Abstract
Purpose
The objective of this study is to evaluate the feasibility of using coated microneedles to deliver vaccines into the oral cavity to induce systemic and mucosal immune responses.
Method
Microneedles were coated with sulforhodamine, ovalbumin and two HIV antigens. Coated microneedles were inserted into the inner lower lip and dorsal surface of the tongue of rabbits. Histology was used to confirm microneedle insertion, and systemic and mucosal immune responses were characterized by measuring antigen-specific immunoglobulin G (IgG) in serum and immunoglobulin A (IgA) in saliva, respectively.
Results
Histological evaluation of tissues shows that coated microneedles can penetrate the lip and tongue to deliver coatings. Using ovalbumin as a model antigen it was found that the lip and the tongue are equally immunogenic sites for vaccination. Importantly, both sites also induced a significant (p < 0.05) secretory IgA in saliva compared to pre-immune saliva. Microneedle-based oral cavity vaccination was also compared to the intramuscular route using two HIV antigens, a virus-like particle and a DNA vaccine. Microneedle-based delivery to the oral cavity and the intramuscular route exhibited similar (p > 0.05) yet significant (p < 0.05) levels of antigen-specific IgG in serum. However, only the microneedle-based oral cavity vaccination group stimulated a significantly higher (p < 0.05) antigen-specific IgA response in saliva, but not intramuscular injection.
Conclusion
In conclusion, this study provides a novel method using microneedles to induce systemic IgG and secretory IgA in saliva, and could offer a versatile technique for oral mucosal vaccination.
Keywords: lip vaccination, mucosal vaccination, oral cavity vaccination, oral HIV, tongue vaccination
1. INTRODUCTION
Mucosal surfaces are the first point of contact and a major portal of pathogen-entry into the human body (1, 2). Mucosal immunity in the form of secretory immunoglobulin A (sIgA) has the potential to neutralize pathogens on mucosal surfaces to prevent their colonization and replication (1). Multiple mucosal sites including the oral cavity mucosa, nasal mucosa, vaginal mucosa and gastrointestinal mucosa have been investigated in an attempt to stimulate mucosal immunity (3). Of these sites the oral cavity is especially attractive. The oral cavity mucosa is easily accessible, has relatively neutral pH and has fewer digestive enzymes than gastrointestinal mucosa making it relatively less harsh to biological molecules, and is also resilient towards potential sensitizers and irritants than for example the nasal mucosa (4). Oral cavity is also richly endowed with a large number of lymphoid tissues that could be harnessed to stimulate robust systemic and mucosal immune responses. For example, two palatine tonsils, two tubal tonsils, an adenoid and a lingual tonsil are anatomically arranged in the oral-nasopharyngeal cavity to form the Waldeyer’s ring, which can potentially mediate strong immunological responses towards antigens delivered to oral cavity tissues (5–7). In addition, dendritic cells and Langerhans cells, which are known to be potent antigen presenting cells have been identified to reside within the uppermost few-hundred micrometers of the oral cavity mucosa (8). Thus, oral mucosa is a potential site to induce secretory immunoglobulin A (sIgA) in the saliva. The potential clinical translation of inducing sIgA in saliva could be to prevent HIV transmission from mother to newborns during breastfeeding (9, 10) or to prevent dental caries (11).
Despite these potential advantages, vaccine delivery to the oral cavity is very challenging due to the stratified squamous epithelium that lines the oral cavity tissues. This stratified layer offers a tough barrier to inward diffusion of topically applied vaccine macromolecules such as, sub-unit protein-based vaccines, DNA vaccines, virus-like particles and inactivated viruses (12). Furthermore the continuous flow of saliva causes rapid removal of the topically applied vaccines (4, 12). To circumvent these delivery barriers, strong adjuvants such as cholera toxin (13, 14) have been used. While no neuronal retrograde transport was observed for cholera toxin when delivered through the sublingual route, its use in humans is questionable due to its acute toxicity. Mucoadhesive patches have also met with only moderate success for oral cavity vaccination (15).
We postulated that to overcome the delivery challenge for oral cavity vaccination, microneedles, which were originally developed for delivery of vaccines and other therapeutics in to the skin (16–22) could be exploited. Arrays of microneedles could potentially be used for targeted delivery of vaccines directly in to the oral cavity mucosa, completely bypassing the diffusion barrier offered by the stratified squamous epithelium. In one such approach, microneedles can be coated with a vaccine film that rapidly dissolves-off upon insertion into the oral tissue thus achieving bolus vaccine delivery. Previous research has shown that microneedles can be coated with a broad range of compounds ranging from small molecules to peptides to proteins to DNA to viruses to insoluble microparticles, thus offering a large flexibility to this vaccine delivery approach (17, 18). Using coated-microneedles vaccines against influenza (21, 23), anthrax (24) and japanese encephalitis (25) have been delivered in to the skin. Due to their micrometer dimensions, coated microneedles also have the potential to allow targeting of the vaccine antigens to dendritic cells and Langerhans cell that reside in the topmost few hundred micrometers of the oral cavity mucosa (8, 26). Furthermore, due to their small size microneedles can be minimally invasive and painless (27).
In this study we provide the first report describing the use of microneedles for vaccine delivery to the oral cavity. Unlike the skin, the oral cavity tissues are soft, flexible and largely unsupported by hard bony surfaces. Furthermore the application area is quite limited, and because of the wet environment in the mouth there exists a risk that the coatings from microneedles can prematurely dissolve into the saliva. Accordingly, we investigated the feasibility of inserting coated microneedles into the inner lip and dorsal surface of the tongue in a rabbit animal model, and quantified the delivery efficiency of microneedle coatings in vivo. Lastly, we evaluated the ability of coated microneedles to induce immune response by delivering three antigen types: a soluble protein subunit vaccine (ovalbumin); and two HIV antigens - a virus-like particle, and a DNA vaccine. Ovalbumin as a model soluble antigen was delivered to the inner lower lip and the dorsal (i.e. superior) surface of the tongue to compare the systemic and mucosal antibody response between these two delivery sites. Next, immune response against two HIV antigens was evaluated, by delivering them to the oral cavity using microneedles, and comparing the response to their intramuscular delivery using a hypodermic needle.
2. MATERIALS AND METHODS
2.1 Microneedle fabrication and coating
Planar 1D arrays with five microneedles in a row were fabricated from 50 µm-thick stainless steel (304) sheets using a wet etch process. Each microneedle measured 700 µm in length and 200 µm in width. 2D microneedle arrays with 50 microneedles were similarly fabricated by etching microneedles in 50 µm-thick stainless steel (304) sheets. The individual microneedles were then manually bent to make them perpendicular to the metal sheet.
Microneedles were coated using a micro-precision dip coating station developed in-house. It comprised of an automated x-y linear computer-controlled stage on to which microneedles were mounted. The coating solution was housed in an orifice in to which the microneedles were dipped through motion control of the x-y stage. The coating solution was composed of 1% (w/v) carboxymethylcellulose sodium salt (low viscosity, USP grade, CarboMer, San Diego, CA, USA), 0.5% (w/v) Lutrol F-68 NF (BASF, Mt. Olive, NJ, USA), and either 2.5 % sulforhodamine (Molecular Probes, Eugene, OR, USA), or 2.5 % ovalbumin (MP Biomedical, Solon, OH, USA) or 0.41 % E2V3 or 0.45 % DNA expressing gp160.
2.2 In vivo assessment of coated microneedle penetration in oral tissues
2.2.1 Microneedle insertions in to the rabbit oral cavity
Use of animals was approved by the Texas Tech University - Animal Care and Use Committee (IACUC). New Zealand White Rabbits (9 weeks old) were anesthetized using ketamine and xylazine injected intramuscularly. 1D microneedle arrays coated with sulforhodamine were inserted into the rabbit dorsal tongue or rabbit inner lower lip for 2 min. A Kelly locking forcep was used for gripping microneedle arrays to help in insertion. To make insertions into the lower lip, the lip was stretched to accommodate the microneedle array. No stretching was required for inserting microneedles in to the tongue.
2.2.2 Imaging and histology
Brightfield and fluorescence micrographs of sulforhodamine-coated microneedles were collected using an Olympus SZX16 stereo microscope with a CCD camera (Leica DC 300, Leica Microsystems, Bannockburn, IL, USA).
Histological examination of rabbit tongue and lip was conducted through frozen sections. After inserting microneedles, rabbits were euthanized, lips and tongue were resected and frozen in OCT compound (Tissue-Tek, 4583, Sakura Finetek, Torrance, CA, USA). The frozen OCT blocks were cut into 10 µm-thick sections using a cryostat (CM1950, Leica, Buffalo Grove, IL,USA). Fluorescence and brightfield micrographs of histological sections of lip and tongue were collected using a Nikon Ti eclipse fluorescent microscope with a CCD camera (Andor DR-328G-C10-SIL, Andor Technology, South Windsor, CT,USA).
2.2.3 Efficiency of delivery
The amount of sulforhodamine coated on unused microneedles (n=5) was obtained by placing freshly-coated microneedles in 500 µl of deionized water (DIW) to dissolve the coatings and then measuring the resulting concentration (C1 in µg/µl). Another set of sulforhodamine-coated microneedles was inserted in to rabbit lip or tongue for 2 min and removed. A total of three 1D arrays were inserted in to the lip or tongue per rabbit (n=3 rabbits). After insertions, a cotton swab pre-soaked in water (by dipping in a tube containing 300 µl water) was gently rubbed on the lip or tongue surface to collect sulforhodamine left on the tissue after microneedle insertions. The swab was placed back in to the 300 µl water tube and sulforhodamine concentration was measured (C2 in µg/µl). The amount of sulforhodamine left attached to the microneedle surface after insertions was similarly obtained by placing used microneedles in 500 µl DIW to dissolve the residual material (C3 in µg/µl). The concentration of sulforhodamine was measured using a fluorescent spectrophotometer (Cary Eclipse, Agilent Technologies, Santa Clara, CA, USA), at excitation and emission of 565 nm and 586 nm, respectively. The final delivery efficiency was calculated by obtaining the ratio of mass of sulforhodamine delivered to that available on unused microneedles: [(C1*500)−(C2*300)−(C3*500)] / (C1*500)
2.3 Evaluation of immune response
2.3.1. Vaccination with ovalbumin
Two groups of New Zealand white rabbits (n=3 per group) were immunized with 125 µg ovalbumin via the lip or the tongue. A total of five microneedle arrays each coated with 25 µg ovalbumin were inserted into the lip or tongue of anesthetized rabbits and held for 2 min. Each group was vaccinated twice, i.e. at week 0 and week 4.
2.3.2. Vaccination with HIV antigens
Two HIV antigens were tested: a virus like particle expressing third hypervariable region (V3) of HIV-1 envelope (Env) as an N-terminal fusion protein on the E2 subunit of the pyruvate dehydrogenase complex of Geobacillus stearothermophilus (E2V3) (28), and a DNA vaccine expressing gp160 (28). Three New Zealand white rabbits were immunized with 95.4 µg of gp160-expressing DNA and 51.6 µg of E2V3 vaccine by using microneedles. The DNA and E2V3 antigens were synthesized as described previously (28). In microneedle group, microneedles coated with E2V3 and gp160-expressing DNA were equally distributed for delivery to the tongue and lip. Three out of six microneedle arrays each coated with 15.9 µg gp160-expressing DNA were inserted into the lip of anesthetized rabbits and the other three were inserted into the tongue. Similarly, a total of six microneedle arrays each coated with 8.6 µg E2V3 were equally distributed into the lip and tongue for delivery via microneedles. The control groups were immunized with the same doses of vaccines by intramuscular injection. Each group was vaccinated twice, i.e. at week 0 and week 4.
2.3.3. Serum and mucosal secretion collection
Blood, saliva secretions were collected from unimmunized rabbits at day 0 and every two weeks thereafter for 8 weeks in both ovalbumin and HIV vaccination studies. All samples were collected while rabbits were under anesthesia. 5–7 ml blood was collected from rabbit ear vein by using a butterfly needle and syringe set. Blood was incubated at 4 °C for 3 h, centrifuged at 2000 g for 10 min and serum was collected and stored at −20 °C until further analysis. Carbamoylcholine chloride (15 µg) (Tocris Bioscience, Minneapolis, MN, USA) was injected intramuscularly to stimulate secretion of saliva and 7–10 ml saliva was collected as the saliva dripped from rabbit's mouth. Saliva was centrifuged at 5000 g for 20 min and supernatant was collected and stored at −20 °C until further analysis.
2.3.4. Enzyme-linked immunosorbent assay (ELISA)
ELISAs were performed to determine anti-ovalbumin IgG antibodies in serum, and anti-ovalbumin IgA antibodies in saliva. Ovalbumin was coated on Nunc MaxiSorp® flat-bottom 96 well plate (Thermo Scientific, Rochester, NY, USA) by adding 50 µl of 5 µg/ml ovalbumin into each well. After incubating the plate at 4°C overnight, the plate was washed 3 times with phosphate buffered saline (PBS) containing 0.5% tween 20 (PBST) using a plate washer (Elx405 BioTek, Winooski, VT, USA). Next, blotting-grade non-fat dry milk (Bio-Rad laboratories, Hercules, CA, USA) dissolved in PBS at 5% (w/v) was used as a blocker (100 µl per well). After incubating the plate for 2 h at room temperature, the plate was washed thrice in PBST. Diluted serum (1:80) or saliva (1:4) were added into wells (50 µl per well). After incubating another 2 h the plate was washed thrice. HRP-conjugated goat-anti-rabbit IgG (Southern Biotech, Birmingham, Alabama, USA) or HRP-conjugated goat-anti-rabbit IgA (Thermo Scientific, Rochester, NY, USA ) was added into the plate to detect IgG and IgA antibodies, respectively in serum and saliva. After 2 h incubation, the plate was washed with PBST and o-phenylenediamine (OPD tablets, Invitrogen, Camarillo, CA, USA) solution was added into the plate. After 6 min reaction time for serum samples or 12 min reaction time for saliva samples, the reaction was stopped with 4N H2SO4 and the plate was read using a microplate reader (Spectramax plus 384, Molecular Devices, Sunnyvale, CA, USA) at 490 nm wavelength.
A similar ELISA analysis was conducted to measure gp140- and V3-specific antibodies resulting from gp160-DNA-based or E2V3 vaccination, except that the plates were coated using a 1 µg/ml solution of HIV-1 Clade B recombinant gp140 (Immune Technology, New York, NY) or 5 µg/ml V3, and a dilution ratio of 1:20 was used for serum samples while a dilution ratio 1:4 was used for saliva samples.
2.3.5. Comparison of invasiveness between microneedle and hypodermic needle insertion in the oral cavity
To obtain a qualitative understanding of the difference in invasiveness from insertion of a microneedle vs a hypodermic needle in the rabbit oral cavity, we compared the bleeding-response between microneedle insertion and insertion of a hypodermic needle (27 gauge) in the lip and tongue of rabbits. The hypodermic needle was inserted approximately 3–5 mm deep to simulate a local hypodermic injection. Insertion of 2D microneedle arrays in the tongue was also evaluated since 2D arrays can enable delivery of a larger dose of the vaccine. 2D arrays were not inserted in the lip due to the smaller area available at the lip surface.
2.3.6. Statistical analysis
Statistical analysis was conducted using SAS 9.3 package. Orthogonal contrast method was incorporated in one-way ANOVA to compare different time points with time zero for each vaccination group. For statistical significance the p-value was set at less than 0.05.
3. RESULTS
3.1 Microneedle arrays can penetrate oral cavity tissues
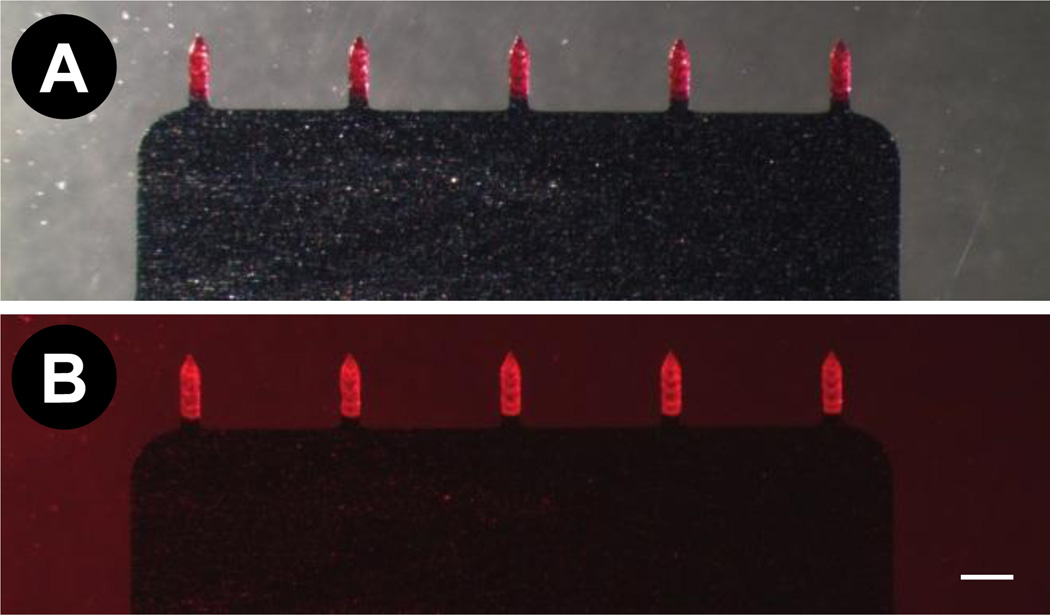
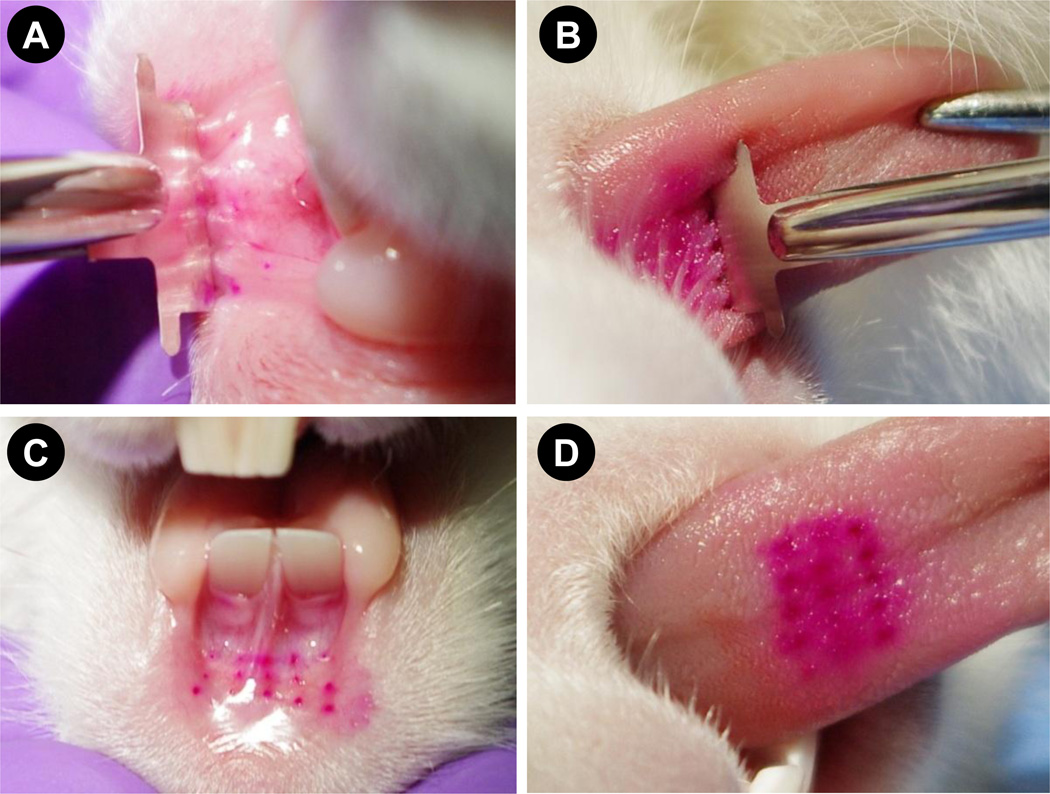
Our first objective was to evaluate the feasibility of coated microneedles to penetrate the oral cavity tissues and deliver the coated material. To assess this, 1D microneedle arrays were coated with sulforhodamine-dye (Fig 1A and B). Insertion of 1D arrays into lips required stretching of the lower lip to accommodate the array (Fig 2A). By stretching the lower lip and supporting it on the fingers it was possible to insert 1D microneedle arrays. The insertion of 1D microneedles into the tongue was relatively easier due to the larger area available and better accessibility than the lips. Unlike the lips, the tongue did not require stretching to insert the microneedle arrays (Fig 2B). Figures 2C and 2D show dots of sulforhodamine localized inside the tissues. These dots correspond to microneedle insertions and demonstrate that sulforhodamine coated onto microneedles was delivered in to the lip and the tongue.
Fig. 1. Sulforhodamine-coated 1D microneedle arrays.
Array visualized using (A) brightfield microscopy, and (B) fluorescence microscopy. Scale bar indicates 500 µm.
Fig. 2. Insertion of sulforhodamine-coated 1D microneedle arrays in rabbit oral cavity tissues.
1D array held in a Kelly locking forcep inserted into (A) stretched lower lip and (B) tongue. Regular array of dots formed by insertion of coated microneedles into rabbit (C) lip and (D) tongue.
3.2 Tissue histology to confirm intra-tissue delivery of coated material
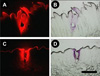
To confirm the delivery of microneedle coatings into the tissues, we next isolated rabbit tongue and lip tissues post-euthanasia and characterized them using histology. Using fluorescence and brightfield microscopy, sulforhodamine dye was detected in tongue tissue as indicated in Fig 3A and Fig 3B. Similarly, sulforhodamine from coated microneedles was also detected in lip histological sections (Fig 3C and 3D). Histological evaluation thus confirmed delivery of microneedle coatings into the lip and tongue tissue of rabbits.
Fig. 3. Histological cryosections of rabbit lip and tongue biopsies after insertion of coated microneedles.
Tongue cryosection visualized using (A) fluorescence microscopy and (B) brightfield microscopy. Lip cryosection visualized using (C) fluorescence microscopy and (D) brightfield microscopy. Scale bar indicates 400 µm.
3.3 Delivery efficiency of microneedle coatings
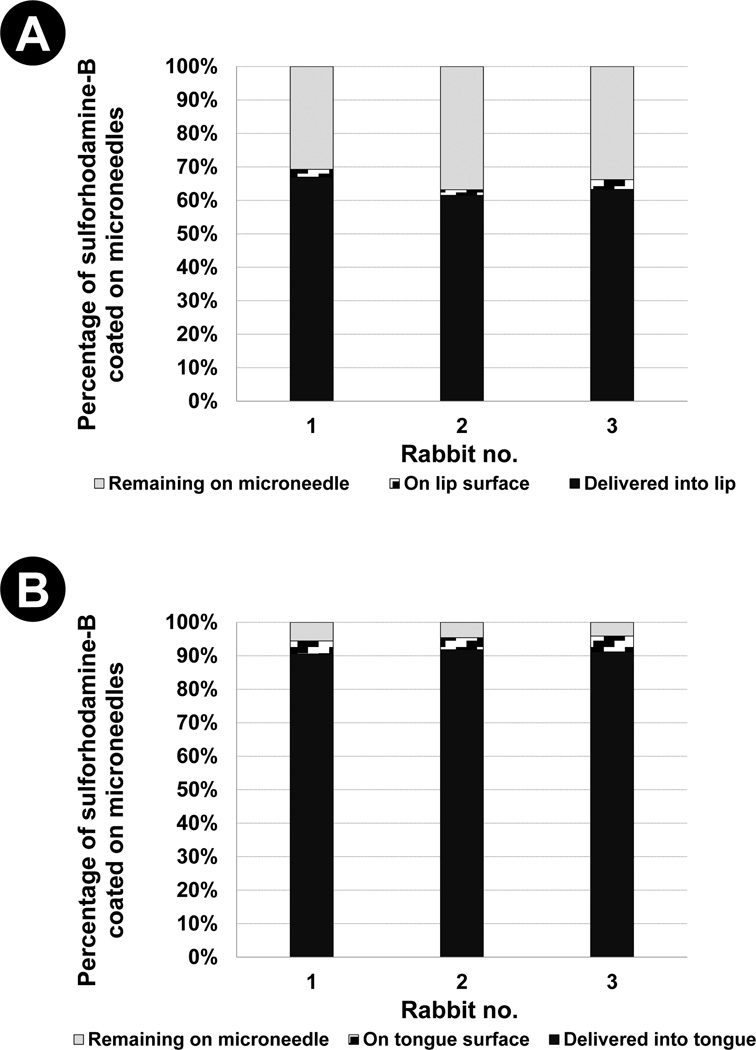
We next quantified the fraction of microneedle coating delivered into the tissues in vivo to assess whether the moist environment of the oral cavity can reduce delivery efficiency by pre-wetting coatings and causing them to be lost on moist tissue surfaces. As shown in Fig 4 the delivery efficiency of coatings was found to be 63.9% ± 6.9% and 91.2% ± 1.6% for the lip and tongue, respectively, after inserting microneedles for 2 min into the respective tissues. It was further observed that a larger fraction of coatings remained attached to the microneedles after insertion into lip (33.8% ± 7.8%) than in the tongue (4.7% ± 1.9%), although the amount of coating lost on the tissue surface was approximately the same (lip: 2.3% ± 1.5%, tongue: 4.1% ± 1.5% ). The delivery efficiency of coated microneedles in the skin has been reported to be 70% (29) and 90% (18). Thus, comparing the delivery efficiency of coatings between the skin and the oral cavity, a similar level of delivery efficiency was achieved. Furthermore, only about 2% – 4% of coated drug was lost on oral cavity tissue surfaces. This suggests that the moist environment of the oral cavity does not hinder the ability to use coated microneedles for therapeutic delivery to the oral cavity.
Fig. 4. Delivery efficiency of coated microneedles.
Percentage of sulforhodamine coated on microneedles that is found in residual coatings, on tissue surface and in tissues, after microneedle insertion into (A) rabbit lip and (B) rabbit tongue.
3.4 Systemic and mucosal immune response induced by delivery of ovalbumin into the lip and the tongue using coated microneedles
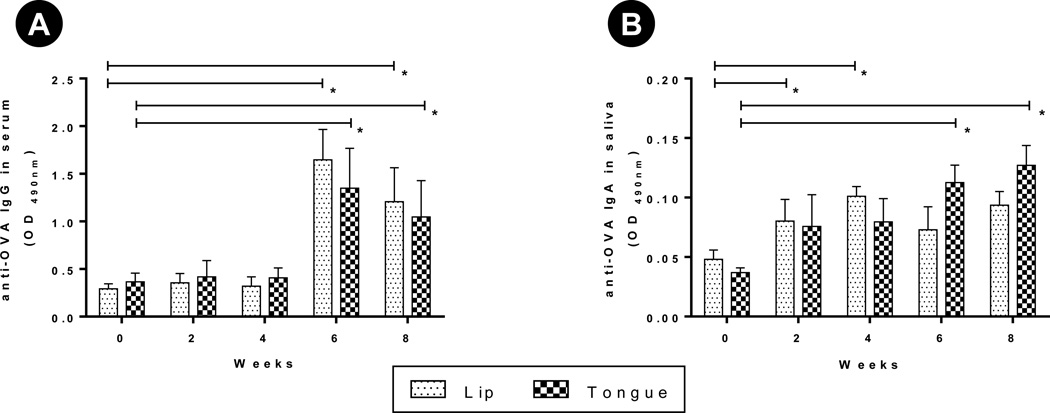
We next asked the question whether delivery of antigens to the oral cavity tissues using coated microneedles can induce systemic and mucosal immune responses, and whether the immune response is different depending on delivery site, i.e., the lip or the tongue. To address these questions we coated microneedles with ovalbumin as a model antigen and inserted coated microneedles into the lip or tongue of rabbits. To assess systemic immune responses, we measured ovalbumin-specific IgG in the serum. A low increase in serum IgG response was seen at weeks 2 and 4 (Fig 5A). This increase is not readily apparent because of the high serum dilution (1:80) used in the ELISA. A lower serum dilution (1:20) ELISA at week 0, 2 and 4 better captures this marginal increase in IgG (see supplementary Fig S1). Following a booster dose at week 4 with ovalbumin-coated microneedles, a significant increase in serum anti-ovalbumin IgG was seen (p < 0.05, Fig 5A). No significant difference was observed in IgG levels for microneedles inserted into the lip vs the tongue (p > 0.05). A direct comparison of serum titration of pooled samples for week 8 further demonstrates that there is no significant difference in ovalbumin-specific IgG generated via vaccination through lip or the tongue (see supplementary Fig S2) suggesting that both sites are equally immunogenic.
Fig. 5. Antibody response after vaccination with ovalbumin-coated microneedles.
Rabbits (n=3 per group) were immunized two times (0 and 4 weeks) by inserting ovalbumin (OVA)-coated microneedles in to their lip or tongue. Serum, saliva were collected on weeks 0, 2, 4, 6 and 8. Serum (1:80), saliva (1:4) were diluted to measure OVA-specific IgG and IgA using ELISA. ELISA result is reported as optical density (OD) measured at 490 nm. (A) OVA-specific IgG in serum, (B) OVA-specific IgA in saliva. Error bar: SE, * : p < 0.05.
To evaluate mucosal response we measured ovalbumin-specific IgA in the saliva. The mean optical density (OD) for ova-specific IgA in saliva increased for both groups and resulted in a significantly higher (p < 0.05) IgA response compared to pre-immune saliva (Fig 5B). Again no significant difference was observed between the lip and tongue as vaccination sites.
3.5 Stimulation of systemic and mucosal immune response to DNA and virus like particle HIV antigens delivered to the oral cavity using coated microneedles
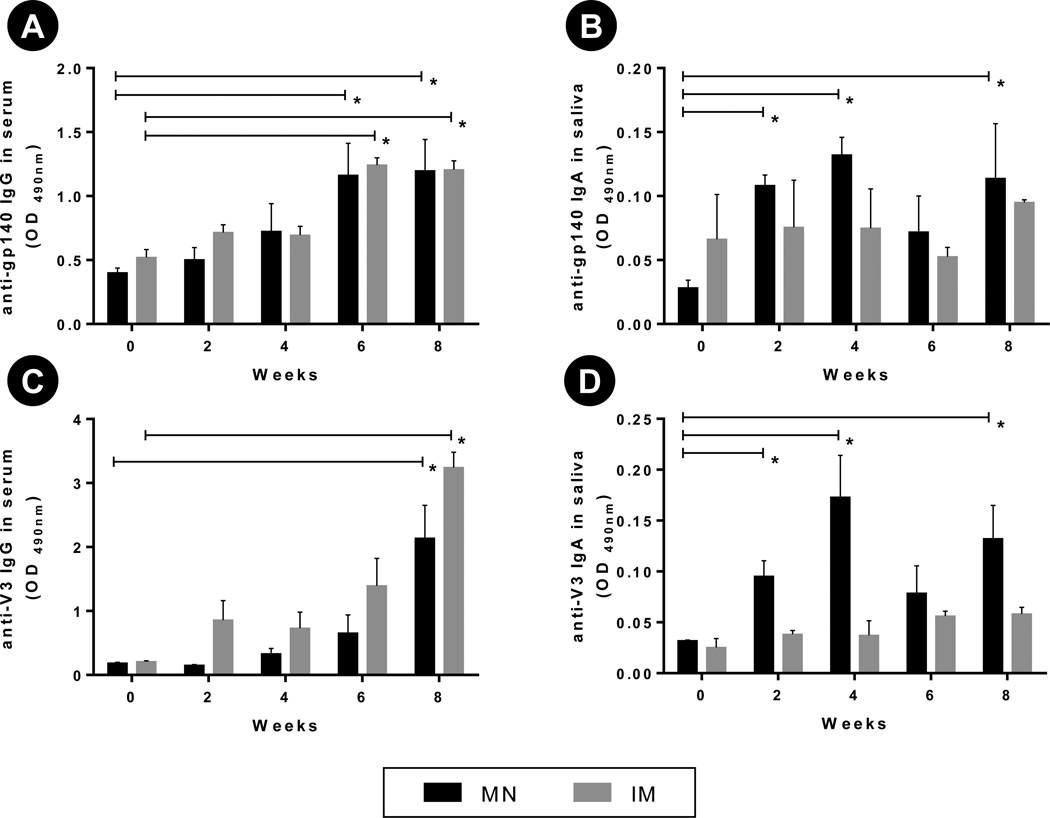
After verification of an immune response using ovalbumin and observing no difference between the lip and the tongue as vaccination sites, we next sought to determine whether a similar mucosal and systemic response could be induced by other antigens upon delivery to the oral cavity. We thus immunized rabbits with two already tested HIV antigens (28), E2V3 - a virus like particle expressing the V3 loop of HIV-1, and a DNA expressing gp160 protein. We focused on the use of HIV antigens for this assessment because a successful stimulation of anti-HIV IgA in saliva that could bind to and neutralize HIV, could in future be of significant benefit in reducing transmission of HIV from infected mothers to newborns during breastfeeding (10). Our previous study (28) has shown that concurrent delivery of these two antigens results in higher immune response than when either antigen is administered alone. Thus, the two antigens were concurrently delivered using coated microneedles, and were equally distributed amongst the lip and the tongue. For comparison, the two antigens were also delivered concurrently via the intramuscular route. After two vaccine doses, both the microneedle-based oral delivery group and the intramuscular delivery group demonstrated a significantly (p < 0.05) enhanced gp140- and V3-specific IgG in serum compared to pre-immunization antibody levels (Fig 6A and Fig 6C). However, no significant differences (p > 0.05) were observed between the oral cavity route and the intramuscular route of immunization in their ability to induce serum IgG specific to V3 and gp160 antigens. This demonstrates the ability of coated microneedle-based oral cavity vaccination to induce similar systemic immunity as when antigens are delivered intramuscularly using a hypodermic needle.
Fig. 6. Antibody response after vaccination with gp160 DNA and E2V3 antigens.
Group of rabbits (n=3 per group) were immunized two times (0, 4 weeks) by inserting DNA-coated microneedles and E2V3-coated microneedles into their lip or tongue. The control groups were intramuscularly injected by the same antigen respectively. Serum (1:20) and saliva (1:4) were diluted to measure gp140- and V3-specific IgG and IgA using ELISA. ELISA result is reported as optical density (OD) measured at 490 nm. (A) gp140-specific IgG in serum, (B) gp140-specific IgA in saliva, (C) V3-specific IgG in serum, and (D) V3-specific IgA in saliva. Error bar: SE, * : p < 0.05.
In contrast, microneedle-based delivery to the oral cavity was superior to the intramuscular route in inducing mucosal immunity as measured via antigen-specific IgA antibodies in saliva. While microneedle-based delivery of gp160 and E2V3 led to a significantly higher (p < 0.05) gp140 and V3 specific IgA (Fig 6B and Fig 6D) in saliva compared to pre-immunization levels, the intramuscular route of vaccination produced only a weak stimulation (p > 0.5) of salivary IgA for both gp160 and V3 antigens (Fig 6B and Fig 6D).
3.6 Comparison of bleeding-response between microneedle and hypodermic needle insertion in the oral cavity
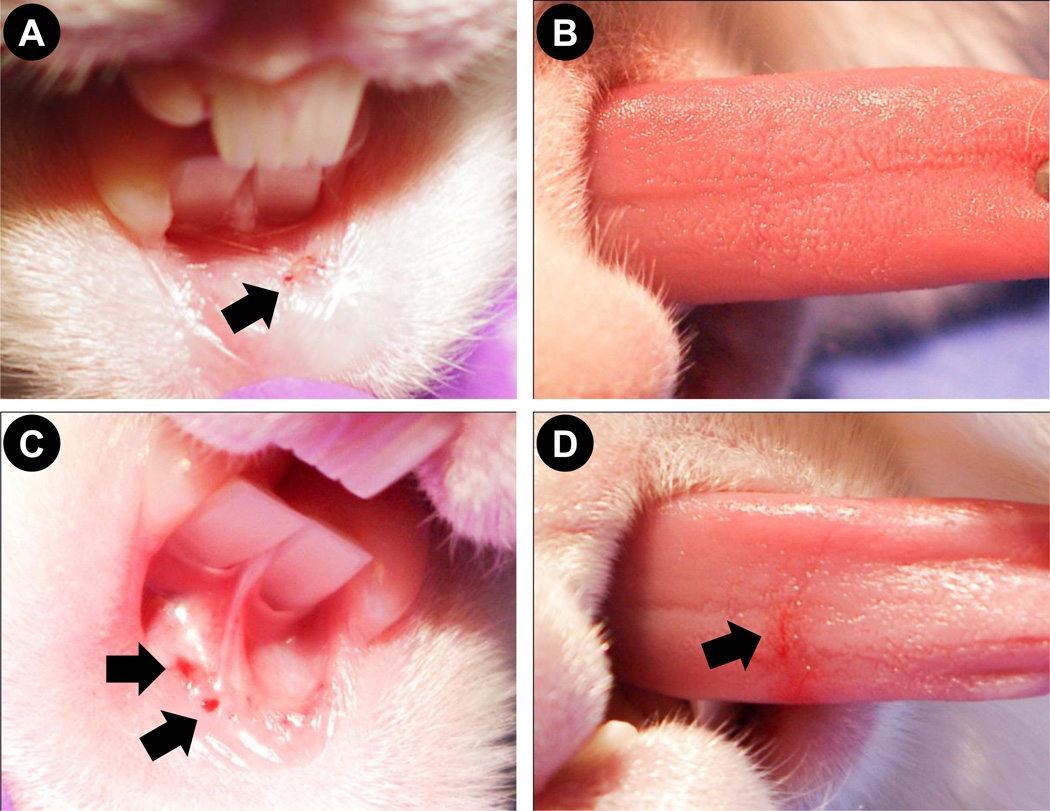
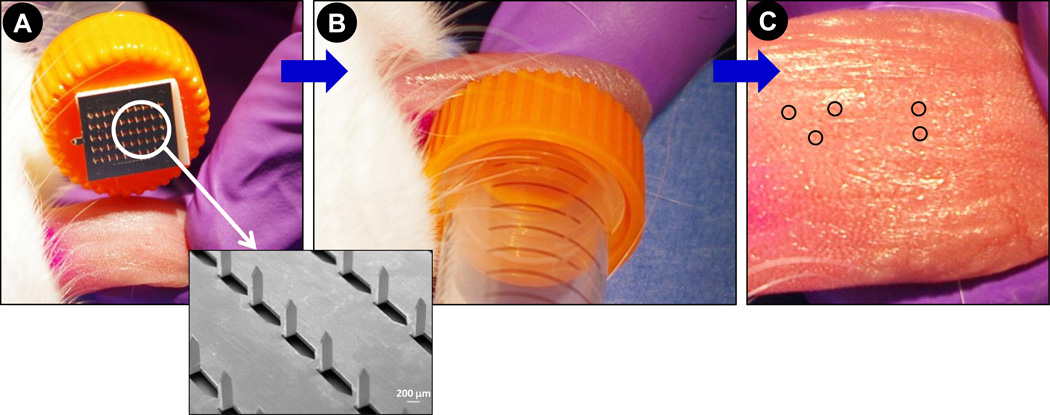
Based on hundreds of 1D coated microneedle insertions performed in the rabbit oral cavity (from this study and other ongoing studies in rabbits in our laboratory) we have not observed a single case of rabbit infection or oral cavity inflammation. On occasion, blood-spotting (approximately 1 µl) can be seen at the place of microneedle insertion. Qualitatively, the frequency of blood-spotting from microneedle insertion in the lip was observed to be higher in lips than the tongue. As seen in Fig 7A, occasional blood-spotting is observed when a microneedle array is inserted into the lip and little to no spotting is observed from microneedle arrays insertion in to the tongue (Fig 7B). In contrast, insertion of a hypodermic needle in the lip (Fig 7C) and the tongue (Fig 7D) results in bleeding suggesting that microneedles are less invasive compared to hypodermic needles. 1D microneedle arrays have a sharp edge, which has the potential to cut the soft tissue of the tongue and lips if excessive force is applied during insertion. We thus evaluated the ability of 2D arrays (inset, Fig 8A) to be inserted in to rabbit tongue and any potential damage to the tissue. We found that 2D microneedle arrays are even less invasive compared to 1D arrays due to the flat base of 2D array, which eliminates secondary damage from applying higher pressure. During our insertion of 2D arrays with 50 microneedles into the rabbit tongue (Fig 8A and 8B), we have never observed blood-spotting (Fig 8C). This study is representative of at least five independent insertions using 2D arrays. The points of microneedle insertion are barely visible and some are encircled in Fig 8C. Due to smaller area of application in the rabbit lip we have been unable to test the 2D arrays in the rabbit lips.
Fig. 7. Effect of microneedle and hypodermic needle insertion in to rabbit oral tissues.
Photographs of tissue surface after (A) microneedle insertion into rabbit lip, (B) microneedle insertion into rabbit tongue, (C) hypodermic needle insertion into rabbit lip, and (D) hypodermic needle insertion into rabbit tongue. Arrows point to blood-spotting.
Fig. 8. Insertion of 2D microneedle array in to rabbit tongue.
Photographs showing (A) a microneedle array containing 50-uncoated microneedles attached to the cap of a 15 ml conical tube, (B) application of 2D array to dorsal surface of rabbit tongue, and (C) dorsal surface of tongue after application of microneedle array. No blood spotting is observed. Inset is a scanning electron micrograph of a portion of the 50-microneedle array. Circles in (C) encircle few of the more visually perceptible microneedle insertion points.
4. DISCUSSION
To the best of our knowledge, this is the first study to apply microneedles for drug or vaccine delivery to the oral cavity. The oral cavity provides multiple constraints to the use microneedles: (i) the oral cavity tissues are soft, thus use of high forces for insertion, such as impacting devices (30) could potentially cause tissue damage and pain, (ii) they have a wet surface, which could prematurely dissolve coatings from microneedles on the tissue surface during insertion, and (iii) unlike the skin, most oral cavity tissues including cheeks, lips and tongue are not supported by hard bony surfaces, making insertion of very large arrays (approximately greater than 2.5 cm × 2.5 cm) difficult. Furthermore, the surface area available for insertions is much smaller compared to the skin. For example, in a stretched state the lower rabbit lip measures approximately 0.8 cm×0.8 cm, while the rabbit tongue measures about 3 cm in length and 1 cm in width. Despite these unique constraints, we have shown that coated microneedle arrays can be reliably inserted into rabbit oral cavity to achieve delivery of the coated substance, and that the moist environment of the oral cavity does not reduce the efficiency of delivery from coated microneedles. We also show that microneedle-based delivery of antigens to the oral cavity can induce both systemic and mucosal immune responses, as measured via antigen-specific antibodies in serum and saliva, respectively.
4.1 Comparison of the lip and the tongue for vaccination
Not many studies have investigated the oral cavity for vaccination. Nonetheless, the buccal surface (15) and the sublingual route (13, 14, 31) has received some attention. However, it is not known what anatomical part of the oral cavity is most suited for vaccine delivery for induction of a strong immune response. Since microneedles can be inserted in practically all accessible parts of the oral cavity, we first compared the tongue and the inner lip of the rabbit with regards to their ability to induce an immune response. Due to the limited opening of the rabbit mouth it was impractical to reliably insert microneedles in their buccal tissue, thus, we were unable to assess it for vaccination. However, in humans where the mouth can open wider, giving full access to the cheeks, we certainly envision that microneedle patches can be applied to buccal oral mucosa in humans.
We first determined the delivery efficiency of ovalbumin as the model antigen in the lip and the tongue. We observed that while the delivery efficiency was high and comparable to that seen in the skin, still the efficiency was higher for the tongue. To understand why a relatively higher amount of coating was left behind on microneedles inserted into the lips we microscopically examined microneedles post-insertion. We observed that the coatings were left partially undissolved on microneedles situated along the outer edges of the 1D arrays. We believe that the reduced efficiency can be attributed to the stretching required to increase surface area of the rabbit lip to make it topologically flat. Despite manual stretching the area available for insertions is still small to reliably insert the microneedles farthest along the outer edges of the 1D array. In addition, as discussed in section 3.6, blood-spotting does occasionally occur from microneedle insertions in to the lip. Presence of microliter amounts of blood can potentially wash away some of the coatings. Indeed, we have observed coatings re-deposited on the microneedle base farther away from the microneedle shafts after insertion. These coatings appear red and were concluded to contain dried blood with ovalbumin. This phenomenon was not observed in delivery to the tongue. Since the inner lip area in humans is larger compared to rabbits, the observed reduction in delivery efficiency to lips may cease to be of concern when translated to humans.
Delivery of ovalbumin to the rabbit lip or the tongue successfully induced anti-ovalbumin IgG in serum and anti-ovalbumin IgA in saliva, demonstrating the potential of microneedle-based vaccination approach to induce both systemic and mucosal arms of the adaptive immunity. Comparing the immune response between groups of rabbits immunized via the lips or the tongue no statistically significant difference (p > 0.05) was observed in ovalbumin-specific IgG and IgA levels, suggesting that both locations are equally immunogenic. Overall, these immunization results demonstrate the ability to induce an immune response against a soluble protein antigen via delivery through the oral cavity using coated microneedles.
4.2 Immune response against HIV DNA vaccine and a virus like particle antigen
Upon successful demonstration that delivery of ovalbumin, a model protein subunit antigen to the oral cavity using microneedles can stimulate systemic and mucosal immunity, we next asked whether the approach is applicable to other antigen types. Since microneedles can be readily coated with DNA, viral particles or micro- and nano-particles (17), the coated microneedle-based approach is very flexible and can potentially be used to deliver different vaccine formulations to the oral cavity tissues. Accordingly we coated microneedles with two well characterized HIV antigens, a DNA expressing gp160, and E2V3 a virus like particle displaying the V3 region of HIV (28). HIV-specific antigen vaccination by microneedles was carried out and compared with the intramuscular route. DNA expressing gp160 and E2V3 virus-like particles have proven capable of inducing systemic IgG immune response and neutralizing antibodies when delivered concurrently via gene gun to the skin and intramuscular injection, respectively (28). However, it is not clear if they can elicit IgA antibodies when administered via the mucosal route. In this study, we postulated that microneedles could assist in oral mucosal vaccination and stimulate both systemic IgG and mucosal IgA production. Not surprisingly, our results show that mucosal vaccination by coated microneedles does indeed generate more antigen-specific IgA in saliva than the intramuscular route of vaccination. Furthermore, analysis of the IgG immune response in serum showed that microneedles could induce a systemic immune response similar to the intramuscular route.
Previous studies investigating the potential of oral cavity for vaccination have typically relied on topical delivery via the sublingual mucosa and have used cholera toxin as a potent but toxic adjuvant to enhance the immune response (13, 31, 32). In contrast, coated microneedles demonstrate the potential to bypass the topical delivery barrier without use of a toxic adjuvant by directly delivering antigens in to the oral cavity mucosa to induce immune responses. While our study provides the basis for using microneedles for vaccine delivery through the oral cavity, additional studies are required to investigate how addition of a safer adjuvant into microneedle coatings can enhance the immune response in comparison to the current use of cholera toxin.
The ability to effectively stimulate antigen-specific IgA in saliva could be helpful in preventing multiple diseases such as dental carries (11), or for stopping transmission of HIV from infected mother to child via breast feeding (9, 10).
4.3 Practical and safety considerations in use of microneedles for oral cavity vaccination
The topology of the human oral cavity is not flat and the available surface area is limited. It is thus important to assess whether 2D microneedle arrays can fit onto human oral tissues to achieve delivery of a therapeutic vaccine dose. Typically a dose of 50–200 µg of vaccine antigen can be coated on a 50 microneedle patch measuring 10 mm×10 mm (17). This patch size should be sufficient to perform immunizations. As shown in Fig 8A this array can be attached to the cap of a standard 15 ml conical tube, which could be replaced by a similarly designed microneedle-applicator, to help reach oral tissues including the buccal tissue. However, further experiments in humans are necessary to validate microneedle insertions in the oral cavity. Regarding discomfort from microneedle application, a previous study (27) has tested similarly-sized microneedles and has demonstrated that microneedles cause minimal to no pain when inserted into the human skin. However, to assess the degree of discomfort if any, which is experienced from insertion of microneedles in the human oral cavity, additional studies are needed.
5. CONCLUSION
This study provides the first demonstration of the use of microneedles for delivery to the oral cavity with specific focus on mucosal vaccination. The coated microneedle approach was evaluated in rabbits to assess the ability of microneedles to insert and deliver coatings into the rabbit lip and tongue, and to produce a mucosal immune response. Stainless steel microneedles were fabricated, coated with sulforhodamine-dye as a model drug, and inserted into rabbit lip and tongue. By imaging the surface of tissues after microneedle insertion and through histological evaluation, microneedle insertion and delivery of coatings into the lip and tongue was validated. Delivery efficiency of coatings was found to be 63.9% and 91.2% for the lips and tongue, respectively. Systemic and mucosal immune responses were measured by delivering three different antigen types: ovalbumin as a model soluble protein antigen; E2V3, an HIV virus-like particle vaccine; and a gp160 HIV DNA vaccine. Antigen-specific IgG antibodies against all three antigens (p < 0.05) were generated in blood demonstrating the potential of systemic immunization using the oral cavity. No statistical difference was observed between the immune response generated using either the lip or the tongue as the site for vaccination using ovalbumin-coated microneedles. Furthermore, no significant difference in serum IgG was observed for E2V3 virus-like particles and gp160-DNA vaccine. sIgA specific to all three antigens was significantly (p<0.05) increased in saliva when the three antigens were delivered to the oral cavity using microneedles, than when delivered intramuscularly using a hypodermic needle. Thus, while the coated microneedle approach of delivering vaccines to the oral cavity could activate both systemic and mucosal immunity, the intramuscular method could only activate systemic immunity. Insertion of hypodermic needles in the rabbit oral cavity induced blood spotting while microneedles exhibited reduced to no bleeding, especially in the rabbit tongue. Altogether, this study demonstrates that coated microneedles can be used for vaccine delivery to the oral cavity to induce both systemic and mucosal immune responses.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institute of Health (1R03DE021667-01A1).
Footnotes
FINANCIAL DISCLOSURE
HSG is a co-inventor of a microneedle coating technology, which has been licensed to a US company. The patent application is still pending in the US patent office. No collaboration or other financial contracts exist between HSG and the licensee. The official technology transfer and license is managed by Georgia Tech Research Corporation.
REFERENCES
- 1.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 2.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 3.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 4.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery--a promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Lu FX, Jacobson RS. Oral mucosal immunity and HIV/SIV infection. J Dent Res. 2007;86:216–226. doi: 10.1177/154405910708600305. [DOI] [PubMed] [Google Scholar]
- 6.Brandtzaeg P. Potential of Nasopharynx-associated Lymphoid Tissue for Vaccine Responses in the Airways. Am J Respir Crit Care Med. 2011;183:1595–1604. doi: 10.1164/rccm.201011-1783OC. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg P. Immune functions of nasopharyngeal lymphoid tissue. Adv Otorhinolaryngol. 2011;72:20–24. doi: 10.1159/000324588. [DOI] [PubMed] [Google Scholar]
- 8.Hasseus B, Jontell M, Bergenholtz G, Dahlgren UI. Langerhans cells from human oral epithelium are more effective at stimulating allogeneic T cells in vitro than Langerhans cells from skin. Clin Exp Immunol. 2004;136:483–489. doi: 10.1111/j.1365-2249.2004.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farquhar C, VanCott T, Bosire R, Bermudez C, Mbori-Ngacha D, Lohman-Payne B, Nduati R, Otieno P, John-Stewart G. Salivary human immunodeficiency virus (HIV)-1-specific immunoglobulin A in HIV-1-exposed infants in Kenya. Clin Exp Immunol. 2008;153:37–43. doi: 10.1111/j.1365-2249.2008.03664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luzuriaga K, Newell ML, Dabis F, Excler JL, Sullivan JL. Vaccines to prevent transmission of HIV-1 via breastmilk: scientific and logistical priorities. Lancet. 2006;368:511–521. doi: 10.1016/S0140-6736(06)69159-9. [DOI] [PubMed] [Google Scholar]
- 11.Koga T, Oho T, Shimazaki Y, Nakano Y. Immunization against dental caries. Vaccine. 2002;20:2027–2044. doi: 10.1016/s0264-410x(02)00047-6. [DOI] [PubMed] [Google Scholar]
- 12.Madhav NV, Shakya AK, Shakya P, Singh K. Orotransmucosal drug delivery systems: a review. J Control Release. 2009;140:2–11. doi: 10.1016/j.jconrel.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Çuburu N, Kweon M-N, Song J-H, Hervouet C, Luci C, Sun J-B, Hofman P, Holmgren J, Anjuère F, Czerkinsky C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;25:8598–8610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 14.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, Czerkinsky C, Kweon MN. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A. 2008;105:1644–1649. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Z, Mumper RJ. Bilayer films for mucosal (genetic) immunization via the buccal route in rabbits. Pharm Res. 2002;19:947–953. doi: 10.1023/a:1016454003450. [DOI] [PubMed] [Google Scholar]
- 16.Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, Babiuk LA, Townsend H, Mutwiri G. Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci USA. 2009;106:18936–18941. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24:1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 18.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21:947–952. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- 20.Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, Stinchcomb AL. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci U S A. 2008;105:2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang C, Compans RW. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikszta JA, Sullivan VJ, Dean C, Waterston AM, Alarcon JB, Dekker JP, 3rd, Brittingham JM, Huang J, Hwang CR, Ferriter M, Jiang G, Mar K, Saikh KU, Stiles BG, Roy CJ, Ulrich RG, Harvey NG. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J Infect Dis. 2005;191:278–288. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- 25.Dean CH, Alarcon JB, Waterston AM, Draper K, Early R, Guirakhoo F, Monath TP, Mikszta JA. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax)-JE) in non-human primates. Hum Vaccin. 2005;1:106–111. doi: 10.4161/hv.1.3.1797. [DOI] [PubMed] [Google Scholar]
- 26.Desvignes C, Esteves F, Etchart N, Bella C, Czerkinsky C, Kaiserlian D. The murine buccal mucosa is an inductive site for priming class I-restricted CD8+ effector T cells in vivo. Clin Exp Immunol. 1998;113:386–393. doi: 10.1046/j.1365-2249.1998.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaworski JP, Krebs SJ, Trovato M, Kovarik DN, Brower Z, Sutton WF, Waagmeester G, Sartorius R, D'Apice L, Caivano A. Co-immunization with multimeric scaffolds and DNA rapidly induces potent autologous HIV-1 neutralizing antibodies and CD8+ T cells. PloS one. 2012;7:e31464. doi: 10.1371/journal.pone.0031464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y-C, Quan F-S, Compans RW, Kang S-M, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, Daddona P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–511. doi: 10.1016/j.jconrel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Shim BS, Choi YK, Yun CH, Lee EG, Jeon YS, Park SM, Cheon IS, Joo DH, Cho CH, Song MS, Seo SU, Byun YH, Park HJ, Poo H, Seong BL, Kim JO, Nguyen HH, Stadler K, Kim DW, Hong KJ, Czerkinsky C, Song MK. Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza. PloS one. 2011;6:e27953. doi: 10.1371/journal.pone.0027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jun S, Clapp B, Zlotkowska D, Hoyt T, Holderness K, Maddaloni M, Pascual DW. Sublingual immunization with adenovirus F protein-based vaccines stimulates protective immunity against botulinum neurotoxin A intoxication. Int Immunol. 2012;24:117–128. doi: 10.1093/intimm/dxr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.