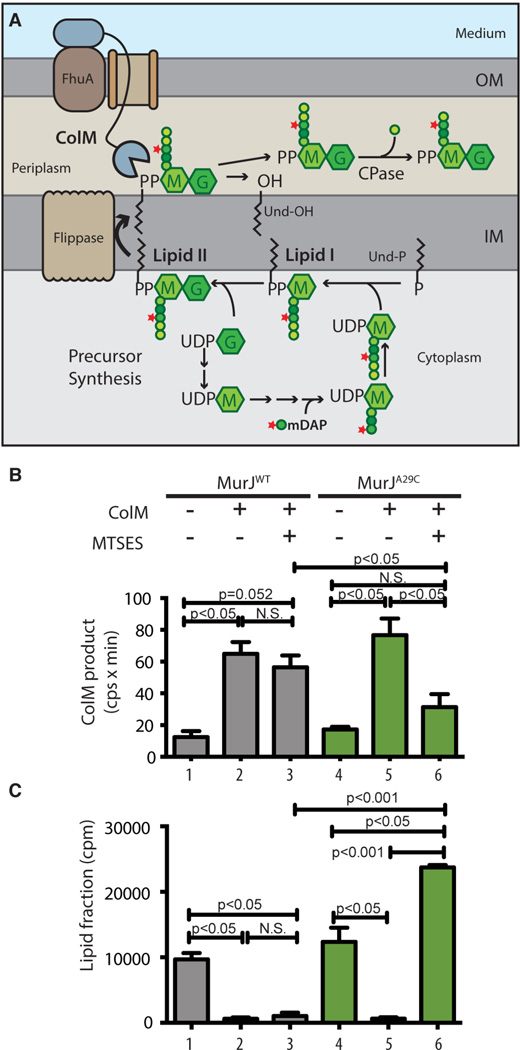
Fig. 1. In vivo assay for lipid II flippase activity.
(A) PG precursor synthesis starts with the conversion of UDP-N-acetylglucosamine (UDP-G) to UDP-N-acetylmuramic acid (UDP-M), followed by the addition of amino acids (represented by colored spheres) to UDP-M to form the pentapeptide (pep5) stem (L-Ala-γ-D-Glu-m-DAP-D-Ala-D-Ala). [3H]-mDAP label is indicated by the star. The UDP-sugars are transferred to undecaprenol-P (Und-P) in the IM to form lipid II, which is flipped across the IM to expose the disaccharide-pep5 (Mpep5-G) for polymerization and crosslinking into PG (not illustrated). Exogenous ColM binds to FhuA and is translocated across the OM presumably through a porin. In the periplasm, ColM cleaves lipid II into undecaprenol (Und-OH) and soluble PP-Mpep5-G, which is further processed by carboxypeptidases (CPase) to produce PP-Mpep4-G. (B-C) Cells of ΔmurJ ΔlysA strains producing FLAGMurJ lacking endogenous Cys residues referred to as MurJWT (NR2592) or its derivative MurJA29C (NR2593) were labeled with [3H]-mDAP. After 15 min, ColM and/or MTSES were added as indicated and growth was continued for 10 min. Samples were then withdrawn and either extracted with hot water alone or sequentially with water then butanol. Hot water extracts were subjected to high-performance liquid chromatography (HPLC) and radiodetection to quantify the labeled ColM product (B); scintillation counting was used to quantify label in the lipid (butanol) fraction (C). See figs. S1–S4 for experimental details and peak identification. Shown are the mean ± SD; N = 3. P value determined with Student’s t test. N.S., not significant.