanies necrotic lesions in PVL (Pierson et al. 2007) with depletion of pyramidal neurons in layer V (Andiman et al. 2010). Glutamate receptor expression is not exclusive to layer V neurons in human cortex (Talos et al. 2006b), but it has been observed that layer V neurons, but not other neurons, heavily express EAAT2 (DeSilva et al. 2012). Therefore, the loss of layer V neurons in the setting of necrotic WMI might be seen as another example of the importance of feedback loops to explain selective vulnerability in the developing brain. Alternative explanations are conceivable, for example retrograde degeneration caused by axonal degeneration in chronic WMI.
Further in vivo studies are needed to verify the direct toxicity of glutamate to preOLs, other OL lineage stages as well as astrocytes (Domingues et al. 2010; Salter and Fern 2008; Shannon et al. 2007). Glutamate antagonists and other agents reduced ibotenate-mediated neonatal rodent white matter necrosis (Bemelmans et al. 2006; Dicou et al. 2003; Dommergues et al. 2000; Husson et al. 2002; Marret et al. 1995; Sfaello et al. 2005). NMDA and AMPA/kainate receptor antagonists prevented myelin loss after perinatal hypoxia-ischemia in the term equivalent rat (Follet et al. 2004; Follet et al. 2000; Manning et al. 2008). However, the efficacy of glutamate receptor antagonists to directly block degeneration of OL lineage cells and axons in vivo remains largely unstudied. Future studies are needed to define whether the protective effects of the AMPA/kainate receptor antagonists against myelin loss involve decreased degeneration of OL lineage cells and/or reduction in neuro-axonal degeneration. An additional unresolved question is whether AMPA/kainate receptor antagonists may cause cerebral hypothermia that might render cerebro-protection via an alternative mechanism.
Role of OL lineage Susceptibility in Acute Hypoxic-Ischemic WMI
The timing of appearance and spatial distribution of susceptible OL lineage cells appears to explain the magnitude and distribution of acute hypoxic-ischemic injury in several experimental models of WMI. The white matter of two day old preterm-equivalent rat pups, for example, displays a much greater susceptibility to hypoxia-ischemia than the white matter in 7 day-old near-term equivalent animals (Back et al. 2002a). A major developmental difference at these two ages is the extent of differentiation of the OL lineage. At postnatal day two, the rat white matter contains predominantly preOLs and, thus, resembles human cerebral white matter during the high-risk period for WMI (Craig et al. 2003). By day 5, in three different strains of rat, the white matter is populated mainly by a more differentiated population of immature OLs, and thus resembles near-term human (Dean et al. 2011). PreOLs are highly susceptible to hypoxiaischemia, whereas earlier and later OL stages are markedly more resistant (Back et al. 2002a; Riddle et al. 2006; Segovia et al. 2008). The enhanced susceptibility of preOLs in vivo is, thus, a stage-specific property that is independent of the postnatal age of the animal or the location of these cells in the forebrain. The increasing developmental resistance of the cerebral white matter to hypoxia-ischemia is related to the onset of preOL differentiation to pre-myelinating immature OLs that display reduced susceptibility to hypoxia-ischemia. Similar to reactive astrocytes, immature OLs in the white matter often display a robust reactive response to cerebral injury, which is common after hypoxia-ischemia at postnatal day 7 in the rat. This response coincides with gray matter injury and secondary injury to axons in the adjacent callosal white matter (Back et al., 2002a). Although reduced myelination has been reported after hypoxia-ischemia in the 5-day old rat, the mechanism is unclear, as these studies did not determine the extent of preOL and OL degeneration in vivo (Follet et al. 2000; Liu et al. 2002). Reduced myelination can occur secondary to neuro-axonal degeneration, which is a prominent feature in perinatal rat models of hypoxia-ischemia between postnatal day 2-7 (Selip 2012; Sizonenko et al. 2005; Sizonenko et al. 2003) and in more severe hypoxia-ischemia in fetal sheep (Petersson et al 2002). Cerebral hypoxic-ischemic injury in rodents is typically more severe than in human where substantial necrotic neuro-axonal injury is mostly associated with cystic WMI, which now occur infrequently.
Caspase-mediated mechanisms of apoptosis contribute at least partially to acute preOL death from hypoxia-ischemia (Back et al. 2002a; Cao et al. 2003; Castillo-Melendez et al. 2004; Ness et al. 2001). The magnitude of caspase-activation differs among studies and appears to be related to the severity of the hypoxic-ischemic insult. For example, in a perinatal rodent model of moderate hypoxia-ischemia, the majority of preOLs degenerated acutely by a mechanism of apparent necrosis that did not involve caspase-3 activation (Back et al. 2002a; Segovia et al. 2008). By contrast, in the preterm sheep fetus, preOL degeneration more commonly involved activation of caspase-3 (Riddle et al. 2006). Similar observations were made in late-gestation sheep (Cao et al. 2003; Castillo-Melendez et al. 2004). Hence, a less severe insult may result in a combination of apoptosis and necrosis whereas more severe insults may be biased toward mostly necrosis (Cheng et al. 1998; Han et al. 2000). The relative extent of apoptotic and necrotic preOL degeneration in most models is difficult to establish due to the lack of histological markers of necrosis. Recent ultrastructural studies identified a spectrum of cell death in O4 antibody-labeled cells that ranged from necrosis to apoptosis (Alix et al. 2012).
Relative Contributions of Hypoxia-Ischemia and OL Lineage Immaturity to Acute WMI
The preterm fetal sheep (0.65 gestation) displays heterogeneous OL lineage maturation in frontal periventricular white matter (Riddle et al 2006), which allowed us to define the relative contributions of oligodendroglial maturational factors and vascular factors to acute WMI. OL lineage maturation in medial periventricular white matter (PVWM) was similar to human (~23-28 weeks gestation) in that preOLs were the major OL stage present. By contrast, lateral PVWM was more differentiated and contained predominantly pre-myelinating and early myelinating immature OLs. Surprisingly, moderate cerebral ischemia did not uniformly damage the PVWM. The medial and lateral PVWM sustained differing degrees of acute injury even though they sustained a similar degree of low flow during prolonged ischemia-reperfusion. Hence, while global ischemia was necessary for WMI, no regional differences in blood flow were found within the PVWM under basal or ischemic conditions to account for the differences in cell death between medial and lateral PVWM. Rather, differences in the topography of WMI were closely associated with the distribution of vulnerable preOLs. Interestingly, in regions of preOL degeneration, other neural cell types (astrocytes, microglia and axons) were markedly more resistant to injury.
Recently, in a fetal rabbit model of placental insufficiency, significant global hypoxiaischemia caused minimal WMI at fetal day 22, but a similar insult three days later in gestation caused pronounced WMI (Buser et al., 2010). The differences in susceptibility of the white matter at these two developmental ages can be accounted for by the appearance of susceptible preOLs at fetal day 25. Taken together, these findings suggest that perturbations in cerebral blood flow are necessary but not sufficient for WMI. The developmental predilection for WMI appears to be related to both the timing of appearance and regional distribution of susceptible preOLs. These findings predict that some near-term infants with delayed OL differentiation and myelination might also be more susceptible to WMI. Interestingly, a more variable degree of WMI was detected in near term sheep after several insults (Clapp III et al. 1988; Ikeda et al. 1999; Mallard et al. 1998; Ohyu et al. 1999; Penning et al. 1994; Raad et al. 1999). Moreover, near term and term infants with congenital heart disease are also at high risk for WMI (McQuillen and Miller 2010). Several clinical and MRI studies support the notion that this susceptibility relates to a delay in brain maturation (Dimitropoulos et al. 2013; Licht et al. 2009; Limperopoulos et al. 2010; Miller 2007). Hence, the targeted death of preOLs from hypoxiaischemia or inflammatory mediators could contribute to the pathogenesis of acute WMI across a broad range of gestational ages and regions of white matter.
Chronic WMI: Myelination Failure and Susceptibility of OLs and Axons Limitations of Current Approaches to Study Myelination Failure
The propensity for disturbances in OL lineage maturation and myelination is the central feature that distinguishes chronic WMI in preterm survivors from other forms of neonatal cerebral injury. Early histopathological descriptions of myelination disturbances analyzed cases where cystic necrotic WMI was associated with diffuse white matter astrogliosis (Billiards et al. 2008; Iida et al. 1995). Myelin loss in association with necrotic foci is related to axonal degeneration and loss of all glial elements. In the studies of Billiards et al. cited above, a third of the cases (6/18) were reported to have chronic foci with cavitation. In the remainder of the cases, the necrosis rarely exceeded 1 mm in diameter and thus could be considered “microcysts” that are typically undetected by MRI. Thus, it is unclear whether these cases are significantly different than current cases of WMI where cystic necrosis occurs in about 5% of cases. More recent MRI studies have analyzed contemporary cohorts of preterm survivors that mostly lack cystic necrotic lesions that exceed 1 mm in diameter. These studies have commonly defined focal or diffuse forms of WMI that were attributed to abnormal “myelination.” Since these studies lack histopathological correlation, the cellular basis for the signal abnormalities in the white matter remains unclear. For example, in subjects with these milder forms of WMI, fractional anisotropy (FA) did not increase in central white matter tracts relative to normal infants (Huppi et al. 2001; Ment et al. 2009; Miller et al. 2002), and this was attributed to abnormal myelination. However, experimental studies in a preterm fetal rabbit model of global cerebral ischemia (Derrick et al. 2004; Drobyshevsky et al. 2007a; Drobyshevsky et al. 2007b) found that the most rapid rise in FA normally occurs prior to myelination at a time when pre-myelinating immature OLs increase in number in central white matter tracts and axons show maturational changes (Drobyshevsky et al. 2005). These experimental findings suggest that MRI-defined WMI in contemporary cohorts of patients may be related to disturbances in OL lineage maturation rather than axonal degeneration, but histopathological confirmation in human is needed.
To define cellular mechanisms of MRI-defined WMI, registration algorithms were developed that permitted MRI data to be aligned at high resolution with the corresponding histopathological data from the same white matter regions (Riddle et. al., 2011). WMI was generated by cerebral ischemia in a preterm fetal sheep model that closely reproduces the spectrum of WMI seen in human preterm survivors (Back et. al. 2012). We identified the histopathological features of three classes of MRI-defined chronic WMI (Figure 2). Each lesion type displayed unique astroglial and microglial responses that corresponded to distinct forms of necrotic or non-necrotic injury. At 1 week after injury, high field MRI (12 Tesla) identified a novel hypo-intense signal abnormality on T2-weighted images that corresponded to diffuse WMI characterized by astrogliosis and myelination disturbances related to arrested maturation of preOLs (Fig. 2; upper panel). This was the major form of WMI identified and comprised nearly 90% of the total volume of WMI. A second minor form of necrotic WMI corresponded to focal hyperintense signal abnormalities on MRI (Fig. 2; middle panel). A third minor form of WMI comprised small foci of necrosis (microcysts) typically less than a millimeter in diameter (Fig. 3; lower panel). Microcysts are not detected at clinical MRI field strengths (1.5 or 3T), but have been resolved by microscopic pathology studies (Pierson et. al., 2007; Buser et. al., 2012). These findings suggest that current clinical MRI field strengths have limited sensitivity to detect early diffuse WMI as well as small foci of necrosis where axonal degeneration occurs.
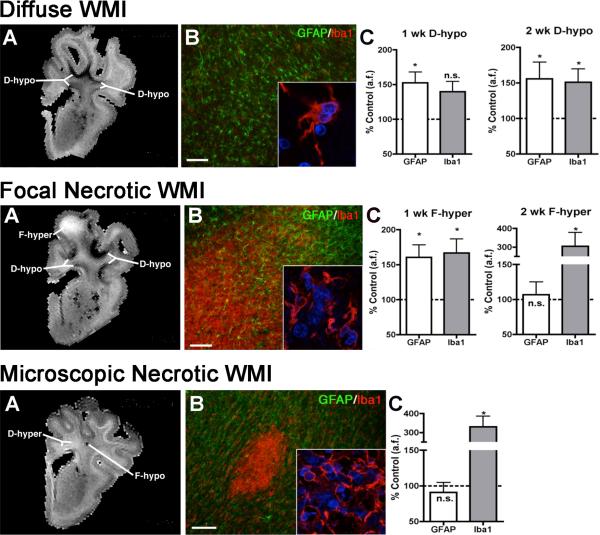
Figure 2.
Three forms of high field MRI-defined perinatal WMI with corresponding histopathological features that were generated in the 0.65 gestation fetal sheep brain at 1 or 2 weeks after global cerebral ischemia (adapted from: Riddle et al., 2011).
Upper Panel. Diffuse WMI in chronic lesions (A) Representative appearance and distribution of diffuse hypointense (D-hypo) lesions seen on a T2w image at 1 week after injury. (B) Diffuse WMI had pronounced astrogliosis defined by immunohistochemical staining of reactive astrocytes with glial fibrillary acidic protein (GFAP; green) and a lesser population of Iba1-labeled microglia/macrophages (red) with a reactive morphology (inset). Nuclei in the inset are visualized with Hoechst 33342 (blue). (C) Quantification of GFAP-labeled astrocytes and Iba1-labeled microglia within MRI-defined WM signal abnormalities at 1 and 2 weeks after global ischemia. The D-hypo lesions had significantly elevated GFAP, consistent with a diffuse astrogliotic response to injury. * p<0.05, n.s., not significant. Bar in B, 100 μm.
Middle Panel. Focal Necrotic WMI. (A) Representative appearance from the largest focal hyperintense (F-hyper) lesion seen on a T2w image at 1 week after injury. These lesions typically localized to subcortical white matter. Note the substantial difference in the F-hyper lesion relative to the diffuse gliotic lesions, which appears much more hypointense (D-hypo). (B) A typical macroscopic necrotic lesion defined by diffuse dense staining for reactive microglia and macrophages with Iba1 (red and inset) and a paucity of GFAP-labeled astrocytes. Nuclei in the inset are visualized with Hoechst 33342 (blue). (C ) F-hyper lesions displayed a progressive decrease in GFAP staining and markedly increased Iba1 labeling for microglia by 2 weeks after global ischemia. * p<0.05. Bar in B, 100 μm.
Lower Panel. Microscopic necrotic WMI. (A) Representative appearance of a focal hypointense (F-hypo) lesion seen on a T2w image at 2 weeks after injury. Note the substantial difference in the F-hypo lesion relative to a diffuse gliotic lesion at 2 weeks, which appears more hyperintense (D-hyper). (B) A typical microscopic necrotic lesion defined by a discrete focus of immunohistochemical staining for reactive microglia and macrophages with Iba1 (red and inset) and a paucity of staining for astrocytes with glial fibrillary acidic protein (GFAP; green). Nuclei in the inset are visualized with Hoechst 33342 (blue). (C) F-hypo lesions had markedly increased Iba1 labeling and no significant difference in GFAP labeling vs. control. * p<0.05. Bar in B, 100 μm.
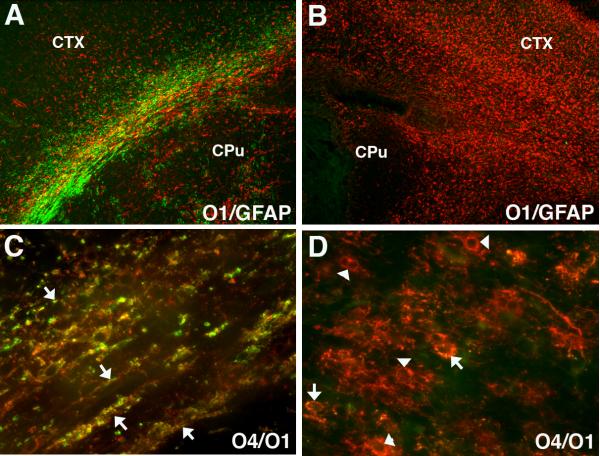
Figure 3.
Numerous late oligodendrocyte progenitors (preOLs) accumulate in chronic myelin-deficient perinatal white matter lesions. Lesions were generated in response to unilateral hypoxia-ischemia in the postnatal day 3 rat with the contralateral hemisphere serving as control (see Segovia et al., 2008). (A) Normal early myelination (O1-antibody; green) in control subcortical white matter (corpus callosum/external capsule) at P10 is seen with low levels of GFAP-labeled astrocytes (red) mostly concentrated over the white matter. (B) Absence of myelin in the contralateral post-ischemic lesion coincided with a diffuse glial scar that stained for GFAP-labeled astrocytes. (C) Early myelination in control white matter at P10 with sheaths (yellow) double-labeled for O4 and O1 antibodies. (D) Absence of myelin in the contralateral lesion coincided with clusters of preOLs (O4+O1-) in maturation arrest (red; arrowheads). Such dense clusters of preOLs are not normally seen in control white matter and are consistent with the pronounced proliferative state that is triggered in response to injury. Peak preOL density can expand roughly 4-fold relative to control. Oligodendroctes (yellow; arrows; O4+O1+) are rarely seen in the lesions. Abbreviations: CTX, cerebral cortex; CPu, caudate putamen.
Role of Axonal Injury in Myelination Failure
The major cell types that may contribute to myelination failure in WMI are the axon and OL lineage cells. During development, small premyelinated axons are particularly susceptible to glutamate-mediated excitotoxicity at sites of contact with OL processes and involve both N-methyl-D-aspartic acid (NMDA) receptors and non-NMDA glutamate receptors (Alix and Fern 2009; Fern et al. 1998). Glutamate-mediated axonal injury appears related to a mechanism of excessive glutamate depletion from OLs and axons (Fern et al. 1998; Fern and Moller 2000; Salter and Fern 2005; Wilke et al. 2004), which appear to be the major sources of extracellular glutamate during energy failure from hypoxia-ischemia (Back et al. 2007). Recently, it was shown that axons also display maturation-dependent vulnerability to oxidative stress and hypoxia-ischemia (Alix et al. 2012). Larger caliber axons, which are preparing to myelinate, are particularly susceptible to injury via axolemma-associated voltage-gated calcium channels, in contrast to smaller caliber unmyelinated axons, which are more resistant.
Axonal injury is a prominent feature of necrotic WMI (Haynes et al. 2008; Kinney and Back 1998). Such necrotic lesions often contain dystrophic axons and axonal spheroids, which degenerate during the early phase of coagulative necrosis (Banker and Larroche 1962; Deguchi et al. 1997; Hirayama et al. 2001; Marin-Padilla 1997). Recent studies support that necrotic lesions are a minor component of WMI in both sheep and human and comprise only about 5% of the total burden of WMI (Buser et al. 2012; Riddle et al. 2011).
Primary axonal injury does not appear to be a major feature of diffuse WMI that occurs during the pre-myelinating phase of white matter development. Diffuse WMI is the major form of WMI seen in preterm survivors (Buser et al. 2012). It is highly enriched in reactive astrocytes and microglia that overlap with areas of myelination failure (see below). During the acute phase of diffuse WMI in preterm fetal sheep, acute axonal injury was low and preOLs were the major cells that degenerated (Riddle et al. 2006). During the chronic phase of WMI from this same fetal sheep preparation (Riddle et al. 2011), no significant axonal degeneration, axonal loss or shift in the distribution of axon calibers was observed by quantitative electron microscopy studies (Riddle et al. 2012). Axonal injury has been observed in regions of chronic human diffuse WMI that are not within regions of necrosis (Haynes et al. 2008), but these dystrophic axons are likely to be related to the degenerating axons in necrotic foci. Hence, the major sites of axonal degeneration are necrotic lesions and axons appear to be mostly intact in diffuse WMI.
Responses of OL Lineage Cells in Chronic Myelination Failure
As discussed above, acute WMI involves pronounced selective degeneration of pre-OLs, with sparing of other neural cell types. These findings, thus, supported the hypothesis that myelination failure arises from a persistent deficit of pre-OLs (Back and Volpe 1997). However, two related sets of observations later challenged this hypothesis and suggested the alternative hypothesis that myelination failure involves a potentially reversible process that is linked to arrested pre-OL maturation in chronic WM lesions. First, in adult WMI, reactive astrocyte-derived hyaluronic acid (HA) accumulates in diffuse WMI and reversibly inhibits preOL differentiation and myelination (Back et al. 2005a; Preston et al. 2013; Sloane et al. 2010). Secondly, despite substantial acute and delayed preOL degeneration after hypoxia-ischemia, surviving preOLs either were not depleted (Billiards et al. 2008) or in preterm-equivalent rats rapidly increased in number to regenerate depleted preOLs (Segovia et al. 2008; Wright et al. 2010; Zhiheng et al. 2009). Significant delayed apoptotic preOL degeneration occurred during the first week after cerebral hypoxia-ischemia in rats, but there was a net increase in total preOLs in chronic lesions due to pronounced expansion of the preOL pool (Segovia et al. 2008). This preOL expansion appeared to be driven mostly by OL progenitors that proliferated locally at the sites of WMI (Segovia et al. 2008) or cortical injury (Sizonenko et al. 2008) rather than from the subventricular zone, where less robust generation of OL lineage cells has been observed (Felling et al. 2006; Yang and Levison 2006; Zaidi et al. 2004). In contrast to normal myelinating white matter (Fig. 3A, C), chronic WMI was characterized by a striking absence of myelinating cells in lesions highly enriched in diffuse reactive astrogliosis (Fig. 3B). However, these lesions were highly enriched in preOLs, but immature OLs were rarely detected (Fig. 3D). Similarly, in preterm fetal sheep (Riddle et al. 2011), preOL expansion was accompanied by a significant blunting of OL maturation by 2 weeks after ischemia, despite a 50% increase in controls. In sheep and human (Buser et al. 2012), progressive preOL accumulation and maturation arrest were significantly associated with the magnitude of astrogliosis, consistent with the notion that arrested preOL differentiation was related to factors derived from reactive gliosis.
PreOL expansion thus compensates for preOL death, but surviving preOLs display persistent arrested differentiation in chronic gliotic lesions. Presently unclear is the temporal evolution of impaired myelination in chronic WMI. What is the period over which the glial scar remodels and preOL arrest persists? Discussed below is the role of potential inhibitory factors that mediate preOL arrest. It is unknown whether myelination is delayed or permanently arrested. Such information is of critical importance to define the potential for regeneration and repair of injured white matter. Recent studies support that viable OLs and myelination are critical for axon survival (Lee et al. 2012), raising the possibility that preOL arrest could also adversely affect the functional integrity of axons in chronic lesions.
Molecular Mechanisms of PreOL Maturation Arrest and Myelination Failure
A number of molecular mechanisms have been identified that delay maturation of the OL lineage (Fancy et al. 2011b; Franklin and Gallo 2014; Kotter et al. 2011). These mechanisms have important therapeutic relevance not only for neonatal WMI, but for a wide variety of disorders of myelination failure where preOL arrest is also implicated—including multiple sclerosis (Preston et al. 2013) and vascular dementias (Back et al. 2011). A complex array of intrinsic, extrinsic and epigenetic factors regulate OPC cell cycle exit, OL lineage progression and myelination (Barres et al. 1993; Emery et al. 2009; Fancy et al. 2010; Silbereis et al. 2010; Sizonenko et al. 2007) (see accompanying article, Franklin and Gallo). Briefly, inhibitors of voltage-activated potassium channels and membrane depolarization block proliferation and differentiation of OL progenitors (Ghiani et al. 1999; Knutson et al. 1997). Sox17 expression regulates cell cycle exit in OPCs, transgenic overexpression promotes OL differentiation (Ming et al. 2013), and enhanced Sox17 expression occurs in OLs in active remyelinating lesions (Moll et al. 2013). Numerous genes are activated by oxidative stress that regulate OL maturation, and oxidative stress promotes global histone acetylation, which may block OL differentiation (French et al. 2009). Post-transcriptional control by microRNAs regulates OL differentiation and OPCs that lack mature microRNAs display arrested maturation (Dugas et al. 2010; Shin et al. 2009). Insulin-like growth factors (IGFs) promote OL lineage cell survival and myelination and protect against OL progenitor loss in fetal and neonatal models of WMI (Guan et al. 2001; Pang et al. 2010b; Wood et al. 2007). Bone morphogenetic proteins both repress OL differentiation and regulate myelin protein expression (See et al. 2004; See and Grinspan 2009). Constitutive activation of the Wnt/beta-catenin pathway in vivo also delays the differentiation of OL progenitors to mature myelinating OLs (Fancy et al. 2011a; Feigenson et al. 2009; Ye et al. 2009).
In human lesions from neonatal WMI (Buser et al. 2012), patients with multiple sclerosis (Back et al. 2005a; Preston et al. 2013) and traumatic spinal cord lesions (Struve et al. 2005), the extracellular matrix (ECM) is a rich source of hyaluronic acid (HA) and one of its receptors, CD44. During chronic human neonatal WMI (Buser et al. 2012), CD44-positive reactive astrocytes synthesize high molecular weight HA (i.e., >106 Da), a non-sulfated, protein-free glycosaminoglycan that accumulates in the ECM (Asher et al. 1991). HA synthases extrude HA molecules into the ECM where they have distinct activities depending on their size (Sherman and Back 2008).
Arrest of preOL maturation is stimulated both in vitro and in vivo by high molecular weight forms of HA (Back et al. 2005a; Sloane et al. 2010) that are digested to bioactive forms by a CNS enriched hyaluronidase, PH20, that is GPI-anchored and has both neutral and acidic pH optima (Preston et al. 2013). PH20 is expressed by OPCs and reactive astrocytes and its expression is particularly elevated in demyelinated lesions. Overexpression of PH20 inhibits preOL differentiation in vitro and HA fragments generated by PH20 block re-myelination in vivo. Pharmacological inhibition of PH20 promotes OL maturation in vitro and myelination in vivo, which is accompanied by enhanced nerve conduction.
Animal Models of WMI Related to Hypoxia
Although hypoxia-ischemia is a major cause of human WMI, the next two sections will review mechanisms through which isolated hypoxia or hyperoxia may contribute independently to disturbances in OL lineage progression and myelination in preterm white matter. Fetal hypoxemia activates a variety of hypoxia-responsive genes that modify responses to oxidative stress (Gunn and Bennet 2009; Trollmann and Gassmann 2009), alters glutamate homeostasis (Fontaine et al. 2008b; Henderson et al. 1998; Lee et al. 2010) and triggers disturbances in oxidative metabolism that affect fetal growth, development and behavior (Henderson et al. 1998; Ireland et al. 2010; Iwamoto et al. 1989; Penninga and Longo 1998; Richardson et al. 1993).
The response of the fetus to acute hypoxemia without ischemia has been extensively studied in preterm fetal sheep. The late gestation fetus is able to adapt to mild to moderate reductions in oxygen tension through a compensatory increase in blood pressure (Gunn and Bennet, 2009). This occurs via a rapid peripheral vasoconstriction that preferentially redirects blood flow and greater oxygen to the brain. The responses of the preterm fetus to acute hypoxia have been studied in preparations where the pregnant ewe inspires a modified air mixture with reduced oxygen content, which renders the fetus hypoxic via placental hypoxemia. Transient hypoxemia triggered a variable amount of white and gray matter injury in midgestation or near term fetal sheep (Penning et al. 1994; Rees et al. 1999; Rees et al. 1997). However, WMI was notably more severe when significant hypotension was observed, which resulted in concomittant cerebral hypoxia and ischemia (Ting et al. 1983).
The near term fetal sheep even appears to fully adapt to chronic hypoxia, albeit with reduced somatic growth. Mid-gestation chronic hypoxemia, as a consequence of chronic placental insufficiency, resulted in widespread disturbances in hippocampal, cerebellar and white matter development (Penning et al. 1994; Rees et al. 1999). A rodent model of chronic placental insufficiency generated by unilateral uterine-artery ligation during late embryonic development resulted in focal white matter injury with reduced myelination (Fontaine et al. 2008a; Olivier et al. 2007; Olivier et al. 2005).
The most widely used postnatal model of chronic hypoxia is the chronic sub-lethal hypoxia model where preterm equivalent neonatal mice continuously inspire 9.5% or 11% oxygen from P3 to at least P11 (Ment et al, 1998). This model was designed to study mechanisms related to neurodevelopmental disabilities associated with chronic lung disease (CLD) (Gray et al. 1995), previously known as bronchopulmonary dysplasia (BPD). CLD is a common disorder of chronic lung immaturity in preterm survivors that is associated with chronic hypoxemia. The actual levels of hypoxemia/ blood oxygen saturation achieved with this model have not been established, due to the challenges of obtaining sufficient blood under hypoxic-conditions from a neonatal mouse to measure arterial blood oxygenation. Moreover, given the protracted nature of the chronic hypoxia, serial measurements over the course of the hypoxic exposure period are clearly needed, since even the physiological responses to brief intermittent hypoxia are known to vary with the maturational state of the animal. Brief intermittent hypoxic exposure in the P2 mouse produces more variable but less severe oxygen desaturation than under the same conditions at P6 (Cai et al, 2011). Theoretical models predict that chronic sublethal hypoxia may result in blood oxygen saturation and content that are perhaps less than half of normal (~40-50%), but these predicted values may be influenced by species-dependent factors (Miller and Granger, 1982). 20-day old kittens exposed to chronic hypoxia (10% oxygen; balanced nitrogen) underwent serial carotid blood sampling during hypoxia and had a decrease in PaO2 that decreased to ~65% of inspired room air values (Baker and McGinty, DJ, 1977). Many compensatory physiological responses may be triggered by chronic hypoxia that include fluctuations in acid-base state, hemoglobin content, cerebral capillary density, lactate levels, core body temperature, metabolic rate, cerebral blood volume, minute ventilation and sympathetic tone---all of which may contribute to the variable responses to hypoxia as the animal matures. Given the lack of detailed physiological characterization of the model, its clinical relevance for human WMI remains to be defined.
Chronic hypoxia results in a spectrum of somatic and cerebral growth retardation (Back et al. 2006b; Farahani et al, 2008; Ment et al, 1998) that appears related to the severity of chronic hypoxia. There are also disturbances in white matter development that include ventriculomegaly, reduction in white and gray matter volumes, reduced myelin basic protein (MBP) expression, and diminished total axon volume (Back et al. 2006b; Ment et al, 1998; Kanaan et al, 2006; Jablonska et al, 2012; Scafidi et al., 2013). However, these CNS abnormalities are not observed in children with CLD in whom other neurological complications are excluded. Overt features of WMI in the human premature infant, such as preOL degeneration and reactive gliosis are also not observed in this model.
The disturbances in murine white matter development induced by chronic hypoxia are also reproduced by treatment of rats with an A1 adenosine receptor (A1AR) agonist during early postnatal life (Turner et al. 2002). Consistent with the notion that A1ARs play a role in hypoxia-induced disturbances in brain growth, hypoxia-induced ventriculomegaly is reduced and white matter preserved in mice lacking A1ARs (Turner et al. 2003). Similar effects are also seen when elevated circulating levels of adenosine occur due to deficiency of adenosine deaminase (Turner et al. 2003). These mice have circulating levels of adenosine that are 100-fold higher than control animals (Blackburn et al. 1998; Blackburn et al. 1995). In further support of the notion that adenosine contributes to hypoxia-induced white matter disturbances, caffeine also prevented hypoxia-induced delayed myelination and brain injury (Back et al. 2006b). Caffeine is a non-selective adenosine antagonist that has also been shown to reduce hypoxic-ischemic injury in neonatal mice (Bona et al. 1995; Bona et al. 1997). In preterm infants, caffeine exposure was associated with improved white matter development as assessed by diffusion-weighted MRI (Doyle et al. 2010). Although clinical data are not entirely consistent, neonatal caffeine therapy was associated with improved motor function in survivors of premature birth (Schmidt et al. 2012).
Recent additional studies have begun to resolve the cellular and molecular mechanisms related to the impaired myelination observed in neonatal rodents after chronic exposure to hypoxia between P3 to P11 (Jablonska et al., 2012). Interestingly, no myelination abnormalities are observed in this model at the conclusion of hypoxia at P11. However, multiple myelin-associated proteins are significantly but transiently reduced within a week after hypoxia (P18). In contrast to the response to hypoxia-ischemia, which targets preOLs, the chronic hypoxia triggered delayed apoptosis of OLs that persisted at least until P18. In chronic survivors at P45, myelin protein levels and the total number of mature CC1+ OLs were significantly elevated, consistent with the generation of new OLs, which was mediated via the FoxO1/p27kip1 pathway. Despite recovery of OLs, chronic structural myelin abnormalities and functional deficits persist in this model (Jablonska et al. 2012; Scafidi et al. 2013).
In contrast to the injury responses to hypoxia-ischemia, exposure to chronic hypoxia does not trigger reactive astrogliosis (Back et al., 2006), but does disrupt glutamate transporter function in astrocytes and JAK/STAT signaling in neonatal white matter (Raymond et al. 2011). Inhibitors of the JAK/STAT pathway also partially reproduced the decreased expression of GLAST and GLT-1 induced by chronic hypoxia.
Mechanisms of WMI Related to Hyperoxia
During brain development, oxidative stress results from the generation of injurious reactive species or oxidants as a consequence of either ischemia-reperfusion or hyperoxia (Blomgren and Hagberg 2006; Perrone et al. 2010). Hyperoxia is a potential complication of neonatal resuscitation and ventilation of the preterm infant during intensive care. Several in vitro studies demonstrated maturation-dependent vulnerability of the OL lineage to hyperoxia where preOLs were more susceptible than mature OLs to caspase-dependent cell death triggered by oxidative stress (Gerstner et al. 2006; Gerstner et al. 2008; Gerstner et al. 2007; Koch et al. 2008). A more recent in vivo analysis of the response to hyperoxia found that a transient reduction in myelination was restored within a week after exposure (Schmitz et al. 2011). A detailed OL lineage analysis found that hyperoxia acutely caused a reduction in total OLs labeled with Olig2 that was related to degeneration of early OL progenitors (NG2+) rather than preOLs. This cell death response was followed by a proliferative response of NG2+ progenitors that resulted in a pronounced early expansion in the pool of mature CC1+ OLs in the white matter. Despite restoration of the mature OL pool, MRI diffusion studies found chronic changes in radial diffusivity in adult white matter consistent with disturbances in myelination. Ultrastructural studies of myelin were not performed. Susceptibility of NG2+ progenitors to hyperoxia was related to early disturbances in glutamate transporter expression and function. Moreover, hyperoxia-exposed astrocytes conferred less in vitro protection to glutamate-mediated OL progenitor degeneration.
Infection, Inflammation and Cerebral WMI
Both experimental and clinical studies have found that neuroinflammatory mediators are detected in association with WMI (Ellison et al. 2005; Folkerth et al. 2004b; Hagberg et al. 2002; Kannan et al. 2012; Lin et al. 2010; Sadowska et al. 2012; Wang et al. 2009; Yoon et al. 1997; Yoon et al. 1996) and can disrupt white matter development in developing animals (Favrais et al. 2011). Necrotizing enterocolitis, which is associated with the release of inflammatory mediators, also has a strong association with WMI (Shah et al. 2008; Volpe 2008b). Recent human studies support that whereas postnatal infections are associated with increased risk for WMI, prenatal risk factors for infection such as chorioamniotis do not correlate with increased risk (Chau et al. 2009; Glass et al. 2008a; Kaukola et al. 2006; Shah et al. 2008).
Inflammatory mediators can promote WMI through vasoactive mechanisms that involve hypoxia-ischemia or via direct or indirect toxicity to OLs or their precursors in developing white matter (reviewed in: Back 2006a). Activated microglia release a number of cytokines that are toxic to cells of the OL lineage (Kaur and Ling 2009). The microglial products TNF-α and interferon-γ (INF-γ) are toxic to OL precursors and mature OLs (Kim et al. 2011; McLaurin et al. 1995; Merrill 1991; Selmaj and Raine 1988; Sherwin and Fern 2005; Vartanian et al. 1995). This toxicity is synergistic (Agresti et al. 1996) and appears to involve mediation by other glia. Astrocytes, for example, modify the mechanisms of microglial-mediated TNF-α toxicity (Li et al. 2008). INF-γ immunopositive cells localize to necrotic foci of PVL (Folkerth et al. 2004b). Indirect toxicity of bacterial endotoxin to OL progenitors occurs via activation of toll-like receptor 4 on activated microglia (He et al. 2010; Lehnardt et al. 2002; Pang et al. 2000; Patrizi et al. 2000) and is mediated by peroxynitrite in the absence of astrocytes (Li et al. 2005). Astrocytes modify endotoxin-induced, microglia-dependent toxicity to preOLs, which is not mediated by peroxynitrite, but is TNFα-dependent (Li et al. 2008). Maternal exposure to endotoxin, without direct fetal exposure, increases CNS microglial activation in the fetus (Hutton et al. 2008). Several microglial-derived inflammatory cytokines have been identified that lead to damage to cerebral white matter through peroxynitrite-mediated toxicity to the OL lineage (Cai et al. 2003; Li et al. 2005; Lin et al. 2004; Olivier et al. 2010; Pang et al. 2010a). Endotoxin further exacerbates WMI via peroxisomal dysfunction and related oxidative stress (Paintlia et al. 2004; Paintlia et al. 2008). Further studies in relevant animal models of WMI are needed to define how neuroinflammatory mediators and cerebral ischemia may interact to enhance or reduce both the acute and delayed phases of WMI (Czeh et al. 2011).
Comparison of Animal Models of Human WMI that Involve Disturbances in Cerebral Oxygenation
Table 1 summarizes major salient features of small and large pre-clinical animal models developed to study the pathogenesis of neonatal WMI related to three distinct disturbances in cerebral oxygenation: hypoxia-ischemia, hypoxia or hyperoxia. As previously reviewed (Back et al., 2012), lissencephalic rodent models have significant limitations for modeling human gyrencephalic WMI. These include a paucity of cerebral white matter, substantial differences in cerebral blood flow and metabolism and a greater susceptibility to concomitant cerebral gray matter injury in hypoxia-ischemia models. Despite these limitations, rodent models are often an attractive system in which to generate initial observations for subsequent validation in larger preclinical animal models that more closely resemble human. Of the large pre-clinical animal models, instrumented fetal sheep preparations have been most widely studied due to their close similarity to preterm human cerebral development (Back et al. 2012). They also offer greater viability and less expense compared to preterm non-human primates that also generate a spectrum of WMI similar to human (Griffith et al. 2012). Preterm fetal sheep hypoxia-ischemia preparations generate a spectrum of WMI similar to human. These similarities include the heightened vulnerability of the preOL in early diffuse WMI, arrested preOL maturation in chronic lesions enriched in reactive astrocytes and microglia and degeneration of all cellular elements in chronic foci of necrosis. WMI is also accompanied by reduced cortical and subcortical gray matter volumes, which are related to dendritic arborization disturbances without diffuse neuronal degeneration (Dean et al., 2013; McClendon et al., 2014).
Table 1.
Major Features of Animal Models Developed to Study WMI Related to Hypoxia-Ischemia, Hypoxia or Hyperoxia.
| INSULT | CLINICAL CONDITIONS ASSOCIATED WITH THE INSULT |
MAJOR HUMAN NEURO- PATHOLOGICAL FEATURES |
CHRONIC HUMAN NEUROLOGICAL SEQUELAE |
RELEVANT ANIMAL MODELS |
MAJOR CELL TYPES WITH ACUTE OR DELAYED CELL DEATH |
CHRONIC GLIAL REACTIVE RESPONSES |
DELAYED MYELINATION |
MYLELNATION / FUNCTIONAL RECOVERY |
|---|---|---|---|---|---|---|---|---|
| HYPOXIA-ISCHEMIA | •complications of prematurity •congenital heart disease |
•focal microscopic necrosis •focal cystic necrosis •diffuse WMI with preOL arrest •cerebral gray matter volume loss |
•cerebral palsy •Disabilities in: - cognition/learning -attention -socialization |
•neonatal P2 rodents (Vannucci model variant) •fetal rabbit •fetal sheep |
•PreOL necrotic>apoptotic death in diffuse WMI •Axons and all glia in necrotic foci |
•OL progenitor proliferation and preOL arrest •diffuse astrogliosis and microgliosis |
• delayed myelination in human, rats and sheep | • degree of myelin recovery in human and animal models is not defined |
| ACUTE or CHRONIC HYPOXIA | • chronic lung disease (CLD) - formerly, bronchopulmonary dysplasia | • no specific forms of pathology | • cognitive/learning disabilities may occur independently of other causes of WMI | •fetal sheep •neonatal mouse models (P3 to P11 11% O2) and saturation of ~40-50% O2 |
•diffuse mature OL apoptosis •PreOLs and axons spared •no necrotic foci |
•no astrogliosis or microgliosis •altered glial glutamate transport (GLAST/GLT-1) |
•early decrease in OLs and myelin markers •increased OLs in adult |
• myelin recovery in mice, but mild myelin changes with higher G-ratio and behavioral deficits |
| CHRONIC HYPEROXIA | •neonatal resuscitation •complicated mechanical ventilation |
• no specific forms of pathology | • retinopathy of prematurity | •P7 rat; 80% O2 •P6 mouse; 80% O2 for 6 or 48 h) |
•PreOLs spared in vivo but not in vitro •neuronal death in rats •NG2+ early OL progenitor apoptosis in mice |
•early decrease in GFAP in mice at P8 but astrogliosis by P12 •transient reduction in GLAST+ cells at P8 but not P12 •Na-dependent transport of D-aspartate reduced at P8 and P12 but not at P15 |
•reduced myelination in rats •delayed CC1 + OL maturation in mice up to P10 |
•early increased OL generation with normal CC1 and olig2 by P12 •decrease in radial diffusivity on MRI in adult mice |
See text for references.
As also summarized in Table 1, models of hypoxia-ischemia, hypoxia and hyperoxia are not equivalent in terms of their clinical basis and do not generate similar forms of WMI. Hypoxia-ischemia models are particularly relevant to WMI in critically ill preterm infants who commonly display disturbances in cerebral autoregulation that compromise cerebral blood flow in the setting of systemic hypotension. Hypoxia-ischemia generates graded cerebral WMI that resembles the classic spectrum of PVL and ranges from diffuse WMI to focal cystic necrotic lesions. Hyperoxia most commonly occurs when elevated oxygen requirements occur as a complication of ventilatory compromise. Such preterm infants are often clinically unstable and at risk for concomitant hypoxia-ischemia and other causes of WMI. Chronic hypoxia typically results from chronic lung disease. Severe chronic hypoxemia in human preterm survivors is presently much less common than in prior decades. Often, preterm survivors with CLD have a history of other forms of WMI arising from other causes. In fact, there are no well-defined human neuropathological features that characterize the cerebral response to isolated chronic hypoxia, because preterm survivors with chronic lung disease have frequently sustained other forms of WMI related to hypoxia-ischemia, hyperoxia, or intraventricular hemorrhagic infarction.
Models of hypoxia-ischemia, hypoxia and hyperoxia also do not generate similar forms of WMI, because of distinct differences in the glial and axonal responses to each insult. In response to hypoxia-ischemia, preOLs degenerate by both excitotoxic mechanisms and by apoptosis. By contrast, chronic hypoxia in rodents triggers delayed apoptosis of CC1+ mature OLs, whereas chronic hyperoxia causes apoptosis of NG2+ early OL progenitors. Hence, although disturbances in myelination are a consequence of all three types of cerebral oxygenation disturbances, the underlying cellular and molecular mechanisms are distinctly different. Consequently, potential therapies for dysmyelination arising from chronic hypoxia or hyperoxia may not, for example, be relevant to hypoxia-ischemic myelination disturbances related to preOL injury and arrest. The distinction between these different forms of WMI is also evident in the disparate responses of other glia. Reactive astrocytes, for example, are common in WMI associated with hypoxia-ischemia, but are not observed even with chronic sublethal hypoxia in rodents. Similar considerations apply when comparing animal models in terms of outcome measures derived from MRI or neurobehavioral studies. Multiple conditions may share similar neuroimaging features on MRI, which underscores the need for histopathological confirmation to define the cellular responses that contribute to MRI signal abnormalities. Similarly, studies of neurobehavioral disturbances require histopathological confirmation, due to the relatively non-specific nature of behavioral disturbances that may present similarly despite disparate forms of WMI.
There has been considerable confusion and uncertainty about the specific postnatal age in the rodent when hypoxia-ischemia best models human WMI, with most researchers choosing to work with animals from P2 through P7. As noted above, the vulnerable stage in the OL lineage that is injured in human and experimental WMI is the preOL. PreOLs predominate in rodent white matter at P2, which thus resembles preterm human white matter (Craig et al. 2003; Dean et al., 2011). By P5, OL development has largely proceeded past the preOL stage (Dean et al. 2011), and so, on the basis of human neuropathological evidence, would not seem to be an appropriate model. Also, initial reports of white matter selectivity of the P7 model, making it appear similar to human WMI, have not been borne out (Selip et al. 2012).
If one accepts that the preOL is an important target cell in WMI, then it is of great importance to characterize the vulnerability of this cell-type in vivo and in vitro. It is recognized, however, that glutamate receptor expression in vitro, especially NMDA receptor expression, may not reflect NMDA receptor expression in vivo (Karadottir et al. 2005; Salter and Fern, 2005; Micu et al. 2006). Although extensive characterization of AMPA receptors has been carried out in rodent models and in the human (see above), the studies in rodent models have not clearly characterized calcium permeable AMPA receptors at P2 when preOLs are most abundant (Talos et al. 2006b). Furthermore, these studies, and a more recent study (Jantzie et al. 2013), are limited by lack of recognition that O4+ cells are not necessarily preOLs; they might also be O1+ immature OLs that also express the O4 antigen. The results obtained may apply to the preOL stage, but this has not been shown. The data of Talos et al. suggest that calcium permeable AMPA receptors might account for the vulnerability of the P7 rodent white matter to hypoxic/ischemic injury, but they do not account necessarily for injury to preOLs. Hence, a careful characterization of glutamate receptor expression specifically on preOLs in developing rodent and human white matter is still needed.
Despite the impressive array of animal models currently available, future studies are critically needed to develop more clinically relevant models of neonatal WMI. All of the models in Table 1 and described elsewhere are over-simplified with respect to the numerous clinico-pathological variables that modify the evolution of human WMI. Although many clinical insults may be recurrent, most models study single or chronic insults. It is increasingly apparent from human clinical studies that multiple systemic factors may contribute to WMI. These include exposure to prenatal or postnatal infection, suboptimal nutrition, exposure to stressful procedures, endocrine imbalances and congenital heart disease. Iatrogenic factors also are increasingly recognized to contribute to WMI. These include acute or recurrent exposure to anesthesia, sedatives, analgesics and neurosteroids. Maternal-fetal exposure to alcohol and drugs of abuse is an additional complicating factor. The susceptibility to WMI is undoubtedly influenced by numerous genetic and epigenetic factors that remain to be defined.
Clinical Implications from Current Mechanisms of Acute and Chronic WMI
Despite the fact that neonatal WMI contributes to a static encephalopathy characterized by cerebral palsy and neurobehavioral abnormalities, there is a broad developmental window over which white matter lesions undergo progressive evolution. Advances in the understanding of the pathogenesis of acute and chronic neonatal WMI suggest promising new directions to prevent initial WMI or promote greater regeneration and repair after injury.
The acute phase of WMI coincides with a sequence of injurious events that include primary energy failure, glutamate-mediated excitotoxicity, free radical generation and mitochondrial dysfunction. Energy failure is triggered by a combination of physiological factors that include hypotension, hypoxemia and glucose consumption. In preterm fetal sheep, the severity of chronic WMI was much more significantly correlated with a lower basal blood glucose level at the time of ischemia than with fetal blood pressure or blood oxygenation (Riddle et al. 2013). Hypoglycemia causes long-term deficits that are associated with MRI-defined injury, especially to neonatal gray and white matter (Barkovich et al. 1998; Burns et al. 2008; Wong et al. 2013). Glucose levels may thus be a useful marker of fetal response to hypoxiaischemia and glucose supplementation may provide protection against energy failure and more severe WMI.
Glutamate is preferentially depleted from preOLs and axons during acute WMI (Back et al. 2007) and microdialysis studies have detected glutamate release in response to preterm fetal ovine hypoxia-ischemia (Fraser et al. 2008; Loeliger et al. 2003). Although hypoxia-ischemia appears to trigger accumulation of glutamate in the extracellular space, glutamate receptor blockade rendered only partial neuroprotection after severe preterm hypoxia-ischemia (George et al. 2012). This is consistent with findings that acute WMI is characterized by a spectrum of necrotic and apoptotic preOL degeneration (Alix et al. 2012). Hence, to achieve greater neuroprotection a combination of approaches may be needed to block both cell death pathways.
One promising approach to block multiple cell death pathways is to combine cerebral hypothermia with neuroprotective agents. In preclinical studies, cerebral hypothermia rendered greater global protection against WMI and preOL degeneration (Barrett et al. 2012). However there is insufficient contemporary clinical data regarding the safety of hypothermia in the human preterm neonate (Gunn and Bennet 2008). Although a variety of agents have shown promising preclinical neuro-protection for cerebral injury in full term neonates, many of these agents have not been extensively evaluated for preterm WMI (Robertson et al. 2012). Agents that may provide at least partial protection against preterm WMI include melatonin (Gressens et al. 2008), erthryopoietin (Juul 2012; Xiong et al. 2011), thyroxine (Vose et al. 2013) and magnesium sulfate (Doyle 2012). One cautionary note is that bedside monitoring to identify preterm infants at high risk for WMI is not presently feasible, thus making it difficult to rapidly identify candidates for therapy.
Improved definition of the timing and mechanisms of free radical-mediated acute WMI would also be a significant advance to design more efficacious anti-oxidant therapies. Despite considerable in vitro evidence that free radicals trigger WMI via maturation-dependent mechanisms, it has been challenging to demonstrate that free radical generation occurs in vivo in the immediate ischemia-reperfusion period. Studies with an umbilical cord occlusion model of WMI in preterm fetal sheep confirmed that significant delayed free radical generation was detected between 4 and 14 hours after ischemia-reperfusion by electron spin resonance-detection of ascorbyl radicals collected by microdialysis (Welin et al. 2005). Recently, a fetal rabbit model of placental abruption detected superoxide formation in the immediate ischemia-reperfusion period, the severity of which was defined by serial MRI diffusion measurements. The frequency of animals with postnatal hypertonia was significantly reduced by a novel superoxide dismutase mimic that also enhanced survival after uterine ischemia (Drobyshevsky et al. 2012). Interestingly, after neonatal hypoxia-ischemia, elevations of other ROS markers in the immediate ischemia-reperfusion period have not been reported (Miller et al. 2005b; Welin et al. 2005), which suggests that early neonatal WMI may be associated with preferential generation of certain injurious free radicals, including the superoxide radical.
There continues to be a significant proportion of preterm infants for whom WMI derives from poorly defined antenatal or perinatal factors. Hence, there is a need for alternative therapies that promote regeneration and repair of chronic WMI that may not be recognized until after birth. Chronic WMI may also coincide with an expanded developmental window during which preOL maturation-arrest persists and confers an enhanced risk for recurrent and potentially more severe WMI. In neonatal rat chronic WMI, preOLs with arrested maturation displayed a markedly increased susceptibility to recurrent hypoxia-ischemia that triggered a massive selective apoptotic degeneration of preOLs (Segovia et al. 2008). Serial neuro-imaging studies are needed to better define the progression of WMI in human preterm infants at risk for recurrent insults (Miller and Ferriero 2009; Miller et al. 2007). Prior studies have identified clinical features that identify infants at risk for exacerbation of initial cerebral injury (e.g., preterm newborns with postnatal sepsis). Recurrent and systemic illness is an important risk factor that may increase susceptibility to progressively more severe WMI (Glass et al., 2008a; Chau et al., 2009).
Future studies are needed in relevant experimental models and from human autopsy studies to define the evolution of cerebral white matter lesions over months to years to identify the relative contributions of dysmyelination and axonal dysfunction to functional disabilities in preterm survivors. Such information is of critical importance to define the period over which chronic reactive gliosis remodels and to identify mechanisms to promote regeneration and repair of WMI. Multiple molecules likely act in concert with other signals in chronic white matter lesions to prevent normal myelination. Like hyaluronic acid accumulation, many of these signals appear to be linked to reactive astrogliosis (Sherman and Back 2008). Reactive astrocytes increase their expression of bone morphogenetic proteins that inhibit OL progenitor differentiation with concurrent promotion of astrocyte differentiation (Wang et al. 2011). Similarly, the Notch ligand Jagged1 is elevated on reactive astrocytes in demyelinating lesions and activates Notch signaling on OL progenitors, preventing their maturation (John et al. 2002). Dysregulation of WNT-beta catenin signaling in OL progenitors promotes preOL arrest, delays normal myelination and disrupts remyelination (Fancy et al. 2011a; Fancy et al. 2009; Feigenson et al. 2009; McClain et al. 2012; Tawk et al. 2011). The glycogen synthase kinase 3β (GSK3β) is a component of the Wnt signaling cascade, which also has been implicated in the regulation of OL progenitor maturation and inhibitors of GSK3β promote remyelination in adult white matter via mechanisms that include decreasing Notch1 signaling (Azim and Butt 2011). Constitutive expression of the epidermal growth factor receptor (EGFR) in neonatal white matter not only promotes pronounced proliferation of OL progenitors (Ivkovic et al. 2008), but enhanced EGFR signaling stimulates adult CNS myelination and remyelination (Aguirre et al. 2007). EGFR activation via an intranasally administered form of EGF reduced OL death from neonatal chronic hypoxia, promoted OL maturation and myelination and lead to functional improvement (Scafidi et al. 2013). Definition of mechanisms of preOL arrest may also accelerate the therapeutic potential of stem cell therapy to promote myelination in chronic neonatal WMI (Goldman et al. 2008; Gupta et al. 2012; Uchida et al. 2012; Webber et al. 2009). The potential benefits of stem cell therapy for cerebral palsy were recently reviewed (Bennet et al. 2012) and addressed in an international workshop, which underscores growing interest and expanding opportunities to develop therapies for neonatal WMI (http://www.cirm.ca.gov/sites/default/files/files/funding_page/CIRM_Cerebral_Palsy_Report.pdf ).
Acknowledgements
Dr. Back is supported by the NIH (National Institutes of Neurological Diseases and Stroke: 1RO1NS054044, R37NS045737; National Institute of Aging: 1R01AG031892-01), the American Heart Association (11GRNT7510072) and the March of Dimes Birth Defects Foundation (# 6-FY11-323). Dr. Rosenberg is supported by the NIH (P30 HD018655, R01 NS066019), the Whitehall Foundation, and the Baby Alex Foundation. We are grateful to Dr. Joseph Volpe for reading several drafts of the manuscript and providing invaluable advice to improve it. We are grateful to Dr. Roger Hohimer, Dr. Scott Rivkees and Dr. Joseph Scafidi for many thoughtful discussions regarding physiological mechanisms of chronic hypoxia-induced WMI.
References
- (CDC) CfDCaP. Economic costs associated with mental retardation, cerebral palsy, hearing loss and vision impairment--United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:57–59. [PubMed] [Google Scholar]
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–28. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Agresti C, D'Urso D, Levi G. Reversible inhibitory effects of interferon-γ and tumor necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. doi: 10.1111/j.1460-9568.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Alix JJ, Fern R. Glutamate receptor-mediated ischemic injury of premyelinated central axons. Ann Neurol. 2009;66:682–93. doi: 10.1002/ana.21767. [DOI] [PubMed] [Google Scholar]
- Alix JJ, Zammit C, Riddle A, Meshul CK, Back SA, Valentino M, Fern R. Central axons preparing to myelinate are highly sensitivity to ischemic injury. Ann Neurol. 2012;72:936–51. doi: 10.1002/ana.23690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C, RA S. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Anderson PJ, De Luca CR, Hutchinson E, Spencer-Smith MM, Roberts G, Doyle LW. Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev Neuropsychol. 2011;36:57–73. doi: 10.1080/87565641.2011.540538. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32:51–8. doi: 10.1053/j.semperi.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Andiman SE, Haynes RL, Trachtenberg FL, Billiards SS, Folkerth RD, Volpe JJ, Kinney HC. The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons. Brain Pathol. 2010;20:803–14. doi: 10.1111/j.1750-3639.2010.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz AM, Hussein A, Alix JJ, Perez-Cerda F, Allcock N, Matute C, Fern R. Functional glutamate transport in rodent optic nerve axons and glia. Glia. 2008;56:1353–67. doi: 10.1002/glia.20703. [DOI] [PubMed] [Google Scholar]
- Asher R, Perides G, Vanderhaeghen J, Bignami A. Extracellular matrix of central nervous system white matter: demonstration of an hyaluronan-protein complex. J Neurosci Res. 1991;28:410–421. doi: 10.1002/jnr.490280314. [DOI] [PubMed] [Google Scholar]
- Azim K, Butt AM. GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia. 2011;59:540–53. doi: 10.1002/glia.21122. [DOI] [PubMed] [Google Scholar]
- Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006a;12:129–40. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- Back SA, Craig A, Luo N, Ren J, Akundi R, Rebeiro I, Rivkees S. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 2006b;60:696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- Back SA, Craig A, Kayton R, Luo NL, Meshul C, Allcock N, Fern R. Hypoxia-Ischemia preferentially triggers glutamate depletion from oligodendroglia and axons in perinatal cerebral white matter. J Cereb Blood Flow Metab. 2007;27:334–347. doi: 10.1038/sj.jcbfm.9600344. [DOI] [PubMed] [Google Scholar]
- Back S, Kroenke C, Sherman L, Lawrence G, Gong X, Taber E, Sonnen J, Larson E, Montine T. White matter lesions defined by diffusion tensor imaging in older adults. Ann Neurol. 2011;70:465–476. doi: 10.1002/ana.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Tuohy T, Chen H, Wallingford N, Craig A, Struve J, Luo N, Banine F, Liu Y, Chang A. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005a;9:966–972. doi: 10.1038/nm1279. others. [DOI] [PubMed] [Google Scholar]
- Back SA, Gan X-D, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chrichton CA, Tam J, Xanthoudakis S, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002a;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Volpe JJ, Kinney HC. Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol. 2002b;61:197–211. doi: 10.1093/jnen/61.2.197. [DOI] [PubMed] [Google Scholar]
- Back SA, Luo NL, Mallinson RA, O'Malley JP, Wallen LD, Frei B, Morrow JD, Petito CK, Roberts CT, Jr., Murdoch GH. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 2005b;58:108–20. doi: 10.1002/ana.20530. others. [DOI] [PubMed] [Google Scholar]
- Back SA, Riddle A, Hohimer AR. Carmichael ST, Chesselet MF, editors. The instrumented fetal sheep as a model of cerebral white matter injury in the preterm infant. Neurotherapeutics, Special Issue: Animal Models of Neurological Disorders. 2012;9:359–70. doi: 10.1007/s13311-012-0108-y. Neurotherapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Volpe JJ. Cellular and molecular pathogenesis of periventricular white matter injury. MRDD Res Rev. 1997;3:96–107. [Google Scholar]
- Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TL, McGinty DJ. Reversal of cardiopulmonary failure during active sleep in hypoxic kittens: implications for sudden infant death. Science. 1977;198:419–421. doi: 10.1126/science.910138. [DOI] [PubMed] [Google Scholar]
- Banker B, Larroche J. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem. 1980;255:2372–2376. [PubMed] [Google Scholar]
- Barkovich AJ, Ali FA, Rowley HA, Bass N. Imaging patterns of neonatal hypoglycemia. AJNR Am J Neuroradiol. 1998;19:523–8. [PMC free article] [PubMed] [Google Scholar]
- Barres B, Schmid R, Sendnte M, Raff M. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Barrett RD, Bennet L, Naylor A, George SA, Dean JM, Gunn AJ. Effect of cerebral hypothermia and asphyxia on the subventricular zone and white matter tracts in preterm fetal sheep. Brain Res. 2012;1469:35–42. doi: 10.1016/j.brainres.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Bassan H, Gauvreau K, Newburger J, Tsuji M, Limperopoulous C, Soul JS, Walter G, Laussen P, Jonas R, du Plessis A. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res. 2005;57:35–41. doi: 10.1203/01.PDR.0000147576.84092.F9. [DOI] [PubMed] [Google Scholar]
- Baud O, Greene A, Li J, Wang H, Volpe JJ, Rosenberg PA. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J Neurosci. 2004a;24:1531–1540. doi: 10.1523/JNEUROSCI.3989-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud O, Haynes R, Wang H, Folkerth RD, Li J, Volpe J, Rosenberg PA. Developmental up-regulation of MnSOD in rat oligodendrocytes confers protection against oxidative injury. Eur J Neurosci. 2004b;19:2669–2681. doi: 10.1111/j.0953-816X.2004.03451.x. [DOI] [PubMed] [Google Scholar]
- Bax M, Tydeman C, Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA. 2006;296:1602–1608. doi: 10.1001/jama.296.13.1602. [DOI] [PubMed] [Google Scholar]
- Beaino G, Khoshnood B, Kaminski M, Pierrat V, Marret S, Matis J, Ledesert B, Thiriez G, Fresson J, Roze JC. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Dev Med Child Neurol. 2010;52:e119–25. doi: 10.1111/j.1469-8749.2010.03612.x. others. [DOI] [PubMed] [Google Scholar]
- Bemelmans A, Husson I, Mailet J, Kosofsky B, Gressens P. Lentiviral-mediated transfer of brain-derived neurotrophic factor is neuroprotective in a mouse model of neonatal excitotoxic damage. J Neurosci Res. 2006;83:50–60. doi: 10.1002/jnr.20704. [DOI] [PubMed] [Google Scholar]
- Bennet L, Tan S, Van den Heuij L, Derrick M, Groenendaal F, van Bel F, Juul S, Back SA, Northington F, Robertson NJ. Cell therapy for neonatal hypoxia-ischemia and cerebral palsy. Ann Neurol. 2012;71:589–600. doi: 10.1002/ana.22670. others. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete J, Seifert G, Steinhäuse rC, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Billiards S, Haynes R, Folkerth R, NS B, Trachtenberg F, Rowitch D, Ligon K, Volpe J, Kinney H. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathology. 2008;18:153–163. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn M, Datta S, Kellems R. Adenosine deaminase-deficient mice generated using a two-stage genetic engineering strategy exhibit a combined immunodeficiency. J Biol Chem. 1998;273:5093–5100. doi: 10.1074/jbc.273.9.5093. [DOI] [PubMed] [Google Scholar]
- Blackburn M, Wakamiya M, Caskey C, Kellems R. Tissue-specific rescue suggests that placental adenosine deaminase is important for fetal development in mice. J Biol Chem. 1995;270:23891–23894. doi: 10.1074/jbc.270.41.23891. [DOI] [PubMed] [Google Scholar]
- Blomgren K, Hagberg H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med. 2006;40:388–97. doi: 10.1016/j.freeradbiomed.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Bona E, Aden U, Fredholm B, Hagberg H. The effect of caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr Res. 1995;38:312–318. doi: 10.1203/00006450-199509000-00007. [DOI] [PubMed] [Google Scholar]
- Bona E, Aden U, Gilland E, Fredholm B, Hagberg H. Neonatal cerebral hypoxia-ischemia: the effects of adenosine receptor antagonists. Neuropharmacology. 1997;36:1327–1338. doi: 10.1016/s0028-3908(97)00139-1. [DOI] [PubMed] [Google Scholar]
- Borges K, Ohlemeyer C, Trotter J, Kettenmann H. AMPA/Kainate receptor activation in murine oligodendrocyte precursor cells leads to activation of a cation conductance, calcium influx and blockade of delayed rectifying K+ channels. Neuroscience. 1994;63:135–149. doi: 10.1016/0306-4522(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Brash AR. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- Brault S, Martinez-Bermudez A, Roberts J, II, Cui Q-L, Fragoso G, Hemdan S, Lui H-N, Gobeil F, Jr, Quiniou C, Kermorvant-Duchemin E. Cytotoxicity of the E2-Isoprostane 152T-IsoP on oligodendrocyte progenitors. Free Radic Biol Med. 2004a;37:358–366. doi: 10.1016/j.freeradbiomed.2004.05.007. others. [DOI] [PubMed] [Google Scholar]
- Brault S, Martinez-Bermudez AK, Roberts J, 2nd, Cui QL, Fragoso G, Hemdan S, Liu HN, Gobeil F, Jr., Quiniou C, Kermorvant-Duchemin E. Cytotoxicity of the E(2)-isoprostane 15-E(2t)-IsoP on oligodendrocyte progenitors. Free Radic Biol Med. 2004b;37:358–66. doi: 10.1016/j.freeradbiomed.2004.05.007. others. [DOI] [PubMed] [Google Scholar]
- Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122:65–74. doi: 10.1542/peds.2007-2822. [DOI] [PubMed] [Google Scholar]
- Buser JR, Segovia KN, Dean JM, Nelson K, Beardsley D, Gong X, Luo NL, Ren J, Wan Y, Riddle A, McClure MM, Ji X, Derrick M, Hohimer AR, Back SA, Tan S. Timing of appearance of late oligodendrocyte progenitors coincides with enhanced susceptibility of preterm rabbit cerebral white matter to hypoxia-ischemia. J Cereb Blood Flow and Metab. 2010;30:1053–1065. doi: 10.1038/jcbfm.2009.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS. Arrested pre-oligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Tuong CM, Gozal D. A neonatal mouse model of intermittent hypoxia associated with features of apnea in premature infants. Respir Physiol Neurobiol. 2011;178:210–217. doi: 10.1016/j.resp.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Pang Y, Lin S, Rhodes P. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- Cao Y, Gunn A, Bennet L, Wu D, George S, Gluckman PD, Shao X-M, Guan J. Insulin-like growth factor (IGF)-1 suppresses oligodendrocyte caspase-3 activation and increases glial proliferation after ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2003;23:739–747. doi: 10.1097/01.WCB.0000067720.12805.6F. [DOI] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostaglandins Other Lipid Mediat. 2002;68-69:325–44. doi: 10.1016/s0090-6980(02)00038-2. [DOI] [PubMed] [Google Scholar]
- Castillo-Melendez M, Chow J, Walker D. Lipid peroxidation, caspase-3 immunoreactivity, and pyknosis in late-gestation fetal sheep brain after umbilical cord occlusion. Pediatr Res. 2004;55:864–871. doi: 10.1203/01.PDR.0000115679.86566.C4. [DOI] [PubMed] [Google Scholar]
- Chau V, Poskitt KJ, McFadden DE, Bowen-Roberts T, Synnes A, Brant R, Sargent MA, Soulikias W, Miller SP. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66:155–64. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- Chavez-Valdez R, Martin LJ, Flock DL, Northington FJ. Necrostatin-1 attenuates mitochondrial dysfunction in neurons and astrocytes following neonatal hypoxia-ischemia. Neuroscience. 2012;219:192–203. doi: 10.1016/j.neuroscience.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J Neurosci. 2004;24:1136–48. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Deshmukh M, D'Costa A, Demaro J, Gidday J, Shah A, Sun Y, Jacquin M, Johnson E, Jr., Holtzman D. Caspase inhibitor affords neuroprotection with delayed adminstration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998;101:1992–1999. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp J, III, Peress N, Wesley M, Mann L. Brain damage after intermittent partial cord occlusion in the chronically instrumented fetal lamb. Am J Obstet Gynecol. 1988;159:504–509. doi: 10.1016/s0002-9378(88)80118-2. [DOI] [PubMed] [Google Scholar]
- Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, Tworetzky W, McElhinney DB, Brown DW, Gholipour A, Kudelski D, Warfield SK, McCarter RJ. Delayed Cortical Development in Fetuses with Complex Congenital Heart Disease. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs281. others. [DOI] [PubMed] [Google Scholar]
- Constantinou JC, Adamson-Macedo EN, Mirmiran M, Fleisher BE. Movement, imaging and neurobehavioral assessment as predictors of cerebral palsy in preterm infants. J Perinatol. 2007;27:225–9. doi: 10.1038/sj.jp.7211664. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative Stress, Glutamate, and Neurodegenerative Disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Craig A, Luo NL, Beardsley DJ, Wingate-Pearse N, Walker DW, Hohimer AR, Back SA. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 2003;181:231–240. doi: 10.1016/s0014-4886(03)00032-3. [DOI] [PubMed] [Google Scholar]
- Czeh M, Gressens P, Kaindl AM. The Yin and Yang of Microglia. Dev Neurosci. 2011;33:199–209. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- de Jesus Domingues AM, Neugebauer KM, Fern R. Identification of four functional NR3B isoforms in the developing white matter reveals unexpected diversity among glutamate receptors. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07212.x. [DOI] [PubMed] [Google Scholar]
- Dean J, McClendon E, Hansen K, Azimi-Zonooz A, Chen K, Riddle A, Gong X, Sharifnia E, Hagen M, Ahmad T, Leigland LA, Hohimer AR, Kroenke CD, Back SA. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci Transl Med. 2013;5:101–111. doi: 10.1126/scitranslmed.3004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J, Moravec M, Grafe M, Abend N, Ren J, Gong X, Volpe J, Jensen F, Hohimer A, Back S. Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human. Dev Neurosci. 2011;33:251–260. doi: 10.1159/000327242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21. doi: 10.1038/nchembio.83. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Degterev A, Maki JL, Yuan J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013;20:366. doi: 10.1038/cdd.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi K, Oguchi K, Takashima S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol. 1997;16:296–300. doi: 10.1016/s0887-8994(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, Burguet A, Roze JC, Matis J, Picaud JC. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123:1485–92. doi: 10.1542/peds.2008-1216. others. [DOI] [PubMed] [Google Scholar]
- Deng W, Rosenberg P, Volpe J, Jensen F. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci USA. 2003;100:6801–6806. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick M, N.L. L, Bregman JC, Jilling T, Ji X, Fisher K, Gladson CL, Beardsley DJ, Murdoch GA, Back SA. Preterm fetal hypoxia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva TM, Borenstein NS, Volpe JJ, Kinney HC, Rosenberg PA. Expression of EAAT2 in neurons and protoplasmic astrocytes during human cortical development. J Comp Neurol. 2012 doi: 10.1002/cne.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva TM, Kabakov AY, Goldhoff PE, Volpe JJ, Rosenberg PA. Regulation of glutamate transport in developing rat oligodendrocytes. J Neurosci. 2009;29:7898–908. doi: 10.1523/JNEUROSCI.6129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva TM, Kinney HC, Borenstein NS, Trachtenberg FL, Irwin N, Volpe JJ, Rosenberg PA. The glutamate transporter EAAT2 is transiently expressed in developing human cerebral white matter. J Comp Neurol. 2007;501:879–90. doi: 10.1002/cne.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicou E, Rangon C, Guimiot F, Spedding M, Gressens P. Positive allosteric modulators of AMPA receptors are neuroprotective against lesions induced by an NMDA agonist in neonatal mouse brain. Brain Res. 2003;970:221–225. doi: 10.1016/s0006-8993(03)02357-6. [DOI] [PubMed] [Google Scholar]
- Dimitropoulos A, McQuillen PS, Sethi V, Moosa A, Chau V, Xu D, Brant R, Azakie A, Campbell A, Barkovich AJ. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81:241–8. doi: 10.1212/WNL.0b013e31829bfdcf. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Etxebarria E, Perez-Samartin A, Matute C. Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia. 2005;52:36–46. doi: 10.1002/glia.20221. [DOI] [PubMed] [Google Scholar]
- Domercq M, Sanchez-Gomez M, Areso P, Matute C. Expression of glutamate transporters in rat optic nerve oligodendrocytes. Eur J Neurosci. 1999;11:2226–2236. doi: 10.1046/j.1460-9568.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Domingues AM, Taylor M, Fern R. Glia as transmitter sources and sensors in health and disease. Neurochem Int. 2010;57:359–66. doi: 10.1016/j.neuint.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Dommergues M-A, Patkai J, Renauld J-C, Evrard P, Gressens P. Proinflammatory cytokines and interleukin-9 exacerbate excitotoxic lesions of the newborn murine neopallium. Ann Neurol. 2000;47:54–63. [PubMed] [Google Scholar]
- Doyle LW, Cheong J, Hunt RW, Lee KJ, Thompson DK, Davis PG, Rees S, Anderson PJ, Inder TE. Caffeine and brain development in very preterm infants. Ann Neurol. 2010;68:734–42. doi: 10.1002/ana.22098. [DOI] [PubMed] [Google Scholar]
- Doyle L. Antenatal magnesium sulfate and neuroprotection. Curr Opin Pediatr. 2012;24:154–159. doi: 10.1097/MOP.0b013e3283504da1. [DOI] [PubMed] [Google Scholar]
- Drobyshevsky A, Luo K, Derrick M, Yu L, Du H, Prasad PV, Vasquez-Vivar J, Batinic-Haberle I, Tan S. Motor deficits are triggered by reperfusion-reoxygenation injury as diagnosed by MRI and by a mechanism involving oxidants. J Neurosci. 2012;32:5500–9. doi: 10.1523/JNEUROSCI.5986-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Derrick M, Prasad P, Ji X, Englof I, Tan S. Fetal brain magnetic resonance imaging response acutely to hypoxia-ischemia predicts postnatal outcome. Ann Neurol. 2007a;61:307–314. doi: 10.1002/ana.21095. [DOI] [PubMed] [Google Scholar]
- Drobyshevsky A, Derrick M, Wyrwicz A, Ji X, Englof I, Ullman L, Zelaya M, Northington F, Tan S. White matter injury correlates with hypertonia in an animal model of cerebral palsy. J Cereb Blood Flow and Metab. 2007b;27:270–281. doi: 10.1038/sj.jcbfm.9600333. [DOI] [PubMed] [Google Scholar]
- Drobyshevsky A, Song S-K, Gamkrelidze G, Wyrwicz A, Derrick M, Meng F, Li L, Ji X, Trommer D, Beardsley D. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzhyna N, Hollensworth S, Kelley M, Wilson G, Ledoux S. Targeting human 8-oxoguanine glycosylase to mitochondria of oligodendroyctes protects against menadione-induced oxidative stress. Glia. 2003;42:370–378. doi: 10.1002/glia.10230. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Barres BA. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison V, Mocatta T, Winterbourn C, Darlow B, Volpe J, Inder T. The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res. 2005;57:282–286. doi: 10.1203/01.PDR.0000148286.53572.95. [DOI] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–85. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy S, Harrington E, Yuen T, Silbereis J, Zhao C, Baranzini S, Bruce C, Otero J, Huang E, Nusse R. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011a;14:1009–1016. doi: 10.1038/nn.2855. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Chan JR, Baranzini SE, Franklin RJ, Rowitch DH. Myelin regeneration: a recapitulation of development? Annu Rev Neurosci. 2011b;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Kotter MR, Harrington EP, Huang JK, Zhao C, Rowitch DH, Franklin RJ. Overcoming remyelination failure in multiple sclerosis and other myelin disorders. Exp Neurol. 2010;225:18–23. doi: 10.1016/j.expneurol.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–85. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahani R, Kanaan A, Gavrialov O, Brunnert S, Douglas RM, Morcillo P, Haddad GG. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr Pulmon. 2008;43:20–28. doi: 10.1002/ppul.20729. [DOI] [PubMed] [Google Scholar]
- Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, Lacaud A, Saliba E, Dammann O, Gallego J. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011;70:550–565. doi: 10.1002/ana.22489. others. [DOI] [PubMed] [Google Scholar]
- Feigenson K, Reid M, See J, Crenshaw EB, 3rd, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42:255–65. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Felling RJ, Snyder MJ, Romanko MJ, Rothstein RP, Ziegler AN, Yang Z, Givogri MI, Bongarzone ER, Levison SW. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci. 2006;26:4359–69. doi: 10.1523/JNEUROSCI.1898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern R, Davis P, Waxman S, Ransom B. Axon conduction and survival in CNS white matter during energy deprivation: a developmental study. J Neurophysiol. 1998;79:95–105. doi: 10.1152/jn.1998.79.1.95. [DOI] [PubMed] [Google Scholar]
- Fern R, Möller T. Rapid ischemic cell death in immature oligodendrocytes: A fatal glutamate release feedback loop. J Neurosci. 2000;20:34–42. doi: 10.1523/JNEUROSCI.20-01-00034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM, Miller SP. Imaging selective vulnerability in the developing nervous system. J Anat. 2010;217:429–35. doi: 10.1111/j.1469-7580.2010.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerth R, Haynes R, Borenstein NS, Volpe JJ, Kinney HC. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol. 2004a;63:990–999. doi: 10.1093/jnen/63.9.990. [DOI] [PubMed] [Google Scholar]
- Folkerth R, Keefe R, Haynes R, Trachtenberg F, Volpe J, Kinney H. Interferon-gamma expression in periventricular leukomalacia in the human brain. Brain Pathol. 2004b;14:265–274. doi: 10.1111/j.1750-3639.2004.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follet PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24:4412–4420. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follet PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury to the developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine RH, Olivier P, Massonneau V, Leroux P, Degos V, Lebon S, El Ghouzzi V, Lelievre V, Gressens P, Baud O. Vulnerability of white matter towards antenatal hypoxia is linked to a species-dependent regulation of glutamate receptor subunits. Proc Natl Acad Sci U S A. 2008b;105:16779–84. doi: 10.1073/pnas.0803004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlie P, Davis P. Prophylactic indomethacin for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2003;88:F464–466. doi: 10.1136/fn.88.6.F464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso G, Martinez-Bermudez A, Lui H-N, Khorchid A, Chemtob S, Mushynski W, Almazan G. Developmental differences in H2O2-induced oligodendrocyte cell death: role of glutathione, mitogen-activated protein kinases and caspase 3. J Neurochem. 2004;90:392–404. doi: 10.1111/j.1471-4159.2004.02488.x. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Gallo V. The translational biology of remyelination: Past, present and future. Glia. 2014 Jan 20; doi: 10.1002/glia.22622. on line. [DOI] [PubMed] [Google Scholar]
- Fraser M, Bennet L, Van Zijl PL, Mocatta TJ, Williams CE, Gluckman PD, Winterbourn CC, Gunn AJ. Extracellular amino acids and lipid peroxidation products in periventricular white matter during and after cerebral ischemia in preterm fetal sheep. J Neurochem. 2008;105:2214–23. doi: 10.1111/j.1471-4159.2008.05313.x. [DOI] [PubMed] [Google Scholar]
- French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res. 2009;87:3076–87. doi: 10.1002/jnr.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli K, Zimmerman R, Jarvik G, Wernovsky G, Kuypers M, Clancy R, Montenegro L, Mahle W, Newman M, Saunders A. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. others. [DOI] [PubMed] [Google Scholar]
- George SA, Barrett RD, Bennet L, Mathai S, Jensen EC, Gunn AJ. Nonadditive neuroprotection with early glutamate receptor blockade and delayed hypothermia after asphyxia in preterm fetal sheep. Stroke. 2012;43:3114–7. doi: 10.1161/STROKEAHA.112.671982. [DOI] [PubMed] [Google Scholar]
- Gerstner B, Buhrer C, Rheinlander C, Polley O, Schuller A, Berns M, Obladen M, Felderhoff-Mueser U. Maturation-dependent oligodendrocyte apoptosis caused by hyperoxia. J Neurosci Res. 2006;84:306–15. doi: 10.1002/jnr.20880. [DOI] [PubMed] [Google Scholar]
- Gerstner B, DeSilva TM, Genz K, Armstrong A, Brehmer F, Neve RL, Felderhoff-Mueser U, Volpe JJ, Rosenberg PA. Hyperoxia causes maturation-dependent cell death in the developing white matter. J Neurosci. 2008;28:1236–45. doi: 10.1523/JNEUROSCI.3213-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner B, Sifringer M, Dzietko M, Schuller A, Lee J, Simons S, Obladen M, Volpe JJ, Rosenberg PA, Felderhoff-Mueser U. Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann Neurol. 2007;61:562–73. doi: 10.1002/ana.21118. [DOI] [PubMed] [Google Scholar]
- Ghiani C, Yuan X, Eisen A, Knutson P, DePinho R, McBain C, Gallo V. Voltage-activated K+ channels and membrane depolarization regulate accumulation of the cyclin-dependent kinase inhibitors P27 Kip1 and p21CIP1 in glial progenitor cells. J Neurosci. 1999;19:5380–5392. doi: 10.1523/JNEUROSCI.19-13-05380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ, Ferriero DM, Miller SP. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008a;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- Glass HC, Fujimoto S, Ceppi-Cozzio C, Bartha AI, Vigneron DB, Barkovich AJ, Glidden DV, Ferriero DM, Miller SP. White-matter injury is associated with impaired gaze in premature infants. Pediatr Neurol. 2008b;38:10–15. doi: 10.1016/j.pediatrneurol.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Schanz S, Windrem MS. Stem cell-based strategies for treating pediatric disorders of myelin. Hum Mol Genet. 2008;17:R76–83. doi: 10.1093/hmg/ddn052. [DOI] [PubMed] [Google Scholar]
- Gray PH, Burns YR, Mohay HA, O'Callaghan MJ, Tudehope DI. Neurodevelopmental outcome of preterm infants with bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. 1995;73:F128–134. doi: 10.1136/fn.73.3.f128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressens P, Schwendimann L, Husson I, Sarkozy G, Mocaer E, Vamecq J, Spedding M. Agomelatine, a melatonin receptor agonist with 5-HT(2C) receptor antagonist properties, protects the developing murine white matter against excitotoxicity. Eur J Pharmacol. 2008;588:58–63. doi: 10.1016/j.ejphar.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Griffith JL, Shimony JS, Cousins SA, Rees SE, McCurnin DC, Inder TE, Neil JJ. MR imaging correlates of white-matter pathology in a preterm baboon model. Pediatr Res. 2012;71:185–191. doi: 10.1038/pr.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J, Bennet L, George S, Wu D, Waldvogel HJ, Gluckman PD, Faull RLM, Crosier PS, Gunn AJ. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow and Metab. 2001;21:493–502. doi: 10.1097/00004647-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Bennet L. Fetal hypoxia insults and patterns of brain injury: insights from animal models. Clin Perinatol. 2009;36:579–93. doi: 10.1016/j.clp.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn AJ, Bennet L. Brain cooling for preterm infants. Clin Perinatal. 2008;35:735–748. doi: 10.1016/j.clp.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M, Taylor H, Drotar D, Schluchter M, Cartar L, Andreias L, Wilson-Costello D, Klein N. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990's. JAMA. 2005;294:318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. MRDD Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Han B, D'Costa A, Back SA, Parsadian M, Patel S, Shah A, Gidday J, Srinvasan A, Deshmukh M, Holtzman D. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis. 2000;7:38–53. doi: 10.1006/nbdi.1999.0275. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 2008;63:656–61. doi: 10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RL, van Leyen K. 12/15-lipoxygenase expression is increased in oligodendrocytes and microglia of periventricular leukomalacia. Dev Neurosci. 2013;35:140–54. doi: 10.1159/000350230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LF, Chen HJ, Qian LH, Chen GY, Buzby JS. Curcumin protects pre-oligodendrocytes from activated microglia in vitro and in vivo. Brain Res. 2010;1339:60–9. doi: 10.1016/j.brainres.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Henderson J, Reynolds J, Dexter F, Atkins B, Hrdy J, Poduska D, Penning D. Chronic hypoxemia causes extracellular glutamate concentration to increase in the cerebral cortex of the near-term fetal sheep. Dev Brain Res. 1998;105:287–293. doi: 10.1016/s0165-3806(97)00192-2. [DOI] [PubMed] [Google Scholar]
- Hirayama A, Okoshi Y, Hachiya Y, Ozawa Y, Ito M, Kida Y, Imai Y, Kohsaka S, Takashima S. Early immunohistochemical detection of axonal damage and glial activation in extremely immature brains with periventricular leukomalacia. Clin Neuropathol. 2001;20:87–91. [PubMed] [Google Scholar]
- Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–23. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Samarasinghe R, Knoch ME, Lewis M, Aizenman E, DeFranco DB. Selective inhibition of mitogen-activated protein kinase phosphatases by zinc accounts for extracellular signal-regulated kinase 1/2-dependent oxidative neuronal cell death. Mol Pharmacol. 2008;74:1141–51. doi: 10.1124/mol.108.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, Kikinis R, Jolesz FA, Volpe JJ. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–60. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- Husson I, Mesples B, Bac P, Vamecq J, Evrard P, Gressens P. Melatonergic neuroprotection of the murine periventricular white matter against neonatal excitotoxic challenge. Ann Neurol. 2002;51:82–92. doi: 10.1002/ana.10072. [DOI] [PubMed] [Google Scholar]
- Hutton LC, Castillo-Melendez M, Smythe GA, Walker DW. Microglial activation, macrophage infiltration, and evidence of cell death in the fetal brain after uteroplacental administration of lipopolysaccharide in sheep in late gestation. Am J Obstet Gynecol. 2008;198:117, e1–11. doi: 10.1016/j.ajog.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Iida K, Takashima S, Ueda K. Immunohistochemical study of myelination and oligodendrocyte in infants with periventricular leukomalacia. Pediatr Neurol. 1995;13:296–304. doi: 10.1016/0887-8994(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Choi BH, Yee S, Murata Y, Quilligan EJ. Oxidative stress, brain white matter damage and intrauterine asphyxia in fetal lambs. Int J Dev Neurosci. 1999;17:1–14. doi: 10.1016/s0736-5748(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Inder T, Mocatta T, Darlow B, Spencer C, Volpe JJ, Winterbourn C. Elevated free radical products in the cerebrospinal fluid of VLBW infants with cerebral white matter injury. Pediatr Res. 2002;52:213–8. doi: 10.1203/00006450-200208000-00013. [DOI] [PubMed] [Google Scholar]
- Ireland Z, Dickinson H, Fleiss B, Hutton LC, Walker DW. Behavioural effects of near-term acute fetal hypoxia in a small precocial animal, the spiny mouse (Acomys cahirinus). Neonatology. 2010;97:45–51. doi: 10.1159/000227293. [DOI] [PubMed] [Google Scholar]
- Itoh T, Beesley J, Itoh A, Cohen AS, Kavanaugh B, Coulter DA, Grinspan JB, Pleasure D. AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem. 2002;81:390–402. doi: 10.1046/j.1471-4159.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Canoll P, Goldman JE. Constitutive EGFR signaling in oligodendrocyte progenitors leads to diffuse hyperplasia in postnatal white matter. J Neurosci. 2008;28:914–22. doi: 10.1523/JNEUROSCI.4327-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto HS, Kaufman T, Keil LC, Rudolph AM. Responses to acute hypoxemia in fetal sheep at 0.6-0.7 gestation. Am J Physiol. 1989;256:H613–20. doi: 10.1152/ajpheart.1989.256.3.H613. [DOI] [PubMed] [Google Scholar]
- Jablonska B, Scafidi J, Aguirre A, Vaccarino F, Nguyen V, Borok E, Horvath TL, Rowitch DH, Gallo V. Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J Neurosci. 2012;32:14775–14793. doi: 10.1523/JNEUROSCI.2060-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LK, Dutton GN. Periventricular leukomalacia: an important cause of visual and ocular motility dysfunction in children. Surv Ophthalmol. 2000;45:1–13. doi: 10.1016/s0039-6257(00)00134-x. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat. 2009;3:5. doi: 10.3389/neuro.05.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia. 2005;49:480–91. doi: 10.1002/glia.20134. [DOI] [PubMed] [Google Scholar]
- Jantzie LL, Talos DM, Jackson MC, Park HK, Graham DA, Lechpammer M, Folkerth RD, Volpe JJ, Jensen FE. Developmental Expression of N-Methyl-D-Aspartate (NMDA) Receptor Subunits in Human White and Gray Matter: Potential Mechanism of Increased Vulnerability in the Immature Brain. Cereb Cortex. 2013 Sep 17; doi: 10.1093/cercor/bht246. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Erichsen MN, Nielsen CW, Stensbol TB, Kehler J, Bunch L. Discovery of the first selective inhibitor of excitatory amino acid transporter subtype 1. J Med Chem. 2009;52:912–5. doi: 10.1021/jm8013458. [DOI] [PubMed] [Google Scholar]
- Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39:2538–43. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John G, Shankar S, Shafit-Zagardo B, Massimi A, Lee S, Raine C, Brosnan C. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- Juul S. Neuroprotective role of erythropoietin in neonates. J Matern Fetal Neonatal Med. 2012;25:105–107. doi: 10.3109/14767058.2012.715025. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kanaan A, Farahani R, Douglas RM, LaManna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1105–R1114. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4:130ra46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen L, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H, Okuda S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog Neurobiol. 1995;46:607–636. doi: 10.1016/0301-0082(95)00016-o. [DOI] [PubMed] [Google Scholar]
- Kaukola T, Herva R, Perhomaa M, Paakko E, Kingsmore S, Vainionpaa L, Hallman M. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res. 2006;59:478–83. doi: 10.1203/01.pdr.0000182596.66175.ee. [DOI] [PubMed] [Google Scholar]
- Kaur C, Ling EA. Periventricular white matter damage in the hypoxic neonatal brain: role of microglial cells. Prog Neurobiol. 2009;87:264–80. doi: 10.1016/j.pneurobio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, Maller-Kesselman J, Silbereis J, Constable RT, Makuch RW. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513–20. 520, e1. doi: 10.1016/j.jpeds.2007.08.009. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Dayani L, Rosenberg PA, Li J. RIP1 kinase mediates arachidonic acid-induced oxidative death of oligodendrocyte precursors. Int J Physiol Pathophysiol Pharmacol. 2010;2:137–147. [PMC free article] [PubMed] [Google Scholar]
- Kim S, Steelman AJ, Koito H, Li J. Astrocytes promote TNF-mediated toxicity to oligodendrocyte precursors. J Neurochem. 2011;116:53–66. doi: 10.1111/j.1471-4159.2010.07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H, Back S. Human oligodendroglial development: relationship to periventricular leukomalacia. Semin Pediatr Neurol. 1998;5:180–189. doi: 10.1016/s1071-9091(98)80033-8. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Panigraphy A, Newburger J, Jonas R, Sleeper L. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Act Neuropathol (Berl) 2005;110:563–578. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- Knutson P, Ghiani CA, Zhou JM, Gallo V, McBain CJ. K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells. J Neurosci. 1997;17:2669–82. doi: 10.1523/JNEUROSCI.17-08-02669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch J, Miles D, Gilley J, Yang C, Kernie S. Brief exposure to hyperoxia depletes the glial progenitor pool and impairs functional recovery after hypoxic-ischemic brain injury. J Cereb Blood Flow Metab. 2008;28:1294–1306. doi: 10.1038/jcbfm.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease--can we wrap it up? Brain. 2011;134:1882–900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- Kriegler S, Chiu S. Calcium signaling of glial cells along the mammalian axons. J Neurosci. 1993;13:4229–4245. doi: 10.1523/JNEUROSCI.13-10-04229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasry O, Shevell MI, Dagenais L. Cross-sectional comparison of periventricular leukomalacia in preterm and term children. Neurology. 2010;74:1386–91. doi: 10.1212/WNL.0b013e3181dad62d. [DOI] [PubMed] [Google Scholar]
- Lee A, Lingwood BE, Bjorkman ST, Miller SM, Poronnik P, Barnett NL, Colditz P, Pow DV. Rapid loss of glutamine synthetase from astrocytes in response to hypoxia: implications for excitotoxicity. J Chem Neuroanat. 2010;39:211–20. doi: 10.1016/j.jchemneu.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison B, Li Y, Lengacher S, Farah M, Hoffman P, Liu Y, Tsinalia A, Jin L, Zhang P-W. Oligodendrolia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M. The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18:522–55. doi: 10.1089/ars.2011.4391. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AM, Chau V, Poskitt KJ, Sargent MA, Lupton BA, Hill A, Roland E, Miller SP. White matter injury in term newborns with neonatal encephalopathy. Pediatr Res. 2009;65:85–9. doi: 10.1203/PDR.0b013e31818912d2. [DOI] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe J, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ramenaden ER, Peng J, Koito H, Volpe JJ, Rosenberg PA. Tumor necrosis factor alpha mediates lipopolysaccharide-induced microglial toxicity to developing oligodendrocytes when astrocytes are present. J Neurosci. 2008;28:5321–30. doi: 10.1523/JNEUROSCI.3995-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36. doi: 10.1016/j.jtcvs.2008.10.025. discussion 536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Jr., Guizard N, McGrath E, Geva J, Annese D. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Chang YC, Wang ST, Lee TY, Lin CF, Huang CC. Altered inflammatory responses in preterm children with cerebral palsy. Ann Neurol. 2010;68:204–12. doi: 10.1002/ana.22049. [DOI] [PubMed] [Google Scholar]
- Lin S, Rhodes P, Lei M, Zhang F, Cai Z. α-Phenyl-n-tert-butyl-nitrone attenuates hypoxic-ischemic white matter injury in the neonatal rat brain. Brain Res. 2004;1007:132–141. doi: 10.1016/j.brainres.2004.01.074. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–22. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Litt J, Taylor H, Klein N, Hack M. Learning disabilities in children with very low birthweight:prevalence, neuropsychological correlates and educational interventions. J Learn Disabil. 2005;8:130–141. doi: 10.1177/00222194050380020301. [DOI] [PubMed] [Google Scholar]
- Liu J, Li J, Qin GL, Chen YH, Wang Q. Periventricular leukomalacia in premature infants in mainland China. Am J Perinatol. 2008;25:535–40. doi: 10.1055/s-0028-1083841. [DOI] [PubMed] [Google Scholar]
- Liu YY, Silverstein FS, Skoff R, Barks JD. Hypoxic-ischemic oligodendroglial injury in neonatal rat brain. Pediatr Res. 2002;51:25–33. doi: 10.1203/00006450-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Loeliger M, Inder T, Cain S, Ramesh RC, Camm E, Thomson MA, Coalson J, Rees SM. Cerebral outcomes in a preterm baboon model of early versus delayed nasal continuous positive airway pressure. Pediatrics. 2006;118:1640–53. doi: 10.1542/peds.2006-0653. [DOI] [PubMed] [Google Scholar]
- Loeliger M, Watson CS, Reynolds JD, Penning DH, Harding R, Bocking AD, Rees SM. Extracellular glutamate levels and neuropathology in cerebral white matter following repeated umbilical cord occlusion in the near term fetal sheep. Neuroscience. 2003;116:705–14. doi: 10.1016/s0306-4522(02)00756-x. [DOI] [PubMed] [Google Scholar]
- Mallard E, Rees S, Stringer M, Cock M, Harding R. Effects of chronic placental insufficiency on brain development in fetal sheep. Pediatr Res. 1998;43:262–270. doi: 10.1203/00006450-199802000-00018. [DOI] [PubMed] [Google Scholar]
- Manning S, Talos D, Zhou C, Selip D, Park H, Park C, Volpe J, Jensen F. NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci. 2008;25:6670–6678. doi: 10.1523/JNEUROSCI.1702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. Developmental neuropathology and impact of perinatal brain damage. II: white matter lesions of the neocortex. J Neuropathol Exp Neurol. 1997;56:219–35. doi: 10.1097/00005072-199703000-00001. [DOI] [PubMed] [Google Scholar]
- Marret S, Mukendi R, Gadisseux J-F, Gressens P, Evrard P. Effect of ibotenate on brain development: an excitotoxic mouse model of microgyria and posthypoxic-like lesions. J Neuropathol Exp Neurol. 1995;54:358–370. doi: 10.1097/00005072-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew AT, Diez-Sebastian J, Rutherford MA, Cowan FM. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr. 2012;161:799–807. doi: 10.1016/j.jpeds.2012.04.054. [DOI] [PubMed] [Google Scholar]
- McClain CR, Sim FJ, Goldman SA. Pleiotrophin suppression of receptor protein tyrosine phosphatase-beta/zeta maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells. J Neurosci. 2012;32:15066–75. doi: 10.1523/JNEUROSCI.1320-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClendon E, Chen K, Gong X, Sharifnia E, Hagen M, Cai V, Shaver DC, Riddle A, Dean JM, Gunn AJ, Mohr C, Kaplan JS, Rossi DJ, Kroenke CD, Hohimer AR, Back SA. Prenatal cerebral ischemia triggers dysmaturation of caudate projection neurons. Ann Neurol. 2014 doi: 10.1002/ana.24100. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Althomsons S, Hyrc K, Choi D, Goldberg M. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- McLaurin J, D'Souza S, Stewart J, Blain M, Beaudet A, Nalbantoglu J, Antel JP. Effect of tumor necrosis factor alpha and beta on human oligodendrocytes and neurons in culture. Int J Dev Neurosci. 1995;13:369–381. doi: 10.1016/0736-5748(95)00012-6. [DOI] [PubMed] [Google Scholar]
- McQuillen P, Miller S. Congenital heart disease and brain development. Ann N Y Acad Sci. 2010;1184:68–86. doi: 10.1111/j.1749-6632.2009.05116.x. [DOI] [PubMed] [Google Scholar]
- Ment L, Schwartz M, Makuch R, Stewart W. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res Dev Brain Res. 1998;111:197–203. doi: 10.1016/s0165-3806(98)00139-4. [DOI] [PubMed] [Google Scholar]
- Ment LR, Hirtz D, Huppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–55. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998-2003. Neonatology. 2010;97:329–38. doi: 10.1159/000260136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE. Effects of interleukin-1 and tumor necrosis factor-α on astrocytes, microglia, oligodendrocytes, and glial precursors in vitro. Dev Neurosci. 1991;13:130. doi: 10.1159/000112150. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp B, McRory J, Rehak R. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. others. [DOI] [PubMed] [Google Scholar]
- Miller DA, Granger WM. A block diagram, graphical and microcomputer analysis of the O2 transport system. Physiologist. 1982;25(2):111–117. [PubMed] [Google Scholar]
- Miller SL, Yan EB, Castillo-Melendez M, Jenkin G, Walker DW. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev Neurosci. 2005a;27:200–10. doi: 10.1159/000085993. [DOI] [PubMed] [Google Scholar]
- Miller SP, Ferriero D. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009;32:496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SP, McQuillen P, Hamrick S, Xu D, Glidden D, Charlton N, Karl T, Azakie A, Ferriero D, Barkovich A. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1971–1973. doi: 10.1056/NEJMoa067393. others. [DOI] [PubMed] [Google Scholar]
- Miller SP, Vigneron D, Henry R, Bohland M, Ceppi-Cozzio C, Hoffman C, Newton N, Partridge JC, Ferriero DM, Barkovich AJ. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn ResonImaging. 2002;16:621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- Miller SP. Newborn brain injury: looking back to the fetus. Ann Neurol. 2007;61:285–7. doi: 10.1002/ana.21124. [DOI] [PubMed] [Google Scholar]
- Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden D, Partridge JC, Perez M, Mukherjee P, Vigneron D, Barkovich AJ. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse neurodevelopmental outcome. J Pediatr. 2005b;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Ming X, Chew LJ, Gallo V. Transgenic overexpression of sox17 promotes oligodendrocyte development and attenuates demyelination. J Neurosci. 2013;33:12528–42. doi: 10.1523/JNEUROSCI.0536-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll NM, Hong E, Fauveau M, Naruse M, Kerninon C, Tepavcevic V, Klopstein A, Seilhean D, Chew LJ, Gallo V. SOX17 is expressed in regenerating oligodendrocytes in experimental models of demyelination and in multiple sclerosis. Glia. 2013;61:1659–72. doi: 10.1002/glia.22547. others. [DOI] [PubMed] [Google Scholar]
- Mronga T, Stahnke T, Goldbaum O, Richter-Landsberg C. Mitochondrial pathway is involved in hydrogen-peroxide-induced apoptotic cell death of oligodendrocytes. Glia. 2004;46:446–455. doi: 10.1002/glia.20022. [DOI] [PubMed] [Google Scholar]
- Murphy T, Miyamoto M, Sastre A, Schnaar R, Coyle J. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989a;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Murphy T, Parikh A, Schnaar R, Coyle JT. Arachidonic acid metabolism in glutamate neurotoxicity. Ann N Y Acad Sci. 1989b;559:474–477. [Google Scholar]
- Ness JK, Romanko MJ, Rothstein RP, Wood TL, Levison SW. Perinatal hypoxia-ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Dev Neurosci. 2001;23:203–208. doi: 10.1159/000046144. [DOI] [PubMed] [Google Scholar]
- Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–89. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyu J, Marumo G, Ozawa H, Takashima S, Nakajima K, Kohsaka S, Hamai Y, Machida Y, Kobayashi K, Ryo E. Early axonal and glial pathology in fetal sheep brains with leukomalacia induced by repeated umbilical cord occlusion. Brain Dev. 1999;21:248–252. doi: 10.1016/s0387-7604(99)00018-2. others. [DOI] [PubMed] [Google Scholar]
- Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci. 1993;13(4):1441–1453. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier P, Baud O, Bouslama M, Evrard P, Gressens P, Verney C. Moderate growth restriction: deleterious and protective effects on white matter damage. Neurobiol Dis. 2007;26:253–63. doi: 10.1016/j.nbd.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Olivier P, Baud O, Evrard P, Gressens P, Verney C. Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol. 2005;64:998–1006. doi: 10.1097/01.jnen.0000187052.81889.57. [DOI] [PubMed] [Google Scholar]
- Olivier P, Loron G, Fontaine RH, Pansiot J, Dalous J, Thi HP, Charriaut-Marlangue C, Thomas JL, Mercier JC, Gressens P. Nitric oxide plays a key role in myelination in the developing brain. J Neuropathol Exp Neurol. 2010;69:828–37. doi: 10.1097/NEN.0b013e3181ea5203. others. [DOI] [PubMed] [Google Scholar]
- Ong WY, Leong SK, Garey LJ, Reynolds R. A light- and electron- microscopic study of GluR4-positive cells in cerebral cortex, subcortical white matter and corpus callosum of neonatal, immature and adult rats. Exp Brain Res. 1996;110 doi: 10.1007/BF00229137. [DOI] [PubMed] [Google Scholar]
- Pagliano E, Fedrizzi E, Erbetta A, Bulgheroni S, Solari A, Bono R, Fazzi E, Andreucci E, Riva D. Cognitive profiles and visuoperceptual abilities in preterm and term spastic diplegic children with periventricular leukomalacia. J Child Neurol. 2007;22:282–8. doi: 10.1177/0883073807300529. [DOI] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK. N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J Neurosci Res. 2004;78:347–61. doi: 10.1002/jnr.20261. [DOI] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: attenuation by N-acetyl cysteine. Exp Neurol. 2008;210:560–76. doi: 10.1016/j.expneurol.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, Lo EH, van Leyen K. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab. 2010;30:1157–67. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallast S, Arai K, Wang X, Lo EH, van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J Neurochem. 2009;111:882–9. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes P. Effects of lipopolysaccharide on oligodendrocyte progenitor cells are mediated by astrocytes and microglia. J Neurosci Res. 2000;62:510–520. doi: 10.1002/1097-4547(20001115)62:4<510::AID-JNR5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Pang Y, Campbell L, Zheng B, Fan L, Cai Z, Rhodes P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. 2010a;166:464–75. doi: 10.1016/j.neuroscience.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Pang Y, Zheng B, Campbell LR, Fan LW, Cai Z, Rhodes PG. IGF-1 can either protect against or increase LPS-induced damage in the developing rat brain. Pediatr Res. 2010b;67:579–84. doi: 10.1203/PDR.0b013e3181dc240f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizi S, Lachance C, Volpe JJ, Follett P, Jensen FE, Rosenberg PA, Vartanian TK. Developing oligodendrocytes are selectivity vulnerable to lipopolysaccharides (LPS) toxicity. Pediatr Res. 2000;47:464A. [Google Scholar]
- Pekcec A, Yigitkanli K, Jung JE, Pallast S, Xing C, Antipenko A, Minchenko M, Nikolov DB, Holman TR, Lo EH. Following experimental stroke, the recovering brain is vulnerable to lipoxygenase-dependent semaphorin signaling. FASEB J. 2012 doi: 10.1096/fj.12-206896. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning D, Grafe J, Hammond R, Matsuda Y, Patrick J, Richardson B. Neuropathology of the near-term and midgestation ovine fetal brain after sustained in utero hypoxemia. Am J Obstet Gynecol. 1994;170:1425–1432. doi: 10.1016/s0002-9378(94)70175-x. [DOI] [PubMed] [Google Scholar]
- Penninga L, Longo L. Ovine placentome morphology: effect of high altitude, long-term hypoxia. Placenta. 1998;19:187–193. doi: 10.1016/s0143-4004(98)90008-x. [DOI] [PubMed] [Google Scholar]
- Perrone S, Negro S, Tataranno ML, Buonocore G. Oxidative stress and antioxidant strategies in newborns. J Matern Fetal Neonatal Med 23 Suppl. 2010;3:63–5. doi: 10.3109/14767058.2010.509940. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–47. doi: 10.1001/jama.284.15.1939. others. [DOI] [PubMed] [Google Scholar]
- Petersson KH, Pinar H, Stopa EG, Faris RA, Sadowska GB, Hanumara RC, Stonestreet BS. White matter injury after cerebral ischemia in ovine fetuses. Pediatr Res. 2002;51:768–776. doi: 10.1203/00006450-200206000-00019. [DOI] [PubMed] [Google Scholar]
- Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, Kinney HC. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–31. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston M, Gong X, Su W, Matsumoto SG, Banine F, Winkler C, Foster S, Xing R, Struve J, Dean J. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013;73:266–80. doi: 10.1002/ana.23788. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad RA, Tan WK, Bennet L, Gunn AJ, Davis SL, Gluckman PD, Johnston BM, Williams CE. Role of the cerebrovascular and metabolic responses in the delayed phases of injury after transient cerebral ischemia in fetal sheep. Stroke. 1999;30:2735–2741. doi: 10.1161/01.str.30.12.2735. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Early oligodendrocyte precursor cells in the human fetal telencephalon. Glia. 2003;41:117–127. doi: 10.1002/glia.10140. [DOI] [PubMed] [Google Scholar]
- Raymond M, Li P, Mangin JM, Huntsman M, Gallo V. Chronic perinatal hypoxia reduces glutamate-aspartate transporter function in astrocytes through the Janus kinase/signal transducer and activator of transcription pathway. J Neurosci. 2011;31:17864–71. doi: 10.1523/JNEUROSCI.3179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees S, Breen S, Loeliger M, McCrabb G, Harding R. Hypoxemia near mid-gestation has long-term effects on fetal brain development. J Neuropathol Exp Neurol. 1999;58:932–945. doi: 10.1097/00005072-199909000-00004. [DOI] [PubMed] [Google Scholar]
- Rees S, Hale N, De Matteo R, Cardamone L, Tolcos M, Loeliger M, Mackintosh A, Shields A, Probyn M, Greenwood D. Erythropoietin is neuroprotective in a preterm ovine model of endotoxin-induced brain injury. J Neuropathol Exp Neurol. 2010;69:306–19. doi: 10.1097/NEN.0b013e3181d27138. others. [DOI] [PubMed] [Google Scholar]
- Rees S, Stringer M, Just Y, Hooper S, Harding R. The vulnerability of the fetal sheep brain to hypoxemia at mid-gestation. Devel Brain Res. 1997;103:103–118. doi: 10.1016/s0165-3806(97)81787-7. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Carmichael L, Homan J, Patrick JE. Cerebral oxidative-metabolism in fetal sheep with prolonged and graded hypoxemia. J Dev Physiol. 1993;19:77–83. [PubMed] [Google Scholar]
- Riddle A, Maire J, Cai V, Nguyen T, Gong X, Hansen K, Grafe M, Hohimer A, Back S. Hemodynamic and metabolic correlates of perinatal white matter injury severity. PLoS One. 2013;8:e82940. doi: 10.1371/journal.pone.0082940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle A, Dean J, JR B, Gong X, Maire J, Chen K, Ahmad T, Chen V, Nguyen T, Kroenke C. Histopathological correlates of MRI-defined chronic perinatal white matter injury. Ann Neurol. 2011;70:493–507. doi: 10.1002/ana.22501. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle A, Luo N, Manese M, Beardsley D, Green L, Rorvik D, Kelly K, Barlow C, Kelly J, Hohimer A. Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. J Neurosci. 2006;26:3045–3055. doi: 10.1523/JNEUROSCI.5200-05.2006. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle A, Maire J, Gong X, Chen K, CD K, AR H, SA B. Differential susceptibility to axonopathy in necrotic and non-necrotic perinatal white matter injury. Stroke. 2012;43:178–184. doi: 10.1161/STROKEAHA.111.632265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson NJ, Tan S, Groenendaal F, van Bel F, Juul SE, Bennet L, Derrick M, Back SA, Valdez RC, Northington F. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J Pediatr. 2012;160:544–552. e4. doi: 10.1016/j.jpeds.2011.12.052. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA, Dai WM, Gan XD, Ali S, Fu J, Back SA, Sanchez RM, Segal MM, Follett PL, Jensen FE. Mature myelin basic protein-expressing oligodendrocytes are insensitive to kainate toxicity. J Neurosci Res. 2003;71:237–245. doi: 10.1002/jnr.10472. others. [DOI] [PubMed] [Google Scholar]
- Sadowska GB, Threlkeld SW, Flangini A, Sharma S, Stonestreet BS. Ontogeny and the effects of in utero brain ischemia on interleukin-1β and interleukin-6 protein expression in ovine cerebral cortex and white matter. Int J Dev Neurosci. 2012;30:457–463. doi: 10.1016/j.ijdevneu.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. The mechanisms of acute ischemic injury in the cell processes of developing white matter astrocytes. J Cereb Blood Flow Metab. 2008;28:588–601. doi: 10.1038/sj.jcbfm.9600555. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gomez M, Alberdi E, Ibarretxe G, Torre I, Matute C. Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. J Neurosci. 2003;23:9519–9528. doi: 10.1523/JNEUROSCI.23-29-09519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gomez M, Alberdi E, Perez-Navarro E, Alberch J, Matute C. Bax and calpain mediate excitotoxic oligodendrocyte death induced via activation of both AMPA and kainate receptors. J Neurosci. 2011;31:2996–3006. doi: 10.1523/JNEUROSCI.5578-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gomez M, Matute C. AMPA and kainate receptors each mediate excitotoxicity in oligodendroglial cultures. Neurobiol Dis. 2000;6:475–485. doi: 10.1006/nbdi.1999.0264. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Scafidi J, Hammond TR, Scafidi S, Ritter J, Jablonska B, Roncal M, Szigeti-Buck K, Coman D, Huang Y, McCarter RJ. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2013 doi: 10.1038/nature12880. others in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafidi J, Fagel DM, Ment LR, Vaccarino FM. Modeling premature brain injury and recovery. Int J Dev Neurosci. 2009;27:863–71. doi: 10.1016/j.ijdevneu.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, Davis PG, Tin W, Moddemann D, Solimano A. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307:275–82. doi: 10.1001/jama.2011.2024. others. [DOI] [PubMed] [Google Scholar]
- Schmitz T, Ritter J, Mueller S, Felderhoff-Mueser U, Chew L-J, Gallo V. Cellular changes underlying hyperoxia-induced delay of white matter development. J Neurosci. 2011;31:4327–4344. doi: 10.1523/JNEUROSCI.3942-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See J, Zhang X, Eraydin N, Mun SB, Mamontov P, Golden JA, Grinspan JB. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol Cell Neurosci. 2004;26:481–92. doi: 10.1016/j.mcn.2004.04.004. [DOI] [PubMed] [Google Scholar]
- See JM, Grinspan JB. Sending mixed signals: bone morphogenetic protein in myelination and demyelination. J Neuropathol Exp Neurol. 2009;68:595–604. doi: 10.1097/NEN.0b013e3181a66ad9. [DOI] [PubMed] [Google Scholar]
- Segovia K, Mcclure M, Moravec M, Luo N, Wang Y, Gong X, Riddle A, Craig A, Struve J, Sherman L. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:517–526. doi: 10.1002/ana.21359. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–48. doi: 10.1016/j.cmet.2008.07.005. others. [DOI] [PubMed] [Google Scholar]
- Selip DB, Jantzie LL, Chang M, Jackson MC, Fitzgerald EC, Boll G, Murphy A, Jensen FE. Regional differences in susceptibility to hypoxic-ischemic injury in the preterm brain: exploring the spectrum from white matter loss to selective grey matter injury in a rat model. Neurol Res Int. 2012:725184. doi: 10.1155/2012/725184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Sfaello I, Baud O, Arzimanoglou A, Gressens P. Topiramate prevents excitotoxic damage in the newborn rodent brain. Neurobiol Dis. 2005;20:837–848. doi: 10.1016/j.nbd.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–5. 175, e1. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Shannon C, Salter M, Fern R. GFP imaging of live astrocytes: regional differences in the effects of ischaemia upon astrocytes. J Anat. 2007;210:684–92. doi: 10.1111/j.1469-7580.2007.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L, Back S. A GAG reflex prevents repair of the damaged CNS. Trends Neurosci. 2008;31:44–52. doi: 10.1016/j.tins.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Sherwin C, Fern R. Acute lipopolysaccharide-mediated injury in neonatal white matter glia: role of TNF-α, IL-β, and calcium. J Immunol 1. 2005;175:155–161. doi: 10.4049/jimmunol.175.1.155. [DOI] [PubMed] [Google Scholar]
- Shin D, Shin JY, McManus MT, Ptacek LJ, Fu YH. Dicer ablation in oligodendrocytes provokes neuronal impairment in mice. Ann Neurol. 2009;66:843–57. doi: 10.1002/ana.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis J, Huang E, Back S, Rowitch D. Toward improved animal models of neonatal white matter injury associate with cerebral palsy. Dis Model Mech. 2010;3:678–688. doi: 10.1242/dmm.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizonenko SV, Bednarek N, Gressens P. Growth factors and plasticity. Semin Fetal Neonatal Med. 2007;12:241–9. doi: 10.1016/j.siny.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Sizonenko SV, Camm EJ, Dayer A, Kiss JZ. Glial responses to neonatal hypoxic-ischemic injury in the rat cerebral cortex. Int J Dev Neurosci. 2008;26:37–45. doi: 10.1016/j.ijdevneu.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Sizonenko SV, Kiss JZ, Inder T, Gluckman PD, Williams CE. Distinctive neuropathologic alterations in the deep layers of the parietal cortex after moderate ischemic-hypoxic injury in the P3 immature rat brain. Pediatr Res. 2005;57:865–72. doi: 10.1203/01.PDR.0000157673.36848.67. [DOI] [PubMed] [Google Scholar]
- Sizonenko SV, Sirimanne E, Mayall Y, Gluckman PD, Inder T, Williams C. Selective cortical alteration after hypoxic-ischemic injury in the very immature rat brain. Pediatr Res. 2003;54:263–9. doi: 10.1203/01.PDR.0000072517.01207.87. [DOI] [PubMed] [Google Scholar]
- Sloane J, Batt C, Ma Y, Harris Z, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci USA. 2010;107:11555–11560. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Pastor S, Gimenez M, Narberhaus A, Falcon C, Botet F, Bargallo N, Mercader JM, Junque C. Patterns of cerebral white matter damage and cognitive impairment in adolescents born very preterm. Int J Dev Neurosci. 2008;26:647–54. doi: 10.1016/j.ijdevneu.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Soria-Pastor S, Padilla N, Zubiaurre-Elorza L, Ibarretxe-Bilbao N, Botet F, Costas-Moragas C, Falcon C, Bargallo N, Mercader JM, Junque C. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009;124:e1161–70. doi: 10.1542/peds.2009-0244. [DOI] [PubMed] [Google Scholar]
- Spittle AJ, Boyd RN, Inder TE, Doyle LW. Predicting motor development in very preterm infants at 12 months' corrected age: the role of qualitative magnetic resonance imaging and general movements assessments. Pediatrics. 2009;123:512–7. doi: 10.1542/peds.2008-0590. [DOI] [PubMed] [Google Scholar]
- Spittle AJ, Brown NC, Doyle LW, Boyd RN, Hunt RW, Bear M, Inder TE. Quality of general movements is related to white matter pathology in very preterm infants. Pediatrics. 2008;121:e1184–9. doi: 10.1542/peds.2007-1924. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Stys PK. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol Med. 2010;16:160–70. doi: 10.1016/j.molmed.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve J, Maher P, Li Y, Kinnery S, Fehlings MG, Kuntz Ct, Sherman LS. Disruption of the hyaluronan-based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia. 2005;52:16–24. doi: 10.1002/glia.20215. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, Goossens V, Roelandt R, Van Hauwermeiren F, Libert C. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Fishman R, Park H, Folkerth R, Follett P, Volpe J, Jensen F. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. J Comp Neurol. 2006a;497:42–60. doi: 10.1002/cne.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Follett PL, Folkerth RD, Fishman RE, Trachtenberg FL, Volpe JJ, Jensen FE. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol. 2006b;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawk M, Makoukji J, Belle M, Fonte C, Trousson A, Hawkins T, Li H, Ghandour S, Schumacher M, Massaad C. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J Neurosci. 2011;31:3729–42. doi: 10.1523/JNEUROSCI.4270-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting P, Yamaguchi S, Bacher J, Killens R, Myers R. Hypoxic-ischemic cerebral necrosis in midgestation sheep fetuses: physiopathological correlations. Exp Neurol. 1983;80:227–245. doi: 10.1016/0014-4886(83)90019-5. [DOI] [PubMed] [Google Scholar]
- Trollmann R, Gassmann M. The role of hypoxia-inducible transcription factors in the hypoxic neonatal brain. Brain Dev. 2009;31:503–9. doi: 10.1016/j.braindev.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Turner C, Seli M, Ment L, Stewart WA, Yan H, Johansson B, Fredholm B, Blackburn M, RIvkees S. A1 adenosine receptors mediate hypoxia-induced ventriculomegaly. Proc Natl Acad Sci USA. 2003;100:11718–11722. doi: 10.1073/pnas.1931975100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C, Yan H, Schwartz M, Othman T, Rivkees S. A1 adenosine receptor activation induces ventriculomegaly and white matter loss. Neuroreport. 2002;13:1199–1204. doi: 10.1097/00001756-200207020-00026. [DOI] [PubMed] [Google Scholar]
- Uchida N, Chen K, Dohse M, Hansen KD, Dean J, Buser JR, Riddle A, Beardsley DJ, Wan Y, Gong X. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med. 2012;4:90–100. doi: 10.1126/scitranslmed.3004371. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leyen K, Arai K, Jin G, Kenyon V, Gerstner B, Rosenberg PA, Holman TR, Lo EH. Novel lipoxygenase inhibitors as neuroprotective reagents. J Neurosci Res. 2008;86:904–9. doi: 10.1002/jnr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–8. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Grootjans S, Callewaert N, Takahashi N. Necrostatin-1 blocks both RIPK1 and IDO: consequences for the study of cell death in experimental disease models. Cell Death Differ. 2013;20:185–7. doi: 10.1038/cdd.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian T, Li Y, Zhao M, Stefanson K. Interferon-γ-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med. 1995;1:732–743. [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the Newborn. W.B. Saunders; Philadelphia: 2008a. pp. 399–407. [Google Scholar]
- Volpe JJ. Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J Pediatr. 2008b;153:160–3. doi: 10.1016/j.jpeds.2008.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose LR, Vinukonda G, Jo S, Miry O, Diamond D, Korumilli R, Arshad A, Zia MT, Hu F, Kayton RJ. Treatment with thyroxine restores myelination and clinical recovery after intraventricular hemorrhage. J Neurosci. 2013;33:17232–46. doi: 10.1523/JNEUROSCI.2713-13.2013. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li J, Follett PL, Zhang Y, Cotanche DA, Jensen FE, Volpe JJ, Rosenberg PA. 12-Lipoxygenase plays a key role in cell death caused by glutathione depletion and arachidonic acid in rat oligodendrocytes. Eur J Neurosci. 2004;20:2049–58. doi: 10.1111/j.1460-9568.2004.03650.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Hellgren G, Lofqvist C, Li W, Hellstrom A, Hagberg H, Mallard C. White matter damage after chronic subclinical inflammation in newborn mice. J Child Neurol. 2009;24:1171–8. doi: 10.1177/0883073809338068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheng X, He Q, Zheng Y, Kim D, Whittemore S, Cao Q. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31:6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber DJ, van Blitterswijk M, Chandran S. Neuroprotective effect of oligodendrocyte precursor cell transplantation in a long-term model of periventricular leukomalacia. Am J Pathol. 2009;175:2332–42. doi: 10.2353/ajpath.2009.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welin AK, Svedin P, Lapatto R, Sultan B, Hagberg H, Gressens P, Kjellmer I, Mallard C. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr Res. 2007;61:153–8. doi: 10.1203/01.pdr.0000252546.20451.1a. [DOI] [PubMed] [Google Scholar]
- Welin A-K, Sandberg M, Lindblom A, Arvidsson P, Nilsson U, Kjellmer I, Mallard C. White matter injury following prolonged free radical formation in the 0.65 gestation fetal sheep brain. Pediatr Res. 2005;58:100–105. doi: 10.1203/01.PDR.0000163388.04017.26. [DOI] [PubMed] [Google Scholar]
- Wernovsky G, Shillingford A, Gaynor J. Central nervous system outcomes in children with complex congenital heart disease. Curr Opin Cardiol. 2005;20:94–99. doi: 10.1097/01.hco.0000153451.68212.68. [DOI] [PubMed] [Google Scholar]
- Wilke S, Thomas R, Allcock N, Fern R. Mechanism of acute ischemic injury of oligodendroglia in early myelinating white matter: the importance of astrocyte injury and glutamate release. J Neuropathol Exp Neurol. 2004;68:872–881. doi: 10.1093/jnen/63.8.872. [DOI] [PubMed] [Google Scholar]
- Wilson-Costello D, Fridedman H, Minich N, Fanaroff A, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- Wong DS, Poskitt KJ, Chau V, Miller SP, Roland E, Hill A, Tam EW. Brain injury patterns in hypoglycemia in neonatal encephalopathy. AJNR Am J Neuroradiol. 2013;34:1456–61. doi: 10.3174/ajnr.A3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TL, Loladze V, Altieri S, Gangoli N, Levison SW, Brywe KG, Mallard C, Hagberg H. Delayed IGF-1 administration rescues oligodendrocyte progenitors from glutamate-induced cell death and hypoxic-ischemic brain damage. Dev Neurosci. 2007;29:302–10. doi: 10.1159/000105471. [DOI] [PubMed] [Google Scholar]
- Wright J, Zhang G, Yu T-S, Kernie S. Age-related changes in the oligodendrocyte progenitor pool influence brain remodeling after injury. Dev Neurosci. 2010;32:499–509. doi: 10.1159/000322081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong T, Qu Y, Mu D, Ferriero D. Erythropoietin for neonatal brain injury: opportunity and challenge. Int J Dev Neurosci. 2011;29:583–591. doi: 10.1016/j.ijdevneu.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Yang Z, Levison SW. Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience. 2006;139:555–64. doi: 10.1016/j.neuroscience.2005.12.059. [DOI] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–38. doi: 10.1038/nn.2333. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Wyeth M, Baltan-Tekkok S, Ransom B. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, Smirnova N, Gazaryan I, Ratan RR, Witztum JL. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann Neurol. 2012 doi: 10.1002/ana.23734. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa M, Back SA, Gan XD, Rosenberg PA, Volpe JJ. Cystine deprivation induces oligodendroglial death: Rescue by free radical scavengers and by a diffusible glial factor. J Neurochem. 1996;67:566–573. doi: 10.1046/j.1471-4159.1996.67020566.x. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, Chi JG. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Yang SH, Jun JK, Kim I-O, Choi J-H, Syn HC. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- Yoshioka A, Hardy M, Younkin DP, Grinspan J, Stern JL, Pleasure D. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors mediate excitotoxicity in the oligodendroglial lineage. J Neurochem. 1995;64:2442–2448. doi: 10.1046/j.1471-4159.1995.64062442.x. [DOI] [PubMed] [Google Scholar]
- Zaidi A, Bessert D, Ong J, Xu H, Barks J, Silverstein F. New oligodendrocytes are generated after neonatal hypoxic-ischemic brain injury in rodents. Glia. 2004;46:380–390. doi: 10.1002/glia.20013. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Aizenman E, DeFranco DB, Rosenberg PA. Intracellular zinc release, 12-lipoxygenase activation and MAPK dependent neuronal and oligodendroglial death. Mol Med. 2007;13:350–5. doi: 10.2119/2007-00042.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Li J, Dong L, Xu P, Chen W, Neve RL, Volpe JJ, Rosenberg PA. Intracellular zinc release and ERK phosphorylation are required upstream of 12-lipoxygenase activation in peroxynitrite toxicity to mature rat oligodendrocytes. J Biol Chem. 2006;281:9460–70. doi: 10.1074/jbc.M510650200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Li J, Jiminez DA, Levitan ES, Aizenman E, Rosenberg PA. Peroxynitrite induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J Neurosci. 2004;24:10616–10627. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiheng H, Liu J, Cheung P-Y, Chen C. Long-term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic-ischemia brain injury. Brain Res. 2009;1301:100–109. doi: 10.1016/j.brainres.2009.09.006. [DOI] [PubMed] [Google Scholar]