Abstract
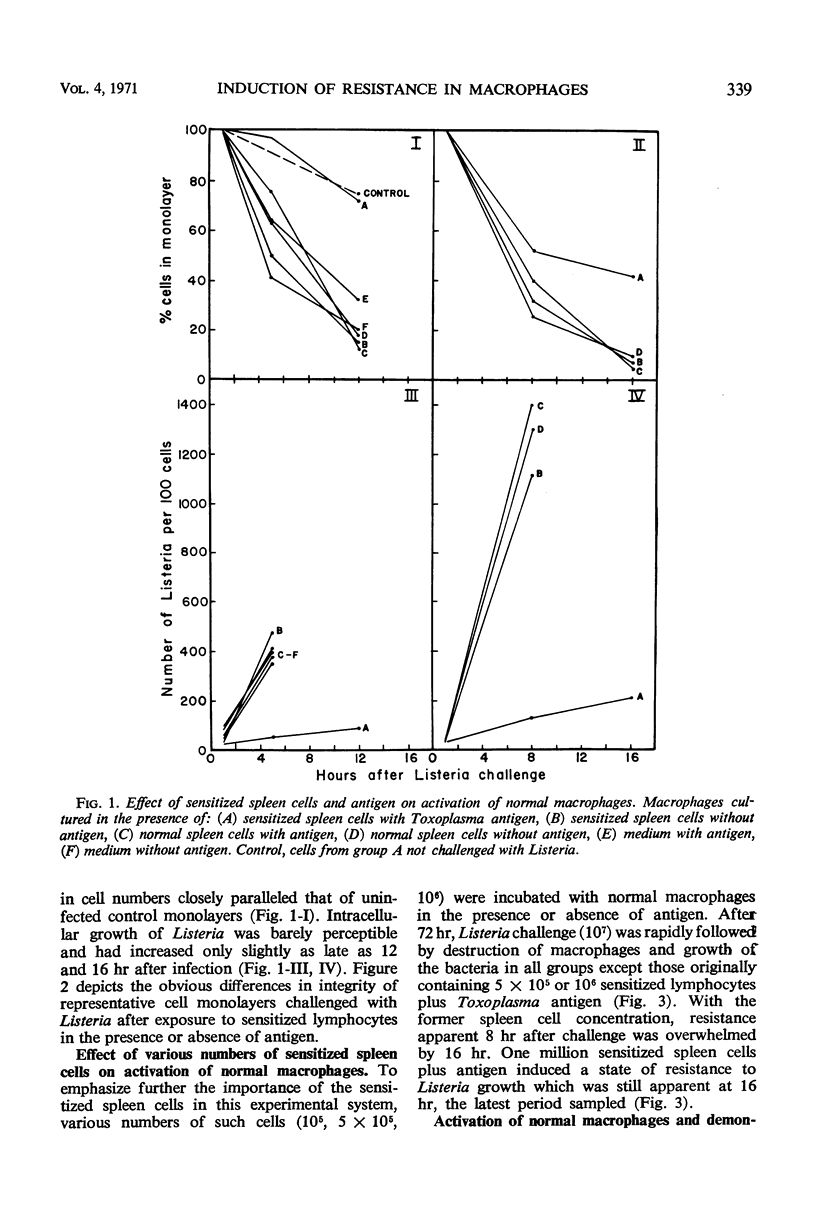
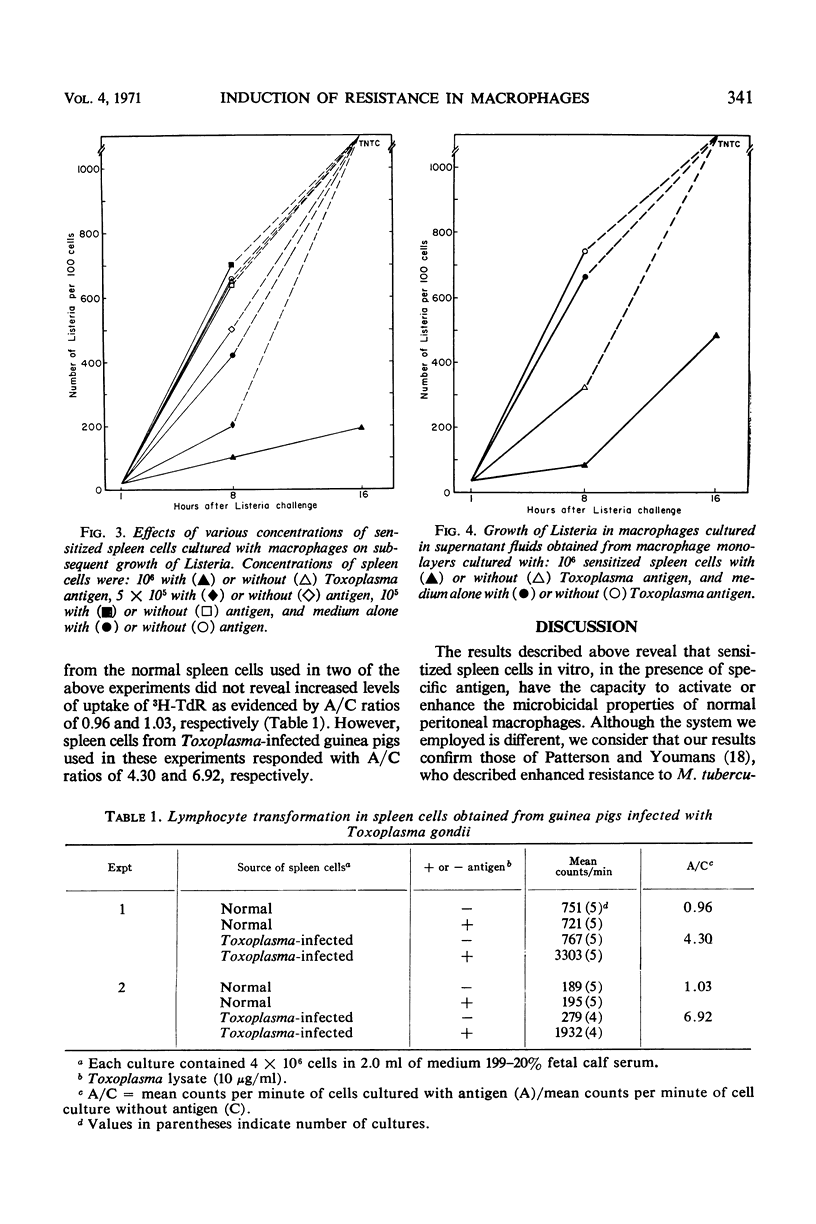
Experiments were carried out to determine whether macrophages can be activated in vitro to resist challenge with heterologous microorganisms. Sensitized spleen cells from guinea pigs chronically infected with Toxoplasma gondii were cultured with normal guinea pig peritoneal macrophages in the presence and absence of Toxoplasma antigen. Macrophage monolayers incubated with sensitized spleen cells and antigen were markedly resistant to challenge from Listeria monocytogenes. Resistance was manifested by prolonged survival of the monolayers and rapid intracellular killing of the bacteria. Macrophages incubated with sensitized spleen cells but in the absence of antigen, as well as macrophages cultured with normal spleen cells, in the presence or absence of antigen, were rapidly destroyed. Sensitized spleen cells responded to the presence of Toxoplasma antigen by increased uptake of tritium-labeled thymidine. Supernatant fluid medium obtained from cultures of macrophages, sensitized spleen cells, and antigen contained a macrophage migration inhibitory factor(s). In addition, these supernatant fluids were capable of inducing increased resistance to Listeria in normal macrophages.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett B., Bloom B. R. Reactions in vivo and in vitro produced by a soluble substance associated with delayed-type hypersensitivity. Proc Natl Acad Sci U S A. 1968 Mar;59(3):756–762. doi: 10.1073/pnas.59.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Coppel S., Youmans G. P. Specificity of the anamnestic response produced by Listeria monocytogenes or Mycobacterium tuberculosis to challenge with Listeria monocytogenes. J Bacteriol. 1969 Jan;97(1):127–133. doi: 10.1128/jb.97.1.127-133.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger G. A., Kolb W. P. Lymphocyte in vitro cytotoxicity: mechanisms of immune and non-immune small lymphocyte mediated target L cell destruction. J Immunol. 1968 Jul;101(1):111–120. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Blazkovec A. A., Lysenko M. G. In Vivo and In Vitro Studies of Delayed-Type Hypersensitivity to Toxoplasma gondii in Guinea Pigs. Infect Immun. 1971 Feb;3(2):260–267. doi: 10.1128/iai.3.2.260-267.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Blazkovec A. A., Lysenko M. G. The use of tissue culture-grown trophozoites of an avirulent strain of Toxoplasma gondii for the immunization of mice and guinea pigs. J Parasitol. 1971 Apr;57(2):386–390. [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Aronson W. J., Remington J. S. Late parasitemia in asymptomatic acquired toxoplasmosis. Ann Intern Med. 1969 Jul;71(1):139–145. doi: 10.7326/0003-4819-71-1-139. [DOI] [PubMed] [Google Scholar]
- Mooney J. J., Waksman B. H. Activation of normal rabbit macrophage monolayers by supernatants of antigen-stimulated lymphocytes. J Immunol. 1970 Nov;105(5):1138–1145. [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMINGTON J. S., MELTON M. L., JACOBS L. Induced and spontaneous recurrent parasitemia in chronic infections with avirulent strains of Toxoplasma gondii. J Immunol. 1961 Nov;87:578–581. [PubMed] [Google Scholar]
- Remington J. S., Bloomfield M. M., Russell E., Jr, Robinson W. S. The RNA of toxoplasma gondii. Proc Soc Exp Biol Med. 1970 Feb;133(2):623–626. doi: 10.3181/00379727-133-34531. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Cavanaugh E. N. Isolation of the encysted form of Toxoplasma gondii from human skeletal muscle and brain. N Engl J Med. 1965 Dec 9;273(24):1308–1310. doi: 10.1056/NEJM196512092732404. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Hackman R. Changes in mouse serum proteins during acute and chronic infection with an intracellular parasite (Toxoplasma gondii). J Immunol. 1965 Dec;95(6):1023–1033. [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Resistance to virus challenge in mice infected with protozoa or bacteria. Proc Soc Exp Biol Med. 1969 Sep;131(4):1184–1188. doi: 10.3181/00379727-131-34066. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Waksman B. H. Cytotoxicity mediated by soluble antigen and lymphocytes in delayed hypersensitivity. I. Characterization of the phenomenon. J Exp Med. 1968 Dec 1;128(6):1237–1254. doi: 10.1084/jem.128.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- Ruskin J., Remington J. S. Immunity and intracellular infection: resistance to bacteria in mice infected with a protozoan. Science. 1968 Apr 5;160(3823):72–74. doi: 10.1126/science.160.3823.72. [DOI] [PubMed] [Google Scholar]
- Ruskin J., Rengton J. S. Role for the macrophage in acquired immunity to phylogenetically unrelated intracellular organisms. Antimicrob Agents Chemother (Bethesda) 1968;8:474–477. doi: 10.1128/AAC.8.4.474. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. Leukotactic factor produced by sensitized lymphocytes. Science. 1969 Mar 7;163(3871):1079–1081. doi: 10.1126/science.163.3871.1079. [DOI] [PubMed] [Google Scholar]



