Abstract
Two parallel phase II trials in adults with hematologic malignancies demonstrated comparable survival after reduced intensity conditioning and transplantation of either two HLA-mismatched umbilical cord blood units or bone marrow from HLA-haploidentical relatives. Donor choice is often subject to physician practice and institutional preference. Despite clear preliminary evidence of equipoise between HLA-haploidentical related donor and double unrelated donor UCB transplantation, the actual prospect of being randomized between these two very different donor sources is daunting to patients and their treating physicians alike. Under these circumstances it is challenging to conduct a phase III randomized trial in which patients are assigned to the umbilical cord blood or haploidentical bone marrow arms. Therefore, we aimed to provide an evidence-based review and recommendations for selecting donors for adults without an HLA-matched sibling or an HLA-matched adult unrelated donor.
Introduction
The case: A 58-year-old gentleman with de novo acute myeloid leukemia (FAB subtype: M2) is enrolled on the South West Oncology Group (SWOG) trial 1203. Cytogenetic tests are consistent with normal karyotype and molecular tests consistent with mutated NPM1 and FLT3-ITD positive. The patient underwent induction therapy and achieved first complete remission. A donor search was initiated soon after diagnosis; the patient and his sibling are fully HLA mismatched. Preliminary search of the adult unrelated donor registries suggest the patient lacks unrelated adult donors who are likely to be HLA-matched at HLA-A, -B, -C and –DRB1. However, several potential mismatched related and mismatched unrelated adult donors and umbilical cord blood (UCB) units are identified. Potential alternative donor options include the following: 1) the recipient’s son, aged 23 years and partially HLA-matched (HLA-haploidentical) to the recipient; 2) an unrelated adult donor who is aged 35 years, mismatched to the recipient at the allele-level at HLA-A with a permissive mismatch at HLA-DPB1. The unrelated donor is medically fit and able to donate in the next 8 weeks; and, 3) three UCB units: Unit 1 has a single mismatch at HLA-A to the recipient with total nucleated cell dose of 3.1 × 107/kg; Unit 2 has a single mismatch at HLA-B with total nucleated cell dose of 3.5 × 107/kg; and Unit 3 is has a single mismatch at each of HLA-A and –DRB1 with total nucleated cell dose of 4.1 × 107/kg.
The treating physician and the patient have decided to proceed with allogeneic hematopoietic cell transplantation and are currently engaged in discussions as to the best alternative donor available. They are particularly interested in a phase III clinical trial conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 1101; NCT0159778) that is open for enrollment. In this trial, using the platform as designed for the earlier parallel phase II trials (BMT CTN 0603 and BMT CTN 0604),1 patients are randomized to either HLA-haploidentical donor or two UCB units. The phase II trials (BMT CTN 0603 and BMT CTN 0604) tested reduced intensity conditioning regimens of similar intensity for adults with hematologic malignancy; BMT CTN 0603 used bone marrow (BM) grafts from HLA-haploidentical related donors and BMT CTN 0604, used mismatched umbilical cord blood (UCB) grafts (co-infusion of two UCB units). The 1-year overall and progression-free survival after haplo-BM transplantation was 62% (95% confidence interval [CI] 44 – 76) and 48% (95% CI 32 – 62), respectively.1 The corresponding probabilities after mismatched UCB transplantation were 54% (95% CI 38 – 67) and 46% (95% CI 31 – 60).1
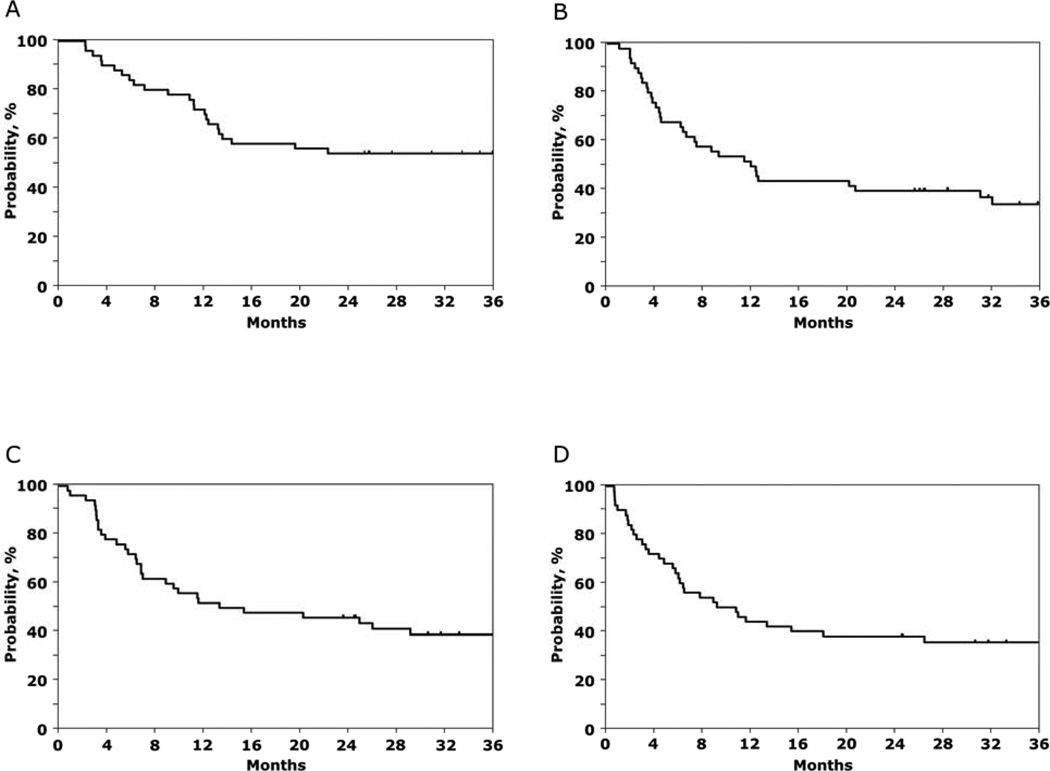
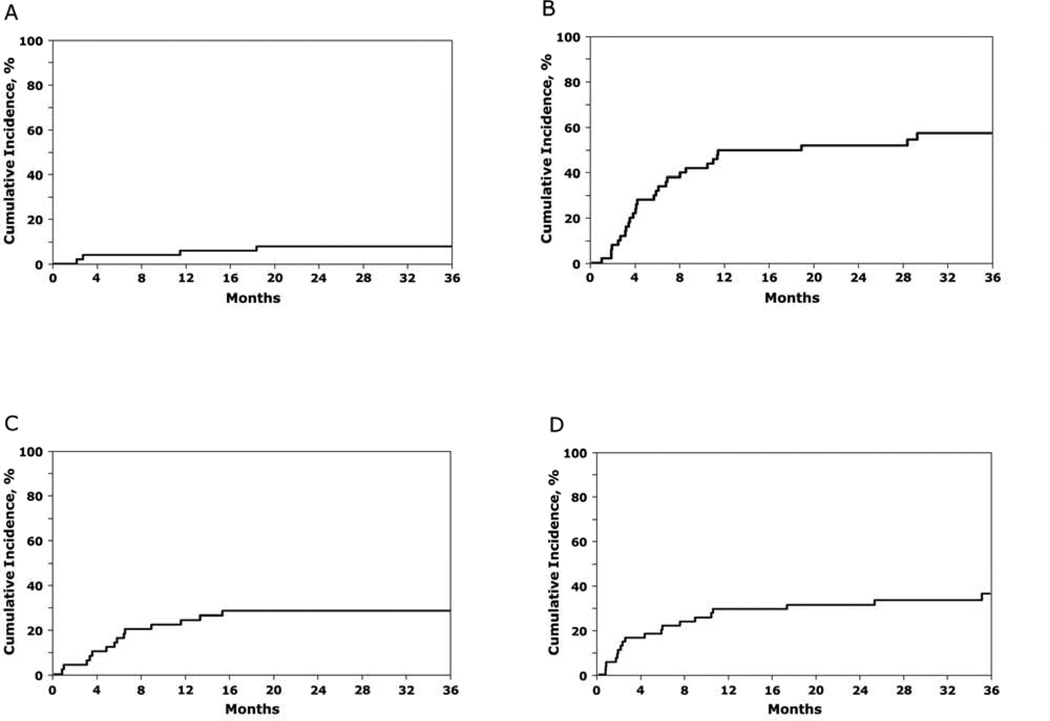
Thirty-four of 50 subjects enrolled on BMT CTN 0603 and twenty-nine of 50 subjects enrolled on BMT CTN 0604 were alive at time of publication of the above report in 2011.1 Surviving subjects were followed up in 2013: 27 of 34 subjects on BMT CTN 0603 and 20 of 29 subjects on BMT CTN 0604 were alive in 2013. The median follow-up of surviving subjects enrolled on BMT CTN 0603 and 0604 was 3 years (range 2 – 4). The 3-year overall and progression-free survivals after haplo-BM transplantation were 54% (95% CI 39 – 67) and 35% (95% CI 21 – 48), respectively (Table 1, Figure 1A, B). The corresponding probabilities after mismatched UCB transplantation were 39% (95% CI 26 – 53) and 36% (95% CI 23 – 49), (Table 1, Figure 1C, D). The pattern of treatment failure differed between the two donor sources (Table 1; Figure 2A – D). Relapse rates were high and non-relapse mortality rates low after haplo-BM transplantation. In contrast, relapse and non-relapse mortality rates were modestly high after mismatched UCB transplantation. There were no reported cases of graft failure with extended follow-up after haplo-BM and UCB transplantation.
Table 1.
The 3-year probabilities of non-relapse mortailty, relapse, progression-free and overall survival
| Donor Type | ||
|---|---|---|
| Double umbilical cord blood BMT CTN 0604 |
Haploidentical bone marrow BMT CTN 0603 |
|
| Non-relapse mortality | 28% (95% CI 15 – 41) | 8% (95% CI 0.4 – 16) |
| Relapse | 36% (95% CI 23 – 50) | 58% (95% CI 43 – 72) |
| Progression-free survival | 36% (95% CI 23 – 49) | 35% (95% CI 21 – 48) |
| Overall survival | 39% (95% CI 26 – 53) | 54% (95% CI 39 – 67) |
Figure 1.
The 3-year probability of overall survival after HLA-haploidentical bone marrow (A), progression-free survival after HLA-haploidentical bone marrow (B), overall survival after double UCB (C) and progression-free survival after double UCB (D) transplantation.
Figure 2.
The 3-year probability of non-relapse mortality after HLA-haploidentical bone marrow (A), relapse after HLA-haploidentical bone marrow (B), non-relapse mortality after double UCB (C) and relapse after double UCB (D) transplantation.
Despite clear preliminary evidence of equipoise between HLA haploidentical related donor and double unrelated donor UCB transplantation, the actual prospect of being randomized to different donor sources has proved to be a challenge. To our knowledge differing patterns of treatment failure on BMTCTN 0603 and 0604 have not been cited as an inhibitory factor for randomization. For patients referred to the larger centers known for their expertise for UCB or haploidentical transplantation, this amounts to selecting the donor source based on center expertise. On the other hand, physician bias relate to their perceived knowledge on the optimal alternative donor for their patient without an HLA-matched sibling or adult unrelated donor. Consequently, accrual to the trial has ramped up slowly; it has taken over a year from opening the trial to achieve projected quarterly accruals. Therefore, in this report, we present guidelines for donor selection based on published current and relevant data.
Donor Sources other an HLA-matched siblings for Hematopoietic Cell Transplantation
When an HLA-matched sibling is not available or not suitable to donate, alternative donors may be considered if the patient is likely to benefit from allogeneic transplantation. Alternative donor sources include HLA-matched or mismatched adult unrelated donors, unrelated umbilical cord blood (UCB) and mismatched family members (haploidentical donor).
Unrelated adult donors
Better supportive care including the selection of unrelated adult donors who are more closely HLA-matched to their recipients have improved survival after allogeneic transplantation for hematologic malignancy. Reports support the general concept that there is a direct association between the number of donor-recipient HLA mismatches and mortality risks. Based on available literature, a fully matched donor is one who is HLA-matched to the recipient at the allele-level at HLA-A, -B, -C and –DRB1.2–4 While some would support including HLA-match status at the HLA-DQ locus, in their definition of a “suitably HLA-matched unrelated donor” it is important to note that over 95% of donor-recipient pairs matched at the allele-level at HLA-A, -B, -C and – DRB1 are also matched at HLA-DQ and, an isolated mismatch at the HLA-DQ locus does not have an adverse effect on survival.4
Other frequently asked questions include whether HLA-match requirements differ when selecting peripheral blood progenitor cells (PBPC) or with reduced intensity transplant conditioning regimens. PBPC grafts differ from BM in that the former contain substantially more cells including CD3+ and CD34+ cells, which might influence the effects of HLA matching. In a recent report, unrelated PBPC transplantations mismatched at a single HLA-locus for HLA-A, -B, -C or –DRB1 were associated with higher mortality compared to transplantations matched at HLA-A, -B, -C and –DRB15 and consistent with that reported for BM grafts.4 As seen with BM transplantations, an isolated mismatch at the HLA-DQ locus was not associated with higher mortality risks. With the increase in numbers of reduced intensity conditioning regimen transplantations for hematologic malignancy it is timely to explore whether HLA-match is associated with survival. In a recent study that explored matching at HLA-A, -B, -C and – DRB1 in 2500 donor-recipient pairs, a single locus HLA-mismatch was associated with higher mortality.6 It is noteworthy that most reduced intensity conditioning regimen transplantations are matched (only 21% were mismatched at a single HLA-locus) and matching at HLA-DQ was not considered in that report. Taken together, the general concept of a direct association between donor-recipient HLA match and survival after unrelated adult donor transplantation holds true for BM and PBPC grafts and, myeloablative and reduced intensity conditioning regimens.
HLA-A, -B, -C and –DRB1 are the high expressing alleles. However, there are several low expressing alleles and matching between donors and recipients does not routinely consider the low expressing alleles. Two recent reports explored the effects of matching at low expression alleles. In the first, Fleischauer and colleagues considered the effects of matching at HLA-DPB1, grouped as matched, permissive or non-permissive mismatch based on T-cell epitope matching.7 In that study, HLA-match was defined as donor-recipient pairs matched at the allele-level at HLA-A, -B, -C, -DRB1 and –DQB1. On the one hand, there were no significant differences in mortality risks between HLA-matched transplantations and transplantations with permissive mismatch at HLA-DPB1 locus. On the other hand, non-permissive mismatch at HLA-DPB1 locus was associated with higher mortality for matched and single HLA-locus mismatch transplantations. Interestingly, permissive mismatch at HLA-DPB1 locus was rather well tolerated with no significant differences in mortality risks between HLA-matched transplantations and single HLA-locus mismatched transplantations. The data support avoiding a non-permissive mismatch at the HLA-DPB1 locus, resulting in better survival. The second report, explored the effect of multiple mismatches at HLA-DP, -DQ and –DRB3/4/5.8 Their findings support the general concept that matching at the low expression alleles can be ignored when donors and recipients are matched at the high expressing alleles. On the other hand, in the presence of a single HLA locus mismatch at any of the high expressing alleles, three or more mismatches at the low expressing alleles is associated with high mortality. Therefore, in the absence of an unrelated donor matched at HLA-A, -B, -C and –DRB1, it is important to consider matching at HLA-DP, DQ and DRB3/4/5.9
Availability of suitably HLA-matched adult unrelated donors
Unfortunately, HLA-matched unrelated donors are not available for all patients even with large unrelated adult donor registries because the polymorphism of HLA genes is extremely high and allelic variation is population-specific.10,11 The Bioinformatics Division of the National Marrow Donor Program, using their donor registry of volunteer donors, recently built mathematical models to predict the likelihood of identifying a suitable adult donor for patients in the United States and considering race/ethnic groups.12 That report12 suggest the ethnic and racial group of the patient influences the likelihood of identifying a suitable donor. An HLA-matched unrelated adult donor can be identified without difficulty for patients with common HLA genotypes. Consequently, about 75% of Caucasians of European descent will find a fully HLA-matched adult donor and another 20%, a donor who is mismatched at a single HLA-locus (HLA-A, or –B, or –C or –DRB1). For persons of other race and/or ethnicity, the likelihood of identifying a fully HLA-matched adult donor is substantially lower and range from 15% to 50%. If a single HLA-mismatch (at HLA-A, -B, -C or –DRB1) can be tolerated, almost all persons will be able to identify an adult donor. It is noteworthy that the mathematical model did not consider matching at the low expression alleles and the likelihood of identifying suitably mismatched unrelated adult donors (i.e., tolerating a single HLA-mismatch at a high expressing alleles (HLA-A, -B, -C or DRB1) and fewer than three mismatches at low expressing alleles (HLA-DP, DQ, DR3/4/5) could be even lower than what is predicted using the current mathematical model which only considered the high expression loci. Clearly, survival after adult unrelated donor transplantation mismatched at a single HLA-locus is lower than that after matched unrelated donor transplantation. While selecting a mismatched adult unrelated donor may be acceptable to several treating physicians, there are others who favor HLA-haploidentical relatives or UCB units.
Further, the time from diagnosis to transplantation for hematologic malignancy adversely affects patient outcomes and any delay incurred in procuring an adult donor unrelated graft is an obstacle to the timing of allogeneic transplantation.13,14 Potential adult unrelated or related donors have to be medically fit and available to donate. While a relative is often available to donate and UCB units readily available from a Cord Blood Bank, the average time taken from identification of an adult unrelated donor to transplantation is approximately 7 weeks. For patients with high-risk hematologic malignancy, 7 weeks could be too long a waiting period. The other important factor to consider is donor attrition with respect to the unrelated adult donor. Attrition rates vary; higher attrition rates are associated with large volume donor centers, donors residing in high population urban areas with large minority and less stable populations.15 Additionally, intrinsic commitment to donation, more realistic expectations, fewer medical concerns, and greater contact with the donor center were all associated with lower attrition.16 Attrition rates among family donors is minimal and for UCB units, none.
Taken together, for patients with common HLA phenotypes, a suitably matched adult donor can usually be identified on the first match run. If one is not able to identify a suitably matched adult donor in a worldwide search (19 million donors), it is unlikely that newly recruited donors will match the patient in a timely manner.12 Thus while every attempt must be made to identify the best HLA-matched donor, delaying transplantation because such a donor is not immediately available is not advisable. Under these circumstances it is recommended that alternative treatment options be evaluated including lowering HLA-match requirements or using another unrelated graft source such as UCB or partially matched family members. The high cost of extensive HLA typing must also be considered. Enlisting the assistance of an HLA expert can help maximize available resources by focusing selection of donors for screening to those most likely to match the patient.
Unrelated Umbilical Cord Blood
UCB units are increasingly used as an alternative donor/graft for unrelated donor transplantation in adults. While the majority of UCB transplantations have been performed in children and adolescents, increasing numbers are now being performed in adults and account for about 10% of allogeneic transplants in adults. UCB units are readily available and generally HLA-match requirements are less stringent than that for the adult grafts (BM or PBPC) making this an attractive alternative option in the absence of a suitably HLA-matched related or unrelated adult donor. There are several reports from transplant registries and single institutions that have retrospectively compared outcomes after HLA-matched and mismatched adult unrelated donor transplantation to that after UCB transplantation, including infusion of two UCB units, with comparable overall and leukemia-free survival.17–19 All these reports included patients in all disease states at transplantation, i.e., first or second complete remission and in relapse at transplantation. It is plausible findings may differ in selected populations. In a recent report,20 older patients (aged 50 years or older) with acute myeloid leukemia in 1st complete remission had significantly higher leukemia-free and overall survival after HLA-matched adult donor transplantation compared to either mismatched adult donor or UCB transplantation. Non-relapse mortality was high after both mismatched adult donor and UCB transplantation accounting for the observed differences in leukemia-free and overall survival after matched and, mismatched adult donor or UCB transplantation. It is noteworthy, that these reports considered matching at HLA-A, -B, -C and –DRB1 at the allele-level and matching at the low expression alleles (for the mismatched adult unrelated donor transplants) was not considered.
A major limitation when considering UCB transplantation, regardless of the patient’s age, is the high non-relapse mortality risk. A substantial proportion of adults in the U.S. are unable to find a single UCB unit with adequate total nucleated cell count and there are several strategies employed to increase the TNC dose delivered. These include the infusion of two UCB units,1,21,22 infusion of UCB unit or units with CD34-selected hematopoietic progenitor cells from a HLA-haploidentical relative23,24 or infusion of ex vivo expanded UCB unit with two non-manipulated UCB units.25,26 These strategies have resulted in markedly improved hematopoietic recovery and in some cases achieving recovery times comparable to that after transplantation of PBPC. To our knowledge none of these early trials with ex vivo expanded products or the co-infusion of hematopoietic progenitor cells from an HLA-haploidentical donor together with an UCB unit have demonstrated a survival advantage compared to infusion of two unmanipulated UCB units. Only with longer follow-up of patients treated with expanded UCB units and the conduct of comparative studies with conventional unrelated adult donors can we determine whether these strategies may be translated to every-day clinical practice and importantly whether there is longer-term survival advantage with this approach as compared to conventional UCB transplants, i.e., infusion of one or two adequately dosed UCB units.
An important difference when selecting volunteer unrelated adult donors and UCB units is the criteria for HLA matching donors to recipients. Unrelated adult donors are selected to be closely HLA-matched to recipients and consider matching at HLA-A, -B, -C and –DRB1 at the allele-level at the very least2–4 whereas UCB units are selected using lower resolution HLA typing (antigen-level) for HLA–A and –B and at the allele-level for HLA-DRB1; HLA-C is not typically considered. However, two recent publications suggest considering allele-level HLA-matching including matching at the HLA-C when selecting UCB units leads to better hematopoietic recovery and lower non-relapse mortality.27,28 It is important to note that the findings of these reports are applicable to the selection of single UCB units for transplantation of patients with hematologic malignancy. The role of better HLA-matched units in the setting of double UCB transplantation (infusion of two UCB units) is not known. In the setting of adult unrelated donor transplantations, differences in mortality risks are negligible when comparing transplantations mismatched at two or more HLA-loci. As most UCB transplantations in adults are already mismatched at two HLA-loci at the lower resolution, only with larger numbers of better-matched UCB transplants can we begin to study the role of HLA-matching in the setting of double UCB transplantation.
Haploidentical donors
Almost all patients will have at least one haploidentical relative and this represents a valid donor source for those who may benefit from allogeneic hematopoietic transplantation. Potential advantages of haploidentical donors include immediate availability and flexible management of graft procurement, avoiding the monetary burden associated with an unrelated donor search and the availability of the donor for post-transplantation cellular therapy. Early studies of HLA-haploidentical stem cell transplantation clearly showed worsening outcomes with increasing HLA disparity between donor and recipient.29,30 These studies generally employed serologic rather than molecular typing and so the extent of mismatch may have been underestimated. While one HLA antigen-mismatched transplants produced outcomes comparable to transplants from HLA-matched siblings, two or three antigen mismatched transplants were associated with high incidences of severe GVHD, non-relapse mortality, and poor survival.29 Increasing HLA mismatch between donor and recipient may be associated with a decreased risk of leukemia relapse,31 especially among patients with poor-risk hematologic malignancies, but this benefit of mismatching is more than offset by the higher incidence of non-relapse mortality.30 Increasing HLA disparity between donor and recipient was associated with an increased risk of graft failure, which was also more common among recipients who had antibodies against donor HLA molecules.32 Taken together, the results of these early studies in HLA-haploidentical stem cell transplantation led to the preferential selection of donors with the least degree of HLA mismatch and the use of donor-specific antibody testing to exclude donors whose grafts were more likely to be rejected.
More recently several groups have demonstrated that graft failure, GVHD and non-relapse mortality rates are acceptable through a variety of strategies that have targeted in vivo or ex vivo graft engineering methods. The Perugia group, uses in vivo graft engineering methods to selectively deplete T-cells that results in very low GVHD rates but high non-relapse mortality rates.33 More recently, they have augmented their strategy with infusion of selective T-cell and NK-cell populations as a means of introducing anti-viral activity to reduce non-relapse mortality.34 Although successful the approach is not used widely. The effect of HLA mismatching between donor and recipient has been revisited in the recent era of improved GVHD prophylaxis for transplantation of HLA-haploidentical bone marrow grafts. Notably in the United States, transplantation of unmanipulated bone marrow from haploidentical related donors is followed by post-transplantation cyclophosphamide (100 mg/kg) after reduced intensity transplant conditioning (TBI 200 cGy, low dose cyclophosphamide and fludarabine) and GVHD prophylaxis with tacrolimus and mycophenolate.35 More recently, two reports from Italy that adopted the approach of transplanting unmanipulated BM from haploidentical related donors also report favorable outcomes.36,37 One approach incorporated high-dose post-transplant cyclophosphamide, cyclosporine and mycophenolate to a myeloablative transplant conditioning regimen36 and the other, five-drug GVHD prophylaxis to myeloablative or reduced intensity transplant conditioning regimens.37 The former report that employed high-dose post-transplant cyclophosphamide demonstrated adequate recovery of immune function as evidenced by CD4+ lymphocyte recovery at day +100 and +150 post-transplantation. Data on immune reconstitution are lacking for the later report.
The effect of HLA mismatching between donor and recipient are mixed with some reporting relevance. For patients receiving posttransplantation cyclophosphamide and reduced intensity conditioning, increasing HLA mismatch between donor and recipient was not found to worsen GVHD or to compromise survival.38 After myeloablative conditioning, HLA-B mismatching was found to compromise outcome, but multiple HLA mismatches between donor and recipient did not have synergistic negative effects on outcome.39 It is therefore possible that improved regimens of GVHD prophylaxis have mitigated the negative impact of HLA mismatching on outcome after T cell-replete, HLA-haploidentical BMT to the point that outcomes of such transplants are comparable to those of transplants from HLA-matched siblings or unrelated donors.40,41
Natural killer cells may play a significant role in the graft-versus-tumor effect of HLA-haploidentical transplantation34 raising the possibility that donors could be selected to optimize natural killer cell alloreactivity to reduce the risk of relapse. The Killer Immunoglobulin-like Receptor, or KIR, genetic locus on chromosome 19q13.4 encodes a set of receptors on NK cells for HLA Class I molecules. KIRs can either be stimulatory or inhibitory to NK cell activation depending on the length of the receptor’s cytoplasmic tail. Specific alleles of HLA Class I molecules engage inhibitory KIRs to prevent NK cell activation and killing. When donor and recipient are HLA-haploidentical, it is possible that the recipient lacks HLA molecules for inhibitory KIRs leading to NK cell alloreactivity and a graft-versus-leukemia effect. Specific incompatibilities in KIR ligands (HLA Class I molecules) between donor and recipient were associated with a reduced risk of relapse of acute myeloid leukemia after myeloablative conditioning and T cell-depleted, HLA-haploidentical stem cell transplants.34,42 However, the presence of T cells in the donor graft and GVHD may nullify the beneficial effect of KIR ligand incompatibility.43 The presence of specific stimulatory KIRs or KIR haplotypes in the donor has been associated with favorable outcomes after HLA-matched unrelated or HLA-haploidentical bone marrow transplantation.44–46 Prospective clinical trials will ultimately be required to determine whether the outcome of HLA-haploidentical transplantation can be improved by selecting donors based upon specific KIR genes or haplotypes.
Summary
Our review of published data support, any one of three following donor options for the case illustrated in this review: haplo-identical relative, HLA-mismatched unrelated adult or UCB. For the case illustrated, we recommend enrolling the patient on the clinical trial (BMT CTN 1101) that randomizes patients to receive BM grafts from a haplo-identical relative or two UCB units. Only through the conduct of well-designed clinical trials, can we be understand and appreciate the complexities of donor choices and its outcome on allogeneic transplantation for hematologic malignancies. There are no on-going trials that compare outcomes after HLA-mismatched adult unrelated donor to that after related mismatched or UCB transplantation.
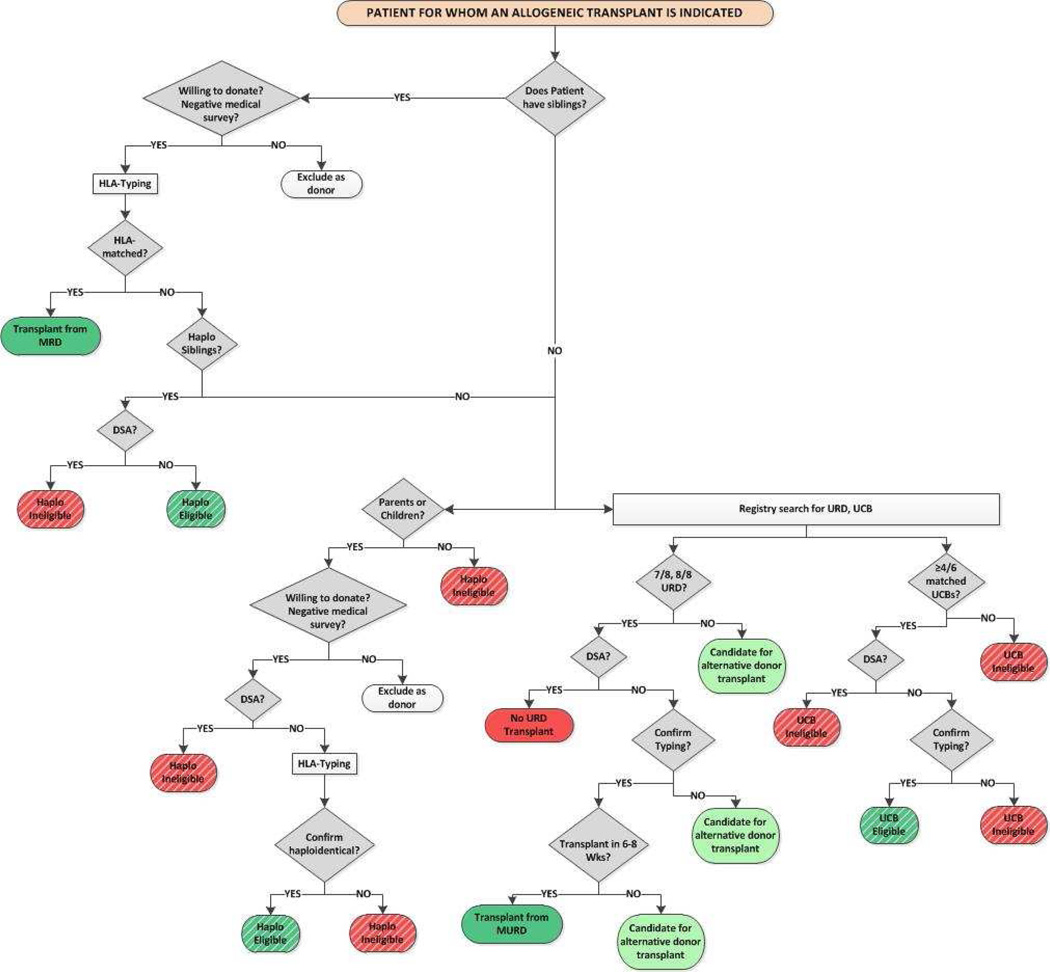
The timing of transplantation is critical for a successful outcome for patients with hematologic malignancy; disease risk, performance score and co-morbidities are associated with survival after transplantation. Patients thought to benefit from transplantation must proceed to this treatment in a timely manner. Consequently, in the absence of HLA-matched sibling, review available HLA typings from other family members (siblings and/or parents) to determine whether haploidentical related donors are available, and initiate an unrelated donor search for either an adult donor or UCB units. Therefore, information on the three alternative donor options are available when counseling patients for alternative donor transplantation (Figure 3). The data to-date support utilizing a HLA-matched adult unrelated donor (matched at the allele-level at HLA-A, -B, -C and –DRB1) when such a donor is available in a timely manner to donate. Barring this, there is little evidence to support the superiority of a HLA-mismatched unrelated donor to a haploidentical relative or UCB units.
Figure 3.
Donor selection algorithm
Abbreviations
MRD = matched related donor; URD = unrelated donor; MURD = HLA-mismatched unrelated donor; UCB = umbilical cord blood; DSA = donor specific antibody
Acknowledgement
This work was supported in part by grant U10 HL069294 from the National Heart Lung and Blood Institute and the National Cancer Institute and grant U24 CA76518 from the National Heart Lung and Blood Institute, the National Cancer Institute and the National Institute for Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
ME drafted the manuscript. PVO, CGB and EJF critically reviewed and edited the manuscript. JW and AM prepared and analyzed follow-up data on patients enrolled on BMT CTN 0603 and 0604. KB is the protocol coordinator for BMTCTN 1101. All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare none
References
- 1.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation: results of parallel phase II trials using HLA-mismatched related bone marrow or unrelated umbilical cord blood grafts. Blood. 2011;118(2):282–286. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morishima Y, Sasazuki T, Inoko H, et al. The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B and HLA-DR matched unrelated donors. Blood. 2002;99(11):420–4206. doi: 10.1182/blood.v99.11.4200. [DOI] [PubMed] [Google Scholar]
- 3.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 5.Woolfrey A, Klein JP, Haagenson M, et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koreth J, Ahn KW, Pidala J, et al. HLA-mismatch is associated with worse outcomes after unrelated donor reduced intensity hematopoietic cell transplantation: A CIBMTR analysis. Blood. 2013;122:547a. doi: 10.1016/j.bbmt.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleischhauer K, Shaw BE, Gooley T, et al. Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated donor haematopoietic cell transplantation: a retrospective study. Lancet Oncol. 2012;13(4):366–374. doi: 10.1016/S1470-2045(12)70004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-Viña MA, Klein JP, Haagenson M, et al. Multiple mismatches at the low expression HLA loci DP, DQ, and DR3/4/5 associate with adverse outcomes in hematopoietic stem cell transplantation. Blood. 2013;121(22):4603–4610. doi: 10.1182/blood-2013-02-481945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spellman SR, Eapen M, Logan BR, et al. A perspective on the selection of unrelated donors and cord blood units for transplantation. Blood. 2012;120(2):259–265. doi: 10.1182/blood-2012-03-379032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beatty PG, Mori M, Milford E. Impact of racial genetic polymorphism on the probability of finding an HLA-matched donor. Transplantation. 1995;60(8):778–783. [PubMed] [Google Scholar]
- 11.Kollman C, Maiers M, Gragert L, et al. Estimation of HLA-A, -B, -DRB1 Haplotype Frequencies Using Mixed Resolution Data from a National Registry with Selective Retyping of Volunteers. Human Immunology. 2007;68(12):950–958. doi: 10.1016/j.humimm.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Garget L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, Rizzo D, Horowitz M, Confer D, Maiers M. HLA match likelihoods for unrelated donor grafts in the U.S. registry. N Engl J Med. 2014 doi: 10.1056/NEJMsa1311707. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lown R, Shaw BE. Beating the odds: factors implicated in the speed and availability of unrelated haematopoietic cell donor provision. Bone Marrow Transplant. 2013;48(2):210–219. doi: 10.1038/bmt.2012.54. [DOI] [PubMed] [Google Scholar]
- 14.Pidala J, Kim J, Schell M, et al. Race/ethnicity affects the probability of finding an HLA-A, -B, -C and –DRB1 allele-matched unrelated donor and likelihood of subsequent transplant utilization. Bone Marrow Transplant. 2013;48(3):346–350. doi: 10.1038/bmt.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myaskovsky L, Switzer GE, Dew MA, Goycoolea JM, Confer DL, Abress L. The association of donor center characteristics with attrition from the National Marrow Donor Registry. Transplantation. 2004;77(6):874–880. doi: 10.1097/01.tp.0000116394.88804.a7. [DOI] [PubMed] [Google Scholar]
- 16.Switzer GE, Dew MA, Goycoolea JM, Myaskovsky L, Abress L, Confer DL. Attrition of potential bone marrow donors at two key decision points leading to donation. Transplantation. 2004;77(10):1529–1534. doi: 10.1097/01.tp.0000122219.35928.d6. [DOI] [PubMed] [Google Scholar]
- 17.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haematopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risk and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119(23):5591–5598. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisdorf D, Eapen M, Ruggeri A, et al. Alternative donor hematopoietic transplantation for patients older than 50 years with AML in first complete remission: A Center for International Blood and Marrow Transplant Research – Eurocord Analysis. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.02.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121(5):752–758. doi: 10.1182/blood-2012-08-449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Besien K, Liu H, Jain N, Stock W, Artz A. Umbilical cord blood transplantation supported by third-party donor cells: rationale, results and applications. Biol Blood Marrow Transplant. 2013;19(5):682–691. doi: 10.1016/j.bbmt.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD and durable remission. Blood. 2011;118(24):6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(20):232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eapen M, Klein JP, Sanz GF, et al. Effect of donor-recipient HLA matching at HLAA, B, C and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol. 2011;12(13):1214–1221. doi: 10.1016/S1470-2045(11)70260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anasetti C, Beatty PG, Storb R, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum.Immunol. 1990;29(2):79–91. doi: 10.1016/0198-8859(90)90071-v. [DOI] [PubMed] [Google Scholar]
- 30.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15(5):1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 31.Kanda Y, Chiba S, Hirai H, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991–2000) Blood. 2003;102(4):1541–1547. doi: 10.1182/blood-2003-02-0430. [DOI] [PubMed] [Google Scholar]
- 32.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N.Engl.J.Med. 1989;320(4):197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 33.Aversa F, Tabilio A, Velardi A, et al. Treatment of high risk acute leukemia with T-cell depleted stem cells from related donors with one fully mismatched haplotype. N Eng J Med. 1998;339(17):1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 34.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 35.Luznik L, O'Donnell PV, Symons HJ, et al. Haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and posttransplant ation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplant cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19(1):117–122. doi: 10.1016/j.bbmt.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Di Bartolomeo P, Santarone S, De Angelis G, et al. Haploidentical unmanipulated GCSF primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121(5):849–857. doi: 10.1182/blood-2012-08-453399. [DOI] [PubMed] [Google Scholar]
- 38.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical BMT with high-dose post-transplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16(4):482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huo MR, Xu Lp, D L, et al. The effect of HLA disparity on clinical outcome after HLA-haploidentical blood and marrow transplantation. Clin Transplant. 2012;26(2):284–291. doi: 10.1111/j.1399-0012.2011.01499.x. [DOI] [PubMed] [Google Scholar]
- 40.Bashey A, Zhang X, Sizemore CA, et al. T-Cell replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310–1316. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 41.Burroughs LM, O'Donnell PV, Sandmaier BM, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(11):1279–1287. doi: 10.1016/j.bbmt.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bishara A, De Santis D, Witt CC, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004;63(3):204–211. doi: 10.1111/j.0001-2815.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 44.Venstrom JM, Pittari G, Gooley TA, et al. HLA-C dependent prevention of leukemia relapse by donor activating KIR2DS1. N.Engl.J.Med. 2012;367(9):805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Symons HJ, Leffell MS, Rossiter ND, et al. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2010;16(4):533–542. doi: 10.1016/j.bbmt.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]