Abstract
Fungi represent emerging infectious threats to human populations worldwide. Mice and other laboratory animals have proved invaluable in modeling clinical syndromes associated with superficial and life-threatening invasive mycoses. This review outlines salient features of common vertebrate animal model systems to study fungal pathogenesis, host antifungal immune responses, and antifungal compounds.
1. Introduction
Fungal pathogens are associated with significant infectious morbidity and mortality in humans and with extinctions in amphibian (i.e. collapse of frog species due to chytridioidomycosis) and mammalian (i.e. white nose bat syndrome in the Northeastern United States) populations (Heitman, 2011; Brown et al., 2012; Fisher et al., 2012). The emergence of Cryptococcus gattii as a primary pathogen in the Pacific Northwest (Byrnes et al., 2011), the description of a novel opportunistic fungus (Emmonsia parva) in South African AIDS patients (Kenyon et al., 2013), and the recent outbreak of fungal meningitis due to contaminated corticosteroid injections (Kainer et al., 2012; Smith et al., 2013) all exemplify novel public health threats posed by fungi.
Superficial and mucosal fungal infections, though rarely life-threatening, affect approximately one-quarter of humans worldwide and cause discomfort, disfigurement, diminished reproductive function, and social isolation. Although life-threatening invasive fungal infections are much less frequent, a recent publication estimated that ~2 million such infections occur annually (Brown et al., 2012). Mortality rates associated with invasive infections remain unacceptably high due to lack of access (in the developing world) and limited efficacy of antifungal drugs, and the presence of significant co-morbidities (e.g. AIDS, receipt of immunosuppression for organ transplantation, receipt of myeloablative therapy for cancer) in many patient groups [reviewed in (Brown et al., 2012)]. Furthermore, no vaccines have been licensed for human use to prevent or mitigate fungal infections.
Over the past 4 to 5 decades, researchers have developed a plethora of animal models to investigate fungal pathogenesis, host immune responses, and to examine antifungal properties of chemical and biological compounds. Although inbred strains of laboratory mice are most commonly used to model clinical syndromes associated with pathogenic fungi, other vertebrate hosts (e.g. rats, guinea pigs, rabbits, and zebrafish) have gained popularity, since each model system affords distinct advantages (e.g. repeated body fluid sampling and drug administration in rabbits, availability of genetically defined strains in mice, non-invasive imaging of the infection process in transparent zebrafish larvae) as well as limitations. The purpose of this review is to provide an overview of common syndromes caused by pathogenic fungi and of relevant vertebrate animal models developed to gain insight into fungal pathogenesis process, host immune responses, and the diagnosis and treatment of fungal infections. Due to the broad nature of the topic, it is not possible to emphasize technical detail in all vertebrate animal models discussed herein and the reader is referred to references in Table I. Several outstanding recent reviews summarize the emergence of fruit flies (Drosophila melanogaster) (Lionakis and Kontoyiannis, 2012), wax moths (Galleria mellonella) (Achterman et al., 2011; Lionakis, 2011), and nematodes (Caenorhabditis elegans) (Muhammed et al., 2012a) as invertebrate mini-hosts that are particularly suited for high-throughput screening of chemical libraries for antifungal drug discovery, high-throughput analysis of fungal mutants, and for host-pathogen studies at ambient temperature (Fuchs and Mylonakis, 2006; Desalermos et al., 2012). Similarly, the review does not aim to summarize the wealth of insight gained from animal models into the pathogenesis, host immune response, diagnosis, and treatment of fungal infections and the reader is referred to recent publications (Sable et al., 2008; Brown, 2011; Steele and Wormley, 2012; Wuthrich et al., 2012; Drew et al., 2013; Lanternier et al., 2013).
Table I.
References for Vertebrate Models of Human Fungal Diseases.
| Mycosis | Human Syndrome | Route of Administration in Animal Models |
Selected References for Vertebrate Animal Models |
|---|---|---|---|
|
Aspergillosis (Aspergillus fumigatus) |
Pulmonary aspergillosis | Intratracheal, intranasal, inhalational |
(Dixon et al., 1989; Sheppard et al., 2004; Clemons and Stevens, 2005; Stephens-Romero et al., 2005; Sheppard et al., 2006a; Desoubeaux and Chandenier, 2012; Muhammed et al., 2012b) |
| Allergic bronchopulmonary aspergillosis |
Sensitization with extract ± inhalational or intratracheal challenge with conidia |
(Kurup et al., 1992; Hoselton et al., 2010; Samarasinghe et al., 2011) | |
| Cerebral aspergillosis | Intracranial | (Chiller et al., 2002) | |
| Aspergillus sinusitis | Intranasal | (Rodriguez et al., 2007) | |
| Aspergillus keratitis | Intraocular | (Leal et al., 2010) | |
|
Blastomycosis (Blastomyces dermatidites) |
Pulmonary blastomycosis | Intratracheal, intranasal | (Williams and Moser, 1987; Stevens, 1997; Sorensen et al., 1999) |
| Not applicable | Subcutaneous, vaccine strain |
(Wuthrich et al., 2000) | |
|
Candidiasis (Candida albicans) |
Disseminated candidiasis | Intravenous | (de Repentigny, 2004; Spellberg et al., 2005; Lionakis et al., 2011; MacCallum, 2012; MacCallum, 2013) |
| Disseminated candidiasis | Via drinking water (+ mucosal damage + immunosuppression) |
(Koh et al., 2008) | |
| Neonatal disseminated | Intraperitoneal in day+2 mouse pups |
(Tsai et al., 2011) | |
| Oropharyngeal candidiasis |
Oropharyngeal | (Samaranayake and Samaranayake, 2001; Bar et al., 2012; Rahman et al., 2012; Solis and Filler, 2012; Mosci et al., 2013) | |
| Vaginal candidiasis | Vaginal | (Fidel et al., 1993; Naglik et al., 2008; Rahman et al., 2012) | |
| Catheter- or denture- associated candidiasis (biofilm) |
Instillation in catheter or dentures |
(Nett et al., 2010; Nett et al., 2012) | |
|
Chromoblastomycosis (Fonsecaea pedrosoi) |
Subcutaneous chromo- blastomycosis |
Intraperitoneal | (Sousa Mda et al., 2011) |
|
Coccidioidomycosis* (Coccidioides immitis, Coccidioides posadasii) |
Pulmonary coccidioidomycosis |
Intratracheal, intranasal, inhalational |
(Sorensen et al., 1999; Clemons et al., 2007; Muhammed et al., 2012b) |
| Coccidioides meningitis | intracisternal | (Williams et al., 1998; Kamberi et al., 2003; Clemons et al., 2007) | |
|
Cryptococcosis (Cryptococcus neoformans, Cryptococcus gattii) |
Pulmonary cryptococcosis | intratracheal or intranasal |
(Torres-Rodriguez et al., 2003; Muhammed et al., 2012b) |
| Cryptococcal meningitis | Intracerebral or intrathecal | (Ding et al., 1997; Najvar et al., 1999; Kirkpatrick et al., 2007; Thompson et al., 2012) | |
|
Dermatophytoses (Epidermophyton, Micro- sporum, Trichophyton sp.) |
Tinea (denotes fungal infection of skin) |
Cutaneous ± tape stripping or skin shaving |
(Hay et al., 1983; Van Cutsem and Janssen, 1984; Van Cutsem, 1989; Saunte et al., 2008; Koga, 2009) |
|
Fusariosis (Fusarium solani, Fusarium oxysporum) |
Fusarium keratitis | Intraocular injection or abrasion + infected contact lens |
(Tarabishy et al., 2008; Sun et al., 2010) |
| Pulmonary fusariosis | intratracheal | (Muhammed et al., 2012b) | |
|
Histoplasmosis (Histoplasma capsulatum) |
Pulmonary histoplasmosis | intranasal | (Sorensen et al., 1999; Muhammed et al., 2012b; Smulian, 2012) |
|
Mucormycosis (Rhizopus oryzae) |
Pulmonary mucormycosis | Intratracheal or intranasal | (Reinhardt et al., 1981; Waldorf et al., 1982; Kamei, 2001; Liu et al., 2010; Muhammed et al., 2012b) |
|
Paracoccidioidomycosis (Paracoccidioides brasiliensis) |
Pulmonary paracoccidioidomycosis |
intranasal | (Brummer et al., 1984; McEwen et al., 1987) |
|
Penicilliosis (Penicillium manefeii) |
Pulmonary penicilliosis | Intratracheal | (Kudeken et al., 1996) |
|
Pytisporosis (Malassezia sp.) |
Tinea versicolor | cutaneous | (Van Cutsem, 1989; Koga, 2009) |
|
Pneumocystosis (Pneumocystis jiroveci) |
Pneumocystis pneumonia | intratracheal | (Dei-Cas et al., 1998) |
|
Sporothricosis (Sprorothrix schenkii) |
Cutaneous sporothricosis (+ lymphatic spread) |
subcutaneous | (Charoenvit and Taylor, 1979; Brito et al., 2007) |
classified as a Biosafety level 3 pathogen and potential agent of bioterrorism; US laboratories working with this organism must be registered with the Select Agent Program under the CDC or US Department of Agriculture.
2. Human Pathogenic Fungi and Clinical Syndromes
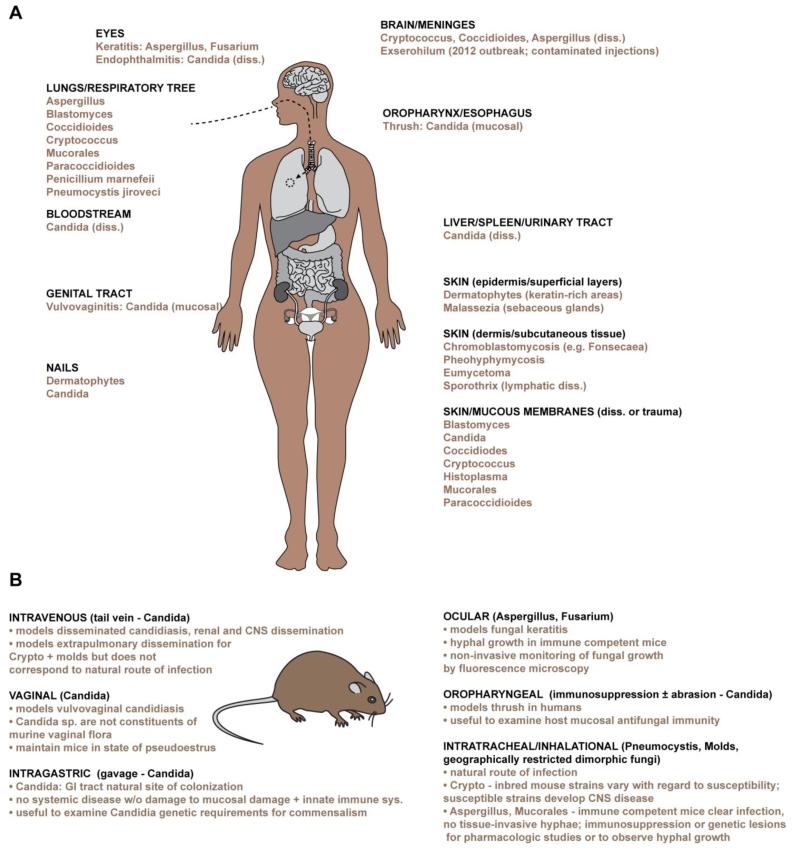
The Earth harbors an estimated 5 million species of fungi that prosper in all natural habitats and collectively play a vital role in decomposing organic matter (Blackwell, 2011). Fungi assume different cellular morphologies. Yeasts form round, oval, or spherical cells that divide by budding, or in rare cases, by fission. Molds form branching tubular filaments, termed hyphae, that grow by elongation and propagate by forming conidia (asexual spores), most of which are dispersed in the air. Dimorphic fungi can switch between morphologic states and typically exist as yeast cells in human tissues and as hyphae within natural environments. Medically relevant yeasts, molds, dimorphic fungi, and the most common sites of clinical disease in humans are summarized in Fig. 1A.
Figure 1.
Medically relevant fungi, syndromes, and murine models of disease.
A, The schematic depicts the primary anatomic sites commonly affected by medically relevant fungal diseases in humans. Most fungal diseases are acquired when infectious particles are inhaled, inoculated via trauma, or penetrate breaches in mucosal integrity. Common sites of fungal dissemination (diss.) are indicated. B, The schematic depicts common routes of fungal administration in vertebrate models of disease. Specific features and experimental considerations associated with each infection route are indicated.
Several hundred species of fungi have been documented to cause superficial, deep tissue, and disseminated diseases in humans. Dermatophytes are the primary causes of superficial infections of the skin (in the epidermal layer) and nails, primarily due to species from the genera Trichophyton, Epidermophyton, and Microsporum (Havlickova et al., 2008). Dermatophytes thrive on human keratin and commonly give rise to infections of the scalp (Tinea capitis, a.k.a as ringworm; over 200 million cases in children worlwide), glabrous skin (Tinea corporis), groin (Tinea cruris), feet (Tinea pedis), and nails (Tinea unguium, a.k.a. onychomycosis; up to a 10% prevalence worldwide, particularly in diabetics and the elderly), yet rarely invade deep tissues or enter the circulation (Achterman and White, 2012). In skin areas rich in sebaceous glands, several species of the lipophilic fungus Malassezia are a common cause of dandruff and seborrheic dermatitis. Subcutaneous infections that extend into the deeper dermal layer (e.g. chromoblastomycosis or phaeohyphomycosis) are typically acquired by traumatic inoculation of fungal elements into human skin and give rise to chronic, slow-growing infections that can be highly destructive at local sites, yet rarely disseminate systemically. A variety of Candida species cause superficial infections of the skin, oral and genital mucosa. For example, 10 million estimated cases of oral or esophageal thrush occur annually, most of which are linked to the HIV pandemic. Vulvovaginal candidiasis is common in adult women, with approximately one-half of women experiencing at least one symptomatic episode during their childbearing years, and an estimated 50 million women that suffer from recurrent disease, defined as four or more episodes annually. Primary immunodeficiency syndromes, particularly defects in the respiratory burst (i.e. chronic granulomatous disease) or in the interleukin-17 (IL-17) cytokine signaling pathway predispose to mucocutaneous candidiasis (Lanternier et al., 2013).
The majority of fungi associated with life-threatening infections are inhaled as infectious propagules, invade sinopulmonary tissues, and in specific instances, disseminate to extrapulmonary sites. Candida species are the major exception to this rule and reside in the gastrointestinal tract as commensal organisms in humans. Of the estimated 2 million annual life-threatening invasive infections, 90% are caused by Candida, Cryptococcus, Aspergillus, and Pneumocystis species (Brown et al., 2012). Geographically restricted dimorphic fungi (i.e Blastomyces dermatiditis, Coccidioides immitis, Histoplasma capsulatum, Paracoccidioides brasiliensis, and Penicillium marnefeii) and non-Aspergillus molds (i.e. Mucorales order; agents of mucormycosis) account for most remaining life-threatening infections.
Candida species are the fourth most common cause of nosocomial bloodstream infections in the United States (Wisplinghoff et al., 2004). Candidemia is typically observed in severely ill patients with breaches in gastrointestinal or mucocutaneous barrier function (e.g. indwelling vascular access catheters or following abdominal surgeries). The pathogenesis of invasive candidiasis typically involves colonization of the gastrointestinal tract or mucosal surface, followed by translocation across damaged barrier structures and into internal organs and compartments such as the bloodstream, mesenteric lymph nodes, spleen, and liver, often in the setting of neutropenia. Fungus-attributable mortality rates for nosocomial candidemia are approximately 40% (Gudlaugsson et al., 2003). The increase in invasive medical procedures, the use of broad-spectrum antibiotics, the advent of intensive care units, and the use of medical therapies that cause damage to barrier function (i.e. mucositis) and neutropenia have all contribute to the emergence of and to the clinical risk of developing invasive candidiasis in modern medical practice. The use of fluconazole for antifungal prophylaxis has led to a gradual increase in clinical isolates of non-albicans Candida species that exhibit drug resistance (e.g. Candida glabrata, Candida krusei).
Cryptococcosis arises from the inhalation of infectious propagules that belong to Cryptococcus neoformans (worldwide distribution) or Cryptococcus gattii (in the Pacific Northwest; see (Byrnes et al., 2011) for review of outbreak). Although most infections resolve in an asymptomatic manner, in susceptible individuals (i.e. patients with AIDS, patients on corticosteroid or other immunosuppressive therapy) local disease typically develops in the lung in the form of pneumonia and can spread to the meninges and central nervous system. The global case-fatality rate for the estimated 1 million cases of cryptococcal meningitis is approximately 60% (Park et al., 2009) and, in developed countries, remains at 15-20% despite receipt of combination therapy (Day et al., 2013). In Sub-Saharan Africa, HIV-related cryptococcosis ranks behind malaria and diarrheal diseases as a cause of death, but ahead of other infectious scourges such as tuberculosis, hepatitis B, and hepatitis C (Park et al., 2009).
Humans inhale conidia of the genus Aspergillus daily and this fungus is associated with invasive and allergenic disease, the latter observed primarily in patients with pre-existing asthma and atopy or in patients with cystic fibrosis. Aspergillus fumigatus represents the most common cause of invasive aspergillosis (IA), a disease initiated by conidial germination into tissue-invasive hyphae that can disseminate and cause vascular thrombosis and tissue infarction at affected sites. IA occurs primarily in individuals with defects in neutrophil number or function, in patients with hematologic malignancies, in hematopoietic and solid organ transplant recipients, and in recipients of immunosuppressive drugs such as corticosteroids. Advances in medical technologies, the development of potent myeloablative and immunosuppressive therapies for cancer and autoimmunity and the increasing use of transplantation for end organ failure, has led to a significant increase in IA incidence, estimated at approximately 200,000 life-threatening infections annually. Despite modern antifungal therapies and improvements in limited diagnostic approaches (i.e. the introduction of fungal surrogate antigen testing), mortality rates associated with IA remain at 30-50% and the disease is usually fatal when diagnosis is delayed or dissemination to extrapulmonary sites has occurred (Steinbach et al., 2012). Beyond sinopulmonary disease, Aspergillus (and Fusarium) species cause fungal keratitis, particularly among agricultural workers in India and Africa, when airborne conidia penetrate and germinate in abraded corneal tissue (Shah et al., 2011).
The global HIV pandemic revealed Pneumocystis jiroveci pneumonia (commonly referred to as PJP or PCP) as one of the most common AIDS-defining illnesses, with an estimated contemporary incidence of 400,000 life-threatening infections worldwide (Brown et al., 2012). PJP is observed in other classes of patients with impaired adaptive immune function, particularly in those receiving corticosteroid or T lymphocyte-directed therapies. Humans are the sole known host of Pneumocystis jiroveci and the initial infection, asymptomatic in immune competent individuals, usually occurs in childhood via aerosol transmission. Since Pneumocystis cannot be cultured in the laboratory, it remains unclear whether clinical disease in immune compromised patients occurs following de novo infection or due to re-activation of latent infection.
3. General Aspects of Vertebrate Fungal Infection Models
3.1. Choice of Vertebrate Fungal Infection Model
The broad range of clinical syndromes associated with human fungal infections has spawned numerous vertebrate animal models to examine fungal pathogenesis and disease progression, host resistance and sterilizing immunity, vaccination strategies, diagnostic tests, and antifungal drugs. The capacity to exert control over experimental variables that include the fungal strain, host species, inoculum size, route of administration, and administration of pharmacotherapies, all represent major advantages of animal experimentation, particularly when combined with statistically validated models that mimic clinical disease states observed in humans. The most commonly used laboratory animals are inbred mice, though fungal infection models have been developed in primates, rabbits, guinea pigs, rats, hamsters, birds, zebrafish, and canines. The widespread availability of defined immunological reagents and murine strains, including the increasing number gene-deficient and transgenic strains (Deepe et al., 2000), and the relative low cost and space associated with colony maintenance compared to larger host species, has propelled the laboratory mouse to the forefront of vertebrate fungal infection models.
In selecting a specific vertebrate model of fungal infection relates, the choice of host, the fungal strain used for inoculation, and the route of administration represent critical variables for experimental outcomes. In general, large animal models allow for more facile and repeated surveillance and collection of tissue, cerebrospinal fluid, and serological samples and the formation of structured fungal biofilms on medical devices. The ability to visualize anatomic detail by computed tomography and to sample the bloodstream repeatedly for the presence of fungal surrogate antigen (i.e. galactomannan) is particularly advantageous to visualize the progression of focal fungal infections and responses to antifungal therapy, as demonstrated in a neutropenic rabbit model of invasive aspergillosis (Walsh et al., 1995; Petraitiene et al., 2002). In rats, placement of a jugular venous catheter in rats facilitates the study of Candida biofilms within a physiologically relevant context that includes vascular sheer forces, antibodies, other serum proteins, as well as infiltrating immune cells. Rat biofilm models using vascular catheters (Nett et al., 2012) or oral dentures (Nett et al., 2010) facilitated the identification of a C. albicans regulatory network that controls biofilm structure and consists of six transcription factors with nearly 1,000 subordinate genes (Nobile et al., 2012). In pharmacologic studies, murine metabolism of antifungal drugs may not resemble the pharmacokinetics or pharmacodynamics observed in humans. For this reason, guinea pigs have been used in studies on the efficacy of voriconazole in treating fungal infections, since the drug undergoes rapid gut mucosal metabolism in outbred mice (Sugar and Liu, 2000; MacCallum and Odds, 2002; Graybill et al., 2003). However, animals that are larger in size than mice impose greater expense and husbandry requirements, require more intense monitoring during experimental infection, have fewer available molecular reagents (e.g. antibodies), and are not available as genetically defined strains, all of which limit the types of studies that can be performed.
In recent years, researchers refined methods for intravital imaging of transparent zebrafish larvae to examine the pathogenesis of invasive candidiasis (i.e. following hindbrain ventricle injection of C. albicans blastoconidia) (Brothers et al., 2011; Brothers and Wheeler, 2012; Brothers et al., 2013). The generation of transgenic lines that express fluorescent proteins in defined innate immune cell populations, the development of fluorescent fungal strains for in vivo applications, and the availability of modified anti-sense oligonucleotides (i.e. Morpholinos) to lower protein expression in zebrafish larvae all contribute to the strength of this animal model, the non-invasive visualization and quantitation of host cell-fungal cell interactions and outcomes within an intact host tissue environment (Tobin et al., 2012). Though the larval zebrafish model lacks adaptive immune cells, this deficiency is not highly relevant for fungal infections that are primarily cleared by the innate immune system.
Laboratory mice represent the host species of choice for most questions related to mycologic infections. However, it is instructive to note important differences between mice (or other model hosts) and humans that must be taken into account when interpreting experimental data. An important example is differences in indigenous fungal flora, particularly for the interpretation of studies of mucosal fungal infections. As mentioned above, healthy human individuals are often in an asymptomatic relationship with Candida albicans and other Candida species on gastrointestinal, epidermal, and genital surfaces. An early study on laboratory mice using culture-based methods suggested that mice are not naturally colonized by human pathogenic Candida species (Savage and Dubos, 1967). However, passage of C. albicans through the murine gastrointestinal tract induces expression of the transcriptional regulator WOR1 and is associated with a phenotypic switch that favors the expression of genes involved in a commensal phenotype (Pande et al., 2013). A contemporary study using high-throughput sequencing methods indicated a rich diversity of over 100 well-annotated fungal species (representing at least 50 genera) within the murine gastrointestinal tract and a predominance of the human pathogen Candida tropicalis (Iliev et al., 2012). Besides the murine gastrointestinal tract, Candida albicans, the most common pathogenic Candida species in humans, also does not appear to colonize the murine reproductive tract and establishment of murine vulvovaginal candidiasis requires prolonged exogenous estrogen administration to maintain an infected doe in a state of pseudoestrus.
Differences in biochemical pathways in laboratory mice and in humans must also be considered when extrapolating experimental murine data into human treatment strategies. For example, murine chronic granulomatous disease (CGD), a genetic defect in NADPH oxidase activity, is associated with susceptibility to invasive aspergillosis and with defects in tryptophan metabolism (i.e. the formation of anti-inflammatory kynurenines) that drives lethal, dysregulated inflammatory responses (Romani et al., 2008). In human CGD patients, NADPH oxidase does not regulate kynurenine formation and therapeutic approaches targeting this pathway are thus not predicted to be effective beyond the murine model (De Ravin et al., 2010).
Inbred mouse strains can vary significantly in their susceptibility to the same fungal inoculum administered via the same route. For example, different inbred mouse strains vary significantly in disease susceptibility when challenged with Cryptoccocus neoformans (Zaragoza et al., 2007) or with Paracoccidioides brasiliensis (Calich et al., 1985). Differences in alternative complement pathway activity segregate with the murine phenotype, underlining the central role of opsonophagocytosis in murine host defense against intravenous cryptoccoccal challenge (Rhodes et al., 1980). This observation extends to the species level with the observation that loss of rat alveolar macrophages, potent anticryptococcal effector cells, coincides with an increase in disease severity, while loss of murine alveolar macrophages, weak or anticryptococcal effector cells, coincides with a reduction in disease severity (Shao et al., 2005).
Similar to immune competent humans, mice are naturally highly resistant to intratracheal challenge with Aspergillus fumigatus conidia and conidia do not form tissue-invasive hyphae within the respiratory tree. Thus, several models of invasive pulmonary aspergillosis have been developed; these rely on administration of different immunosuppressive agents (i.e. corticosteroids or myelotoxic chemotherapy) or on genetic deficiency of antifungal defense mechanisms such as NADPH oxidase. In these different murine models, the pathogenesis and disease progression is distinct and reflects the underlying injury to host immune function (Balloy et al., 2005). In chemotherapy-treated mice, unchecked fungal growth, dissemination, and destruction of parenchymal architecture by invasive hyphae is the primary mechanism of tissue injury and death and murine survival is prolonged by administration of the antifungal drug amphotericin B. In corticosteroid-treated mice, fungal growth is significantly reduced in comparison to chemotherapy-treated mice, and the massive influx of functionally impaired neutrophils triggers dysregulated responses associated with tissue damage, hypoxia, and immunopathology and lack of responsiveness to amphotericin B administration (Balloy et al., 2005; Grahl et al., 2011).
In turn, variations in host tissue microenvironments associated with different models of invasive aspergillosis impact fungal growth and virulence. The Aspergillus fumigatus secondary metabolite gliotoxin has effects on NF-κb-dependent host cell apoptosis and on phagocyte NADPH oxidase function. Investigating the pathogenesis of gliotoxin-producing and non-producing isogenic strains of A. fumigatus, a series of studies demonstrated that the secondary metabolite contributes to virulence in a non-neutropenic murine model of disease but not in neutropenic murine models (Kupfahl et al., 2006; Sugui et al., 2007; Spikes et al., 2008). These data are consistent with the notion that neutrophils represent the primary target of gliotoxin and indicates that fungal factors can contribute to disease outcomes in specific settings of host immune damage. Thus, when the administration of immunosuppressive agents (e.g. corticosteroids, cyclophosphamide) forms the basis for an animal model of invasive mycoses, it is imperative not to extrapolate data to other susceptible or non-susceptible host states in the absence of experimental confirmation.
3.2. Routes of Administration in Vertebrate Animal Models
The route of administration represents a critical variable in vertebrate animal models of fungal disease. In most instances, the inoculum should be administered via the physiologically relevant route of infection (Figure 1). Vertebrate animal models have utilized a plethora of injection and infection sites to model systemic (intravenous, intraperitoneal), pulmonary (intranasal, intratracheal, inhalational), mucosal (oropharyngeal, vaginal), gastric (i.e. by gavage), superficial (i.e. skin abrasion and local application), dermal (subcutaneous), ocular (corneal), and central nervous system (intracranial, intracisternal, or intrathecal) mycoses (see Table I and Fig. 1B).
Systemic fungal diseases can be modeled by intravenous injection of fungal cells, though this route bypasses mucosal host defenses that are typically breached prior to the development of fungemia. Koh and colleagues demonstrated that mucosal damage and neutropenia are both required for C. albicans dissemination from the murine gastrointestinal tract (Koh et al., 2008). The major target organ of systemic candidiasis represents the kidney in mice, and similar to humans, mice succumb to progressive sepsis and renal failure (Spellberg et al., 2005; Lionakis et al., 2011) due to ineffective fungal clearance by resident renal macrophages and the relative sluggish recruitment of infiltrating neutrophils and monocytes compared to the spleen and liver, leading to pseudohyphal tissue invasion, uncontrolled inflammation, and organ failure (Lionakis et al., 2011; Majer et al., 2012; Lionakis et al., 2013; Ngo et al., 2014).
Fungal diseases acquired by inhalation of infectious propagules can be recapitulated by intranasal or intratracheal injection of a liquid fungal suspension or inhalation of dry fungal cells in murine models. The advantage of using liquid fungal suspensions for intranasal or intratracheal infection is that the inoculum can be precisely quantified and calibrated for minimal inter-experimental variability. In addition, instillation permits a wide range of experimental inocula to be administered and, combined with intratracheal delivery, assures that the actual dose reaches the lungs. The use of liquid suspensions for pulmonary delivery is also practical for organisms (e.g. Cryptococcus neoformans) from which it is prohibitive to isolate pure cultures of spores.
Although intranasal delivery of fungal cells is commonly used to induce pulmonary disease, in part because the technique is easy to learn and execute (Machholz et al., 2012), a disadvantage of this method is that delivery of fungal cells to the lungs can be variable, particularly since fungal cells often fail to reach terminal airways. In rodent intranasal infection models, fungal lesions are likely to arise from Aspergillus fumigatus conidia deposited in larger airways rather than in alveoli, the characteristic site of human disease (Tang et al., 1993; Shibuya et al., 1999; Steinbach et al., 2004). An alternative to intranasal infection is to insert a catheter beyond the vocal cords to facilitate intratracheal delivery of fungal cells in a process akin to endotracheal intubation. In recent years, several non-surgical approaches have been refined to enable the delivery of aqueous solutions of fungal cells under visual guidance into the trachea [see videos in (Hasenberg et al., 2011; Rayamajhi et al., 2011; Cai and Kimura, 2013) for non-surgical approaches to intratracheal delivery]. These advances are likely to replace techniques that rely on surgical incision of the trachea for instillation of fungal cells [see videos in (Helms et al., 2010; Reddy et al., 2012)]. Dispersion of fungal cells into the pulmonary parenchyma may be enhanced by brief mechanical ventilation (Hasenberg et al., 2011) or by use of a microsprayer attached to the syringe tip (Kelly et al., 2008). The detergent Tween-20 is commonly used to prepare fungal cell suspensions for intranasal and intratracheal delivery. Inclusion of Tween-20 in aqueous suspensions alters the surface charge of A. fumigatus conidia (Stephens-Romero et al., 2005), though the physiologic relevance of this observation remains undefined.
A major advantage of inhalational infection methods using a sealed chamber is that airborne fungal conidia can delivered homogenously into pulmonary tissue, either by aerosolization of an aqueous solution (Steinbach et al., 2004; Sheppard et al., 2006a) or by insufflation of a conidial lawn grown on an agar plate (Stephens-Romero et al., 2005). The latter technique recapitulates human infection faithfully since inhaled conidia are not solubilized in solutions that typically contain detergents. However, experimental flexibility is limited since it is difficult to vary the infectious dose and since the number of mice that can be fitted into the specialized inhalation chamber is modest. The standardization of pulmonary infection by inhalation of an aqueous solution is particularly suited for pharmacologic studies since the course of invasive aspergillosis is highly reproducible between experiments and among different laboratories (Patterson, 2005; Sheppard et al., 2006a).
Extrapulmonary dissemination does not occur in immune competent murine models of aspergillosis and mucormycosis, though it is observed in murine models of coccidioidomycosis and cryptococcosis. In vulnerable human populations, dissemination and spread to the central nervous system (CNS) is a feared and devastating complication of pulmonary disease caused by these agents. Thus, researchers have developed techniques to inject fungal cells into the CNS, particularly to conduct pharmacologic studies. An advantage of direct inoculation into the CNS is that mice do not have to receive exogenous immunosuppression to develop extrapulmonary disease. To model fungal meningitis in a reproducible manner, delivery of the inoculum into the cerebrospinal fluid (CSF) represents the preferred route and has been achieved in a murine model of coccidioidal meningitis by creating a skin incision over the lumbar vertebrae and advancing a catheter into the subarachnoid space to inject arthroconidia, spherules, or endospores into the CSF (Kamberi et al., 2003). The recent outbreak of fungal meningitis due to contaminated corticosteroid injections, primarily caused by the dematiaceous mold Exserohilum rostratum, underscores the relevance of this model for studies that seek to enhance the diagnosis and treatment of fungal meningitis (Kainer et al., 2012; Smith et al., 2013).
Similar to cryptococcal disease in humans, mice infected via intrapharyngeal aspiration with C. neoformans strain H99 develop meningoencephalitis and this cerebral tropism underlies the cause of death in the animals (Ngamskulrungroj et al., 2012). In HIV-infected individuals, C. neoformans primarily causes meningoencephalitis and only one-third of patients present with pulmonary infections. In contrast, C. gattii causes cryptococosis primarily in non-HIV infected individuals and the lung is the most common site of infection at the time of clinical presentation. Murine infection with C. gattii strain R265 closely mimics this observation in the murine intrapharyngeal aspiration model, with a higher lung fungal burden compared to C. neoformans strain H99 and less frequent dissemination to the CNS despite a more fulminant disease and accelerated murine mortality (Ngamskulrungroj et al., 2012).
Although the intraperitoneal infection route does not recapitulate a physiologic route of disease acquisition in humans, a murine model of chromoblastomycosis using this route of disease yielded important immunologic insights that can be translated for therapeutic purposes in humans (Sousa Mda et al., 2011). Fonsecaea pedrosoi, a causative agent of chromoblastomycosis, is typically associated with chronic skin and soft tissue infections that respond poorly to antifungal therapies, cryotherapy, and to surgical management (La Hoz and Baddley, 2012). The intraperitoneal murine model revealed that fungal clearance is defective and that the underlying mechanism relates to an absence of myeloid differentiation factor 88 (MyD88; i.e. an adaptor of Toll-like receptor and IL-1 and IL-18 receptor signal transduction) co-stimulation of the immune response. Thus, although F. pedrosoi stimulates the C-type lectin receptor Mincle to trigger innate immune responses, these are insufficient to clear the infection (Sousa Mda et al., 2011). Restoration of pattern recognition receptor signaling by administration of the Toll-like receptor 7 agonist imiquinod, an activator of MyD88 signaling, is therapeutic in mice. This approach could be readily adapted and tested as a treatment strategy for human disease.
3.3. Fungal Strain Selection in Vertebrate Animal Models
For most pathogenic fungi, studies have borne out that host responses to individual strains differ in magnitude and in quality. For example, a recent study quantified variations in immune responses to three commonly used and sequenced A. fumigatus clinical isolate strains in a murine pulmonary infection model (Rizzetto et al., 2013). The authors found that A. fumigatus strain CEA10 caused murine death and that this phenotype coincided with a higher frequency of neutrophils within the airway fluid and a greater induction of specific pro-inflammatory cytokines compared to the Af293 and Af300 strains, consistent with the notion that CEA10 elicited dysregulated inflammatory responses that contribute to inflammatory pathology. The molecular mechanisms that underlie strain-specific differences in pathogenesis and host responses remain largely undefined. Several authors have suggested that the rate of in vivo fungal germination (when applicable) and growth in the context of mammalian tissue environments provides a barometer for virulence in animal studies (Paisley et al., 2005; Rhodes, 2006).
A subset of C. albicans strains from different clades induce host signals via the C-type lectin receptor dectin-1 that contribute to host protection and survival in a murine model of systemic candidiasis. Other C. albicans strains do not induce host protective responses via dectin-1, irrespective of the murine host strain examined (Marakalala et al., 2013). Dectin-1 represents the prototypic and best-characterized C-type lectin receptor involved in antifungal immunity; it binds and recognizes fungal β-glucan carbohydrate moieties and activates spleen tyrosine kinase (Syk) and CARD9 signaling components that are critical for eliciting T helper 17- and IL-17-dependent immune responses (LeibundGut-Landmann et al., 2007). Mendelian defects in this signaling cascade underlie human susceptibility to mucocutaneous candidiasis and to dermatophytosis (Lanternier et al., 2013). Although dectin-1-dependent and -independent phenotypes of C. albicans strains were surprisingly not linked to differences in β-glucan exposure in vivo, it is possible that differences in other cell surface immunoreactive polymers may account for the observed phenotypic differences among C. albicans strains. Potential strain-dependent differences in the fungal cell wall components chitin and mannan may contribute to these observations. The recent in vivo functional characterization of signaling receptors [i.e. dectin-2/CLEC4e (Saijo et al., 2010) and dectin-3/CLECsf8 (Zhu et al., 2013)] that recognize C. albicans mannans indicates that multiple receptors could functionally compensate for dectin-1 in host defense, with the contribution of each receptor defined by the strain-specific content of immunoreactive compounds within the C. albicans cell wall. Thus, studies on fungal virulence and on host resistance mechanisms need to consider that molecular and strain-specific differences in fungal cell composition can account for significant experimental variation.
In addition, C. albicans strains differ with respect to pathogenesis at different portals of infection. For example, the strain SC5314, a clinical blood stream isolate and poor colonizer of mucosal surfaces, does not induce vulvovaginal candidiasis in female mice maintained in a state of pseudoestrus (Rahman et al., 2012). This complicates studies of fungal pathogenesis in this and other mucosal models of candidiasis since SC5314 and derivative strains represent the most common background for the construction of mutant strains (Naglik et al., 2008). In contrast, other C. albicans strains (e.g. 529L) permit persistent fungal colonization in two commonly used inbred mouse strains, namely Balb/c and C57BL/6 mice (Rahman et al., 2012). The recent recognition that C. albicans can form structured biofilms within the murine vaginal mucosa will undoubtedly inform studies on the genetic requirements for biofilm formation and its role in pathogenesis on biotic surfaces versus abiotic medical devices (Harriott et al., 2010).
3.4. Experimental Readouts in Vertebrate Animal Models
Classical experimental parameters that are almost universally measured in vertebrate animal models of fungal infection include organ fungal burden, organ histopathology, and survival, though restrictions often preclude monitoring the latter endpoint due to ethical concerns about the humane care of animals. Since animals are typically euthanized when they reach predefined surrogate endpoints mandated by Institutional Animal Care and Use Committees (e.g. 20% weight loss), it is important to clarify the criteria for euthanasia and define these on quantitative terms whenever possible. Ideally, researchers without a priori knowledge about the treatment and control groups of mice should determine whether criteria for euthanasia are met on an individual basis.
Although the determination of fungal tissue burden is straightforward for yeast cells (e.g. Cryptococcus neoformans) and a sensitive measure for the progression of infectious process, quantitation of fungal hyphae is more cumbersome and a universal standard does not exist (Clemons and Stevens, 2009). CFU determinations do not scale linearly scale with hyphal burden in infected tissues. Thus, for the prototypic filamentous organism, A. fumigatus, several alternate methods have been developed. These include determination of tissue chitin content, though chitin deposition is not synonymous with the presence of viable organism. Quantitative measurements of fungal DNA (using the 18S rRNA gene as a target) have been developed using polymerase chain reaction-based methods on DNA extracted from homogenized lung tissue (Bowman et al., 2001; Sheppard et al., 2006b). The release of fungal antigens (i.e. galactomannan for Aspergillus species, polysaccharide antigen for Histoplama capsulatum) during vertebrate tissue invasion by enzyme-linked immunoabsorbent assays also provides a quantitative readout for fungal tissue burden in murine experiments (Wheat et al., 1986; Connolly et al., 2000; Sheppard et al., 2006b).
The expanding murine immunological toolbox to monitor the recruitment and functional activation of immune cells as well as the fate of fungal cells in mammalian tissues informs immunologic studies that seek to identify the molecular and cellular basis of antifungal immunity. Standard immunologic assays measure the production of cytokines and other inflammatory mediators in host tissues (e.g. by ELISA) and quantify host leukocyte populations that reside in or are recruited to portals of infection (e.g. by flow cytometry of enumerated single cell suspensions from infected organs). Recently, the dynamics of fungus-specific CD4 T cell responses have been analyzed using mice that uniformly express a T cell receptor (TCR) transgene in CD4 T cells; these transgenic CD4 T cells bind an antigenic epitope that is derived from A. fumigatus (Rivera et al., 2006) or that is shared among the dimorphic fungi B. dermatidites, C. posadasii, and H. capsulatum (Wuthrich et al., 2011b). These mouse strains facilitate the adoptive transfer of congenically marked TCR-transgenic CD4 T cells into hosts, and transferred CD4 T cells undergo priming, activation, functional differentiation, contraction, and memory formation (Rivera et al., 2006; Rivera et al., 2011; Wuthrich et al., 2011a; Wang et al., 2014).
In a model of murine blastomycosis, the formation of vaccine-induced T-helper (Th) 17 CD4 T cells is critical is both necessary and sufficient to confer vaccine immunity following challenge with a lethal inoculum. Mechanistically, vaccine-induced Th17 cells appear to act by enhancing the antifungal activity of neutrophils and macrophages at the portal of infection and this effect can be reversed by blocking the Th17 effector cytokine IL-17A or its cognate receptor (Wuthrich et al., 2011a). The induction of protective immunity relies on the recognition of vaccine yeast by C-type lectin receptors dectin-1 and dectin-2 (H. capsulatum, C. posadasii) or dectin-2 alone (B. dermatidites) (Wang et al., 2014). Although vaccine yeast strains are not safe for human use, particularly in immune compromised patients, they are a valuable reagent to decipher molecular and cellular requirements for vaccine-induced protection and sterilizing immunity. For candidiasis, a vaccine formulation of recombinant Als3 (a fungal adhesin) and of the adjuvant alum, is protective in a preclinical murine model and has advanced to clinical trials. Vaccine-induced formation of Th1 and Th17 CD4 T cells and subsequent enhancement of innate immune antifungal effector activity appears to be its relevant mechanism of action (Lin et al., 2009).
On the fungal side, researchers have developed fungal strains that emit genetically encodable luminescence and fluorescence signals (Doyle et al., 2006; Brock et al., 2008; Brothers et al., 2011). These advances permit intravital imaging using fluorescence microscopy in transparent zebrafish larvae and using charge coupled device cameras in mice. The technologies differ with regard to requirements for exogenous substrate (i.e. luciferin for bioluminesce, no exogenous substrate for imaging of fluorescent proteins) and with regard to spatial resolution and imaging depth. Bioluminescent tools can be applied to image intact mice during systemic (Doyle et al., 2006) and oropharyngeal candidiasis (Mosci et al., 2013), while fluorescence is generally restricted to transparent animals or to externalized organs.
A novel experimental read-out relates to the functional outcomes of fungal cell-host cell interactions at portals of infection. Jhingran and colleagues developed a fluorescent Aspergillus reporter (FLARE) strain that incorporates a two-component sensor mechanism that alters its fluorescence emission in response to fungal viability (Jhingran et al., 2012). FLARE conidia emit two fluorescence signals when the cells are viable and lose one fluorescent signal upon fungal cell killing in host leukocytes. This principle enables researchers to sort host leukocytes on the basis of conidial uptake and killing and to perform cell profiling in these distinct populations. Since FLARE conidia report the outcome of individual fungal cell-host cell encounters with single event resolution by flow cytometry, imaging cytometry, or fluorescence microscopy, this fungal strain can be applied to interrogate genetic, immunologic, and pharmacologic manipulations on anticonidial activity in defined host cell populations (Jhingran et al., 2012). Thus, newer experimental readouts that measure innate and adaptive fungus-specific responses in defined cell populations are supplementing traditional experimental parameters of fungal disease states.
4. Conclusions
The goal of this review has been to provide an overview of human diseases associated with medically relevant fungi and of vertebrate animal models used to study human mycoses. The choice of model host immune status and species, route of infection, and fungal strain all represent important variables that have a profound impact on experimental data and outcomes. The conclusions that can be drawn from animal studies depend on a critical understanding of the inherent limitations and parallels of common vertebrate models of fungal infection to human disease states. The emergence of fungal pathogens as causes of infectious morbidity and mortality over the past 2-3 decades will likely spur further growth and improvement in this field and lead to new advances in our understanding of fungal pathogenesis, host defense mechanisms, diagnostics, therapeutics, and vaccination strategies.
Acknowledgments
I thank Kyoko Kurosawa (Fig. 1) for graphical support. T.M.H. acknowledges the Robert Sinskey Foundation, and NIH grants RO1 AI093808 and R21 AI105617 for supporting work in his laboratory. I apologize to my colleagues whose important contributions to vertebrate animal models of fungal infection I was not able to cite owing to space constraints.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achterman RR, Smith AR, Oliver BG, White TC. Sequenced dermatophyte strains: growth rate, conidiation, drug susceptibilities, and virulence in an invertebrate model. Fungal genetics and biology. 2011;48:335–41. doi: 10.1016/j.fgb.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achterman RR, White TC. A foot in the door for dermatophyte research. PLoS pathogens. 2012;8:e1002564. doi: 10.1371/journal.ppat.1002564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balloy V, Huerre M, Latge JP, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun. 2005;73:494–503. doi: 10.1128/IAI.73.1.494-503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar E, Gladiator A, Bastidas S, Roschitzki B, Acha-Orbea H, Oxenius A, LeibundGut-Landmann S. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. Journal of immunology. 2012;188:5636–43. doi: 10.4049/jimmunol.1200594. [DOI] [PubMed] [Google Scholar]
- Blackwell M. The fungi: 1, 2, 3 … 5.1 million species? American journal of botany. 2011;98:426–38. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- Bowman JC, Abruzzo GK, Anderson JW, Flattery AM, Gill CJ, Pikounis VB, Schmatz DM, Liberator PA, Douglas CM. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrobial agents and chemotherapy. 2001;45:3474–81. doi: 10.1128/AAC.45.12.3474-3481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito MM, Conceicao-Silva F, Morgado FN, Raibolt PS, Schubach A, Schubach TP, Schaffer GM, Borba CM. Comparison of virulence of different Sporothrix schenckii clinical isolates using experimental murine model. Medical mycology. 2007;45:721–9. doi: 10.1080/13693780701625131. [DOI] [PubMed] [Google Scholar]
- Brock M, Jouvion G, Droin-Bergere S, Dussurget O, Nicola MA, Ibrahim-Granet O. Bioluminescent Aspergillus fumigatus, a new tool for drug efficiency testing and in vivo monitoring of invasive aspergillosis. Applied and environmental microbiology. 2008;74:7023–35. doi: 10.1128/AEM.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers KM, Gratacap RL, Barker SE, Newman ZR, Norum A, Wheeler RT. NADPH oxidase-driven phagocyte recruitment controls Candida albicans filamentous growth and prevents mortality. PLoS pathogens. 2013;9:e1003634. doi: 10.1371/journal.ppat.1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers KM, Newman ZR, Wheeler RT. Live imaging of disseminated candidiasis in zebrafish reveals role of phagocyte oxidase in limiting filamentous growth. Eukaryotic cell. 2011;10:932–44. doi: 10.1128/EC.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers KM, Wheeler RT. Non-invasive imaging of disseminated candidiasis in zebrafish larvae. Journal of visualized experiments. 2012:e4051. doi: 10.3791/4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD. Innate antifungal immunity: the key role of phagocytes. Annual review of immunology. 2011;29:1–21. doi: 10.1146/annurev-immunol-030409-101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science translational medicine. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- Brummer E, Restrepo A, Stevens DA, Azzi R, Gomez AM, Hoyos GL, McEwen JG, Cano LE, de Bedout C. Murine model of paracoccidioidomycosis. Production of fatal acute pulmonary or chronic pulmonary and disseminated disease: immunological and pathological observations. Journal of experimental pathology. 1984;1:241–55. [PubMed] [Google Scholar]
- Byrnes EJ, 3rd, Bartlett KH, Perfect JR, Heitman J. Cryptococcus gattii: an emerging fungal pathogen infecting humans and animals. Microbes and infection / Institut Pasteur. 2011;13:895–907. doi: 10.1016/j.micinf.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Kimura S. Noninvasive intratracheal intubation to study the pathology and physiology of mouse lung. Journal of visualized experiments. 2013:e50601. doi: 10.3791/50601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calich VL, Singer-Vermes LM, Siqueira AM, Burger E. Susceptibility and resistance of inbred mice to Paracoccidioides brasiliensis. British journal of experimental pathology. 1985;66:585–94. [PMC free article] [PubMed] [Google Scholar]
- Charoenvit Y, Taylor RL. Experimental sporotrichosis in Syrian hamsters. Infect Immun. 1979;23:366–72. doi: 10.1128/iai.23.2.366-372.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller TM, Luque JC, Sobel RA, Farrokhshad K, Clemons KV, Stevens DA. Development of a murine model of cerebral aspergillosis. The Journal of infectious diseases. 2002;186:574–7. doi: 10.1086/341567. [DOI] [PubMed] [Google Scholar]
- Clemons KV, Capilla J, Stevens DA. Experimental animal models of coccidioidomycosis. Annals of the New York Academy of Sciences. 2007;1111:208–24. doi: 10.1196/annals.1406.029. [DOI] [PubMed] [Google Scholar]
- Clemons KV, Stevens DA. The contribution of animal models of aspergillosis to understanding pathogenesis, therapy and virulence. Medical mycology. 2005;43(Suppl 1):S101–10. doi: 10.1080/13693780500051919. [DOI] [PubMed] [Google Scholar]
- Clemons KV, Stevens DA. Conventional or molecular measurement of Aspergillus load. Medical mycology. 2009;47(Suppl 1):S132–7. doi: 10.1080/13693780802213340. [DOI] [PubMed] [Google Scholar]
- Connolly P, Wheat LJ, Schnizlein-Bick C, Durkin M, Kohler S, Smedema M, Goldberg J, Brizendine E, Loebenberg D. Comparison of a new triazole, posaconazole, with itraconazole and amphotericin B for treatment of histoplasmosis following pulmonary challenge in immunocompromised mice. Antimicrobial agents and chemotherapy. 2000;44:2604–8. doi: 10.1128/aac.44.10.2604-2608.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JN, Chau TT, Lalloo DG. Combination antifungal therapy for cryptococcal meningitis. The New England journal of medicine. 2013;368:2522–3. doi: 10.1056/NEJMc1305981. [DOI] [PubMed] [Google Scholar]
- De Ravin SS, Zarember KA, Long-Priel D, Chan KC, Fox SD, Gallin JI, Kuhns DB, Malech HL. Tryptophan/kynurenine metabolism in human leukocytes is independent of superoxide and is fully maintained in chronic granulomatous disease. Blood. 2010;116:1755–60. doi: 10.1182/blood-2009-07-233734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Repentigny L. Animal models in the analysis of Candida host-pathogen interactions. Current opinion in microbiology. 2004;7:324–9. doi: 10.1016/j.mib.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Deepe GS, Jr., Romani L, Calich VL, Huffnagle G, Arruda C, Molinari-Madlum EE, Perfect JR. Knockout mice as experimental models of virulence. Medical mycology. 2000;381(Suppl):87–98. [PubMed] [Google Scholar]
- Dei-Cas E, Brun-Pascaud M, Bille-Hansen V, Allaert A, Aliouat EM. Animal models of pneumocystosis. FEMS immunology and medical microbiology. 1998;22:163–8. doi: 10.1111/j.1574-695X.1998.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Desalermos A, Fuchs BB, Mylonakis E. Selecting an invertebrate model host for the study of fungal pathogenesis. PLoS pathogens. 2012;8:e1002451. doi: 10.1371/journal.ppat.1002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoubeaux G, Chandenier J. A nebulized intra-tracheal rat model of invasive pulmonary aspergillosis. Methods in molecular biology. 2012;845:511–8. doi: 10.1007/978-1-61779-539-8_36. [DOI] [PubMed] [Google Scholar]
- Ding JC, Bauer M, Diamond DM, Leal MA, Johnson D, Williams BK, Thomas AM, Najvar L, Graybill JR, Larsen RA. Effect of severity of meningitis on fungicidal activity of flucytosine combined with fluconazole in a murine model of cryptococcal meningitis. Antimicrobial agents and chemotherapy. 1997;41:1589–93. doi: 10.1128/aac.41.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DM, Polak A, Walsh TJ. Fungus dose-dependent primary pulmonary aspergillosis in immunosuppressed mice. Infect Immun. 1989;57:1452–6. doi: 10.1128/iai.57.5.1452-1456.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TC, Nawotka KA, Kawahara CB, Francis KP, Contag PR. Visualizing fungal infections in living mice using bioluminescent pathogenic Candida albicans strains transformed with the firefly luciferase gene. Microbial pathogenesis. 2006;40:82–90. doi: 10.1016/j.micpath.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Drew RH, Townsend ML, Pound MW, Johnson SW, Perfect JR. Recent advances in the treatment of life-threatening, invasive fungal infections. Expert opinion on pharmacotherapy. 2013;14:2361–74. doi: 10.1517/14656566.2013.838217. [DOI] [PubMed] [Google Scholar]
- Fidel PL, Lynch ME, Sobel JD. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:1990–5. doi: 10.1128/iai.61.5.1990-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–94. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs BB, Mylonakis E. Using non-mammalian hosts to study fungal virulence and host defense. Current opinion in microbiology. 2006;9:346–51. doi: 10.1016/j.mib.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, Hohl TM, Cramer RA. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS pathogens. 2011;7:e1002145. doi: 10.1371/journal.ppat.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill JR, Najvar LK, Gonzalez GM, Hernandez S, Bocanegra R. Improving the mouse model for studying the efficacy of voriconazole. The Journal of antimicrobial chemotherapy. 2003;51:1373–6. doi: 10.1093/jac/dkg261. [DOI] [PubMed] [Google Scholar]
- Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. Attributable mortality of nosocomial candidemia, revisited. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2003;37:1172–7. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Jr., Noverr MC. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156:3635–44. doi: 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenberg M, Kohler A, Bonifatius S, Jeron A, Gunzer M. Direct observation of phagocytosis and NET-formation by neutrophils in infected lungs using 2-photon microscopy. Journal of visualized experiments. 2011 doi: 10.3791/2659. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(Suppl 4):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- Hay RJ, Calderon RA, Collins MJ. Experimental dermatophytosis: the clinical and histopathologic features of a mouse model using Trichophyton quinckeanum (mouse favus) The Journal of investigative dermatology. 1983;81:270–4. doi: 10.1111/1523-1747.ep12518292. [DOI] [PubMed] [Google Scholar]
- Heitman J. Microbial Pathogens in the Fungal Kingdom. Fungal biology reviews. 2011;25:48–60. doi: 10.1016/j.fbr.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms MN, Torres-Gonzalez E, Goodson P, Rojas M. Direct tracheal instillation of solutes into mouse lung. Journal of visualized experiments: JoVE. 2010 doi: 10.3791/1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoselton SA, Samarasinghe AE, Seydel JM, Schuh JM. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Medical mycology. 2010;48:1056–65. doi: 10.3109/13693786.2010.485582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, Rotter JI, Wang HL, McGovern DP, Brown GD, Underhill DM. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–7. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhingran A, Mar KB, Kumasaka DK, Knoblaugh SE, Ngo LY, Segal BH, Iwakura Y, Lowell CA, Hamerman JA, Lin X, Hohl TM. Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung. Cell reports. 2012;2:1762–73. doi: 10.1016/j.celrep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainer MA, Reagan DR, Nguyen DB, Wiese AD, Wise ME, Ward J, Park BJ, Kanago ML, Baumblatt J, Schaefer MK, Berger BE, Marder EP, Min JY, Dunn JR, Smith RM, Dreyzehner J, Jones TF, Tennessee Fungal Meningitis Investigation, T. Fungal infections associated with contaminated methylprednisolone in Tennessee. The New England journal of medicine. 2012;367:2194–203. doi: 10.1056/NEJMoa1212972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamberi P, Sobel RA, Clemons KV, Stevens DA, Pappagianis D, Williams PL. A murine model of coccidioidal meningitis. The Journal of infectious diseases. 2003;187:453–60. doi: 10.1086/367961. [DOI] [PubMed] [Google Scholar]
- Kamei K. Animal models of zygomycosis--Absidia, Rhizopus, Rhizomucor, and Cunninghamella. Mycopathologia. 2001;152:5–13. doi: 10.1023/a:1011900630987. [DOI] [PubMed] [Google Scholar]
- Kelly MM, McNagny K, Williams DL, van Rooijen N, Maxwell L, Gwozd C, Mody CH, Kubes P. The lung responds to zymosan in a unique manner independent of toll-like receptors, complement, and dectin-1. American journal of respiratory cell and molecular biology. 2008;38:227–38. doi: 10.1165/rcmb.2007-0045OC. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Bonorchis K, Corcoran C, Meintjes G, Locketz M, Lehloenya R, Vismer HF, Naicker P, Prozesky H, van Wyk M, Bamford C, du Plooy M, Imrie G, Dlamini S, Borman AM, Colebunders R, Yansouni CP, Mendelson M, Govender NP. A dimorphic fungus causing disseminated infection in South Africa. The New England journal of medicine. 2013;369:1416–24. doi: 10.1056/NEJMoa1215460. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick WR, Najvar LK, Bocanegra R, Patterson TF, Graybill JR. New guinea pig model of Cryptococcal meningitis. Antimicrobial agents and chemotherapy. 2007;51:3011–3. doi: 10.1128/AAC.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H. [Animal model for superficial mycosis] Japanese journal of medical mycology. 2009;50:85–9. doi: 10.3314/jjmm.50.085. [DOI] [PubMed] [Google Scholar]
- Koh AY, Kohler JR, Coggshall KT, Van Rooijen N, Pier GB. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS pathogens. 2008;4:e35. doi: 10.1371/journal.ppat.0040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudeken N, Kawakami K, Kusano N, Saito A. Cell-mediated immunity in host resistance against infection caused by Penicillium marneffei. Journal of medical and veterinary mycology. 1996;34:371–8. doi: 10.1080/02681219680000671. [DOI] [PubMed] [Google Scholar]
- Kupfahl C, Heinekamp T, Geginat G, Ruppert T, Hartl A, Hof H, Brakhage AA. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Molecular microbiology. 2006;62:292–302. doi: 10.1111/j.1365-2958.2006.05373.x. [DOI] [PubMed] [Google Scholar]
- Kurup VP, Mauze S, Choi H, Seymour BW, Coffman RL. A murine model of allergic bronchopulmonary aspergillosis with elevated eosinophils and IgE. Journal of immunology. 1992;148:3783–8. [PubMed] [Google Scholar]
- La Hoz RM, Baddley JW. Subcutaneous fungal infections. Current infectious disease reports. 2012;14:530–9. doi: 10.1007/s11908-012-0275-3. [DOI] [PubMed] [Google Scholar]
- Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, Puel A. Primary immunodeficiencies underlying fungal infections. Current opinion in pediatrics. 2013;25:736–47. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SM, Jr., Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS pathogens. 2010;6:e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nature immunology. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE, Jr., Spellberg B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS pathogens. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS. Drosophila and Galleria insect model hosts: new tools for the study of fungal virulence, pharmacology and immunology. Virulence. 2011;2:521–7. doi: 10.4161/viru.2.6.18520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS, Kontoyiannis DP. Drosophila melanogaster as a model organism for invasive aspergillosis. Methods in molecular biology. 2012;845:455–68. doi: 10.1007/978-1-61779-539-8_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS, Lim JK, Lee CC, Murphy PM. Organ-specific innate immune responses in a mouse model of invasive candidiasis. Journal of innate immunity. 2011;3:180–99. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, Wan W, Lee CC, Lim JK, Rivollier A, Yang JC, Laird GM, Wheeler RT, Alexander BD, Perfect JR, Gao JL, Kullberg BJ, Netea MG, Murphy PM. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. The Journal of clinical investigation. 2013;123:5035–51. doi: 10.1172/JCI71307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Spellberg B, Phan QT, Fu Y, Fu Y, Lee AS, Edwards JE, Filler SG, Ibrahim AS. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. The Journal of clinical investigation. 2010;120:1914–24. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum DM. Mouse intravenous challenge models and applications. Methods in molecular biology. 2012;845:499–509. doi: 10.1007/978-1-61779-539-8_35. [DOI] [PubMed] [Google Scholar]
- MacCallum DM. Mouse model of invasive fungal infection. Methods in molecular biology. 2013;1031:145–53. doi: 10.1007/978-1-62703-481-4_17. [DOI] [PubMed] [Google Scholar]
- MacCallum DM, Odds FC. Influence of grapefruit juice on itraconazole plasma levels in mice and guinea pigs. The Journal of antimicrobial chemotherapy. 2002;50:219–24. doi: 10.1093/jac/dkf103. [DOI] [PubMed] [Google Scholar]
- Machholz E, Mulder G, Ruiz C, Corning BF, Pritchett-Corning KR. Manual restraint and common compound administration routes in mice and rats. Journal of visualized experiments: JoVE. 2012 doi: 10.3791/2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer O, Bourgeois C, Zwolanek F, Lassnig C, Kerjaschki D, Mack M, Muller M, Kuchler K. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS pathogens. 2012;8:e1002811. doi: 10.1371/journal.ppat.1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, Mora-Montes HM, Kerrigan A, Netea MG, Murray GI, Maccallum DM, Wheeler R, Munro CA, Gow NA, Cramer RA, Brown AJ, Brown GD. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS pathogens. 2013;9:e1003315. doi: 10.1371/journal.ppat.1003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen JG, Bedoya V, Patino MM, Salazar ME, Restrepo A. Experimental murine paracoccidiodomycosis induced by the inhalation of conidia. Journal of medical and veterinary mycology: bi-monthly publication of the International Society for Human and Animal Mycology. 1987;25:165–75. doi: 10.1080/02681218780000231. [DOI] [PubMed] [Google Scholar]
- Mosci P, Pericolini E, Gabrielli E, Kenno S, Perito S, Bistoni F, d’Enfert C, Vecchiarelli A. A novel bioluminescence mouse model for monitoring oropharyngeal candidiasis in mice. Virulence. 2013;4 doi: 10.4161/viru.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammed M, Coleman JJ, Mylonakis E. Caenorhabditis elegans: a nematode infection model for pathogenic fungi. Methods in molecular biology. 2012a;845:447–54. doi: 10.1007/978-1-61779-539-8_31. [DOI] [PubMed] [Google Scholar]
- Muhammed M, Feldmesser M, Shubitz LF, Lionakis MS, Sil A, Wang Y, Glavis-Bloom J, Lewis RE, Galgiani JN, Casadevall A, Kontoyiannis DP, Mylonakis E. Mouse models for the study of fungal pneumonia: a collection of detailed experimental protocols for the study of Coccidioides, Cryptococcus, Fusarium, Histoplasma and combined infection due to Aspergillus-Rhizopus. Virulence. 2012b;3:329–38. doi: 10.4161/viru.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik JR, Fidel PL, Jr., Odds FC. Animal models of mucosal Candida infection. FEMS microbiology letters. 2008;283:129–39. doi: 10.1111/j.1574-6968.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najvar LK, Bocanegra R, Graybill JR. An alternative animal model for comparison of treatments for cryptococcal meningitis. Antimicrobial agents and chemotherapy. 1999;43:413–4. doi: 10.1128/aac.43.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett JE, Marchillo K, Andes DR. Modeling of fungal biofilms using a rat central vein catheter. Methods in molecular biology. 2012;845:547–56. doi: 10.1007/978-1-61779-539-8_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett JE, Marchillo K, Spiegel CA, Andes DR. Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun. 2010;78:3650–9. doi: 10.1128/IAI.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamskulrungroj P, Chang Y, Sionov E, Kwon-Chung KJ. The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio. 2012:3. doi: 10.1128/mBio.00103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. The Journal of infectious diseases. 2014;209:109–19. doi: 10.1093/infdis/jit413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–38. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisley D, Robson GD, Denning DW. Correlation between in vitro growth rate and in vivo virulence in Aspergillus fumigatus. Medical mycology. 2005;43:397–401. doi: 10.1080/13693780400005866. [DOI] [PubMed] [Google Scholar]
- Pande K, Chen C, Noble SM. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nature genetics. 2013;45:1088–91. doi: 10.1038/ng.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Patterson TF. The future of animal models of invasive aspergillosis. Medical mycology. 2005;43(Suppl 1):S115–9. doi: 10.1080/13693780400029429. [DOI] [PubMed] [Google Scholar]
- Petraitiene R, Petraitis V, Groll AH, Sein T, Schaufele RL, Francesconi A, Bacher J, Avila NA, Walsh TJ. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrobial agents and chemotherapy. 2002;46:12–23. doi: 10.1128/AAC.46.1.12-23.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman D, Mistry M, Thavaraj S, Naglik JR, Challacombe SJ. Murine model of concurrent oral and vaginal Candida albicans colonisation. Methods in molecular biology. 2012;845:527–35. doi: 10.1007/978-1-61779-539-8_38. [DOI] [PubMed] [Google Scholar]
- Rayamajhi M, Redente EF, Condon TV, Gonzalez-Juarrero M, Riches DW, Lenz LL. Non-surgical intratracheal instillation of mice with analysis of lungs and lung draining lymph nodes by flow cytometry. Journal of visualized experiments: JoVE. 2011 doi: 10.3791/2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AT, Lakshmi SP, Reddy RC. Murine model of allergen induced asthma. Journal of visualized experiments: JoVE. 2012:e3771. doi: 10.3791/3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt DJ, Licata I, Kaplan W, Ajello L, Chandler FW, Ellis JJ. Experimental cerebral zygomycosis in alloxan-diabetic rabbits: variation in virulence among zygomycetes. Sabouraudia. 1981;19:245–56. doi: 10.1080/00362178185380421. [DOI] [PubMed] [Google Scholar]
- Rhodes JC. Aspergillus fumigatus: growth and virulence. Medical mycology. 2006;44(Suppl 1):S77–81. doi: 10.1080/13693780600779419. [DOI] [PubMed] [Google Scholar]
- Rhodes JC, Wicker LS, Urba WJ. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect Immun. 1980;29:494–9. doi: 10.1128/iai.29.2.494-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, Coward JW, Iwakura Y, Pamer EG. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. The Journal of experimental medicine. 2011;208:369–81. doi: 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant’Angelo DB, Pamer EG. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity. 2006;25:665–75. doi: 10.1016/j.immuni.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Rizzetto L, Giovannini G, Bromley M, Bowyer P, Romani L, Cavalieri D. Strain dependent variation of immune responses to A. fumigatus: definition of pathogenic species. PloS one. 2013;8:e56651. doi: 10.1371/journal.pone.0056651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez TE, Falkowski NR, Harkema JR, Huffnagle GB. Role of neutrophils in preventing and resolving acute fungal sinusitis. Infect Immun. 2007;75:5663–8. doi: 10.1128/IAI.01542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–5. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- Sable CA, Strohmaier KM, Chodakewitz JA. Advances in antifungal therapy. Annual review of medicine. 2008;59:361–79. doi: 10.1146/annurev.med.59.062906.071602. [DOI] [PubMed] [Google Scholar]
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–91. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Samaranayake YH, Samaranayake LP. Experimental oral candidiasis in animal models. Clinical microbiology reviews. 2001;14:398–429. doi: 10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarasinghe AE, Hoselton SA, Schuh JM. A comparison between intratracheal and inhalation delivery of Aspergillus fumigatus conidia in the development of fungal allergic asthma in C57BL/6 mice. Fungal biology. 2011;115:21–9. doi: 10.1016/j.funbio.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunte DM, Hasselby JP, Brillowska-Dabrowska A, Frimodt-Moller N, Svejgaard EL, Linnemann D, Nielsen SS, Haedersdal M, Arendrup MC. Experimental guinea pig model of dermatophytosis: a simple and useful tool for the evaluation of new diagnostics and antifungals. Medical mycology. 2008;46:303–13. doi: 10.1080/13693780801891732. [DOI] [PubMed] [Google Scholar]
- Savage DC, Dubos RJ. Localization of indigenous yeast in the murine stomach. Journal of bacteriology. 1967;94:1811–6. doi: 10.1128/jb.94.6.1811-1816.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Sachdev A, Coggon D, Hossain P. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. The British journal of ophthalmology. 2011;95:762–7. doi: 10.1136/bjo.2009.169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Mednick A, Alvarez M, van Rooijen N, Casadevall A, Goldman DL. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. Journal of immunology. 2005;175:3244–51. doi: 10.4049/jimmunol.175.5.3244. [DOI] [PubMed] [Google Scholar]
- Sheppard DC, Graybill JR, Najvar LK, Chiang LY, Doedt T, Kirkpatrick WR, Bocanegra R, Vallor AC, Patterson TF, Filler SG. Standardization of an experimental murine model of invasive pulmonary aspergillosis. Antimicrobial agents and chemotherapy. 2006a;50:3501–3. doi: 10.1128/AAC.00787-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DC, Marr KA, Fredricks DN, Chiang LY, Doedt T, Filler SG. Comparison of three methodologies for the determination of pulmonary fungal burden in experimental murine aspergillosis. Clin Microbiol Infect. 2006b;12:376–80. doi: 10.1111/j.1469-0691.2005.01349.x. [DOI] [PubMed] [Google Scholar]
- Sheppard DC, Rieg G, Chiang LY, Filler SG, Edwards JE, Jr., Ibrahim AS. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrobial agents and chemotherapy. 2004;48:1908–11. doi: 10.1128/AAC.48.5.1908-1911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Takaoka M, Uchida K, Wakayama M, Yamaguchi H, Takahashi K, Paris S, Latge JP, Naoe S. Histopathology of experimental invasive pulmonary aspergillosis in rats: pathological comparison of pulmonary lesions induced by specific virulent factor deficient mutants. Microbial pathogenesis. 1999;27:123–31. doi: 10.1006/mpat.1999.0288. [DOI] [PubMed] [Google Scholar]
- Smith RM, Schaefer MK, Kainer MA, Wise M, Finks J, Duwve J, Fontaine E, Chu A, Carothers B, Reilly A, Fiedler J, Wiese AD, Feaster C, Gibson L, Griese S, Purfield A, Cleveland AA, Benedict K, Harris JR, Brandt ME, Blau D, Jernigan J, Weber JT, Park BJ, Multistate Fungal Infection Outbreak Response, T. Fungal infections associated with contaminated methylprednisolone injections. The New England journal of medicine. 2013;369:1598–609. doi: 10.1056/NEJMoa1213978. [DOI] [PubMed] [Google Scholar]
- Smulian AG. Invasive models of histoplasmosis. Methods in molecular biology. 2012;845:519–25. doi: 10.1007/978-1-61779-539-8_37. [DOI] [PubMed] [Google Scholar]
- Solis NV, Filler SG. Mouse model of oropharyngeal candidiasis. Nature protocols. 2012;7:637–42. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen KN, Clemons KV, Stevens DA. Murine models of blastomycosis, coccidioidomycosis, and histoplasmosis. Mycopathologia. 1999;146:53–65. doi: 10.1023/a:1007081707287. [DOI] [PubMed] [Google Scholar]
- Sousa Mda G, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, Yamasaki S, Taylor PR, Almeida SR, Brown GD. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell host & microbe. 2011;9:436–43. doi: 10.1016/j.chom.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Ibrahim AS, Edwards JE, Jr., Filler SG. Mice with disseminated candidiasis die of progressive sepsis. The Journal of infectious diseases. 2005;192:336–43. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- Spikes S, Xu R, Nguyen CK, Chamilos G, Kontoyiannis DP, Jacobson RH, Ejzykowicz DE, Chiang LY, Filler SG, May GS. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. The Journal of infectious diseases. 2008;197:479–86. doi: 10.1086/525044. [DOI] [PubMed] [Google Scholar]
- Steele C, Wormley FL., Jr. Immunology of fungal infections: lessons learned from animal models. Current opinion in microbiology. 2012;15:413–9. doi: 10.1016/j.mib.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Steinbach WJ, Benjamin DK, Jr., Trasi SA, Miller JL, Schell WA, Zaas AK, Foster WM, Perfect JR. Value of an inhalational model of invasive aspergillosis. Medical mycology. 2004;42:417–25. doi: 10.1080/13693780410001712034. [DOI] [PubMed] [Google Scholar]
- Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. The Journal of infection. 2012;65:453–64. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Stephens-Romero SD, Mednick AJ, Feldmesser M. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect Immun. 2005;73:114–25. doi: 10.1128/IAI.73.1.114-125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DA. Animal models of blastomycosis. Seminars in respiratory infections. 1997;12:196–7. [PubMed] [Google Scholar]
- Sugar AM, Liu XP. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Medical mycology. 2000;38:209–12. doi: 10.1080/mmy.38.3.209.212. [DOI] [PubMed] [Google Scholar]
- Sugui JA, Pardo J, Chang YC, Zarember KA, Nardone G, Galvez EM, Mullbacher A, Gallin JI, Simon MM, Kwon-Chung KJ. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryotic cell. 2007;6:1562–9. doi: 10.1128/EC.00141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chandra J, Mukherjee P, Szczotka-Flynn L, Ghannoum MA, Pearlman E. A murine model of contact lens-associated fusarium keratitis. Investigative ophthalmology & visual science. 2010;51:1511–6. doi: 10.1167/iovs.09-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CM, Cohen J, Krausz T, Van Noorden S, Holden DW. The alkaline protease of Aspergillus fumigatus is not a virulence determinant in two murine models of invasive pulmonary aspergillosis. Infect Immun. 1993;61:1650–6. doi: 10.1128/iai.61.5.1650-1656.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabishy AB, Aldabagh B, Sun Y, Imamura Y, Mukherjee PK, Lass JH, Ghannoum MA, Pearlman E. MyD88 regulation of Fusarium keratitis is dependent on TLR4 and IL-1R1 but not TLR2. Journal of immunology. 2008;181:593–600. doi: 10.4049/jimmunol.181.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GR, 3rd, Wiederhold NP, Najvar LK, Bocanegra R, Kirkpatrick WR, Graybill JR, Patterson TF. A murine model of Cryptococcus gattii meningoencephalitis. The Journal of antimicrobial chemotherapy. 2012;67:1432–8. doi: 10.1093/jac/dks060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, May RC, Wheeler RT. Zebrafish: a see-through host and a fluorescent toolbox to probe host-pathogen interaction. PLoS pathogens. 2012;8:e1002349. doi: 10.1371/journal.ppat.1002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rodriguez JM, Morera Y, Baro T, Corominas JM, Castaneda E. Pathogenicity of Cryptococcus neoformans var. gattii in an immunocompetent mouse model. Medical mycology. 2003;41:59–63. doi: 10.1080/mmy.41.1.59.63. [DOI] [PubMed] [Google Scholar]
- Tsai NY, Laforce-Nesbitt SS, Tucker R, Bliss JM. A murine model for disseminated candidiasis in neonates. Pediatric research. 2011;69:189–93. doi: 10.1203/PDR.0b013e318206fd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem J. Animal models for dermatomycotic infections. Current topics in medical mycology. 1989;3:1–35. doi: 10.1007/978-1-4612-3624-5_1. [DOI] [PubMed] [Google Scholar]
- Van Cutsem J, Janssen PA. Experimental systemic dermatophytosis. The Journal of investigative dermatology. 1984;83:26–31. doi: 10.1111/1523-1747.ep12261652. [DOI] [PubMed] [Google Scholar]
- Waldorf AR, Halde C, Vedros NA. Murine model of pulmonary mucormycosis in cortisone-treated mice. Sabouraudia. 1982;20:217–24. doi: 10.1080/00362178285380321. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Garrett K, Feurerstein E, Girton M, Allende M, Bacher J, Francesconi A, Schaufele R, Pizzo PA. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography, a novel, noninvasive method for measuring responses to antifungal therapy. Antimicrobial agents and chemotherapy. 1995;39:1065–9. doi: 10.1128/aac.39.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lebert V, Hung CY, Galles K, Saijo S, Lin X, Cole GT, Klein BS, Wuthrich M. C-type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of north america. Journal of immunology. 2014;192:1107–19. doi: 10.4049/jimmunol.1302314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat LJ, Kohler RB, Tewari RP. Diagnosis of disseminated histoplasmosis by detection of Histoplasma capsulatum antigen in serum and urine specimens. The New England journal of medicine. 1986;314:83–8. doi: 10.1056/NEJM198601093140205. [DOI] [PubMed] [Google Scholar]
- Williams JE, Moser SA. Chronic murine pulmonary blastomycosis induced by intratracheally inoculated Blastomyces dermatitidis conidia. The American review of respiratory disease. 1987;135:17–25. doi: 10.1164/arrd.1987.135.1.17. [DOI] [PubMed] [Google Scholar]
- Williams PL, Sobel RA, Sorensen KN, Clemons KV, Shuer LM, Royaltey SS, Yao Y, Pappagianis D, Lutz JE, Reed C, River ME, Lee BC, Bhatti SU, Stevens DA. A model of coccidioidal meningoencephalitis and cerebrospinal vasculitis in the rabbit. The Journal of infectious diseases. 1998;178:1217–21. doi: 10.1086/515689. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- Wuthrich M, Deepe GS, Jr., Klein B. Adaptive immunity to fungi. Annual review of immunology. 2012;30:115–48. doi: 10.1146/annurev-immunol-020711-074958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Filutowicz HI, Klein BS. Mutation of the WI-1 gene yields an attenuated blastomyces dermatitidis strain that induces host resistance. The Journal of clinical investigation. 2000;106:1381–9. doi: 10.1172/JCI11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, Klein B. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. The Journal of clinical investigation. 2011a;121:554–68. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Hung CY, Gern BH, Pick-Jacobs JC, Galles KJ, Filutowicz HI, Cole GT, Klein BS. A TCR transgenic mouse reactive with multiple systemic dimorphic fungi. Journal of immunology. 2011b;187:1421–31. doi: 10.4049/jimmunol.1100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Alvarez M, Telzak A, Rivera J, Casadevall A. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect Immun. 2007;75:2729–39. doi: 10.1128/IAI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity. 2013;39:324–34. doi: 10.1016/j.immuni.2013.05.017. [DOI] [PubMed] [Google Scholar]