Abstract
Evidence suggests that noradrenergic signaling may play a role in mediating alcohol-drinking behavior in both rodents and humans. We have investigated this possibility by administering clonidine to alcohol-drinking rats selectively bred for alcohol preference (P line). Clonidine is an α2-adrenergic receptor agonist which, at low doses, inhibits noradrenergic signaling by decreasing norepinephrine release from presynaptic noradrenergic neurons. Adult male P rats were given 24-h access to food and water and scheduled access to a 15% (v/v) alcohol solution for 2 h daily. Rats received intraperitoneal (IP) injections with clonidine (0, 10, 20, 40, or 80 µg/kg body weight [BW], 10–11 rats/treatment group) once/day at 30 min prior to onset of the daily 2-h alcohol access period for 2 consecutive days. Clonidine, in doses of 40 or 80 µg/kg BW, significantly reduced alcohol intake on both days of treatment (p < 0.001). Two weeks later, rats were treated with clonidine for 5 consecutive days and clonidine, in doses of 40 or 80 µg/kg BW, reduced alcohol intake on all 5 treatment days (p < 0.001). Clonidine did not alter water consumption during the daily 2-h free-choice between alcohol and water. In a separate group of male P rats, clonidine (40 µg/kg BW) suppressed intake of a saccharin solution (0.04 g/L). These results are consistent with and complement our previous findings that the α1-adrenergic receptor antagonist, prazosin, decreases voluntary alcohol drinking in alcohol-preferring rats, but suggests that effects of clonidine may not be specific for alcohol. The results suggest that although activation of the noradrenergic system plays an important role in mediating voluntary alcohol drinking, care is needed in selecting which drugs to use to suppress central noradrenergic signaling in order to maximize the selectivity of the drugs for treating alcohol-use disorders.
Keywords: clonidine, alcohol, ethanol, norepinephrine, noradrenergic, P rat
Introduction
It has been suggested that excessive noradrenergic activation which accompanies anxiety and hyperarousal may contribute to increased alcohol drinking in an effort to self-medicate, since alcohol is sympatho-suppressive, anxiolytic, and sedating (Edwards, Chandler, Hensman, & Peto, 1972; Koob & LeMoal, 1997; Kushner, Sher, & Beitman, 1990; Kushner, Sher, & Erickson, 1999; Shirao et al., 1988). Numerous lines of evidence support this view: a) anxiety is associated with increased brain noradrenergic and associated sympathoadrenal activation (Kopin, 1984; Sullivan, Coplan, Kent, & Gorman, 1999), b) “anxious” rats consume more alcohol than “non-anxious” rats (Spanagel et al., 1995), c) blocking norepinephrine biosynthesis decreases alcohol self-administration by rodents (Amit, Brown, Levitan, & Ogren, 1977; Brown, Amit, Levitan, Ogren, & Sutherland, 1977; Davis, Smith, & Werner, 1978), d) alcoholics commonly state that relief of anxiety is an important reason for drinking (e.g., Edwards et al., 1972), e) alcoholism co-occurs at high rates with anxiety disorders (Kushner et al., 1990), suggesting that the two disorders represent manifestations of similar underlying mechanisms (Merikangas, Risch, & Weissman, 1994; Merikangas et al., 1998; Sinha, Robinson, & O'Malley, 1998), f) patients with co-morbid anxiety and alcoholism more frequently report that they use alcohol to control anxiety and panic symptoms as compared to other reasons for alcohol use (Kushner et al., 1990), g) increased sympathetic activation is seen during periods of increased anxiety and during prolonged alcohol abstinence (Ehrenreich et al., 1997; Sullivan et al., 1999), and h) increased sympathoadrenal activation and anxiety-like behavior is observed for long periods following termination of chronic alcohol consumption in rats (Rasmussen, Mitton, Green, & Puchalski, 2001; Rasmussen, Wilkinson, & Raskind, 2006). Taken together, these findings suggest that excessive sympathetic activation may contribute not only to maintenance of alcohol drinking and alcohol abuse but may also be one of the aversive physiological events that occur during alcohol withdrawal and abstinence that increases risk of relapse to alcohol drinking (Koob & LeMoal, 1997).
We previously tested the hypothesis that noradrenergic activation promotes and maintains alcohol drinking by assessing whether alcohol drinking in rats is decreased by prazosin treatment. Prazosin is a drug that is centrally active when administered peripherally and that decreases brain noradrenergic signaling by blocking postsynaptic α1-adrenergic receptors. Prazosin dose-dependently reduced withdrawal-induced operant self-administration of alcohol in alcohol-dependent Wistar rats (Walker, Rasmussen, Raskind, & Koob, 2008). Prazosin also suppressed voluntary alcohol drinking by rats selectively bred for alcohol preference (P line) when administered either acutely (Rasmussen, Alexander, Raskind, & Froehlich, 2009) or chronically (Froehlich, Hausauer, Federoff, Fischer, & Rasmussen, 2013; Froehlich, Hausauer, & Rasmussen, 2013). The ability of prazosin to reduce alcohol drinking has been confirmed in humans; Simpson and colleagues (2009) reported that prazosin decreased relapse alcohol drinking in treatment-seeking alcohol-dependent men. These results from both rodents and humans provide compelling evidence that noradrenergic activation plays an important role in mediating alcohol drinking and alcohol relapse.
If alcohol drinking is due in part to activation of the noradrenergic system, any drug that decreases noradrenergic signaling might be expected to decrease alcohol drinking. We recently determined that combined treatment of P rats with both prazosin and propranolol (which block α1- and β-adrenergic receptors, respectively) decreased alcohol drinking more effectively than treatment with either drug alone (Rasmussen, Beckwith, Kincaid, & Froehlich, 2014), suggesting that α1- and β-adrenergic receptors may have complementary roles in facilitating alcohol drinking. This suggests that administration of an α2-adrenergic receptor agonist such as clonidine, which can decrease noradrenergic signaling by decreasing norepinephrine release from presynaptic terminals (Aghajanian & VanderMaelen, 1982; Starke, Montel, Gayk, & Merder, 1974) and thus decrease the amount of norepinephrine available for binding to either α1- or β-adrenergic post-synaptic receptors, may be especially effective in decreasing alcohol drinking. Accordingly, in the present study we evaluated the effect of clonidine on voluntary alcohol drinking in selectively bred alcohol-preferring (P) rats.
Materials and methods
Animals
Male P rats (n = 51 in Study 1; n = 20 in Study 2) from the 60th generation of selective breeding for alcohol preference served as subjects. The rats were individually housed in stainless-steel hanging cages in an isolated vivarium with controlled temperature (21 ± 1 °C) and a 12-h light/dark cycle (lights off at 1000 hours). Standard rodent chow (Laboratory Rodent Diet #7001, Harlan Teklad, Madison, WI) and water were available ad libitum at all times throughout the study. All experimental procedures were approved by the Indiana University Institutional Animal Care and Use Committee and conducted in strict compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Six months prior to onset of the current study, the rats in Study 1 were treated acutely with intra-peritoneal (IP) prazosin for 2 consecutive days and then, 3 weeks later, for 5 consecutive days (Rasmussen et al., 2009). The rats were then held for 6 months without drug treatment prior to onset of the current clonidine study. During this time, all rats received 2-h access to alcohol (15 % v/v) for 2 h/day, 5 days/week until the current investigation. The rats were 13 months old with a mean body weight of 679 g at the start of clonidine treatment.
Drugs
Clonidine hydrochloride (Sigma-Aldrich Co., St. Louis, MO) was dissolved in 0.9% NaCl. In Study 1, each rat received an IP injection of saline vehicle or 10, 20, 40, or 80 µg clonidine/mL saline/kg BW. In Study 2, each rat received an IP injection of saline vehicle or 40 µg clonidine/mL saline/kg BW.
Study 1: effects of clonidine treatment on alcohol intake
The alcohol solution was prepared by diluting 95% alcohol (ethanol; Decon Laboratories Inc., King of Prussia, PA) with distilled, deionized water to make a 15% (v/v) solution. Alcohol (15% v/v) and water were presented in calibrated glass drinking tubes, with positions of the tubes alternated daily to control for potential side preferences. Daily fluid intakes were recorded to the nearest mL. Alcohol intake was converted from mL alcohol/kg BW to g alcohol/kg BW.
During the week prior to clonidine administration, baseline alcohol and water intakes during the daily 2-h alcohol-access period (1100–1300 hours) were calculated for each rat over 4 consecutive days and the rats were ranked in descending order in terms of average daily alcohol consumption. The rats were assigned to drug treatment groups in a manner that ensured that the groups did not differ in baseline alcohol intake prior to clonidine administration. Specifically, the top 5 alcohol drinkers were randomly assigned to the vehicle or one of four clonidine-treatment groups (10, 20, 40, or 80 µg clonidine/kg BW), followed by the next 5 highest alcohol drinkers likewise randomly assigned, etc.
2-day clonidine treatment
To reduce stress associated with IP drug administration, all rats were handled as if they were going to receive an IP injection for 5 consecutive days prior to onset of drug treatment, and all rats received an IP injection of vehicle on the day preceding onset of drug treatment. Clonidine in doses of 10, 20, 40, or 80 µg/kg BW (n = 10–11/dose) or an equivalent volume of vehicle (n = 10–11) was administered 30 min prior to the daily 2-h alcohol-access period (1100–1300 hours, beginning 1 h after lights out) on each of 2 consecutive days.
5-day clonidine treatment
After completion of the 2-day clonidine treatment regimen, rats continued to receive 2-h (1100–1300 h) daily access to alcohol 5 days/week for 3 weeks prior to initiating 5 days of clonidine treatment. To reduce the stress associated with IP drug administration, all rats were again handled as if they were going to receive an IP injection for 5 days prior to treatment and were given an IP injection of vehicle on the day preceding onset of 5 days of drug treatment. Average daily alcohol and water intakes were determined in the week preceding drug treatment and the rats were rank ordered, based on alcohol intake, and re-assigned to treatment groups as previously described. Clonidine, in doses of 10, 20, 40, or 80 µg/kg BW, or an equivalent volume of vehicle, was administered 30 min prior to the daily 2-h alcohol access period on each of 5 consecutive days.
Study 2: effect of clonidine treatment on saccharin intake
A separate group of 20 male P rats were each provided access to a saccharin solution (0.04 g/L) during a 2-h daily access period (1100–1300 hours, beginning one hour after lights out) 5 days/week for 16 days until saccharin intake stabilized (as determined by 2-way ANOVA with repeated measures on days 13–16, with no significant effect of day on saccharin intake). Food and water were freely available for 24 hours/day throughout this study. Saccharin and water intakes were recorded daily and body weights were recorded twice weekly. The rats were 4 months old and had a mean body weight of 455 g at the start of clonidine treatment.
The 0.04 g/L saccharin solution was prepared by dissolving 99+% saccharin (Sigma-Aldrich Co., St. Louis, MO) in distilled, deionized water. Saccharin and water were presented in calibrated glass drinking tubes, and daily fluid intakes were recorded to the nearest mL. Saccharin and water intakes are reported in mL/kg BW to control for size-based variation in fluid requirements/gastric capacity.
Baseline saccharin intake was calculated for each rat during the 2 days prior to clonidine treatment. Rats were ranked in descending order in terms of average daily saccharin consumption and assigned to treatment groups (vehicle or 40 µg clonidine/kg BW) in a manner that ensured that the groups did not differ in baseline saccharin intake prior to clonidine administration, as described for alcohol intake in Study 1.
To reduce the stress associated with IP drug administration, all rats were handled as if they were going to receive an IP injection for 5 consecutive days prior to initial drug treatment. Clonidine (40 µg/kg BW; n = 10) or an equivalent volume (1 mL) of saline vehicle (n = 10) was administered 30 min prior to the daily 2-h saccharin vs. water access period on each of 3 consecutive days.
Data analyses
In Study 1, alcohol intakes during drug treatments were analyzed by 2-way mixed analysis of variance (ANOVA) with repeated measures (dose × day with repeated measures on day) followed, when justified by determination of significant main effects or interactions in the ANOVA, by pairwise multiple comparisons using Fisher’s least significant difference (LSD) tests. Alcohol intakes on 2 post-treatment days were likewise analyzed by 2-way mixed ANOVA with repeated measures on day, followed by pairwise multiple comparisons with Fisher’s LSD tests. Alcohol intakes within the pre-treatment day were similarly analyzed, but with 1-way ANOVA. As discussed in the Results section, Kruskal-Wallis 1-way ANOVA on ranks was used to analyze water intake. In Study 2, saccharin intakes on the 3 treatment days were likewise analyzed by 2-way mixed ANOVA with repeated measures on day, followed by pairwise multiple comparisons with Fisher’s LSD tests. All analyses were conducted using the SigmaStat 3.5 program (Systat Software, Inc., Chicago, IL) with significance accepted at p < 0.05. Data are presented as mean ± SE.
Results
Daily alcohol intake during the 2-h alcohol-access period prior to onset of drug treatment in Study 1 averaged 1.3 ± 0.2 g/kg BW/2 h, which is lower than the intake seen in some of our previous studies using 2-h alcohol access in P rats. This is likely because the rats in the current study were older and larger than those we have previously used (which have averaged only about 350–400 g BW and have consumed approximately 2.0 g alcohol/kg BW during 2 h of alcohol access). Weight gain above 400 g in rats is due to addition of fat; fat tissue has poor blood supply, low water content, and only 10–20% of the alcohol concentration found in blood, so reporting alcohol intake in g/kg BW in fat rats underestimates alcohol intake (Bloom, Lad, Pittman, & Rogers, 1982; Westerfeld & Schulman, 1959; York, 1983).
Effect of 2-day clonidine treatment on alcohol and water intakes
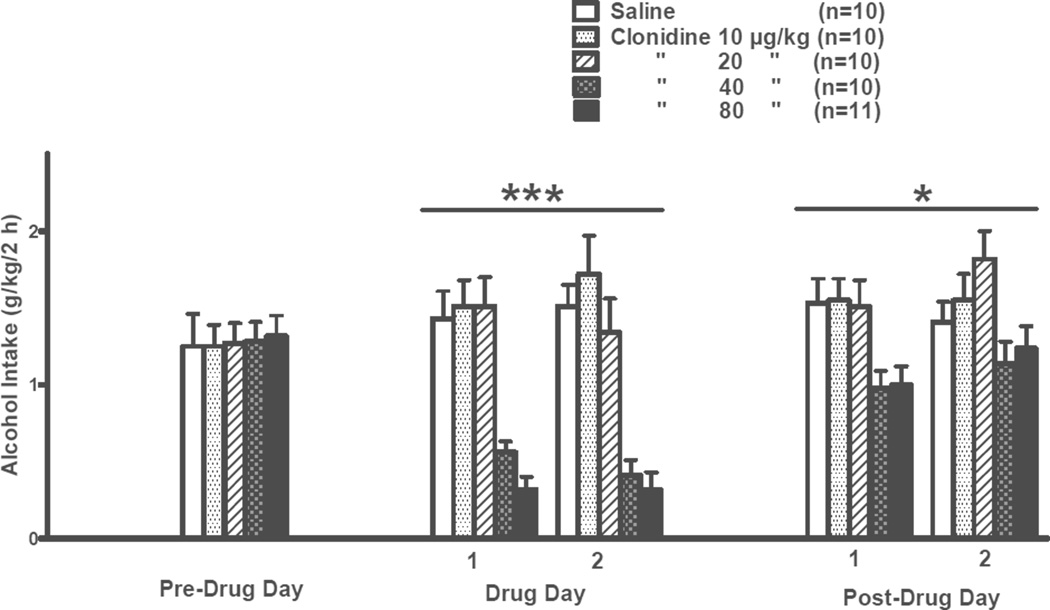
The effects of clonidine treatment on alcohol intake during the daily 2-h freechoice between alcohol and water in a 2-day treatment trial are presented in Fig. 1. There was no significant difference among treatment groups on the pre-drug day. During the 2 treatment days there was a significant effect of dose [F(4,46) = 17.95, p < 0.001] but no significant effect of day and no significant dose × day interaction. Further pairwise comparisons using the Fisher’s LSD test revealed that alcohol intake was decreased (p < 0.001) by clonidine at doses of 40 or 80 µg/kg relative to treatment with vehicle, independent of day. On post-treatment days 1 and 2, there was a significant effect of dose [F(4,46) = 4.51, p < 0.01] but no significant effect of day and no significant dose × day interaction. Further pairwise comparisons using the Fisher’s LSD test revealed that alcohol intake was decreased (p ≤ 0.05) by previous treatment with clonidine 40 or 80 µg/kg relative to previous treatment with vehicle, independent of day.
Figure 1. INITIAL 2-DAY TREATMENT.
Effects of clonidine (10, 20, 40, 80 µg/kg BW, IP) on 2-h alcohol intake by adult male P rats. Clonidine was injected on each of 2 consecutive days (Days 1 and 2) at 30 min prior to onset of a 2-h free-choice between alcohol (15% v/v) and water. ***p < 0.001 vs. saline control treatment, independent of drug treatment day; *p < 0.05 vs. previous saline control treatment, independent of post-drug day.
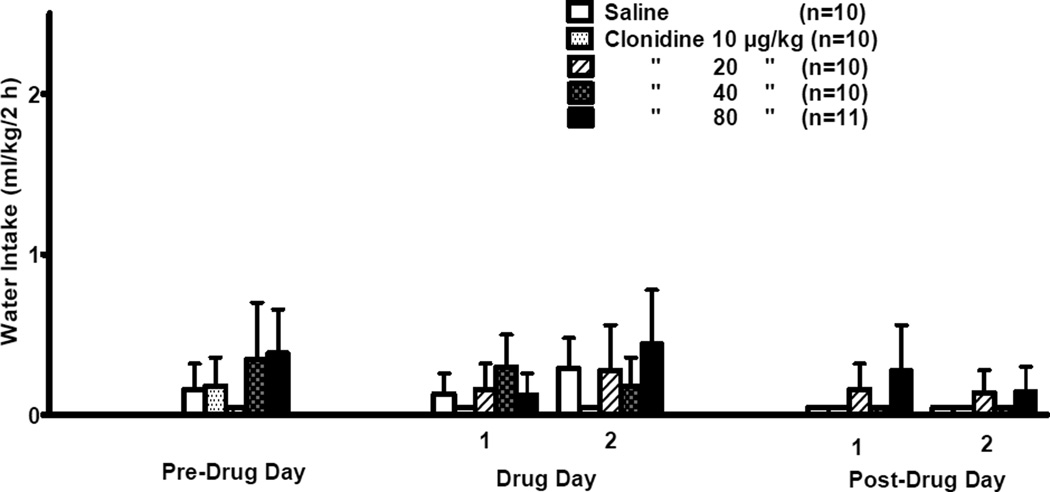
The effect of clonidine on water intake during the daily 2-h free-choice between alcohol and water is presented in Fig. 2. No commonly used data transformations were effective in achieving homogeneity of variance and normal distribution of the water data. Since non-parametric 2-way ANOVA with repeated measures tests are not available, 5 separate non-parametric 1-way ANOVA analyses were conducted, i.e., one each for the Pre-Drug Day, Drug Days 1 and 2, and Post-Drug Days 1 and 2. Kruskal-Wallis 1-way ANOVA on ranks indicated that water intake was not significantly different among the treatment groups on any day.
Figure 2. INITIAL 2-DAY TREATMENT.
Effects of clonidine (10, 20, 40, 80 µg/kg BW, IP) on 2-h water intake by adult male P rats. Clonidine was injected on each of 2 consecutive days (Days 1 and 2) at 30 min prior to onset of a 2-h free-choice between alcohol (15% v/v) and water.
There was no significant difference in 24-h water intake (data not shown) among treatment groups on the pre-drug day. During the 2 treatment days there was a significant effect of dose [F(4,46) = 28.53, p < 0.001] but no significant dose × day interaction (average water intake for the vehicle-treated or 10, 20, 40, or 80 µg/kg clonidine-treated group was 32.5 ± 1.8, 32.0 ± 1.8, 39.5 ± 4.1, 46.9 ± 2.9, and 65.5 ± 2.1 mL/kg/24 h, respectively). Pairwise comparisons using the Fisher’s LSD test revealed that 24-h water intake was increased (p ≤ 0.001) by clonidine at doses of 40 or 80 µg/kg relative to treatment with vehicle, independent of day. On post-treatment days 1 and 2, there was no significant effect of dose or day and no significant dose × day interaction (average 24-h water intake for the vehicle-treated or 10, 20, 40, or 80 µg/kg clonidine-treated group was 34.4 ± 1.9, 34.8 ± 1.9, 34.9 ± 3.5, 32.1 ± 1.8, or 34.3 ± 1.4 mL/kg/24 h, respectively).
Subsequent 5-day clonidine treatment effects on daily alcohol and water intakes
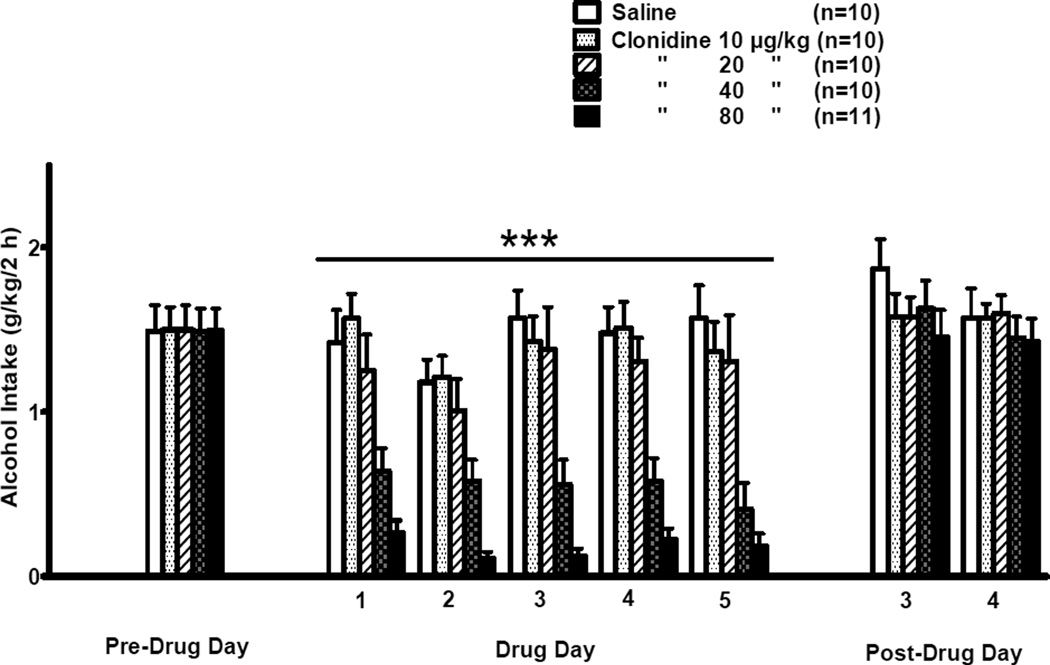
The effects of clonidine treatment on alcohol intake during the daily 2-h free-choice between alcohol and water in the 5-day treatment trial are presented in Fig. 3. Pre-drug alcohol intake was determined on a Friday, drug days 1–5 occurred on Monday–Friday, and the 2 post-drug days occurred on the Monday and Tuesday following termination of drug treatment. There was no significant difference in alcohol intake among treatment groups on the pre-drug day. During the 5 treatment days there was a significant effect of dose [F(4,46) = 22.18, p < 0.001] and a significant effect of day [F(4,184) = 3.36, p < 0.05] on alcohol intake, but no significant dose × day interaction. Pairwise comparisons using the Fisher’s LSD test revealed that alcohol intake was decreased (p < 0.001), independent of day, by clonidine at doses of 40 or 80 µg/kg relative to treatment with vehicle, independent of day. Alcohol intake was decreased (p < 0.05) by treatment with clonidine 80 µg/kg relative to treatment with clonidine 40 µg/kg. On post-treatment days 3 and 4, there were no significant effects of dose or day on alcohol intake and no significant dose × day interaction.
Figure 3. SUBSEQUENT 5-DAY TREATMENT.
Effects of clonidine (10, 20, 40, 80 µg/kg BW, IP) on 2-h alcohol intake by adult male P rats. Clonidine was injected on each of 5 consecutive days (Days 1–5) at 30 min prior to onset of a 2-h free-choice between alcohol (15% v/v) and water. Alcohol was not available on weekends. The Pre-Drug Day was a Friday preceding Drug Days 1–5 (Monday–Friday of the following week) and Post-Drug Days 3 and 4 were on Monday and Tuesday of the week following termination of clonidine treatment. ***p < 0.001 vs. saline control treatment, independent of drug treatment day.
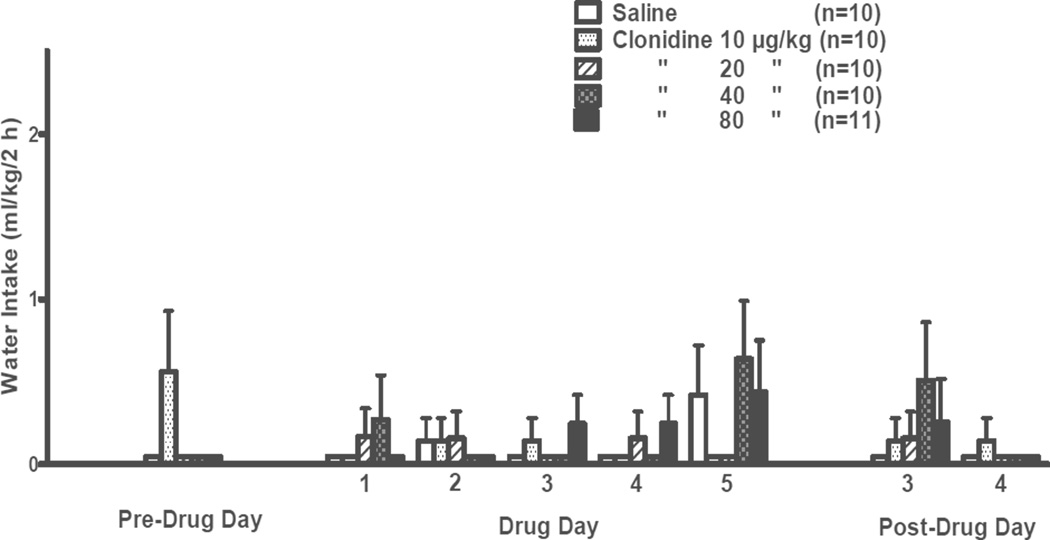
The effects of clonidine on water intake during the daily 2-h free-choice between alcohol and water in this 5-day treatment trial are presented in Fig. 4. As in the previous 2-day treatment trial, no commonly used data transformations were effective in achieving homogeneity of variance and normal distribution of the water data, so eight separate non-parametric 1-way ANOVA analyses were conducted, i.e., one each for the Pre-Drug Day, Drug Days 1–5, and Post-Drug Days 3 and 4. Kruskal-Wallis 1-way ANOVA on ranks indicated that water intake was not significantly different among the treatment groups on any day.
Figure 4. SUBSEQUENT 5-DAY TREATMENT.
Effects of clonidine (10, 20, 40, 80 µg/kg BW, IP) on 2-h water intake by adult male P rats. Clonidine was injected on each of 5 consecutive days (Days 1–5) at 30 min prior to onset of a 2-h free-choice between alcohol (15% v/v) and water. Alcohol was not available on weekends. The Pre-Drug Day was a Friday preceding Drug Days 1–5 (Monday–Friday of the following week) and Post-Drug Days 3 and 4 were on Monday and Tuesday of the week following termination of clonidine treatment.
Alcohol preference ratios during the 5 days of daily 2-h free-choice between alcohol and water were significantly different among the treatment groups [F(4,46) = 8.55, p < 0.001], with significant dose × day interactions [F(16,184) = 1.82, p < 0.05]. Pairwise comparisons using the Fisher’s LSD test revealed that alcohol preference was decreased by clonidine treatment at a dose of 80 µg/kg, relative to treatment with vehicle, on days 1 (p < 0.05), 2 (p < 0.001), 3 (p < 0.001), 4 (p < 0.01), and 5 (p < 0.01). Treatment with clonidine at a dose of 40 µg/kg likewise decreased alcohol preference on days 3 (p < 0.05) and 5 (p < 0.01).
There was no significant difference in 24-h water intake (data not shown) among treatment groups on the pre-drug day. During the 5 treatment days there was an error in 24-h water intake determinations for day 5, so the 24-h intake analyses included only days 1–4. For these 4 treatment days there was a significant effect of dose [F(4,46) = 60.20, p < 0.001] but no significant dose × day interaction (average water intake for the vehicle-treated or 10, 20, 40, or 80 µg/kg clonidine-treated group was 35.0 ± 1.2, 32.5 ± 1.5, 38.4 ± 1.2, 50.9 ± 2.1, or 64.1 ± 2.2 mL/kg/24 h, respectively). Pairwise comparisons using the Fisher’s LSD test revealed that 24-h water intake was increased (p < 0.001) by clonidine at doses of 40 or 80 µg/kg relative to treatment with vehicle, independent of day. On post-treatment days 3 and 4, there was no significant effect of dose or day and no significant dose × day interaction (average 24-h water intake for the vehicle-treated or clonidine 10, 20, 40, or 80 µg/kg clonidine-treated group was 29.8 ± 2.7, 28.7 ± 1.6, 33.5 ± 1.6, 27.4 ± 3.6, or 35.5 ± 3.0 mL/kg/24 h, respectively).
Study 2: clonidine effects on saccharin intake
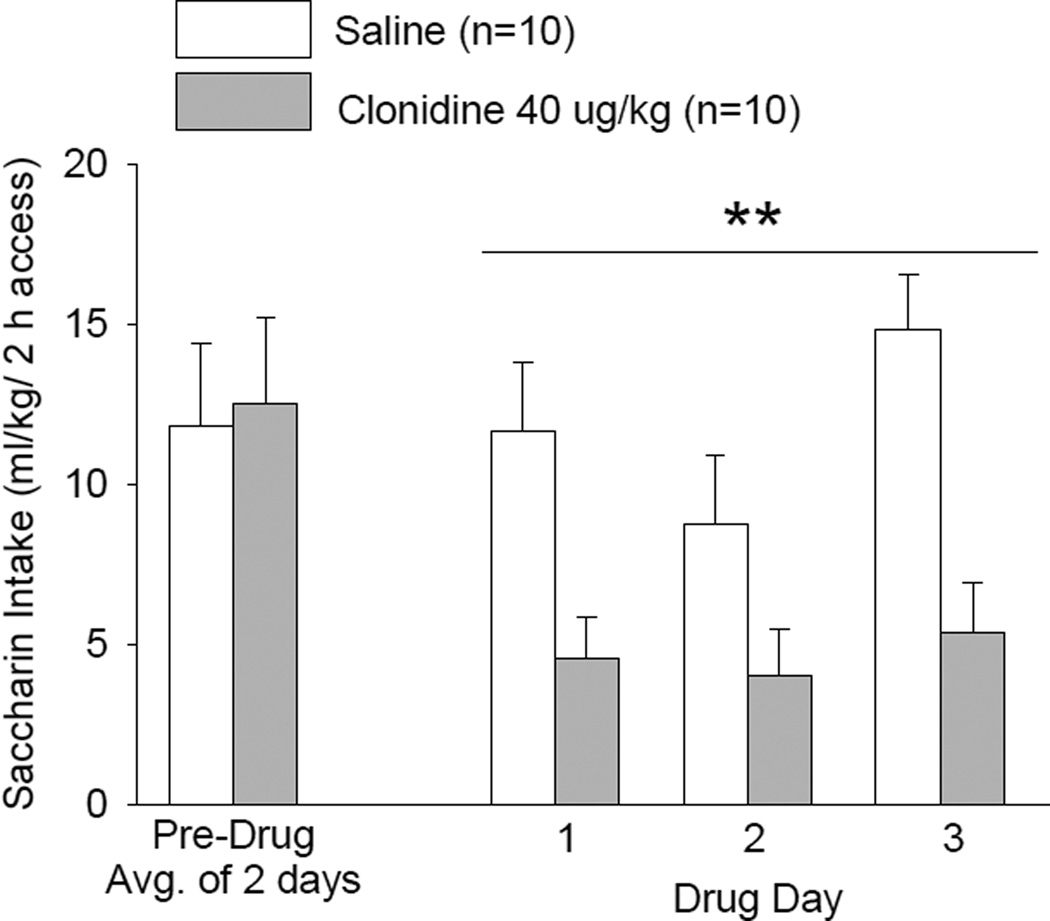
The effect of clonidine 40 µg/kg treatment on intake of saccharin solution during 2-h trials on each of 3 consecutive days is presented in Fig. 5. There was no significant difference in pre-drug saccharin intake among treatment groups. During the 3 treatment days there was a significant effect of clonidine treatment [F(1,18) = 12.97, p < 0.01] and a significant effect of day [F(2,36) = 3.97, p < 0.05] on saccharin intake, but no significant treatment × day interaction. Pairwise comparisons using the Fisher’s LSD test revealed that saccharin intake was decreased (p < 0.01) independent of day by clonidine treatment relative to treatment with saline vehicle.
Figure 5. EFFECTS OF CLONIDINE ON SACCHARIN INTAKE.
Clonidine (40 µg/kg BW, IP) was injected on each of 3 consecutive days at 30 min prior to onset of a 2-h free-choice between water and saccharin solution (0.04 g/L). **p < 0.01 vs. saline control treatment, independent of treatment day.
There was no significant difference in water intake between treatment groups on the pre-drug day. During the 3 treatment days, there was no significant effect of treatment or day and no treatment × day interaction effect on water intake. The 3-day average water intakes were 3.1 ± 0.7 mL/2 h for the saline-treated group and 5.2 ± 1.2 mL/2 h for the clonidine-treated group.
Discussion
Clonidine, the α2-adrenergic receptor agonist which in moderate doses decreases noradrenergic signaling by stimulating inhibitory pre-synaptic α2-adrenergic receptors (Aghajanian & VanderMaelen, 1982; Starke, Montel, Gayk, & Merder, 1974), decreased voluntary alcohol drinking in a dose-dependent manner when administered to P rats for either 2 or 5 consecutive days. These results are consistent with our reports that prazosin, which decreases noradrenergic signaling by blocking post-synaptic α1-adrenergic receptors, decreases alcohol drinking in P rats when administered acutely (Rasmussen et al., 2009) or chronically (Froehlich, Hausauer, Federoff, et al., 2013;). The fact that two different pharmacologic interventions that decrease noradrenergic signaling, but via different mechanisms, are both capable of suppressing alcohol drinking is consistent with evidence that activation of the noradrenergic system plays a key role in mediating voluntary alcohol drinking. These results are also consistent with reports that: a) the α2-adrenergic receptor agonist, lofexidine, reduces operant self-administration of alcohol by Wistar rats (Lé, Harding, Juzytsch, Funk, & Shaham, 2005), b) clonidine and another α2-adrenergic receptor agonist, guanfacine, decrease alcohol drinking by food-restricted rats selectively bred for alcohol drinking (descendants of the Finnish Alko Alcohol (AA) rats) (Opitz, 1990), c) depletion of brain norepinephrine decreases alcohol drinking (Amit, Brown, Levitan, & Ogren, 1977; Brown et al., 1977) and alcohol self-administration in unselected rats (Davis et al., 1978), and d) prazosin reduces acute withdrawal-induced operant self-administration of alcohol by alcohol-dependent Wistar rats (Walker, Rasmussen, Raskind, & Koob, 2008).
Clonidine plasma half-life is approximately 24 h in humans (Arndts, Doevendans, Kirsten, & Heintz, 1983) but less than 1 h in rats (Hui et al., 2007). Following intravenous administration of clonidine doses similar to those administered IP in the current study, clonidine is rapidly distributed throughout the rat brain (with peak levels within 2 min) and then rapidly eliminated from the brain (with a brain tissue half-life of approximately 30–50 min) (Conway & Jarrott, 1980). Consequently, clonidine was administered IP at 30 min before the start of alcohol access in the current study, consistent with the 30 min lead time for IP administration commonly used for other investigations of clonidine effects on rat behaviors (e.g., Shaham, Highfield, Delfs, Leung, & Stewart, 2000).
Clonidine treatment decreased voluntary alcohol drinking without significantly changing water drinking during the daily 2-h free-choice between alcohol and water. A compensatory increase in water intake did not accompany the clonidine-induced decrease in 2-h alcohol intake even though we previously demonstrated that increases in water intake normally accompanied reductions in 2-h alcohol intake produced by prazosin (Rasmussen et al., 2009), and clonidine did increase subsequent water intake as revealed by increased 24-h water intake. The lack of acute increase in water intake during the 2-h free choice between alcohol and water in clonidine-treated rats suggests a possible confound due to potential acute antidipsogenic effects of clonidine (Fregly, Kelleher, & Greenleaf, 1981).
The lowest dose of clonidine to suppress alcohol drinking (40 µg/kg) had previously been reported to produce no motor impairment in the rotarod test (De Luca, Nunes de Souza, Yada, & Meyer, 1999) and not to compromise operant responding for food (Shaham et al., 2000), suggesting that the decrease in alcohol intake after treatment with this dose of clonidine was not due to sedation or motor impairment. To test this further, we assessed the effect of this dose of clonidine on intake of a palatable saccharin solution and found that saccharin intake was reduced as well as alcohol intake. This suppression of saccharin intake may in part reflect motor or hypnotic effects, or may be due to a more generalized suppression in intake of reinforcing substances. At the highest clonidine dose used in the current study (80 µg/kg BW), it is likely that acute hypotensive/sedating effects, which are known to be associated with high doses of clonidine, contributed to the profound clonidine-induced suppression of alcohol consumption. It has been reported that when a bolus intravenous (IV) injection of clonidine was followed immediately (within 10 min) by an IV injection of 1 g alcohol/kg BW, clonidine acutely increased alcohol-induced sedation by an α2A-adrenergic receptor-mediated mechanism (Bender & Abdel-Rahman, 2009). Hence, it also is possible that the high (80 µg/kg) dose of clonidine enhanced the acute sedating effects of alcohol, thus leading to a cessation of alcohol drinking early in the 2-h alcohol-access period. Recent evidence suggests that some α2-agonist effects, including sedation, can be mediated by α2-adrenoceptor receptors located on non-adrenergic cells rather than by autoreceptor-mediated inhibition of norepinephrine release (Gilsbach et al., 2009).
In the present study, alcohol intake remained suppressed for 24 h following termination of 2 days of clonidine treatment. This is not likely due to the continued presence of circulating clonidine since the half-life of clonidine is less than 1 h in rats (Hui et al., 2007). The continued suppression of alcohol intake following termination of clonidine treatment could reflect acute clonidine-induced alterations in α-adrenergic receptors. With more prolonged clonidine treatment (5 consecutive days), suppression of alcohol drinking was maintained over the course of treatment, indicating that drug tolerance to clonidine did not develop. In the 5-day treatment paradigm, the last day of clonidine treatment was Friday, and the first day of post-drug testing did not occur until the following Monday, so the duration of suppression of drinking could not be comprehensively assessed. However, alcohol drinking was no longer suppressed at 72 h after the final clonidine administration, indicating no long-term residual effects of the drug.
It has been suggested that noradrenergic signaling may play a key role in mediating both reinforcement by and relapse to various drugs of abuse. This view is supported by evidence that clonidine attenuates symptoms associated with short-term opiate (Gold & Pottash, 1981), alcohol (Björkqvist, 1975), and nicotine (Glassman, Jackson, Walsh, Roose, & Rosenfeld, 1984) withdrawal in humans. For example, clonidine decreases craving, anxiety, tension, irritability, and restlessness associated with nicotine withdrawal (Glassman et al., 1984). More recently, administration of clonidine or other α2-adrenergic receptor agonists (e.g., lofexidine, guanfacine, guanabenz) has been demonstrated to attenuate stress-induced reinstatement of opiate, cocaine, and alcohol seeking in rats (Erb et al., 2000; Highfield, Yap, Grimm, Shalev, & Shaham, 2001; Lé et al., 2005; Shaham et al., 2000). These results are consistent with evidence that blocking post-synaptic α1-adrenergic receptors with prazosin decreases consumption of cocaine, opiates, nicotine, and alcohol (Greenwell, Walker, Cottone, Zorrilla, & Koob, 2009; Rasmussen et al., 2009; Simpson et al., 2009; Villégier, Lotfipour, Belluzzi, & Leslie, 2007; Wee, Mandyam, Lekic, & Koob, 2008; Zhang & Kosten, 2005, 2007) and with the findings of the current study that inhibiting noradrenergic signaling with the α2-adrenergic receptor agonist, clonidine, decreases voluntary alcohol consumption.
Overall, these results suggest that drugs that suppress noradrenergic signaling could be effective pharmacotherapeutic agents for the treatment of alcohol-use disorders, but care will be required to choose the agents that will be particularly useful in reducing alcohol drinking without introducing potentially deleterious side effects. More work is needed in order to identify the optimal combination of agents that are selective for specific α2-adrenergic receptor subtypes, or agents with mixed adrenergic receptor activity, that are most effective for decreasing alcohol intake. An assessment of the ability of various noradrenergic agents to decrease alcohol drinking is likely to result in the establishment of a hierarchy of drug efficacy within this class of drugs that hold promise as potential pharmacotherapeutic agents for the treatment of alcohol abuse and alcoholism.
Acknowledgments
This material is based upon work supported in part by resources from the VA Puget Sound Health Care System, Seattle, Washington, and by NIH Grants AA017839, AA10567, and AA13881 (DDR); AA10709 and AA007611 (JCF); and AA018604 (JCF and DDR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Amit Z, Brown ZW, Levitan DE, Ogren SO. Noradrenergic mediation of the positive reinforcing properties of ethanol: I. Suppression of ethanol consumption in laboratory rats following dopamine-beta-hydroxylase inhibition. Archives Internationales de Pharmacodynamie et de Thérapie. 1977;230:65–75. [PubMed] [Google Scholar]
- Arndts D, Doevendans J, Kirsten R, Heintz B. New aspects of the pharmacokinetics and pharmacodynamics of clonidine in man. European Journal of Clinical Pharmacology. 1983;24:21–30. doi: 10.1007/BF00613922. [DOI] [PubMed] [Google Scholar]
- Bender TS, Abdel-Rahman AA. Alpha2A-adrenergic receptor signaling underlies synergistic enhancement of ethanol-induced behavioral impairment by clonidine. Alcoholism: Clinical and Experimental Research. 2009;33:408–418. doi: 10.1111/j.1530-0277.2008.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkqvist SE. Clonidine in alcohol withdrawal. Acta Psychiatrica Scandinavica. 1975;52:256–263. doi: 10.1111/j.1600-0447.1975.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Bloom F, Lad P, Pittman Q, Rogers J. Blood alcohol levels in rats: non-uniform yields from intraperitoneal doses based on body weight. British Journal of Pharmacology. 1982;75:251–254. doi: 10.1111/j.1476-5381.1982.tb08780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZW, Amit Z, Levitan DE, Ogren SO, Sutherland EA. Noradrenergic mediation of the positive reinforcing properties of ethanol. II. Extinction of ethanol-drinking behavior in laboratory rats by inhibition of dopamine-beta-hydroxylase. Implications for treatment procedures in human alcoholics. Archives Internationales de Pharmacodynamie et de Thérapie. 1977;230:76–82. [PubMed] [Google Scholar]
- Conway EL, Jarrott B. Clonidine distribution in the rat: temporal relationship between tissue levels and blood pressure response. British Journal of Pharmacology. 1980;71:473–478. doi: 10.1111/j.1476-5381.1980.tb10960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WM, Smith SG, Werner TE. Noradrenergic role in the self-administration of ethanol. Pharmacology, Biochemistry, and Behavior. 1978;9:369–374. doi: 10.1016/0091-3057(78)90298-8. [DOI] [PubMed] [Google Scholar]
- De Luca LA, jr, Nunes de Souza RL, Yada MM, Meyer EW. Sedation and need-free salt intake in rats treated with clonidine. Pharmacology, Biochemistry, and Behavior. 1999;62:585–589. doi: 10.1016/s0091-3057(98)00215-9. [DOI] [PubMed] [Google Scholar]
- Edwards G, Chandler J, Hensman C, Peto J. Drinking in a London suburb II. Correlates of trouble with drinking among men. Quarterly Journal of Studies on Alcohol. 1972;6(Suppl. 6):94–119. [PubMed] [Google Scholar]
- Ehrenreich H, Schuck J, Stender N, Pilz J, Gefeller O, Schilling L, et al. Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcoholism: Clinical and Experimental Research. 1997;21:1285–1293. [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Fregly MJ, Kelleher DL, Greenleaf JE. Antidipsogenic effect of clonidine on angiotensin II-, hypertonic saline-, pilocarpine- and dehydration-induced water intakes. Brain Research Bulletin. 1981;7:661–664. doi: 10.1016/0361-9230(81)90114-3. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fischer SM, Rasmussen DD. Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcoholism: Clinical and Experimental Research. 2013a;37:1552–1560. doi: 10.1111/acer.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Rasmussen DD. Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcoholism: Clinical and Experimental Research. 2013b;37:1763–1770. doi: 10.1111/acer.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsbach R, Röser C, Beetz N, Brede M, Hadamek K, Haubold M, et al. Genetic dissection of alpha2-adrenoceptor functions in adrenergic versus noradrenergic cells. Molecular Pharmacology. 2009;75:1160–1170. doi: 10.1124/mol.109.054544. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Jackson WK, Walsh BT, Roose SP, Rosenfeld B. Cigarette craving, smoking withdrawal, and clonidine. Science. 1984;226:864–866. doi: 10.1126/science.6387913. [DOI] [PubMed] [Google Scholar]
- Gold MS, Pottash AC. The neurobiological implication of clonidine HCl. Annals of the New York Academy of Sciences. 1981;362:191–202. doi: 10.1111/j.1749-6632.1981.tb12809.x. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacology, Biochemistry, and Behavior. 2009;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfield D, Yap J, Grimm JW, Shalev U, Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Hui YH, Huang NH, Ebbert L, Bina H, Chiang A, Maples C, et al. Pharmacokinetic comparisons of tail-bleeding with cannula- or retro-orbital bleeding techniques in rats using six marketed drugs. Journal of Pharmacological and Toxicological Methods. 2007;56:256–264. doi: 10.1016/j.vascn.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kopin IJ. Avenues of investigation for the role of catecholamines in anxiety. Psychopathology. 1984;17(Suppl 1):83–97. doi: 10.1159/000284081. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Beitman BD. The relation between alcohol problems and the anxiety disorders. The American Journal of Psychiatry. 1990;147:685–695. doi: 10.1176/ajp.147.6.685. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Erickson DJ. Prospective analysis of the relation between DSM-III anxiety disorders and alcohol use disorders. The American Journal of Psychiatry. 1999;156:723–732. doi: 10.1176/ajp.156.5.723. [DOI] [PubMed] [Google Scholar]
- Lé AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Risch NJ, Weissman MM. Comorbidity and co-transmission of alcoholism, anxiety and depression. Psychological Medicine. 1994;24:69–80. doi: 10.1017/s0033291700026842. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stevens DE, Fenton B, Stolar M, O'Malley S, Woods SW, et al. Co-morbidity and familial aggregation of alcoholism and anxiety disorders. Psychological Medicine. 1998;28:773–788. doi: 10.1017/s0033291798006941. [DOI] [PubMed] [Google Scholar]
- Opitz K. The effect of clonidine and related substances on voluntary ethanol consumption in rats. Drug and Alcohol Dependence. 1990;25:43–48. doi: 10.1016/0376-8716(90)90139-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcoholism: Clinical and Experimental Research. 2009;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcoholism: Clinical and Experimental Research. 2001;25:999–1005. [PubMed] [Google Scholar]
- Rasmussen DD, Beckwith LE, Kincaid CL, Froehlich JC. Combining the α1-adrenergic receptor antagonist, prazosin, with the β-adrenergic receptor antagonist, propranolol, reduces alcohol drinking more effectively than either drug alone. Alcohol: Clinical and Experimental Research. 2014;38:1532–1539. doi: 10.1111/acer.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during "abstinence". Alcohol. 2006;38:173–177. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. The European Journal of Neuroscience. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shirao I, Tsuda A, Ida Y, Tsujimaru S, Satoh H, Oguchi M, et al. Effect of acute ethanol administration on noradrenaline metabolism in brain regions of stressed and nonstressed rats. Pharmacology, Biochemistry, and Behavior. 1988;30:769–773. doi: 10.1016/0091-3057(88)90097-4. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, et al. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcoholism: Clinical and Experimental Research. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Robinson J, O'Malley S. Stress response dampening: effects of gender and family history of alcoholism and anxiety disorders. Psychopharmacology. 1998;137:311–320. doi: 10.1007/s002130050624. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stöhr T, Shoaib M, Holsboer F, et al. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology. 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Starke K, Montel H, Gayk W, Merder R. Comparison of the effects of clonidine on pre- and postsynaptic adrenoceptors in the rabbit pulmonary artery. Alpha-sympathomimetic inhibition of neurogenic vasoconstriction. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1974;285:133–150. doi: 10.1007/BF00501149. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biological Psychiatry. 1999;46:1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Lotfipour S, Belluzzi JD, Leslie FM. Involvement of apha1-adrenergic receptors in tranylcypromine enhancement of nicotine self-administration in rat. Psychopharmacology. 2007;193:457–465. doi: 10.1007/s00213-007-0799-7. [DOI] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. European Neuropsychopharmacology. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfeld WW, Schulman MP. Metabolism and caloric value of alcohol. Journal of the American Medical Association. 1959;170:197–203. doi: 10.1001/jama.1959.63010020007016. [DOI] [PubMed] [Google Scholar]
- York JL. Increased responsiveness to ethanol with advancing age in rats. Pharmacology, Biochemistry, and Behavior. 1983;19:687–691. doi: 10.1016/0091-3057(83)90346-5. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biological Psychiatry. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Previous exposure to cocaine enhances cocaine self-administration in an alpha 1-adrenergic receptor dependent manner. Neuropsychopharmacology. 2007;32:638–645. doi: 10.1038/sj.npp.1301120. [DOI] [PubMed] [Google Scholar]