Abstract
Environmental complexity (EC) is a powerful, stimulating paradigm that engages animals through a variety of sensory and motor pathways. Exposure to EC (30 days) following 12 days of wheel running preserves hippocampal neuroplasticity in male rats neonatally exposed to alcohol during the third-trimester equivalent (binge-like exposure on postnatal days [PD] 4–9). The current experiment investigates the importance of various components of EC (physical activity, exploration, social interaction, novelty) and examines whether neonatal alcohol exposure affects how male rats interact with their environment and other male rats. Male pups were assigned to 1 of 3 neonatal conditions from PD 4–9: suckle control (SC), sham-intubated (SI), or alcohol-exposed (AE, 5.25 g/kg/day). From PD 30–42 animals were housed with 24-h access to a voluntary running wheel. The animals were then placed in EC from PD 42–72 (9 animals/cage, counterbalanced by neonatal condition). During EC, the animals were filmed for five 30-min sessions (PD 42, 48, 56, 64, 68). For the first experiment, the videos were coded for distance traveled in the cage, overall locomotor activity, time spent near other animals, and interaction with toys. For the second experiment, the videos were analyzed for wrestling, mounting, boxing, grooming, sniffing, and crawling over/under. AE animals were found to be less active and exploratory and engaged in fewer mounting behaviors compared to control animals. Results suggest that after exposure to wheel running, AE animals still have deficits in activity and social behaviors while housed in EC compared to control animals with the same experience.
Keywords: social interaction, locomotor, FASD, development
Introduction
Maternal alcohol consumption is a leading cause of preventable mental disability in children (Centers for Disease Control [CDC], 2013). The umbrella term Fetal Alcohol Spectrum Disorders (FASD) includes a wide range of physical, emotional, behavioral, cognitive, and social deficits associated with prenatal alcohol exposure (Clarke & Gibbard, 2003). Despite increased public awareness regarding the dangers of drinking during pregnancy, the current prevalence of FASDs has been estimated to be as high as 5% of live births in the United States (CDC, 2013; May et al., 2009). The influence of FASD is extensive and often results in serious neurodevelopmental deficits affecting cognition and behavior throughout life. Similarly, alcohol exposure has widespread effects on the brain, with the prefrontal cortex, hippocampus, and cerebellum being some of the more vulnerable regions. Developmental alcohol exposure has been shown to lead to decreased gray matter and basal ganglia volume in humans (Mattson et al., 1996; Mattson, Schoenfeld, & Riley, 2001), impaired cortical plasticity (Rema & Ebner, 1999), reduced cortical and hippocampal dendritic complexity and spine density (Hamilton, Akers, et al., 2010; Hamilton, Criss, & Klintsova, unpublished data; Whitcher & Klintsova, 2008), decreased hippocampal adult neurogenesis (Choi, Allan, & Cunningham, 2005; Hamilton, Boschen, Goodlett, Greenough, & Klintsova, 2012; Helfer, Goodlett, Greenough, & Klintsova, 2009; Klintsova et al., 2007), impaired CA1 long-term potentiation (Puglia & Valenzuela, 2010a,b), and reduced cerebellar synapse per Purkinje cell number (Klintsova, Matthews, Goodlett, Napper, & Greenough, 1997). In addition, numerous behavioral deficits are observed, including motor deficits and impaired performance on hippocampal-associated spatial and executive functioning tasks (reviewed in Klintsova, Hamilton, & Boschen, 2013).
Limited pharmacological or behavioral therapies are available for the treatment of children with FASD, though evidence suggests that early interventions result in better outcomes for these children later in life (CDC, 2013). Within the rodent literature, both exercise and exposure to a complex environment have been shown to be beneficial to both the healthy and damaged brain. Housing in classic environmental complexity (EC) paradigms results in increased cortical thickness, enhanced dendritic branching in the frontal, temporal, and occipital cortices, and visual cortex synaptogenesis in the normal rat brain (Greenough & Volkmar, 1973; Greenough, Volkmar & Juraska, 1973; Rosenzweig, Bennett, & Krech, 1964; Turner & Greenough, 1985). Additionally, EC influences various components of cellular neuroplasticity including neurotrophin release, vesicle-docking proteins, and postsynaptic receptor expression (Olson, Eadie, Ernst, & Christie, 2006). These variables may contribute to the beneficial effect of EC on the alcohol-damaged brain. Our lab has demonstrated that a combination of wheel running (WR) followed by environmental complexity enhances hippocampal adult neurogenesis and cortical dendritic complexity in animals exposed to alcohol from PD 4–9 (third-trimester equivalent) (Hamilton et al., 2012; Hamilton, Criss, & Klintsova, unpublished data). In addition, this intervention ameliorates alcohol-associated impairments in contextual fear conditioning, contextual pre-exposure facilitation effect (CPFE) training, and trace eye-blink conditioning (Hamilton et al., 2014; Schreiber et al., 2013).
The design of an effective behavioral intervention for children with FASD is critical for responding to the problem of maternal drinking in our society. The use of rodent models is key in establishing procedures that show the greatest benefit in the least amount of time. The use of a diverse range of EC protocols in the literature makes it difficult to determine which components of the cage contribute most to the beneficial effects. Recent literature on EC paradigms in mice suggests that aerobic exercise is the key factor contributing to the beneficial effects observed (Kobilo et al., 2011; Mustroph et al., 2012). However, EC paradigms that do not include an element that specifically targets increasing aerobic exercise, such as a running wheel, still produce strong neuroplastic changes (Bredy, Humpartzoomian, Cain, & Meaney, 2003; Ehninger & Kempermann, 2003; Fabel et al., 2009; Greenough & Volkmar, 1973; Greenough, Volkmar, & Juraska, 1973; Parks, McMechan, Hannigan, & Berman, 2008; Rema & Ebner, 1999; Rosenzweig et al., 1964; Schapiro, 2002; Turner & Greenough, 1985). Additionally, our lab uses an intervention that includes wheel running immediately prior to, but separate from, the EC paradigm, and we have found that EC is able to support long-lasting neuroplastic alterations that may have been initiated during WR.
The current experiment seeks to further understand the influence of various components of EC on animal behavior and whether neonatal alcohol exposure affects how the rats interact with their environment and other animals. Four factors involved in our EC paradigm were considered: 1) physical activity, 2) exploratory behavior, 3) novel enrichment items, and 4) social interactions. The first experiment investigated locomotor and exploratory behavior of rats during 4 weeks in EC by assessing the 4 different factors of the intervention listed above. The second experiment examined the specific social interactions between individual animals while in the EC cage.
Materials and methods
Animals
Rats were bred at the University of Delaware’s Office of Laboratory Animal Medicine Facility. On PD 3, 8 litters were culled to 8 pups each (6 male, 2 female when possible). The timeline of the experiment is displayed in Fig. 1A. On PD 4, pups were randomly assigned to 1 of 3 experimental groups: suckle control (SC), sham-intubated (SI), and alcohol-exposed (AE). A split-litter design was used so that SI and AE animals were represented in the same litter. Pups remained with the dam and littermates until weaning on PD 23. Following weaning, rats were housed in groups of 3 same-sex animals in standard cages (17 cm high × 145 cm long × 24 cm wide) until PD 30 (counterbalanced for litter and neonatal condition), when they were placed either into wheel running followed by environmental complexity (described in Environmental Complexity section) or maintained in social housing (animals used for other studies reported elsewhere). In total, 18 male rats were used for the current study, 6 per neonatal condition (AE, SI, SC). The animals were housed in a 12/12-h light/dark cycle (lights on at 9:00 AM). All procedures were carried out in accordance with NIH Animal Care Guidelines and the animal use protocol approved by University of Delaware Institutional Animal Care and Use Committee.
Figure 1.
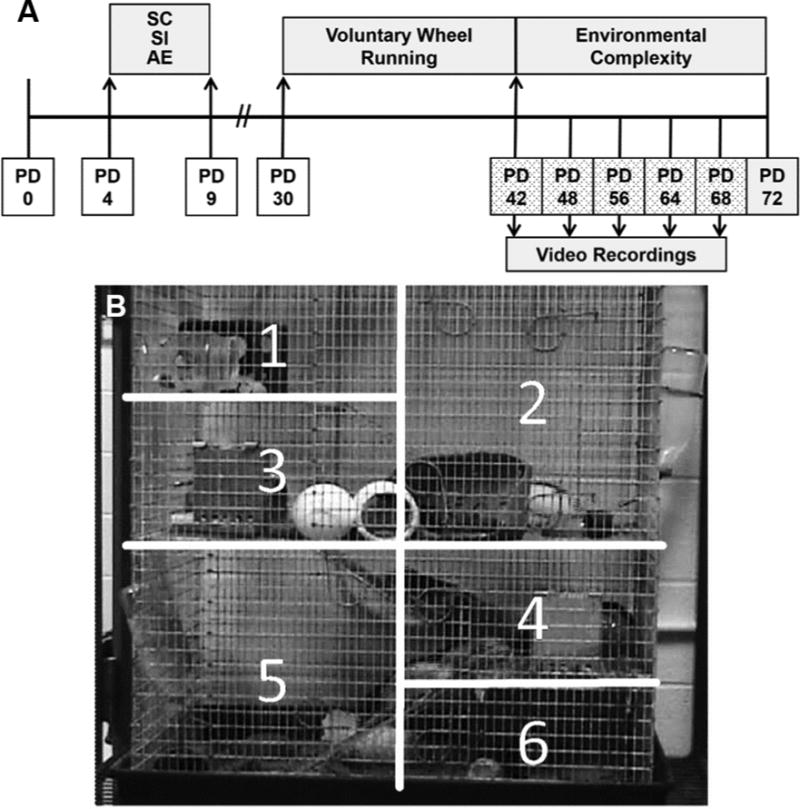
A) Experimental Timeline. Rats were exposed to 5.25 g/kg/day alcohol in a binge-like manner on PD 4–9 and weaned on PD 23. AE: Alcohol-exposed; SI: Sham-intubated; SC: Suckle control (undisturbed). The rats were then housed 3/cage with voluntary access to a running wheel (WR) on PD 30–42 and then housed in environmental complexity (EC) on PD 42–72. Sessions were taped on PD 42, 48, 56, 64, and 68 following the toy change. B) The EC cage was visually split into 6 quadrants on a computer monitor during video analysis to analyze exploratory behavior.
Neonatal alcohol exposure paradigm
On PD 4–9, AE pups were exposed to alcohol in a binge-like manner (5.25 g/kg/day; Fig. 1A). Alcohol was administered in an 11.9% v/v milk solution in 2 doses, 2 h apart via temporary intragastric intubations. On PD 4, 2 supplemental doses of milk formula were administered, 2 h and 4 h following the second alcohol dose to compensate for reduced calorie intake from the dam by AE pups. For the remaining days (PD 5–9), milk formula was administered only once, 2 h following the second alcohol exposure. Sham-intubated (SI) pups received intubations without milk or alcohol solution. SC animals were undisturbed apart from daily weighing (PD 4–9).
Blood alcohol concentrations (BACs)
On PD 4, blood samples were obtained from SI and AE pups for BAC analysis. Blood was collected via tail clip 90 min following the second alcohol exposure. Samples from the AE group were centrifuged (15,000 rpm/15 min), and the plasma was collected and stored at −20°C . Plasma was analyzed for BAC using an Analox GL5 Alcohol Analyzer (Analox Instruments, Boston, MA). Blood samples from the SI group were not analyzed for alcohol concentration; the tail clip was performed in the SI pups to control for the stress of the blood collection procedure.
Voluntary wheel running
From PD 30–42, rats were housed for 12 days in cages with 24-h voluntary access to stainless-steel running wheels (Fig. 1A). Animals were housed 3 per cage, counterbalanced for litter and neonatal condition (same groups as described in the Animals section). The total running distance across each day was determined by the number of wheel revolutions registered by a mechanical counter attached to each of the wheels and recorded daily. Wheel revolutions were checked daily at 9:00 AM. As animals were housed 3 per cage, the exact distance run by each rat could not be recorded. Previous studies from our lab housed AE, SI, and SC animals separately in the wheel running condition (3/cage), and no effect of neonatal treatment was found on distance run per day (Helfer et al., 2009). The rats were also regularly observed running together in the wheel. Based on these observations, wheel-running totals were analyzed by cage, not by individual rat.
Environmental complexity (EC)
From PD 42–72, rats were housed in EC cages (Fig. 1). Two identical EC cages were used in this experiment. Each EC cage consisted of a 30″ × 18″ × 36″ 3-story galvanized steel cage with 3 ramps, 2 balconies, and a full middle floor. The floor of the cage was a drop-in 3½-inch plastic pan filled with wood chip bedding. Each cage housed 9 male animals (3 animals/neonatal condition) and was equipped with a variety of toys and novelty objects. The toys were changed at 9:00 AM every 2nd day. Every 4th day at 9:00 AM, the cages were cleaned by removing the animals (~10 min), replenishing food, water, and bedding, and replacing all of the toys with novel items.
Video recording
On PD 42, 48, 56, 62, and 68, the 2 EC cages (Cage A and Cage B) were videotaped to assess behavior of individual rats during EC exposure (Fig. 1A). To allow for reliable identification of individual animals, animals were marked with a unique nontoxic paint color 15 min prior to recording. Sessions were videotaped during the first 2 h (9:00–11:00 AM) of the light cycle. To control for the potential additive stressors of cage cleaning, rats were only observed on dates when only the toys were replaced. On each scheduled day, four 30-min sessions were collected (2 sessions/cage/day). To control for time differences of recording, the order of cage videotaped first was alternated. For example, on PD 42 the cage recording order was ABAB and on PD 48 the order was BABA. Due to the animals entering the sleeping phase of their daily cycle during the second hour, only data from the first hour of recording (one 30-min session per cage) are reported.
Locomotor and exploratory behavior analysis
Each 30-min session was coded by 2 experimenters blind to each animal’s neonatal condition (> 85% inter-rater agreement). Rats were observed individually across each session. Four activities were coded: 1) the number of cage quadrants traversed per minute (Fig. 2A), 2) frequency of movement (Fig. 2B), 3) frequency of sharing a cage quadrant with other animals (Fig. 2C), and 4) frequency of interaction with toys (Fig. 2D). The number of cage quadrants traversed was determined by splitting the cage into 6 quadrants (Fig. 1B) and tallying the number of quadrants crossed through. The number of quadrants was totaled for each rat across each session. At each minute interval, the recording was paused and the movement of the animal was determined by whether it was in motion or not (defined by ≥ 3 paws in motion). At each minute pause it was also noted whether the animal occupied a quadrant with another rat (yes or no) and whether the animal was interacting with a novelty object (physical contact required, yes or no).
Figure 2.
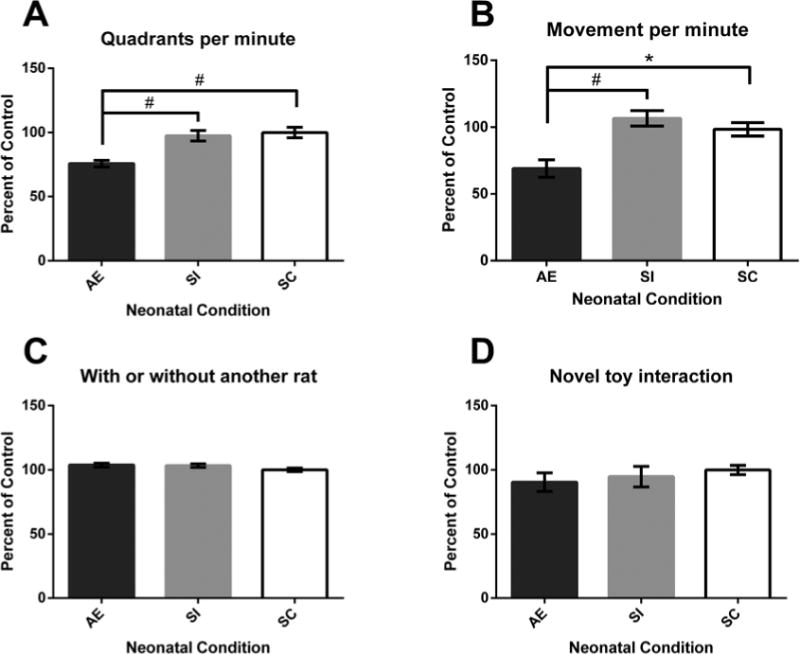
Experiment 1: Activity Behaviors. A) AE animals traversed fewer quadrants per minute compared to SI and SC animals. B) Alcohol exposure (AE) rats were less likely to be moving at each minute compared to sham-intubated (SI) and suckle control (SC) animals. C) and D) No significant effect of Neonatal Condition was found with whether or not the rat was with another animal in the same quadrant, or for interaction with toys. *p < 0.05, #p < 0.01. Values shown as mean percent of control ± SEM.
Social behavior analysis
Each 30-min session was coded by 2 experimenters blind to each animal’s neonatal condition (> 85% inter-rater agreement). Rats were observed for 6 social behaviors: wrestling, mounting, boxing, grooming, sniffing, and crawling over/under. The operational definition for each behavior was adapted from papers by Meaney & Stewart (1981) and Hamilton, Akers, et al. (2010). Wrestling was measured as 2 or more animals rolling and tumbling with one another. In most cases, the behavior ended with one rat positioned over another with its forepaws placed on the second animal (“pinning”). Mounting was measured as one rat approaching another from the rear and placing its forepaws on the back of the other rat. Following initial contact, animals would assume either an on-top posture (one animal positioned over another animal’s back with its forepaws on the second animal) or an on-back posture (one animal would be positioned over the other animal’s stomach with its forepaws on the second animal). Boxing differed from wrestling as 2 animals stood upright on their hind paws facing one another and used their forepaws to make pawing or punching movements toward the other animal. Grooming was defined as 1 animal vigorously cleaning another animal’s fur using a combination of its mouth and forepaws. Sniffing required nose-to-fur contact for a minimum of 2 sec. Any use of the forepaws was deemed grooming. Crawl Over/Under required 1 rat to be stationary and for the other animal to physically crawl over or under the stationary rat.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 21 (2012). Weights data were analyzed using a repeated-measures analysis of variance (ANOVA). Prior to statistical analysis, activity and social data were transformed into percent of control of SC due to differences in baseline levels of activity in each cage. The results for both experiments were analyzed using repeated-measures analysis of covariance (ANCOVA; described in Results) with Cage as the covariate. For the behavior of crawling over/under, a linear mixed model with Cage as a covariate was used to account for missing data values.
Results
Weights
Animal weights were compared for PD 4, 9, 30, 42, and 72 (Table 1). The influence of neonatal treatment on body weights was determined using a repeated-measures analysis of variance (ANOVA) with Neonatal Condition (AE, SI, SC) as the between-subjects factor and Postnatal Day as the within-subjects factor. Weights from the neonatal period (PD 4 and 9) were analyzed separately from adolescent and adult weights (PD 30, 42, and 72). For PD 4 and 9, a main effect of Postnatal Day (F[1,15] = 1225.716, p < 0.01) was found. In addition, there was a Day × Neonatal Condition interaction (F[1,15] = 15.220, p < 0.01). Univariate ANOVAs revealed no effect of Neonatal Condition on PD 4 but a significant main effect of Neonatal Condition on PD 9 (F[1,15] = 3.872, p < 0.05). A post hoc Tukey’s test revealed a specific significant decrease in the body weights of AE animals compared to SI animals (p < 0.05). For PD 30, 42, and 72, the repeated-measures ANOVA found a main effect of Postnatal Day (F[1,15] = 2250.541, p < 0.01), but neither a main effect of Neonatal Condition nor a Condition × Postnatal Day interaction was evident.
Table 1.
Weights and BACs. Average body weights (g ± SEM) are given for alcohol-exposed (AE), sham-intubated (SI), and suckle control (SC) animals. Data are reported across the first and last day of the dosing procedure (PD 4 and PD 9), first and last days of wheel running exposure (PD 30 and PD 42), and the last day of environmental complexity (PD 72). *AE weighed significantly less than SI animals (p < 0.05). BACs were taken from blood samples collected 90 min following the second alcohol dose (reported in mg/dL ± SEM).
| Weights (g) | BACs (mg/dL) | |||||
|---|---|---|---|---|---|---|
| PD 4 | PD 9 | PD 30 | PD 42 | PD 72 | ||
| AE | 11.2 ± 0.4 | 17.6 ± 4.0* | 102.3 ± 4.0 | 184.7 ± 6.6 | 358.8 ± 10.8 | 427.7 ± 10.6 |
| SI | 11.2 ± 0.6 | 20.2 ± 0.6 | 107 ± 5.3 | 183.5 ± 10.5 | 346.5 ± 14.1 | N/A |
| SC | 9.9 ± 0.2 | 19.1 ± 0.4 | 99.2 ± 1.6 | 185.0 ± 3.2 | 361.3 ± 7.3 | N/A |
Blood alcohol concentrations (BACs)
BACs were obtained from samples collected 90 min after the second alcohol dose on PD 4. BACs ranged from 392.80 to 456.40 mg/dL with an average BAC of 427.73 ± 10.64 mg/dL (Table 1). This range is comparable to previously published BACs using the same alcohol exposure paradigm (Hamilton et al., 2012; Helfer et al., 2009).
Wheel running activity
Number of running wheel revolutions was recorded daily at 9:00 AM from PD 30–42. Running distance ranged from 1.73 to 4.14 miles per 24-h period with the average running distance being 2.81 ± 0.37 miles per 24-h period.
Locomotor and exploratory behavior analysis
Data were transformed into percent of control based on the mean of SC behavior per cage. Dates of recording and cages were combined for analysis. Raw means per cage can be found in Supplemental Table 1. A repeated-measures analysis of covariance (ANCOVA) was performed for each variable with Cage as a covariate to control for differences in activity levels between the cages. No main effect of day of recording was found for any of the activity variables.
Total number of quadrants traversed
To determine exploratory behavior across the session, the number of cage quadrants traversed was tallied across each minute for each animal. Results revealed a main effect of neonatal treatment (F[2,14] = 13.092, p = 0.001), with AE animals exploring fewer quadrants of the cage compared to both SI and SC animals (p = 0.001 and 0.003) (Fig. 2A). No effect of Cage or Cage by Neonatal Treatment interaction was found (p < 0.05).
Movement
To determine locomotor activity across the session, at each minute, animals were rated as either stationary or mobile (1–30). A main effect of neonatal treatment was found (F[2,14] = 10.930, p = 0.001), with AE animals being significantly less likely to be moving than SI or SC animals (p = 0.002 and 0.011) (Fig. 2B). There was no main effect of Cage or Cage by Neonatal Treatment interaction (p > 0.05).
Sharing quadrant with other rats
To assess how likely animals were to be near other rats while in EC, animals were coded for whether or not they co-occupied a quadrant with another rat or were alone. No main effects of Neonatal Condition or Cage were found (p > 0.05) (Fig. 2C); in addition, no interactions were found.
Novel toy interaction
To assess the frequency of novel toy interaction during EC exposure, the animals were coded for whether or not they were in contact with a toy at each minute (1–30) across the session. No main effects of Neonatal Condition or Cage or interactions were found (p > 0.05) (Fig. 2D).
Social behavior analysis
Data were transformed into percent of control based on the mean of SC behavior per cage, and dates of recording and cages were combined for analysis. Raw means per cage can be found in Supplemental Table 1. Repeated-measures ANCOVAs were run using Cage as the covariate to determine the influence of Neonatal Condition on each behavior. No effect of day of recording was found for any of the social behaviors.
Wrestling
Results revealed a main effect of Neonatal Condition (F[2,14] = 3.789, p = 0.048) on the frequency of wrestling behavior. Pairwise comparisons (Bonferroni Adjustment) revealed that AE animals engaged in fewer wrestling behaviors than SI animals (p = 0.069) but did not differ significantly from SC rats (Fig. 3A). SI and SC rats were not significantly different. No main effect of Cage or Cage by Neonatal Treatment interaction was found.
Figure 3.
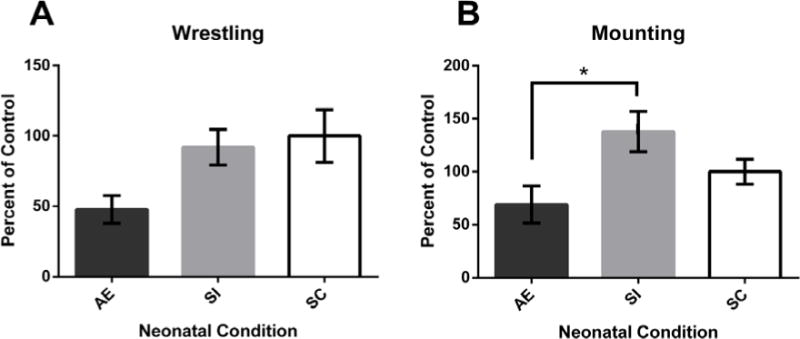
Experiment 2: Social Behaviors. A main effect of neonatal treatment was found for wrestling (A), though post hoc tests were not significant (AE vs. SI, p = 0.069). Alcohol-exposed (AE) animals were significantly less likely to engage in mounting (B) behaviors compared to sham-intubated (SI) rats. *p < 0.05. Values shown as mean percent of control ± SEM.
Mounting
Results revealed a main effect of Neonatal Condition (F[2,14] = 4.394, p = 0.033) on the frequency of mounting behavior. Further analysis using a pairwise comparison (Bonferroni Adjustment) revealed that AE animals engaged in significantly fewer mounting behaviors than SI animals (p = 0.031) but did not statistically differ from SC rats (Fig. 3B). SI and SC rats did not significantly differ. No main effects of Cage or interactions were found.
Other social behaviors
For sniffing and grooming, results revealed no main effect of Neonatal Condition or Cage (F < 1, p > 0.05). In addition, no interactions were found for either variable. No grooming was recorded for either cage on PD 42 so this day was excluded from statistical analyses. For crawling over/under, a mixed linear model analysis with Cage as a covariate was run to account for missing values from Cage B on PD 64 (no crawling behaviors recorded for SC). No main effect of Neonatal Condition was found (p > 0.05) but a trending effect of Cage was reported (t[8.99] = 2.08, p = 0.069), with Cage A engaging in more crawling behaviors than Cage B. Boxing was not analyzed due to the low frequency of this behavior during the recordings (3 occurrences total across days).
Discussion
The current study analyzed activity and social behavior in an environmentally complex (EC) cage in rats neonatally exposed to alcohol (AE), and is the first study to observe how AE animals interact with the environment and other rats while living in the EC cage. This study is distinct from other published data in that the animals were observed in their home environment while engaging in social behaviors with familiar animals compared to being placed in a novel cage with a “stranger” rat. The current experiments demonstrate that AE rats interact with their environment and their cage mates differently than do control animals. Specifically, AE animals were less active and exploratory compared to control animals and were less likely to engage in social behaviors such as mounting and wrestling (n.s., p = 0.069 vs. SI). Interestingly, the activity and social deficits observed in the AE rats in the current experiments were maintained across the entire time spent in the intervention (more than 3 weeks). Although AE animals get the most benefit from exposure to EC following WR in neuroanatomical measures (compared to WR followed by standard housing [SH; Hamilton et al., 2012]), they have decreased levels of exploration, mounting, and wrestling (n.s., p = 0.069 vs. SI) for the duration of housing in EC, suggesting that EC exposure was not sufficient to ameliorate deficits in exploratory and social behaviors. The results presented here provide novel insight into how rats utilize their environment and interact with one another while housed in the EC intervention following exposure to a running wheel (an intervention that has proven beneficial both at the anatomical and at the behavioral level).
The first experiment addressed how alcohol exposure affects overall locomotor activity, exploratory behavior, time spent occupying the same quadrant as another animal, and interaction with novelty items. AE animals were less likely to be in motion and explored fewer quadrants of the cage compared to control animals (Fig. 2A & B). These results were unexpected given that AE animals are often found to display increased locomotor activity in an open field (Gilbertson & Barron, 2005; Kelly, Hulsether, & West, 1987; Melcer, Gonzalez, Barron, & Riley, 1994; Melcer, Gonzalez, & Riley, 1995; Schneider, Moore, & Adkins, 2011). However, locomotor activity reductions have been reported in AE mice placed in a novel environment (Kleiber, Wright, & Singh, 2011). Numerous variables might contribute to the decreased locomotor activity observed in AE animals in the current study. First, AE rats might display increased novelty- or handling-induced anxiety caused by handling for paint marking and changing of the enrichment items (Cullen, Burne, Lavidis, & Moritz, 2013; Kleiber et al., 2011; Zhou, Wang, & Zhu, 2010). Second, circadian rhythms in AE animals could be altered, causing AE rats to enter a sleep cycle at a different time than control animals (Farnell et al., 2008). However, Sakata-Haga and colleagues (2006) found no effect on circadian rhythms or feeding behavior in rats exposed to alcohol during the same developmental window. Third, alcohol exposure could induce motor or balance deficits in AE animals due to cerebellar damage (Klintsova, Matthews, Goodlett, Napper, & Greenough, 1997; Thomas, Burchette, Dominguez, & Riley, 2000); AE animals might restrict themselves to a smaller area of the cage to cope with impairments in maneuvering in a cage filled with toys and other animals. Finally, all animals used in this study were exposed to 12 days of voluntary wheel running prior to housing in the EC cage, and it is possible that wheel running alone is a sufficient intervention to reduce locomotor activity in AE animals to below control levels. Exercise has been shown to reduce motor activity in other models (Duman, Schlesinger, Russell, & Duman, 2008; Hoffman, Thorén, & Ely, 1987). In this view, the amount of locomotor behavior observed in this study could be a positive effect of the intervention rather than hypoactivity signifying a lack of exploratory behavior in the AE rats. Alternately, wheel running could have reversed more severe hypoactivity in the AE rats, or potentially had no effect on activity measures. As all animals were recorded after wheel running, the exact impact of exercise on the behaviors observed cannot be known. Further work is needed to determine which, if any, of these variables could be contributing to the observed results.
With recent work proposing physical exercise to be the most crucial feature of the EC cage for mice, the current study demonstrates that this proposal is not entirely warranted for AE rats (Choi et al., 2005; Kobilo et al., 2011; Mustroph et al., 2012). The experimental setup used in the current study (12 days of WR followed by 30 days of EC) has been previously shown to result in significant neuroanatomical and behavioral benefits compared to SH or WR/SH in AE animals (Hamilton et al., 2012; Hamilton et al., 2014; Hamilton, Criss, & Klintsova, unpublished data). While the EC cage allows for increased physical activity through exploration of the cage and enrichment items, the exercise during such exploration is not as rigorous as during exposure to a running wheel. Additionally, AE rats show decreased exploratory behavior and mobility in the EC cage. Thus, the AE animals display robust neuroanatomical benefits while engaging in less physical activity than control animals, making it possible that aerobic exercise is not the only important variable of EC for AE rats. Other factors, such as increased social interaction (compared to SH) or exposure to novelty might be critical to the benefits of EC in animals that naturally form large social groups, such as rats or macaque monkeys. Previous work in macaques highlights the importance of peer interactions in an environmentally enriched setting (Schapiro, 2002), and social isolation rearing has long been known to have detrimental effects on behavior, neuroanatomy, and brain function in rats (Fone & Porkess, 2008). Comparatively, male mice are more likely than rats to live in breeding pairs or small groups and thus the social aspects of EC might not be as beneficial or could even serve as a stressor for these animals. It is important to note that the current study used rats that had previously been housed for 12 days in cages with 24-h access to wheel running, making the role of physical activity difficult to parse out from the current results, as intense exercise may have long-lasting effects on neurotrophin levels and microvasculature that continue to influence neuroplasticity after placement in the EC cage (Berchtold, Chinn, Chou, Kesslak, & Cotman, 2005; Klintsova et al., 2013; Rasmussen et al., 2009; Sato, Ogoh, Hirasawa, Oue, & Sadamoto, 2011; Van der Borght et al., 2009). Wheel running may “prime” the brain to be more responsive to the novelty and social interactions provided by EC (Cotman & Berchtold, 2002). Future studies will focus on animals in EC without prior exposure to exercise.
To parse out the impact of social interactions in EC, the second experiment examined stereotypical social behaviors between male rats in the EC cage. Housing in the EC cage offers increased chances for natural social interactions compared to other experimental paradigms as there are 9 rats per EC cage compared to 3 rats in our lab’s SH condition. We observed that male AE animals engaged less in the social behaviors of wrestling (n.s, p = 0.069 vs. SI) and mounting (Fig. 3). Both of these behaviors are important developmental behaviors and may have a role in establishing a stable social hierarchy within a group of rats (Meaney & Stewart, 1981; Yamada-Haga, 2002). Wrestling and mounting behaviors were further coded to determine the dominant or subordinate roles of the animals during the interaction (“pinner” vs. “pinned”), but no effect of neonatal treatment was observed (data not shown). These results demonstrate that AE animals are just as likely to be near other animals in the same quadrant (Experiment 1), although the number of actual social interactions is reduced. These results are supported by previous rodent and human studies. Work with prenatal alcohol exposure models has reported similar decreases in play fighting behaviors and social recognition in male rats (Kelly & Dillingham, 1994; Kelly, Leggett, & Cronise, 2009; Middleton, Varlinskaya, & Mooney, 2012; Mooney & Varlinskaya, 2011). Additionally, children with FASD exhibit impaired social functioning independent of IQ (Thomas, Kelly, Mattson, & Riley, 1998). Developmental alcohol exposure affects areas of the brain such as the prefrontal cortex and amygdala, which are important for the processing of social cues. Such damage may contribute to the impaired impulse control and social deficits observed in children with FASD throughout life. In particular, rodent models of developmental alcohol exposure result in increased caspase-3 expression in the central nucleus of the amygdala (Mitchell & Snyder-Keller, 2003), long-term reductions to amygdalar opioid signaling (Lugo, Wilson, & Kelly, 2006), and altered synaptic connectivity and neuronal loss in the amygdala (Balaszczuk, Bender, Pereno, & Beltramino, 2011; Cullen et al., 2013; Zhou et al., 2010) and the prefrontal cortex (Hamilton, Whitcher, & Klintsova, 2010; Ikonomidou et al., 2000; Lawrence, Otero, & Kelly, 2012; Whitcher & Klintsova, 2008). Dysfunction of these brain areas could contribute to disruptions in social and play behavior observed in AE rats. Future work should examine amygdala function and anatomy in animals exposed to alcohol specifically during the third-trimester equivalent, as most of the current literature uses perinatal or adolescent models of alcohol exposure. The social behavior deficits observed in these experiments could be correlated with alterations to the amygdala-prefrontal cortex circuit. Other further extensions of this work include examination of how alcohol exposure affects social behavior of female rats housed in EC, as sexually dimorphic effects of alcohol exposure on social behaviors have been previously reported (Meyer & Riley, 1986), with female rats prenatally exposed to alcohol showing more masculinized behaviors, suggesting that hormonal levels in utero may be affected by alcohol administration. Other work using an artificial-rearing model of third trimester-equivalent exposure found that while males displayed fewer active social behaviors, females engaged in more active behaviors with a same-sex rat (Kelly & Dillingham, 1994). While sex differences in social behavior were beyond the scope of the current study, future work will determine if female AE rats housed in a complex environment would show similar results.
Occurrences of social behaviors in both control and AE animals remained consistent across the days of recording (over 3 weeks of EC housing). Previous work has suggested that social and play behaviors decrease in frequency as animals pass from adolescence into adulthood (Meaney & Stewart, 1981; Panksepp, 1981). The consistency in frequency of social behaviors found in the current study is likely due to the animals being just past the “peak” age of expression of play behavior (PD 32–40) (Panksepp, 1981), and possibly due to facilitation of social behaviors by exposure to the cage filled with novel items. Handling and marking of the animals might also have encouraged social interactions and re-establishment of social hierarchies. Importantly, the reduction in exploratory and social behaviors observed in AE animals in this study remained consistent across days of recording. As discussed above, EC is a powerful intervention which can result in neuroplastic and behavioral alterations in AE animals (Hamilton et al., 2014; Hannigan & Berman, 2000; Hannigan, O’leary-Moore, & Berman, 2007; Schreiber et al., 2013). Our lab has demonstrated that the paradigm used in the current study (30 days of EC following 12 days of WR) significantly benefits neuroanatomical measures, such as hippocampal adult neurogenesis and dendritic complexity, in AE animals compared to AE rats that were housed in 12 days of WR followed by 30 days of SH (Hamilton et al., 2012; Hamilton, Criss, & Klintsova, unpublished data). The fact that there was no change in social or activity behavior across the time spent in EC suggests that, while EC does positively influence performance on learning and memory tasks, social and activity behaviors do not seem to be influenced. If EC did influence social and locomotor behavior in AE animals, the frequency of exploratory and play behaviors might have become more similar to the control animals toward the end of EC housing instead of displaying consistent deficits. These data suggest that EC is not a global intervention and might not affect all brain areas equally. However, it is important to note that the current experimental design cannot definitively conclude that housing in EC did not affect locomotor and social behaviors compared to social housing, as these control groups were not accounted for in this study. Future neuroanatomical work will investigate regions directly involved in social recognition and behavior, including the medial and orbitofrontal prefrontal cortex, hippocampus, perirhinal cortex, and amygdala (Adolphs, 2001; Amodio & Frith, 2006; Lai, Ramiro, Yu, & Johnston, 2005). These results underscore the necessity to continue preventive measures for maternal drinking, as post-exposure interventions might not have equal effectiveness on all areas of function, particularly for high doses such as the binge-like exposure modeled in this study.
In summary, the current study found that third trimester-equivalent binge-like alcohol exposure results in less exploratory behavior and reduced locomotor activity in male rats housed in a complex environment following 12 days with access to a running wheel. In addition, AE rats engage in fewer “play fighting” social interactions such as mounting and wrestling (n.s., p = 0.069 vs. SI), compared to controls while housed in EC. Continued exposure to EC did not affect activity and social behavior deficits in AE animals. These experiments are the first to investigate how male rats neonatally exposed to alcohol interact with the environment and other rats while housed in an EC cage. Future work will focus on investigation of whether voluntary exercise affects AE rats’ behavior in EC, how AE impacts neuroanatomy in brain regions involved in social behavior, and if the results translate to female animals. Evaluation of how AE animals react to new situations and the types of social interactions they have with other animals will help researchers develop more focused behavioral therapies to target FASD.
Supplementary Material
Supplementary Table 1. A) Raw average data from Experiment 1 (Activity Behavior) by Neonatal Treatment and Cage. Quadrants/Min refers to how many cage quadrants (Fig. 1) the animal passed through per minute. Data for Movement, With/Without Another Rat, and Toys expressed as a value from 0–1. The data were coded at each minute mark as 0 = no or 1 = yes based on whether the animal was or was not engaged in that activity. B) Raw average data from Experiment 2 (Social Behavior) by Neonatal Treatment and Cage. Data are expressed as an average of each behavior across the 30-min session. Values were transformed into a percent of control per cage for statistical analyses to account for the significant difference in the number of behaviors performed between Cage A and Cage B. Data are averaged across recording sessions because no effect of Day of Recording was found. SC = Suckle Control, SI = Sham-Intubated, AE = Alcohol-Exposed.
Acknowledgments
The authors would like to thank Mia Castiglione, Eliza Hetterly, and Sam Modlin for their assistance with data collection, and all undergraduate research assistants for help with animal generation and care. This work was supported by NIAAA grant AA009838 to AYK and NIH/NIGMS COBRE: The Delaware Center for Neuroscience Research 1P20GM103653 – 01A1 to AYK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews. Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Balaszczuk V, Bender C, Pereno GL, Beltramino CA. Alcohol-induced neuronal death in central extended amygdala and pyriform cortex during the postnatal period of the rat. International Journal of Developmental Neuroscience. 2011;29:733–742. doi: 10.1016/j.ijdevneu.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- CDC. Fetal Alcohol Spectrum Disorders (FASDs) 2013. [Google Scholar]
- Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcoholism: Clinical and Experimental Research. 2005;29:2053–2062. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- Clarke ME, Gibbard WB. Overview of fetal alcohol spectrum disorders for mental health professionals. The Canadian Child and Adolescent Psychiatry Review = La Revue Canadienne De Psychiatrie De l’Enfant Et De l’Adolescent. 2003;12:57–63. [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neuroscience. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cullen CL, Burne TH, Lavidis NA, Moritz KM. Low dose prenatal ethanol exposure induces anxiety-like behaviour and alters dendritic morphology in the basolateral amygdala of rat offspring. PloS One. 2013;8:e54924. doi: 10.1371/journal.pone.0054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Research. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cerebral Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Frontiers in Neuroscience. 2009;3:50. doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnell YZ, Allen GC, Nahm SS, Neuendorff N, West JR, Chen WA, et al. Neonatal alcohol exposure differentially alters clock gene oscillations within the suprachiasmatic nucleus, cerebellum, and liver of adult rats. Alcoholism: Clinical and Experimental Research. 2008;32:544–552. doi: 10.1111/j.1530-0277.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents – relevance to developmental neuropsychiatric disorders. Neuroscience and Biobehavioral Reviews. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Gilbertson RJ, Barron S. Neonatal ethanol and nicotine exposure causes locomotor activity changes in preweanling animals. Pharmacology, Biochemistry, and Behavior. 2005;81:54–64. doi: 10.1016/j.pbb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Experimental Neurology. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR, Juraska JM. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Experimental Neurology. 1973;41:371–378. doi: 10.1016/0014-4886(73)90278-1. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, et al. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behavioural Brain Research. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Boschen KE, Goodlett CR, Greenough WT, Klintsova AY. Housing in environmental complexity following wheel running augments survival of newly generated hippocampal neurons in a rat model of binge alcohol exposure during the third trimester equivalent. Alcoholism: Clinical and Experimental Research. 2012;36:1196–1204. doi: 10.1111/j.1530-0277.2011.01726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Jablonski SA, Schiffino FL, St Cyr SA, Stanton ME, Klintsova AY. Exercise and environment as an intervention for neonatal alcohol effects on hippocampal adult neurogenesis and learning. Neuroscience. 2014;265:274–290. doi: 10.1016/j.neuroscience.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC Layer II/III pyramidal neurons. Synapse. 2010;64:127–135. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan JH, Berman RF. Amelioration of fetal alcohol-related neurodevelopmental disorders in rats: exploring pharmacological and environmental treatments. Neurotoxicology and Teratology. 2000;22:103–111. doi: 10.1016/s0892-0362(99)00050-1. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, O’leary-Moore SK, Berman RF. Postnatal environmental or experiential amelioration of neurobehavioral effects of perinatal alcohol exposure in rats. Neuroscience and Biobehavioral Reviews. 2007;31:202–211. doi: 10.1016/j.neubiorev.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Helfer JL, Goodlett CR, Greenough WT, Klintsova AY. The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Research. 2009;1294:1–11. doi: 10.1016/j.brainres.2009.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P, Thorén P, Ely D. Effect of voluntary exercise on open-field behavior and on aggression in the spontaneously hypertensive rat (SHR) Behavioral and Neural Biology. 1987;47:346–355. doi: 10.1016/s0163-1047(87)90461-4. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Dillingham RR. Sexually dimorphic effects of perinatal alcohol exposure on social interactions and amygdala DNA and DOPAC concentrations. Neurotoxicology and Teratology. 1994;16:377–384. doi: 10.1016/0892-0362(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Hulsether SA, West JR. Alterations in sensorimotor development: relationship to postnatal alcohol exposure. Neurotoxicology and Teratology. 1987;9:243–251. doi: 10.1016/0892-0362(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Leggett DC, Cronise K. Sexually dimorphic effects of alcohol exposure during development on the processing of social cues. Alcohol and Alcoholism. 2009;44:555–560. doi: 10.1093/alcalc/agp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber ML, Wright E, Singh SM. Maternal voluntary drinking in C57BL/6J mice: advancing a model for fetal alcohol spectrum disorders. Behavioural Brain Research. 2011;223:376–387. doi: 10.1016/j.bbr.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Hamilton GF, Boschen KE. Long-term consequences of developmental alcohol exposure on brain structure and function: therapeutic benefits of physical activity. Brain Sciences. 2013;3:1–38. doi: 10.3390/brainsci3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2007;31:2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Matthews JT, Goodlett CR, Napper RM, Greenough WT. Therapeutic motor training increases parallel fiber synapse number per Purkinje neuron in cerebellar cortex of rats given postnatal binge alcohol exposure: preliminary report. Alcoholism: Clinical and Experimental Research. 1997;21:1257–1263. [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learning & Memory. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WS, Ramiro LL, Yu HA, Johnston RE. Recognition of familiar individuals in golden hamsters: a new method and functional neuroanatomy. The Journal of Neuroscience. 2005;25:11239–11247. doi: 10.1523/JNEUROSCI.2124-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RC, Otero NK, Kelly SJ. Selective effects of perinatal ethanol exposure in medial prefrontal cortex and nucleus accumbens. Neurotoxicology and Teratology. 2012;34:128–135. doi: 10.1016/j.ntt.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo JN, Jr, Wilson MA, Kelly SJ. Perinatal ethanol exposure alters metenkephalin levels of male and female rats. Neurotoxicology and Teratology. 2006;28:238–244. doi: 10.1016/j.ntt.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1996;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Research & Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Hormones and Behavior. 1981;15:197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Melcer T, Gonzalez D, Barron S, Riley EP. Hyperactivity in preweanling rats following postnatal alcohol exposure. Alcohol. 1994;11:41–45. doi: 10.1016/0741-8329(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Melcer T, Gonzalez D, Riley EP. Locomotor activity and alcohol preference in alcohol-preferring and -nonpreferring rats following neonatal alcohol exposure. Neurotoxicology and Teratology. 1995;17:41–48. doi: 10.1016/0892-0362(94)00051-e. [DOI] [PubMed] [Google Scholar]
- Meyer LS, Riley EP. Social play in juvenile rats prenatally exposed to alcohol. Teratology. 1986;34:1–7. doi: 10.1002/tera.1420340102. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Varlinskaya EI, Mooney SM. Molecular substrates of social avoidance seen following prenatal ethanol exposure and its reversal by social enrichment. Developmental Neuroscience. 2012;34:115–128. doi: 10.1159/000337858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell ES, Snyder-Keller A. c-fos and cleaved caspase-3 expression after perinatal exposure to ethanol, cocaine, or the combination of both drugs. Brain Research. Developmental Brain Research. 2003;147:107–117. doi: 10.1016/j.devbrainres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol and social behavior: effects of age, sex, and timing of exposure. Behavioural Brain Research. 2011;216:358–364. doi: 10.1016/j.bbr.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Developmental Psychobiology. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Parks EA, McMechan AP, Hannigan JH, Berman RF. Environmental enrichment alters neurotrophin levels after fetal alcohol exposure in rats. Alcoholism: Clinical and Experimental Research. 2008;32:1741–1751. doi: 10.1111/j.1530-0277.2008.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. Ethanol acutely inhibits ionotropic glutamate receptor-mediated responses and long-term potentiation in the developing CA1 hippocampus. Alcoholism: Clinical and Experimental Research. 2010a;34:594–606. doi: 10.1111/j.1530-0277.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. Repeated third trimester-equivalent ethanol exposure inhibits long-term potentiation in the hippocampal CA1 region of neonatal rats. Alcohol. 2010b;44:283–290. doi: 10.1016/j.alcohol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental Physiology. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Rema V, Ebner FF. Effect of enriched environment rearing on impairments in cortical excitability and plasticity after prenatal alcohol exposure. The Journal of Neuroscience. 1999;19:10993–11006. doi: 10.1523/JNEUROSCI.19-24-10993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Krech D. Cerebral effects of environmental complexity and training among adult rats. Journal of Comparative and Physiological Psychology. 1964;57:438–439. doi: 10.1037/h0046387. [DOI] [PubMed] [Google Scholar]
- Sakata-Haga H, Dominguez HD, Sei H, Fukui Y, Riley EP, Thomas JD. Alterations in circadian rhythm phase shifting ability in rats following ethanol exposure during the third trimester brain growth spurt. Alcoholism: Clinical and Experimental Research. 2006;30:899–907. doi: 10.1111/j.1530-0277.2006.00105.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. The Journal of Physiology. 2011;589:2847–2856. doi: 10.1113/jphysiol.2010.204461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro SJ. Effects of social manipulations and environmental enrichment on behavior and cell-mediated immune responses in rhesus macaques. Pharmacology, Biochemistry, and Behavior. 2002;73:271–278. doi: 10.1016/s0091-3057(02)00779-7. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychology Review. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber WB, St Cyr SA, Jablonski SA, Hunt PS, Klintsova AY, Stanton ME. Effects of exercise and environmental complexity on deficits in trace and contextual fear conditioning produced by neonatal alcohol exposure in rats. Developmental Psychobiology. 2013;55:483–495. doi: 10.1002/dev.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Burchette TL, Dominguez HD, Riley EP. Neonatal alcohol exposure produces more severe motor coordination deficits in high alcohol sensitive rats compared to low alcohol sensitive rats. Alcohol. 2000;20:93–99. doi: 10.1016/s0741-8329(99)00080-4. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcoholism: Clinical and Experimental Research. 1998;22:528–533. [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Research. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Kóbor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C, Van der Zee EA, et al. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19:928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008;62:566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Haga Y. Characteristics of social interaction between unfamiliar male rats (Rattus norvegicus): comparison of juvenile and adult stages. Journal of Ethology. 2002;20:55–62. [Google Scholar]
- Zhou R, Wang S, Zhu X. Prenatal ethanol exposure attenuates GABAergic inhibition in basolateral amygdala leading to neuronal hyperexcitability and anxiety-like behavior of adult rat offspring. Neuroscience. 2010;170:749–757. doi: 10.1016/j.neuroscience.2010.07.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. A) Raw average data from Experiment 1 (Activity Behavior) by Neonatal Treatment and Cage. Quadrants/Min refers to how many cage quadrants (Fig. 1) the animal passed through per minute. Data for Movement, With/Without Another Rat, and Toys expressed as a value from 0–1. The data were coded at each minute mark as 0 = no or 1 = yes based on whether the animal was or was not engaged in that activity. B) Raw average data from Experiment 2 (Social Behavior) by Neonatal Treatment and Cage. Data are expressed as an average of each behavior across the 30-min session. Values were transformed into a percent of control per cage for statistical analyses to account for the significant difference in the number of behaviors performed between Cage A and Cage B. Data are averaged across recording sessions because no effect of Day of Recording was found. SC = Suckle Control, SI = Sham-Intubated, AE = Alcohol-Exposed.


