Summary
Routine necropsies of 27 asymptomatic juvenile chinchillas revealed a high prevalence of gastric ulcers with microscopic lymphoplasmacytic gastroenteritis and typhlocolitis. Polymerase chain reaction (PCR) analysis using Campylobacter genus-specific partial 16S rRNA primers revealed the presence of Campylobacter spp. DNA in the feces of 12 of 27 animals (44.4%). Species-specific partial 16S rRNA PCR and sequencing confirmed that these animals were colonized with C. lanienae, a gram-negative, microaerophilic bacterium that was first identified on routine fecal screening of slaughterhouse employees and subsequently isolated from feces of livestock. C. lanienae was isolated from the feces of six PCR-positive animals and identified with species-specific PCR and full 16S rRNA sequencing. Phylogenetic analysis showed that these isolates clustered with C. lanienae strain NCTC 13004. PCR analysis of DNA extracted from gastrointestinal tissues revealed the presence of C. lanienae DNA in the cecum and colon of these chinchillas. Gastrointestinal lesions were scored and compared between C. lanienae-positive and C. lanienae-negative animals. There was no correlation between colonization status and lesion severity in the stomach, liver, duodenum, or colon. Possible routes of C. lanienae infection in chinchillas could include waterborne transmission and fecal-oral transmission from wild mice and rats or livestock. Based on these findings, the authors conclude that C. lanienae colonizes the lower bowel of chinchillas in the absence of clinical disease. This is the first report of C. lanienae in any rodent species. C. lanienae isolates from different mammalian species demonstrate heterogeneity by 16S rRNA sequence comparison. Analysis using rpoB suggests that isolates and clones currently identified as C. lanienae may represent multiple species or subspecies.
Keywords: Campylobacter lanienae, chinchilla, DNA sequence analysis, 16S ribosomal RNA, rpoB
Introduction
Chinchillas (Chinchilla laniger) are medium-sized, crepuscular, herbivorous rodents whose large and easily accessible tympanic bullae facilitate studies of bacterial otitis media and normal auditory physiology (Donnelly and Quimby, 2002). Recently, an investigator at our institution ordered several dozen chinchillas for use in ex vivo drug delivery studies for the treatment of otitis media. The animals were clinically normal throughout the studies, but upon routine postmortem examinations for surveillance purposes, the chinchillas had a high prevalence of grossly evident gastrointestinal lesions characterized microscopically as ulcerative and microscopic lymphoplasmacytic gastroenteritis and typhlocolitis. Subsequent review of gastrointestinal tissue sections in our archives revealed similar findings in chinchillas from a neighboring institution. Ulcerative gastritis has also been reported anecdotally in published literature (Kennedy, 1952; Donnelly and Quimby, 2002). However, no definitive etiologic agent for ulcerative gastroenteritis has been identified in chinchillas. Because infection with Helicobacter spp. or Campylobacter spp. can cause such a broad spectrum of gastrointestinal diseases with and without clinical signs in a wide range of host species, the authors were interested in exploring the role of these organisms as potential causes of the aforementioned gastrointestinal lesions present in chinchillas.
Materials and methods
Animals
Twenty-seven, six-month-old male chinchillas were purchased from a commercial vendor and housed in a vivarium at Massachusetts Institute of Technology in Cambridge, Massachusetts, in accordance with The Guide for Care and Use of Laboratory Animals (National Research Council, 2010). All animal facilities were accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The animals were pair housed in conventional rabbit cages (Allentown, Incorporated, Allentown, NJ, USA) in an individually ventilated cubicle and offered chlorinated water in sipper bottles and a pelleted guinea pig diet (LabDiet 5025, Purina, St. Louis, MO, USA) ad libitum. The cubicle was maintained on a 12h:12h light:dark cycle with temperature between 19°C and 21°C (66°F and 70°F) and relative humidity between 30% and 70%. One animal had fight wounds and a poor appetite for twenty-four hours prior to euthanasia, but no significant pathology was found on necropsy. All other animals were clinically normal prior to euthanasia. All animals were allowed to acclimate to the vivarium for at least three days before use, and all experimental protocols were approved by the Massachusetts Institute of Technology Committee on Animal Care.
Necropsy
The chinchillas were sedated with ketamine (35 mg/kg) and xylazine (5 mg/kg) administered by intraperitoneal injection before euthanasia with pentobarbital (120 mg/kg) administered by intracardiac injection. After euthanasia, the animals’ heads were removed for use in an ex vivo study, and routine necropsy was performed on the remainder of the carcasses. The gastrointestinal tracts were dissected out of the carcasses, and fecal pellets were removed both transmurally through incisions and also by milking the fecal pellets aborally to the transected end of the rectum. Feces were submitted for trichrome staining to detect Giardia spp. and fecal flotation to detect helminth ova and protozoal oocysts. Fecal pellets were frozen at −80°C either in brucella broth containing 30% glycerol for microaerobic culture (Whary and Fox, 2004) or without brucella broth for molecular diagnostics. Tissue samples from the stomach, liver, duodenum, cecum, and colon were either fixed in buffered formalin for histopathology or frozen at −80°C, either with or without brucella broth. In addition, tissues from all organs from six of the animals were archived in 10% neutral-buffered formalin for future possible use. Due to the scope of research performed in the authors’ laboratories, at this time, animals were not surveyed for potential bacterial or viral enteropathogens except for those in the genera Helicobacter and Campylobacter.
Histology
Formalin-fixed tissues were routinely processed and embedded in paraffin according to accepted histologic technique. Four-micrometer-thick sections were stained with either hematoxylin and eosin (H&E) or Warthin-Starry (W-S) silver stain. Lesions in the stomach, intestine, and liver were scored, according to previously defined criteria, using an ascending scale from 0 to 4, based on the degree of lesion severity: 0 (absent), 1 (mild), 2 (moderate), 3 (marked), and 4 (severe) (Boivin et al., 2003; Rogers et al., 2005; Rogers et al., 2007). H&E-stained stomach sections were used to score lesions in the corpus for inflammation, epithelial defects, atrophy, hyperplasia, mucous metaplasia, intestinal metaplasia, and dysplasia (Rogers et al., 2005). Intestinal lesions were scored for inflammation, epithelial defects, hyperplasia, and dysplasia (Boivin et al., 2003). For the liver, a hepatitis index was calculated by combining individual scores for lobular, portal, and interface hepatitis, as well as the number of lobes (out of a total of four) that contained five or more inflammatory lesions. Hepatitis was defined by a hepatitis index equal to or greater than 4 (Rogers et al., 2007). All lesion scoring was performed in a blinded fashion by two diplomates of the American College of Veterinary Pathologists.
DNA extraction for 16S rRNA PCR analyses
DNA was extracted from fecal pellets using the QIAamp® DNA Stool Mini Kit (Qiagen, Valencia, CA, USA). For tissue samples (stomach, liver, duodenum, cecum, and colon) and individual bacterial colonies, DNA was extracted with the High Pure PCR Template Preparation Kit (Roche Molecular Biochemicals, Indianapolis, IN, USA). The DNA concentration of each final product was verified by measuring the optical density of each solution with a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Campylobacter spp. and Helicobacter spp. detection
PCR analyses on DNA recovered from feces were performed with the Expand High Fidelity PCR System (Roche Molecular Biochemicals, Indianapolis, IN, USA). PCR assays of DNA extracted from tissue samples and single isolated bacterial colonies were performed with Illustra PuReTaq Ready-To-Go™ PCR Beads (GE Healthcare, Waukesha, WI, USA). Genus-specific 16S rRNA PCR assays for Helicobacter spp. (C97/C05 primers, 1200 bp amplicon) (Shen et al., 2001) and Campylobacter spp. (C99/C98 primers, 300 bp amplicon) (Shen et al., 2001) and species-specific 16S rRNA PCR for C. lanienae (CLAN76F/CLANL521021R primers, 920 bp amplicon) (Inglis and Kalischuk, 2003) were performed as previously described. Gel electrophoresis was performed on 2% agarose gel. Gels were imaged with ultraviolet light using the G:Box gel imaging system (Syngene, Frederick, MD, USA).
Analysis of PCR products
Products from partial 16S rRNA PCR reactions of DNA extracted from feces were amplified using the primers and protocols listed above, purified with the QIAGEN DyeEx 2.0 Spin Kit (Qiagen, Valencia, CA, USA), and sequenced with the Applied Biosystems® 3500 Genetic Analyzer (Life Technologies Corporation, Carlsbad, CA, USA). Pre-sequencing amplification was performed using one of the following primer sets: genus-specific 16S rRNA primers for Campylobacter spp. (C99/C98) (Shen et al., 2001) or species-specific 16S rRNA primers for C. lanienae (CLAN76F/CLANL521021R) (Inglis and Kalischuk, 2003). Sequences were analyzed by BLAST analysis at NCBI.
Statistical analysis
Animals were separated into two groups based on PCR-based detection or absence of C. lanienae DNA in DNA samples extracted from feces. Histologic lesion scores for each tissue site (stomach, liver, duodenum, cecum, and colon) were analyzed using GraphPad Prism software (GraphPad Software, Incorporated, La Jolla, CA, USA). The groups were compared using a non-parametric Mann-Whitney U test with a two-tailed P value. Results were considered significant if P < 0.05.
Microaerobic culture and isolation
Fecal pellets were stored in brucella broth at −80°C until culture. For culture, frozen feces from six animals that were PCR-positive for C. lanienae were thawed and homogenized in 1 mL brucella broth. Each sample was streaked on a blood agar plate containing 20 mg/L cefoperazone, 10 mg/L vancomycin, and 2 mg/L amphotericin B (CVA agar) (BD BBL™ Campy CVA Agar, BD, Sparks, MD, USA) and incubated at 37°C for 72 hours in a microaerobic gas mixture (10% CO2, 10% H2, 80% N2) (Whary and Fox, 2004). After 72 hours, one single colony with similar morphology to C. lanienae (Logan et al., 2000) was selected from each animal’s plate and passaged once on CVA agar as described above. These plates were then collected for biochemical characterization and full 16S rRNA sequencing.
Biochemical analysis
Passaged colonies were incubated on CVA agar before being collected for biochemical profiling. Metabolic characteristics were determined using the API® Campy bacterial identification system (bioMérieux, Durham, NC, USA), used according to manufacturer instructions.
16S rRNA gene sequencing
The 16S rRNA genes of isolates were amplified by PCR using the primers F24 (9–27F) 5’-GAGTTTGATYMTGGCTCAG-3’ and Y36 (1525–1541R) 5’-GAAGGAGGTGWTCCADCC-3’. PCR conditions were as previously described (Dewhirst et al., 2010). Amplicons were sequenced by MacroGen (Cambridge, MA, USA) using their standard protocol for PCR amplicons. The following six short 16S rRNA sequencing primers were designed for use with commercial sequencing companies that routinely run their sequencing reactions at 50°C: AE67 (13–17F) 5’-TYGATYMTGGCTCAG-3’; AE53 (520–531F) 5’-AGCAGCCGCGGT-3’; AE55 (1100–1113F) 5’-YAACGAGCGMAAC-3’; AF48 (1527–1541R) 5’-GAAGGAGGTGWTCCA-3’; AE58 (1101–1113R) 5’-GGTTKCGCTCGTT-3’; and AE50 (521–533R) 5’-TKACCGCGGCTGC-3’. These primers were designed using the program Oligo (Molecular Biology Insights, Cascade, CO, USA). For each of the six target positions, three to four primers of varying lengths with annealing temperatures near 50°C were synthesized and validated for use in PCR and sequencing. The best performing sequencing primers were chosen based on examination of sequencing electropherograms.
rpoB gene sequencing
A portion of the rpoB gene was sequenced using primers and conditions recommended by Korczak et al (Korczak et al., 2006).
Sequence analysis and tree construction
The 16S rRNA sequences were entered and aligned using a campylobacter and helicobacter 16S rRNA analysis program (Paster and Dewhirst, 1988). Phylogenetic trees were constructed using neighbor-joining analysis. The chinchilla isolate rpoB sequence and twelve campylobacter reference rpoB sequences, downloaded from NCBI, were aligned and trees constructed using neighbor-joining analysis.
Results
Necropsy and histology
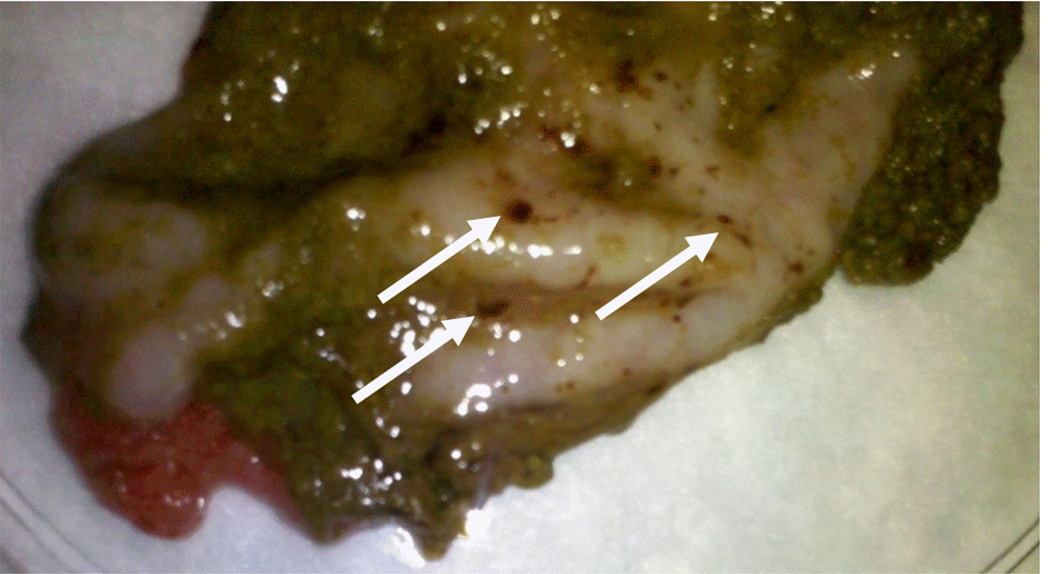
The authors noted a high prevalence of varying degrees of gross gastric ulcerations and microscopic lymphoplasmacytic gastroenteritis and typhlocolitis in unmanipulated asymptomatic chinchillas housed at MIT. Six out of twenty-seven animals had gross gastric ulcers on necropsy examination; Figure 1 shows a representative necropsy image from an animal with gastric ulcerations. The remainder of the gastrointestinal tract in all animals was grossly normal. Table 1 lists the prevalence of predominate microscopic lesion types by anatomic location. In general, lesions were composed of mild to marked, plasmacytic to lymphoplasmacytic gastritis, enteritis, typhlitis, and colitis and random hepatocellular hyalinosis. These lesions affected between 66.7% and 100.0% of the animals, depending on the tissue. W-S staining of gastric tissues did not reveal organisms compatible with Helicobacter spp. by size or morphology on the surface of gastric epithelia or within gastric crypts. In addition, all animals were negative for fecal helminth ova and Giardia oocysts.
Fig. 1.
Stomach tissue from a chinchilla with gross multifocal ulcerative gastritis. This animal had no signs of clinical disease prior to necropsy despite gross postmortem evidence of gastric ulcerations. White arrows indicate hemorrhagic gastric ulcerations.
Table 1.
Prevalence of microscopic gastrointestinal lesions at necropsy. Results are based on a colony of 27 animals. All animals were clinically normal at the time of euthanasia with the exception of one animal with fight wounds and dermatitis.
| Tissue | Microscopic Lesion | # Animals Affected/Total | Prevalence |
|---|---|---|---|
| Stomach | Lymphoplasmacytic mucosal gastritis | 18/27 | 66.7% |
| Liver | Random hepatocellular hyalinosis | 26/27 | 96.3% |
| Duodenum | Plasmacytic mucosal enteritis | 27/27 | 100.0% |
| Cecum | Plasmacytic mucosal typhlitis | 24/27 | 88.9% |
| Colon | Lymphoplasmacytic mucosal colitis | 18/27 | 66.7% |
Lesion scoring and statistical analysis
Samples from stomach, duodenum, cecum, and colon were scored from 0 (normal) to 4 (severe pathology) as previously described (Boivin et al., 2003). A similar scoring system was used for liver samples. Animals were separated into two groups based on PCR-based detection or absence of C. lanienae DNA in the feces, and lesion scores were then compared between the two groups. The presence of C. lanienae-like DNA in the feces of these animals did not significantly correlate with the presence of gastrointestinal pathology; PCR analysis of DNA extracted from fecal samples did not detect C. lanienae DNA in some animals with severe pathology, whereas PCR analysis detected C. lanienae DNA in fecal samples of some animals with minimal to mild pathology. Therefore, the authors conclude that it is unlikely that colonization with C. lanienae is responsible for the gastrointestinal pathology seen in these chinchillas.
PCR analysis of DNA extracted from feces
High fidelity PCR assays using Campylobacter genus-specific primers (C99/C98, 320 bp amplicon) (Shen et al., 2001) revealed that 12 of 27 animals (44.4%) were colonized with a bacterium in the genus Campylobacter, according to PCR results using DNA extracted from fecal samples. To identify the bacterium, PCR products were sequenced and compared to the BLAST database. The PCR products had >99.5% sequence similarity (215/216 bases) with GenBank sequence HQ628642.1, C. lanienae 16S rRNA. To confirm bacterial identity, the DNA extracted from fecal samples was subjected to a second high fidelity PCR assay using C. lanienae-specific primers (CLAN76F/CLANL521021R, 920 bp amplicon) (Inglis and Kalischuk, 2003). The species-specific PCR confirmed that 12 of 27 animals (44.4%) were fecal-positive for C. lanienae, and subsets of fecal-positive and fecal-negative animals identified using the two different primer pairs were identical. 16S rRNA from the species-specific PCR was also sequenced and compared to the BLAST database. The products had >99.8% sequence similarity (866/867 bases) to GenBank sequence AY288304.1, C. lanienae 16S rRNA, which supported our previous results. These findings confirmed fecal shedding of C. lanienae in 44.4% of our chinchillas. There was no molecular evidence of any other campylobacters in any of the tested samples.
All fecal samples were negative for Helicobacter spp. DNA using a genus-specific PCR assay (C97/C05, 1200 bp amplicon, data not shown) (Shen et al., 2001).
PCR analysis of DNA extracted from gastrointestinal tissues
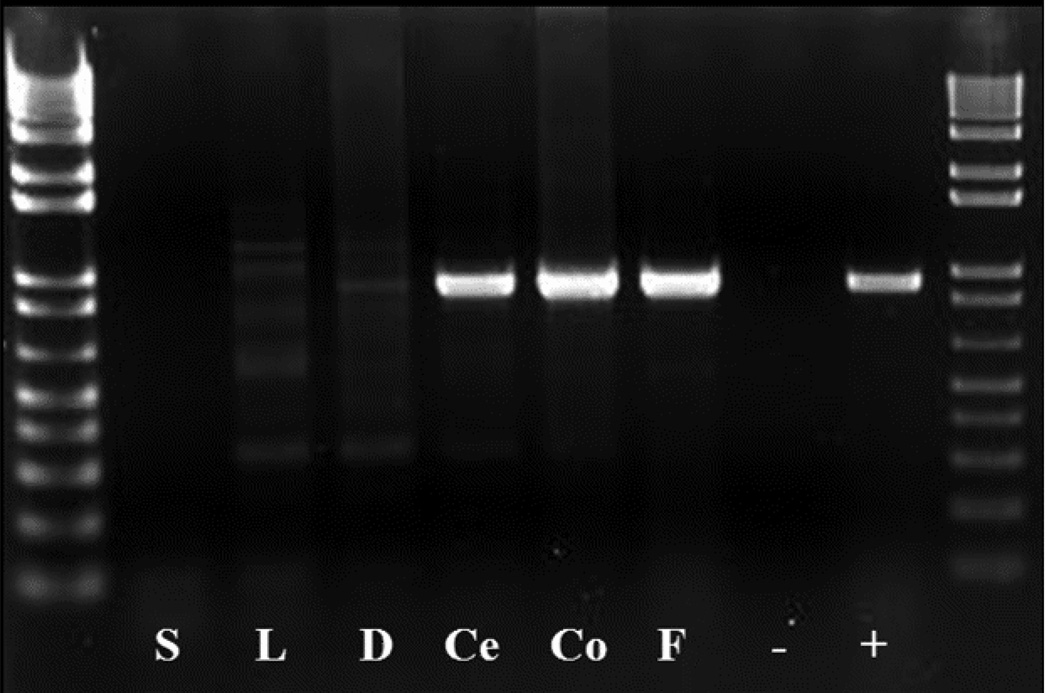
To elucidate preferred colonization sites of C. lanienae, DNA was extracted from the stomach, liver, duodenum, cecum, and colon of three animals in which C. lanienae DNA was detected in feces using PCR. DNA from these gastrointestinal tissues was subjected to PCR using C. lanienae-specific primers and PCR reagent beads. Representative results from one animal are shown in Figure 2. C. lanienae DNA was amplified in 100% (3/3) of the cecum and colon samples and confirmed in the feces of all three animals, but no DNA was identified in the stomach, liver, or duodenum. These findings are consistent with studies in other species of livestock that report selective colonization of C. lanienae in the lower bowel (Inglis et al., 2005; Shin and Lee, 2009).
Fig. 2.
PCR analysis of DNA extracted from gastrointestinal tissues, using C. lanienae-specific primers. Representative results for one fecal DNA-positive animal using C. lanienae-specific primers (CLAN76F/CLAN521021R, 920 bp). C. lanienae-like DNA was detected in the cecum, colon, and feces. No DNA was detected in samples from the stomach, liver, or duodenum. S: stomach, L: liver, D: duodenum, Ce: cecum, Co: colon, F: feces. Data were replicated in three separate experiments.
Tissue samples from the stomach, liver, duodenum, cecum, and colon were all negative for Helicobacter spp. DNA using a genus-specific PCR assay (C97/C05, 1200 bp amplicon, data not shown) (Shen et al., 2001).
Bacterial isolation
Feces from six randomly selected PCR-positive animals were plated on CVA agar, cultured under microaerobic conditions, and passaged once on CVA agar for isolation. Single colonies with morphology consistent with C. lanienae and other campylobacters (Gram-negative curved rods growing in 1–2 mm, translucent colonies) were selected for DNA extraction and PCR analysis with C. lanienae-specific primers. No other campylobacters were found using culture-based methods.
Biochemical analysis
All six strains of C. lanienae were shown to be catalase-positive, urease-negative, oxidase-positive, nitrate-reducing, negative for hydrogen sulfite production, negative for hippurate hydrolysis, unable to grow in 1% glycine, and thermotolerant, growing at both 37°C and 42°C (Table 2). These data are consistent with the biochemical results obtained previously when the type strain of C. lanienae was described (Logan et al., 2000), with the exception of variable sensitivity to cephalothin: three of six strains were sensitive to cephalothin, whereas the type strain was reported to be resistant (Logan et al., 2000).
Table 2.
Results of API testing. Biochemical analysis was performed on isolates from six animals that were fecal PCR positive for C. lanienae-like bacteria. Results are consistent with biochemical characteristics of the type strain of C. lanienae, described previously (Logan et al., 2000), with the exception of sensitivity to cephalothin: three of our isolates were sensitive to cephalothin, whereas the type strain was resistant.
| Accession number |
Catalase | Urease | Oxidase | Indoxyl acetate hydrolysis |
Nitrate reduction |
Esterase | γ-glutamyl transpeptidase |
Hippurate hydrolysis |
TTC1 reduction |
|---|---|---|---|---|---|---|---|---|---|
| 11-0152 | + | − | + | − | + | − | − | − | − |
| 11-0231 | + | − | + | − | + | − | − | − | − |
| 11-0232 | + | − | + | − | + | − | − | − | − |
| 11-0763 | + | − | + | − | + | − | − | − | − |
| 11-0781 | + | − | + | − | + | − | − | − | − |
| 11-0885 | + | − | + | − | + | − | − | − | − |
| NCTC 130042 | + | − | + | − | + | ND3 | ND | − | ND |
| Accession number |
H2S production |
Growth at 37°C |
Growth at 42°C |
Growth at 25°C |
Growth in 1% glycine |
Cephalothin sensitivity |
Nalidixic acid sensitivity |
||
|---|---|---|---|---|---|---|---|---|---|
| 11-0152 | − | + | + | − | − | R | R | ||
| 11-0231 | − | + | + | − | − | S | R | ||
| 11-0232 | − | + | + | − | − | S | R | ||
| 11-0763 | − | + | + | − | − | R | R | ||
| 11-0781 | − | + | + | − | − | S | R | ||
| 11-0885 | − | + | + | − | − | R | R | ||
| NCTC 130042 | − | + | + | − | − | R | R | ||
Triphenyl tetrazolium chloride
Type strain of C. lanienae
Test not performed
Phylogenetic analysis
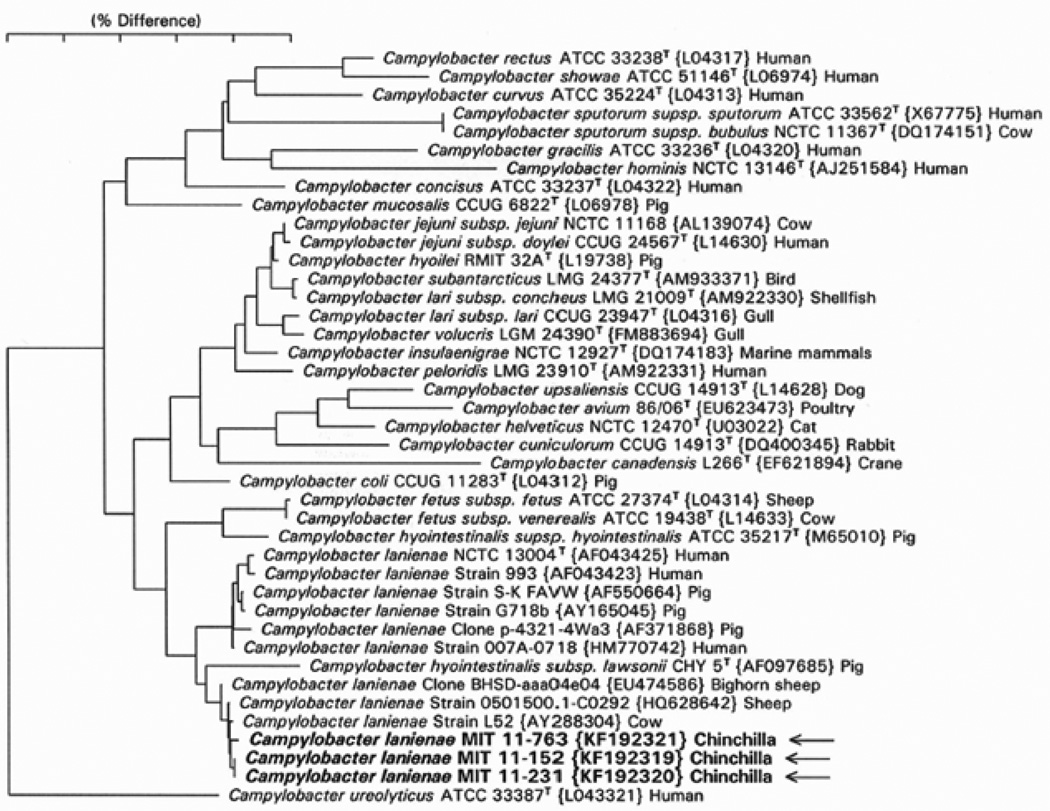
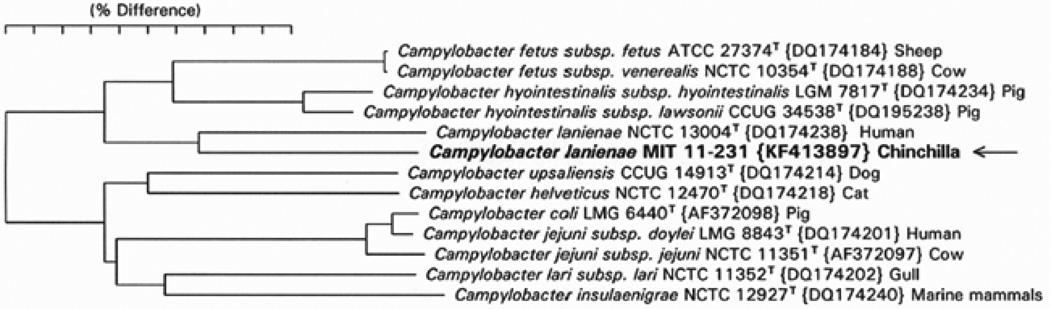
Full-length amplicons of the 16S rRNA gene from three isolates were sequenced and compared to sequences at NCBI by BLAST. The isolate sequences were highly similar to several C. lanienae sequences at NCBI. Figure 3 shows a 16S rRNA phylogenetic tree comparing the three chinchilla isolates with 16S rRNA from other known Campylobacter species. All three of our isolates clustered with C. lanienae strains from a sheep and a cow and a clone from bighorn sheep. The C. lanienae type strain from a human (Logan et al., 2000) forms a slightly separate cluster with other human and pig strains and clone sequences. Because 16S rRNA sequences are known to poorly differentiate certain Campylobacter species, the sequence of a well-studied rpoB fragment was sequenced (Korczak et al., 2006). Figure 4 shows a phylogenetic tree of one of the chinchilla C. lanienae isolates, the type strain of C. lanienae, and the eleven most closely related Campylobacter species by rpoB sequence similarity. The tree was produced using the neighbor-joining analysis method. The chinchilla isolate branches most closely to the human type strain but is only 86.9% similar.
Fig. 3.
Phylogenetic tree for Campylobacter spp. based on 16S rRNA sequence comparison. Full-length 16S rRNA sequences were compared using neighbor-joining analysis. The bar represents 5% sequence divergence. The closest named species type strain of the three chinchilla isolates is that of C. lanienae. Notice that strains previously identified as C. lanienae fall into two clusters separated by the type strain of C. hyointestinalis subsp. lawsonii.
Fig. 4.
Phylogenetic tree for Campylobacter spp. based on a fragment of the rpoB gene. The sequences of PCR-amplified fragments of rpoB genes from several campylobacters were compared using neighbor-joining analysis. The chinchilla isolate is closest to the type strain of C. lanienae but has only 86.9% sequence identity. This is a greater separation than seen for subspecies pairs of C. fetus, C. hyointestinalis, and C. jejuni.
Discussion
Discrepancies in C. lanienae speciation by molecular methods: 16S rRNA sequencing versus rpoB sequencing
PCR and sequence analysis revealed that 12 of 27 (44.4%) of the chinchillas were infected with C. lanienae-like bacteria by 16S rRNA sequence analysis. These animals were clinically normal but had significant gastrointestinal pathology comprising varying degrees of gross ulcerative lesions and microscopic lymphoplasmacytic gastroenteritis and typhlocolitis. The authors determined that C. lanienae-like bacteria preferentially colonized the cecum and colon of chinchillas; however, the severity of these lesions did not correlate with C. lanienae infection. The chinchilla isolates clearly fall within a cluster of previously identified C. lanienae isolates and clones by 16S rRNA sequence analysis as shown in Figure 3. However, C. lanienae isolates fall into two sub-clusters, with human and pig sequences in one sub-cluster and cow, sheep, and chinchilla sequences in a second sub-cluster. Furthermore, the type strain of Campylobacter hyointestinalis subsp. lawsonii branches between the two C. lanienae sub-clusters. Because 16S rRNA sequences are known to poorly resolve some Campylobacter species, the authors sequenced the rpoB gene, which has been shown to be useful for resolving campylobacter phylogeny (Korczak et al., 2006). As shown in Figure 4, the rpoB sequence of chinchilla isolate MIT 11–231 is most closely related to the C. lanienae type strain from humans but differs by 13.1% sequence identity. A full analysis of rpoB and possibly additional genes from C. lanienae-like strains isolated from cows, sheep, pigs, and humans is warranted for resolving the phylogeny and taxonomy of these campylobacters, but these studies are beyond the scope of the current investigation. It is possible that the cow, sheep, and chinchilla C. lanienae-like isolates represent one or more novel species or subspecies distinct from the human type strain of C. lanienae. In this manuscript, the authors elected to use the term “C. lanienae-like” to refer to the chinchilla strain and all other isolates and clones (other than the human type strain) that fall into the C. lanienae group by 16S rRNA similarity to indicate their unresolved taxonomic status.
C. lanienae host range and lack of association with pathology observed in chinchillas
C. lanienae-like organisms have been detected in manure from dairy cattle (Guévremont et al., 2008), beef cattle (Inglis and Kalischuk, 2003; Inglis et al., 2003; Inglis and Kalischuk, 2004), swine (Sasaki et al., 2003; Guévremont et al., 2008), and sheep (Oporto and Hurtado, 2011). The type strain of C. lanienae was isolated from the feces of slaughterhouse employees in the UK (Logan et al., 2000). However, C. lanienae-like organisms have never been isolated from humans or animals with evidence of gastrointestinal pathology. Previous studies have demonstrated that C. lanienae-like organisms colonize the cecum, descending colon, and rectum of beef cattle (Inglis et al., 2005) and the colon of pigs (Shin and Lee, 2009). In our findings, PCR analysis of DNA extracted from gastrointestinal tissues and feces confirmed the presence of C. lanienae-like 16S rDNA in these chinchillas, but the presence of the bacterial DNA in feces did not correlate with an increased severity of gastrointestinal lesions in infected animals. If these C. lanienae-like organisms are pathogenic in chinchillas, it is possible that early infection could lead to gastroenteritis with subsequent loss of colonization. Alternatively, infected animals might only shed C. lanienae-like bacteria intermittently. Either possibility would explain the lack of detectable bacteria in selected animals despite significant intestinal pathology, if these lesions are indeed due to the C. lanienae-like organisms. It is also possible that the C. lanienae-like bacterium is a non-pathologic commensal organism in chinchillas, although further studies are indicated to determine the full extent of this microbe’s pathogenicity. Regardless of its pathogenicity, however, the C. lanienae-like bacterium appears to exhibit a colonization preference for the cecum and colon of chinchillas, similar to its colonization niche in cattle and swine (Inglis et al., 2005; Shin and Lee, 2009).
The findings of gastric ulcers and histological ulcerative gastritis are consistent with Helicobacter pylori-associated peptic ulcer disease in humans and H. mustelae-associated gastric ulcers in ferrets (Whary and Fox, 2004). However, Helicobacter spp. bacteria were not detected in these chinchillas with Helicobacter genus-specific PCR primers. Other infectious organisms or an immune-mediated allergic-based inflammation may be responsible for the gastric or lower bowel lesions, such as Acinetobacter lwoffii infection in mice leads to immune-mediated gastritis (Rathinavelu et al., 2003).
The role of bacteria outside the Helicobacter and Campylobacter genera, such as Salmonella spp. or Escherichia coli, in the gastrointestinal pathology seen in these animals was not investigated in this study, due to the research foci of the authors’ laboratories. In addition, the authors did not attempt to isolate any viral pathogens from these animals. It is possible that these lesions could be secondary to infection with a bacterial or viral pathogen; however, in the authors’ experience, the lesions seen in these animals are more consistent with an autoimmune etiology than with an infectious etiology. Further studies are necessary to determine the cause of the gastrointestinal lesions seen in these chinchillas.
Single nucleotide polymorphisms in C. lanienae 16S rRNA necessitate use of a modified reverse primer for PCR assays
It is worth noting a discrepancy in our PCR findings with previously published data. Initial partial 16S rRNA sequencing of PCR products using Campylobacter genus-specific primers (C99/C98) (Shen et al., 2001) suggested that these chinchillas were infected with C. lanienae. The authors then attempted to confirm the identity of this bacterium with the species-specific primers (CLAN76F/CLAN52R) described when the type strain was first identified (Logan et al., 2000). However, these primers did not amplify any genomic sequences in our samples. On further literature review, the authors discovered that others had experienced similar difficulties with the original primer pair due to the presence of three single nucleotide polymorphisms within the annealing region of the reverse primer (Inglis and Kalischuk, 2003). Subsequently, using the slightly modified primer pair (CLAN76F/CLAN521021R) (Inglis and Kalischuk, 2003), the authors were able to confirm the similarity of our C. lanienae-like strains to C. lanienae-like strains from cows; the Inglis strain L52 does not appear to be available in a public-type culture collection. This slight difference in 16S rRNA sequence noted by Inglis is the first hint that C. lanienae-like organisms isolated or detected by PCR from different mammalian species may not represent the same taxa.
Biochemical testing distinguishes C. lanienae-like isolates from C. hyointestinalis subsp. lawsonii
As shown in Figure 3, full 16S rRNA sequencing showed that in addition to the NCTC 13004 type strain of C. lanienae, our C. lanienae-like isolates also clustered closely with C. hyointestinalis subsp. lawsonii CHY5T. In addition, our partial 16S rRNA BLAST sequencing results revealed that the 320-bp amplicon obtained using the CLAN76/CLAN521021R primers had 98% sequence identity with C. hyointestinalis. Our C. lanienae-like strains were negative for hydrogen sulfide production, negative for growth at 25°C, and negative for growth in 1% glycine, whereas C. hyointestinalis is positive for hydrogen sulfide production, grows variably at 25°C, and is capable of growth in 1% glycine (Logan et al., 2000). These biochemical testing results confirm that our strains are indeed more closely related to C. lanienae than to C. hyointestinalis, reinforcing the importance of biochemical testing in differentiating closely related bacterial isolates.
Possible routes of C. lanienae infection in farm-raised chinchillas
It remains to be determined how these chinchillas became colonized with an organism that has only previously been documented in livestock and humans. On corresponding with the production facility owner, it was noted that the chinchillas’ water source consisted of chlorinated water from an underground well. Low-level chlorination of drinking water has been shown to be ineffective in preventing the colonization of feedlot cattle with C. jejuni (Besser et al., 2005) and the C. jejuni colonization of broiler chickens (Stern et al., 2002). Furthermore, waterborne transmission has also been implicated in the colonization of chicken flocks with both C. jejuni (Pearson et al., 1993) and Campylobacter coli (Pérez-Boto et al., 2010). Waterborne transmission has also been implicated in several outbreaks of campylobacterioisis in humans; four outbreaks and 77 cases of illness in the United States in 2007–2008 were attributed to waterborne transmission of Campylobacter spp. in drinking water (Brunkard et al., 2011). C. jejuni is frequently detected in raw milk, the second most common cause of Campylobacter-induced diarrhea behind consumption of raw poultry meat (Wysok et al., 2011). The chinchilla production facility is located across the road from a small dairy farm, and the two facilities are connected by an above-ground stream. Given the possibility that these chinchillas are being infected with C. lanienae-like bacteria after drinking inadequately chlorinated contaminated ground water, the authors attempted to obtain manure samples from the adjoining dairy farm to test the cattle for C. lanienae-like bacteria. Unfortunately, the farm owner did not grant us permission to visit his facility. It is also possible that local vermin populations could be responsible for transmitting these campylobacters. C. coli, C. jejuni, and C. hyointestinalis have been isolated from wild mice, and C. coli has been isolated from wild rats (Meerburg et al., 2006). Further studies are needed to determine whether or not mice and rats are able to be colonized by C. lanienae or related bacteria.
Conclusions
Our findings are significant because this is the first report of infection with a C. lanienae-like bacterium or any other bacterium in the order Campylobacterales (specifically, Campylobacter spp. and Helicobacter spp.) in chinchillas. Given that C. lanienae was originally isolated from humans (Logan et al., 2000), zoonotic transmission of C. lanienae-like bacteria is likely possible. Also, because co-colonization with C. jejuni and a C. lanienae-like bacterium has been documented in cattle (Inglis et al., 2005), it is conceivable that horizontal transfer of virulence factors and genes encoding for bacterial resistance could occur between these two Campylobacter species. Our findings should prompt additional studies regarding other potential hosts of C. lanienae-like bacteria to broaden our understanding of the epidemiology and pathogenic potential of these campylobacters. The phylogeny of C. lanienae-like isolates from humans, pigs, sheep, cows, chinchillas, and potentially other mammals requires examination using rpoB and possibly other genes to resolve taxonomic issues unresolved by 16S rRNA and phenotypic analyses used to date.
Impacts.
This is the first report of the zoonotic bacterium Campylobacter lanienae in chinchillas and in any rodent host species. C. lanienae does not appear to be associated with background gastrointestinal inflammation in chinchillas, consistent with findings in other host species.
Using partial 16S rRNA-based sequencing, C. lanienae chinchilla isolates cluster phylogenetically with cattle and sheep isolates with >99% sequence identity, while the human-derived type strain of C. lanienae clusters with isolates from swine.
Using an rpoB-based sequencing approach, our chinchilla isolates of C. lanienae cluster with the type strain but have only 86.9% sequence identity, suggesting that rpoB sequence analysis provides higher resolution for speciating campylobacters than does partial 16S rRNA sequencing.
Acknowledgments
Special thanks to Alison Hayward, Catrina Wong, Wayne Au, and Homer Chang for their assistance in acquiring the tissue and fecal samples used in this study. The authors would also like to thank Zhongming Ge, Ellen Buckley, Carolyn Madden, Kvin Lertpiriyapong, and Laura Cacioppo for their assistance in performing the aforementioned laboratory techniques. This work was supported by a Ruth L. Kirschstein National Research Service Award, grants T32-OD007036 (JGF), R01-OD011141 (JGF), and P30-ES02109 (JGF) from the National Institutes of Health.
Footnotes
Conflict of interest
None of the authors have a financial conflict of interest with respect to the information provided here.
References
- Besser TE, Lejeune JT, Rice DH, Berg J, Stillborn RP, Kaya K, Bae W, Hancock DD. Increasing prevalence of Campylobacter jejuni in feedlot cattle through the feeding period. Appl. Environ. Microbiol. 2005;71:5752–5758. doi: 10.1128/AEM.71.10.5752-5758.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- Brunkard JM, Ailes E, Roberts VA, Hill V, Hilborn ED, Craun GF, Rajasingham A, Kahler A, Garrison L, Hicks L, Carpenter J, Wade TJ, Beach MJ, Yoder JS. Surveillance for waterborne disease outbreaks associated with drinking water – United States, 2007–2008. MMWR Surveill. Summ. 2011;60:38–68. [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly TM, Quimby FW. Biology and diseases of other rodents. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory Animal Medicine. 2nd Edn. San Diego, CA: Academic Press; 2002. pp. 286–291. [Google Scholar]
- Fox JG, Shen Z, Muthupalani S, Rogers AR, Kirchain SM, Dewhirst FE. Chronic hepatitis, hepatic dysplasia, fibrosis, and biliary hyperplasia in hamsters naturally infected with a novel helicobacter classified in the H. bilis cluster. J. Clin. Microbiol. 2009;47:3673–3681. doi: 10.1128/JCM.00879-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guévremont E, Normand V, Lamoureux L, Côté C. Genetic detection of Campylobacter lanienae in fecal matter and stored manure from swine and dairy cattle. Foodborne Pathog. Dis. 2008;5:361–364. doi: 10.1089/fpd.2007.0054. [DOI] [PubMed] [Google Scholar]
- Inglis GD, Kalischuk LD. Use of PCR for direct detection of Campylobacter species in bovine feces. Appl. Environ. Microbiol. 2003;69:3435–3447. doi: 10.1128/AEM.69.6.3435-3447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis GD, Kalischuk LD. Direct quantification of Campylobacter jejuni and Campylobacter lanienae in feces of cattle by real-time quantitative PCR. Appl. Environ. Microbiol. 2004;70:2296–2306. doi: 10.1128/AEM.70.4.2296-2306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis GD, Kalischuk LD, Busz HW. A survey of Campylobacter species shed in faeces of beef cattle using polymerase chain reaction. Can. J. Microbiol. 2003;49:655–661. doi: 10.1139/w03-087. [DOI] [PubMed] [Google Scholar]
- Inglis GD, Kalischuk LD, Busz HW, Kastelic JP. Colonization of cattle intestines by Campylobacter jejuni and Campylobacter lanienae. Appl. Environ. Microbiol. 2005;71:5145–5153. doi: 10.1128/AEM.71.9.5145-5153.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AH. Chinchilla Diseases and Ailments. Toronto, Canada: Fur Trade Journal of Canada; 1952. [Google Scholar]
- Korczak BM, Stieber R, Emler S, Burnens AP, Frey J, Kuhnert P. Genetic relatedness within the genus Campylobacter inferred from rpoB sequences. Int. J. Syst. Evol. Microbiol. 2006;56:937–945. doi: 10.1099/ijs.0.64109-0. [DOI] [PubMed] [Google Scholar]
- Logan JM, Burnens A, Linton D, Lawson AJ, Stanley J. Campylobacter lanienae sp. nov., a new species isolated from workers in an abattoir. Int. J. Syst. Evol. Microbiol. 2000;50:865–872. doi: 10.1099/00207713-50-2-865. [DOI] [PubMed] [Google Scholar]
- Meerburg BG, Jacobs-Reitsma WF, Wagenaar JA, Kijlstra A. Presence of Salmonella and Campylobacter spp. in wild small mammals on organic farms. Appl. Environ. Microbiol. 2006;72:960–962. doi: 10.1128/AEM.72.1.960-962.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D. C: National Academy Press; 2010. [Google Scholar]
- Oporto B, Hurtado A. Emerging thermotolerant Campylobacter species in healthy ruminants and swine. Foodborne Pathog. Dis. 2011;8:807–813. doi: 10.1089/fpd.2010.0803. [DOI] [PubMed] [Google Scholar]
- Paster BJ, Dewhirst FE. Phylogeny of campylobacters, wolinellas, Bacteroides gracilis, and Bacteroides ureolyticus by 16S ribosomal ribonucleic acid sequencing. Int. J. Syst. Bacteriol. 1988;38:56–62. [Google Scholar]
- Pearson AD, Greenwood M, Healing TD, Rollins D, Shahamat M, Donaldson J, Colwell RR. Colonization of broiler chickens by waterborne Campylobacter jejuni. Appl. Environ. Microbiol. 1993;59:987–996. doi: 10.1128/aem.59.4.987-996.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Boto D, García-Pena FJ, Abad-Moreno JC, Hurtado-Pizarro MD, Pérez-Cobo I, Echeita MA. Drinking water as the source of Campylobacter coli infection in grandparent heavy breeders. Avian Pathol. 2010;39:483–487. doi: 10.1080/03079457.2010.518138. [DOI] [PubMed] [Google Scholar]
- Rathinavelu S, Zavros Y, Merchant JL. Acinetobacter lwoffii infection and gastritis. Microbes Infect. 2003;5:651–657. doi: 10.1016/s1286-4579(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–10715. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- Rogers AB, Theve EJ, Feng Y, Fry RC, Taghizadeh K, Clapp KM, Boussahmain C, Cormier KS, Fox JG. Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res. 2007;67:11536–11546. doi: 10.1158/0008-5472.CAN-07-1479. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Fujisawa T, Ogikubo K, Ohzono T, Ishihara K, Takahashi T. Characterization of Campylobacter lanienae from pig feces. J. Vet. Med. Sci. 2003;65:129–131. doi: 10.1292/jvms.65.129. [DOI] [PubMed] [Google Scholar]
- Shen Z, Feng Y, Dewhirst FE, Fox JG. Coinfection of enteric Helicobacter spp. and Campylobacter spp. in cats. J. Clin. Microbiol. 2001;39:2166–2172. doi: 10.1128/JCM.39.6.2166-2172.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E, Lee Y. Comparison of three different methods for campylobacter isolation from porcine intestines. J. Microbiol. Biotechnol. 2009;19:647–650. [PubMed] [Google Scholar]
- Stern NJ, Robach MC, Cox NA, Musgrove MT. Effect of drinking water chlorination on Campylobacter spp. colonization of broilers. Avian Dis. 2002;46:401–404. doi: 10.1637/0005-2086(2002)046[0401:EODWCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Whary MT, Fox JG. Natural and experimental Helicobacter infections. Comp. Med. 2004;54:128–158. [PubMed] [Google Scholar]
- Wysok B, Wiszniewska-Łaszczych A, Uradziński J, Szteyn J. Prevalence and antimicrobial resistance of Campylobacter in raw milk in the selected areas of Poland. Pol. J. Vet. Sci. 2011;14:473–477. doi: 10.2478/v10181-011-0070-3. [DOI] [PubMed] [Google Scholar]