Abstract
Functional changes in basal ganglia circuitry are responsible for the major clinical features of Parkinson’s disease (PD). Current models of basal ganglia circuitry can only partially explain the cardinal symptoms in PD. We used functional MRI to investigate the causal connectivity of basal ganglia networks from the substantia nigra pars compacta (SNc) in PD in the movement and resting state. In controls, SNc activity predicted increased activity in the supplementary motor area, the default mode network, and dorsolateral prefrontal cortex, but, in patients, activity predicted decreases in the same structures. The SNc had decreased connectivity with the striatum, globus pallidus, subthalamic nucleus, thalamus, supplementary motor area, dorsolateral prefrontal cortex, insula, default mode network, temporal lobe, cerebellum, and pons in patients compared to controls. Levodopa administration partially normalized the pattern of connectivity. Our findings show how the dopaminergic system exerts influences on widespread brain networks, including motor and cognitive networks. The pattern of basal ganglia network connectivity is abnormal in PD secondary to dopamine depletion, and is more deviant in more severe disease. Use of functional MRI with network analysis appears to be a useful method to demonstrate basal ganglia pathways in vivo in human subjects.
Keywords: Parkinson’s Disease, Basal ganglia circuits, Dopaminergic deficits, Granger Causality analysis, Substantia nigra
Introduction
Dysfunction of basal ganglia (BG) circuitry is responsible for the development of the cardinal features in Parkinson’s disease (PD) [7]. The most accepted BG-thalamo-cortical indirect and direct pathways model provides an explanation for the origin of akinesia in PD, but has difficulty explaining other features such as rigidity, tremor, and some non-motor symptoms [8,18,19]. As most anatomical methods are invasive and can not be applied to human subjects, our knowledge about BG circuitry has been mainly from animal studies, which may not be appropriate for humans. Additionally, animal PD models can present some parkinsonism symptoms, but the pathogenesis of these models is different from idiopathic PD. Thus, these animal models may not really reflect PD-related BG circuitry changes.
The development of functional neuroimaging techniques provides useful methods to analyze effective network connectivity [9]. Granger causality analysis (GCA) is a promising effective connectivity analysis method for inferring directions of neural interactions and information flow directly from neuroimaging data [10], which can estimate the directionality of modulation from recorded time series across all nodes of a network without a priori assumptions [5,11]. In this study, we used functional MRI (fMRI) and GCA method to investigate causal connections within BG circuitry. We anticipated that this study would help to improve the BG model, and provide more information about PD-related neural networks changes.
Material and methods
We studied 16 PD patients (10 male, 6 female; mean age 58.1 ± 4.8 years, disease duration 24.6 ± 7.7 months), and 16 age and sex matched healthy participants. The subjects were all right-handed. The diagnosis of idiopathic PD was based on medical history, physical, neurological, and laboratory examinations. None of the patients had ever had a dopaminergic or other anti-parkinsonian drug treatment (de novo). Akinesia was the predominant symptom in every patient and all patients had at most a mild tremor. The average Unified Parkinson’s Disease Rating Scale (UPDRS) [15] score was 21.5 ± 7.1, and Hoehn and Yahr disability scale [12] was 1.4 ± 0.5. Mini-Mental State Exam was ≥27 in all subjects. The experiments were performed according to the Declaration of Helsinki and were approved by the Institutional Review Board. All subjects gave their written informed consent for the study.
The subjects were at either resting or movement state during fMRI. In the resting state, subjects were instructed to keep their eyes closed, to remain motionless, and to not think of anything. In the movement state, the subjects were asked to perform a self-paced motor task, during which they briskly tapped their right index finger at an interval of 2s. Because we focused on effective connectivity of brain networks, the subjects maintained finger tapping during the entire movement scanning session. Before the scanning, the subjects were trained to execute the finger tapping at the required interval. An electrical response button was used to record the intervals between finger movements during scanning.
fMRIs were performed on a 3T Siemens Sonata scanner. High-resolution anatomical images were acquired with 3D-MPRAGE sequence (TR = 2530 ms, TE = 3.39 ms, 128 axial slices, 1.33 mm thickness, field of view (FOV) = 256 mm). Blood-oxygen-level dependent (BOLD) data were acquired with gradient-echo echo-planar sequences. Whole brain fMRI scanning with a relatively long acquisition time (TR = 2000 ms, TE = 30 ms, 33 axial slices, 3.5 mm thickness, FOV = 220 mm) was performed for GCA analysis of connectivity between BG and cortical areas and cerebellum. Because of the close distances among the BG nuclei, the influences between these nuclei must happen in very short time. Thus, we used a much shorter acquisition time (TR =400 ms), and only covered BG regions (7 axial slices, 3.5 mm thickness, Supplementary Fig.1), to increase the sensitivity of GCA analysis to detect time-directed associations among BG nuclei. Each subject had 4 fMRI scans: resting state with long TR, resting state with short TR, movement state with long TR, and movement state with short TR. Each fMRI scan lasted for 8 min.
Healthy subjects had one scanning session, whereas patients had two sessions, in the clinical off and on states. Patients were first scanned without taking levodopa (off state). After the first session, levodopa was administered orally as 250 mg Madopar (200 mg levodopa/50 mg benserazide, Roche company, Shanghai). The second session was performed 60 min after levodopa had been given, when all patients achieved clinical on state (average UPDRS score was 14.3 ± 4.8).
The intervals between the movements during fMRI scanning were recorded and compared between groups (two-sample t-test). Preprocessing of fMRI data was using SPM8. Data were slice-time corrected, aligned to the first image of each session, and co-registered to high-resolution anatomical images. After spatial normalization, all images were resampled into voxels that were 3×3×3 mm in size, and smoothed with a 4 mm Gaussian smoothing kernel. Linear drift was removed. Nuisance covariates including the three translational and three rotational head-motion estimates, and global mean signal were regressed. Because GCA using relatively low lag orders, operate on high frequency deflections in time-course data, our data were not low-pass filtered [11].
Because the substantia nigra pars compacta (SNc) is the primary area of pathological changes in PD, we chose the SNc bilaterally as regions of interest (ROIs) for GCA analysis. The SNc appears as a strong hypointense signal intensity on T2-weighted images in the mesencephalon so that its borders are easily identifiable. We defined the center of the ROI of SNc as x = 10 (right side), or −10 (left side); y = −12; z = −11 (MNI coordinates), and the radius as 3 mm, as with these parameters we could ascertain that the ROI was located in the SNc and did not extend to any adjacent areas [16].
We used signed path coefficient to reveal the Granger causality between the SNc and whole brain areas [7], with Resting-State fMRI Data Analysis Toolkit (REST, http://www.restfmri.net). We estimated time-directed prediction between BOLD time-series across a lag of one TR (2000 ms for the long TR data or 400 ms for the short TR data) in order to maximize the temporal resolution of our estimates of neural influence. We first identified both SNc to other region and other regions to SNc causal connectivity in each condition in each group (one-sample t-test). Then, second-level random-effect voxel-wise comparisons were applied to compare the GCA results: (1) between movement and resting condition to identify movement-induced changes (paired t-test); (2) between control and patients in the off state to explore PD-related modulations (two-sample t-test); and (3) between patients in the on and off state to examine the dopaminergic effects on BG networks (paired t-test). To explore whether the networks changes correlate with the disease severity, a correlation analysis of GCA results versus the UPDRS score was performed in patients in the off state. A false discovery rate (FDR) corrected threshold of p < 0.05 was used for all statistical analysis.
Results
The mean between tapping interval across the group of controls and patients was 2.04 ± 0.16s and 2.03 ± 0.17s, respectively. There was no significant difference between the two groups (p = 0.730, two-sample t-test). To exclude possible influence of fatigue, a repeated-measures ANOVA was performed, and found that the tapping intervals were stable across the movement session in the patients (repeated-measures ANOVA, p = 0.644). Therefore, motor performance had no effect on our results in the movement state.
In the current study, positive/negative results of GCA analysis indicate that the increased activity in the SNc predicts subsequent increasing/decreasing activity in the corresponding areas. The results between the SNc and other BG nuclei are from the short TR fMRI data, whilst the results between the SNc and cortical areas and cerebellum are from the long TR data. In order to show the findings clearly and concisely, the results from the short and long TRs are presented together. The BG nuclei that had connectivity with the SNc were similar in the short and long TR sessions.
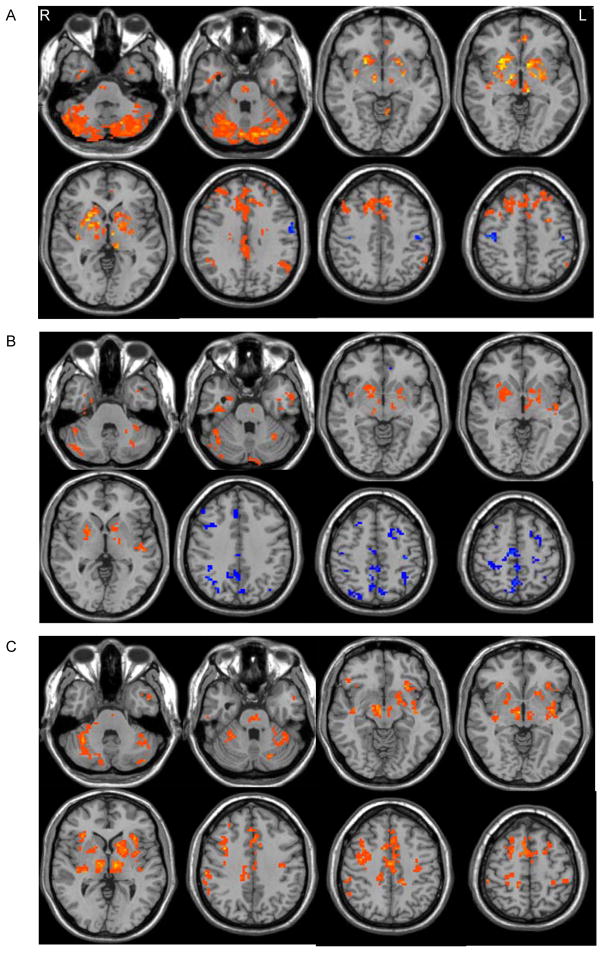
In the resting state, in both controls (Fig. 1a) and patients in the off state (Fig. 1b), both sides of SNc had positive influences on the contralateral SNc, medulla, pons, and bilateral putamen, caudate nucleus, external globus pallidus (GPe), internal globus pallidus (GPi), thalamus, and subthalamic nucleus (STN), insula, temporal cortex, and cerebellum. In contrast, the pattern of influences from the SNc to the cortical motor areas was different between the two groups. While both groups showed negative connectivity with the bilateral primary motor cortex (M1), the SNc had positive connectivity with the bilateral supplementary motor area (SMA), cingulate motor area (CMA), and premotor cortex (PMC) in controls (Fig. 1a), but had negative connectivity with these regions in patients (Fig. 1b). The SNc positively connected with the posterior cingulate cortex (PCC), precuneus, medial prefrontal cortex (MPFC), ventral anterior cingulate cortex (vACC), and bilateral inferior parietal lobe (IPL) in controls; these regions compose the so-called “default mode network” (DMN) [22]. The SNc had positive connectivity with the dorsolateral prefrontal cortex (DLPFC) in controls, but negatively connected with the DMN and DLPFC in patients.
Figure 1. GCA results from the left SNc in the resting state.
Brain regions receiving significant positive (hot color) or negative (cold color) influences from the left SNc in healthy controls (A), and PD patients (B) in the resting state (one-sample t-test, p < 0.05, FDR corrected).
C: Brain regions having significant influences on the left SNc in healthy subjects in the resting state (one-sample t-test, p < 0.05, FDR corrected).
In both groups, the SNc received positive influences from other BG regions (including the contralateral SNc, bilateral putamen, caudate nucleus, GPe, GPi, thalamus, and STN), cortical motor areas (including the SMA, CMA, bilateral M1, and PMC), DMN, DLPFC, insula, tempotal cortex, cerebellum, pons, and medulla in both movement and resting states (Fig. 1c).
In controls, the connectivity from the SNc during movement were similar to that in the resting state; only that the SNc had positive effect on the bilateral M1 (Supplementary Fig. 2a). In patients, during movement, the SNc had positive connectivity not only with other BG regions, temporal cortex, insula, and cerebellum, but also with cortical motor areas (including the SMA, CMA, bilateral M1, and PMC), DLPFC, and DMN (except the vACC) (Supplementary Fig. 2b).
The SNc had increased connectivity with the contralateral SNc, SMA, bilateral M1, PMC, DLPFC, insula, putamen, GPe, GPi, thalamus, temporal lobe, cerebellum, and brainstem in the movement condition than in the resting state in both groups. The connectivity between the SNc and the vACC was increased in controls, but was decreased in patients during movement compared to that in the resting state (Supplementary Fig. 3).
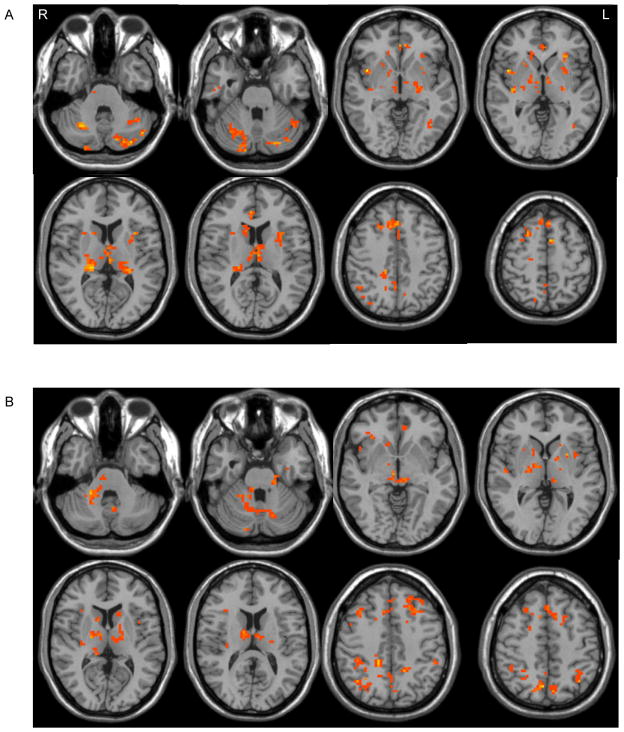
In the resting state, the SNc had decreased connectivity with the SMA, DLPFC, insula, caudate nucleus, putamen, GPe, GPi, STN, thalamus, DMN, temporal lobe, cerebellum, and pons in patients in the off state than that in controls (Fig. 2a). In the movement state, besides these differences, the SNc showed less connectivity with the left M1 (contralateral to the movement side) in patients compared to controls. The SNc had more connectivity with the SMA, DLPFC, insula, caudate nucleus, putamen, GPe, GPi, STN, thalamus, DMN, cerebellum, and pons in the on state than that in the off state in patients (Fig. 2b).
Figure 2. Differences between PD patients and controls, and between on and off state.
A: Brain regions receiving significantly increased influences from the left SNc in healthy controls than in PD patients in the resting state (two-sample t-test, p < 0.05, FDR corrected).
B: Brain regions receiving significantly increased influences from the left SNc in PD patients when on compared to off in the resting state (paired t-test, p < 0.05, FDR corrected).
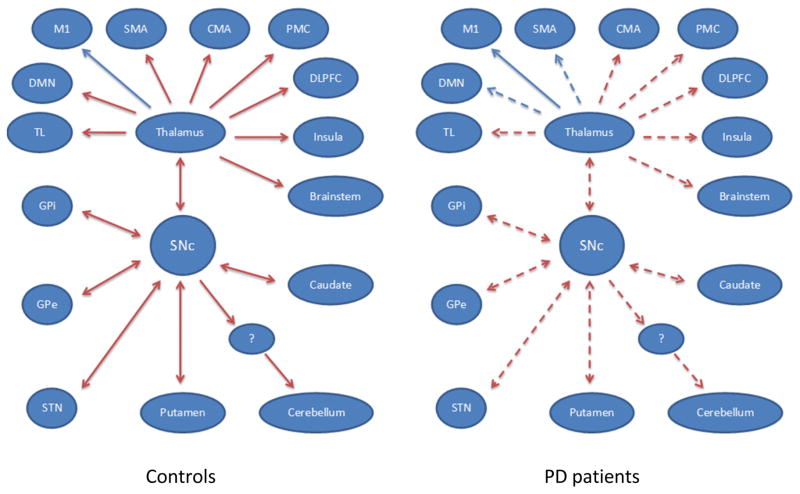
GCA results were negatively correlated with the UPDRS scores in the SMA, bilateral M1, PMC, DLPFC, medial prefrontal cortex, vACC, insula, caudate nucleus, putamen, GPe, GPi, STN, thalamus, temporal lobe, and cerebellum in patients in the off state, which means as the UPDRS increased, the influences from the SNc to these regions are weakened. A graphical model of the pattern of connectivity in the SNc in controls and patients, and the difference between the two groups is shown in Fig. 3.
Figure 3. The different pattern of connectivity in the SNc in healthy controls and PD patients in the resting state.
Red/blue lines indicate positive/negative influences of the SNc with other brain regions. The arrows indicate the directionality of influences between the SNc and other regions. The dotted lines indicate decreased connectivity from the SNc to the corresponding brain regions in PD patients compared to healthy controls.
Abbreviations: CMA, cingulate motor area; DLPFC, dorsolateral prefrontal cortex; DMN, default mode network; GPe, external globus pallidus; GPi, internal globus pallidus; M1, primary motor cortex; PMC, premotor cortex; SMA, supplementary motor area; SNc, substantia nigra pars compacta; STN, subthalamic nucleus; TL, temporal lobe. The question mark indicates uncertain brain region.
Discussion
The present study, for the first time, identified brain regions whose activity follows or predicts activations in the SNc, and how these “causal” connections were abnormal in PD. The novel finding is that the SNc has not only reciprocal influences with other BG nuclei, cortical motor areas, and brainstem, but also with the DMN, DLPFC, temporal cortex, and cerebellum. These connections are disordered in PD secondary to dopamine deficiency.
The SNc had increased connectivity with the regions in the BG-thalamo-cortical motor loop (i.e. M1, SMA, PMC, putamen, GPe, GPi, STN, thalamus, and brainstem) during movement compared to the resting state, which suggests that the motor loop needs stronger dopaminergic influences in order to maintain movement execution. In controls, the SNc had a positive effect on the SMA, and had a negative effect on the M1 in the resting state; but had positive effect on both the SMA and M1 during movement. Thus, a function of the dopaminergic system in the baseline condition is likely to facilitate the motor preparation networks in order to prepare for the coming movements, and to inhibit the motor execution networks to restrict the unnecessary movements. In contrast, the dopaminergic system facilitates both motor preparation and execution networks to maintain motor execution.
In the resting state, the SNc had negative influence on the SMA in PD, but had positive influence on the SMA in controls. During movement, the influence from the SNc to the SMA and M1 was also weaker in PD than in controls. Thus, the effect from the dopaminergic system to the output cortical target of BG-thalamo-cortical motor loop is weakened or even reversed, which in turn impairs function of cortical motor areas [2,7,]. Because the SMA is important in motor preparation and initiation [13], and the M1 is critical in motor execution, dysfunction of this motor circuit should impair the ability to initiate or execute movements, which is likely a crucial reason for motor deficits in PD, like akinesia and bradykinesia.
The SNc showed less influence to the lower brainstem in PD than that in controls. The pathological changes of PD may initially appear at the brainstem below the SN, and extend into the midbrain and neocortex gradually [4]. Because the brainstem is an outflow target of BG motor circuit [8], the disrupted BG-brainstem interaction might also be involved in the development of parkinsonian motor signs.
The SNc also had connectivity with many regions out of the BG motor loop. The caudate nucleus and DLPFC are critical for the oculomotor and dorsolateral prefrontal loops; the caudate nucleus is a part of the orbitofrontal loop; the temporal lobe, insula, ventral striatum, and ACC are involved in the limbic loop. The reduced influences from the dopaminergic system may disrupt the functions of these loops, and contribute to some non-motor symptoms in PD, like cognitive, psychiatric, or autonomic impairments.
The function of DMN is thought to facilitate cognitive performance [22], while malfunction of DMN has been related with cognitive impairment [21]. The relationship between the BG or dopaminergic system and DMN has been rarely studied [14,17]. We demonstrate that there is a reciprocal causal connection between the SNc and DMN, moreover, dopamine depletion induces decreased influence from the dopaminergic system to the DMN, which in turn may disrupt the function of DMN. Cognitive problems also exist in many PD patients [1]. The malfunctioning or functional disconnection of the DMN has been observed in PD during cognitive tasks [6,24]. It may therefore be hypothesized that the dysfunction of the DMN secondary to dopamine deficiency may play an important role in cognitive impairment in PD.
We found a reciprocal connectivity between the SNc and cerebellum. Recent studies have elucidated that the cerebellum and BG have substantial two-way anatomical connections [3]. The causal effect from the SNc to cerebellum was decreased in PD, which is likely a reflection of abnormal signals from the BG influencing cerebellar function. Because the cerebellum and BG are two major subcortical structures that influence multiple aspects of motor cognitive and affective behavior [23], weakened striatum-cerebellar connectivity might contribute substantially to the clinical problems in PD [25,26].
Levodopa usage increased connectivity between the SNc and extensive regions, thus, observed connectivity changes in PD are secondary to dopamine deficiency. Administration of levodopa partially restores normal pattern of interactions within BG circuits. The connectivities between the SNc and many areas were negatively correlated with the UPDRS scores in PD. Thus, as the disorder progresses, the pattern of BG network connectivity becomes more abnormal. The degree of BG network changes is likely a reflection of the level of dopaminergic system impairment. Whether various symptoms and their severity are associated with particular connectivity changes is worth further investigations.
GCA can not tell whether the influence from a region to another region is direct, or by way of other area(s). Thus, our study is still not enough to establish an anatomical model of BG loops. Animal studies [18,20], and diffusion tensor imaging study on human [16] approved that the SNc anatomically connects with the striatum, GPe, GPi, STN, and thalamus. Combining anatomical knowledge with our findings, we setup a preliminary model to show the network connectivity from the SNc in normals and PD (Fig. 3). We only chose the SNc as ROIs, but some other areas are also important in BG loops, like the striatum, GPe, GPi, and STN. Investigation of connectivity from these regions should provide further insights to our understanding of BG circuits.
Due to technical limitation, tremor was not measured during scanning, the confound of tremor was not removed. As our patients had most a mild tremor, tremor should have no significant influence on our findings. However, recording tremor and removing tremor related variance is benefit to imaging analysis in PD, which will be performed in our future studies.
In conclusion, our findings demonstrate that the combination of fMRI and network analysis is likely a useful tool to establish the BG pathways model in vivo in human subjects. The causal connectivity from the substantia nigra to many brain networks suggests that the dopaminergic system exerts influences on extensive brain functions, both motor and cognitive behaviors. The pattern of this connectivity is abnormal in PD secondary to dopamine depletion, and becomes more significant as the disorder progresses. The disrupted BG networks may contribute to the motor and some non-motor impairments in PD.
Supplementary Material
Depiction of imaging volume used to collect fMRI data with short TR (400 ms).
GCA results from the left SNc during movement performance. Brain regions receiving significant positive (hot color) or negative (cold color) influences from the left SNc in healthy controls (A), and PD patients (B) during movement performance (one-sample t-test, p < 0.05, FDR corrected).
The comparison of GCA results between the movement and resting state. Brain regions receiving significantly increased (hot color) or decreased (cold color) influences from the left SNc in healthy controls (A), and PD patients (B) in the movement state compared to the resting state (paired t-test, p < 0.05, FDR corrected).
Acknowledgments
This work was supported by grants from the National Science Foundation of China (grant numbers 30870693 and 81071012).
References
- 1.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Risk of dementia in Parkinson’s disease. A community-based, prospective study. Neurology. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 3.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–6. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Del Tredici K, Rüba U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Hamilton JP, Thomason ME, Gotlib IH, Saad ZS, Cox RW. Granger Causality via Vector Auto-Regression Tuned for FMRI Data Analysis. Proc Intl Soc Mag Reson Med. 2009;17:1718. [Google Scholar]
- 6.Delaveau P, Salgado-Pineda P, Fossati P, Witjas T, Azulay JP, Blin O. Dopaminergic modulation of the default mode network in Parkinson's disease. Eur Neuropsychopharmacol. 2010;20:784–92. doi: 10.1016/j.euroneuro.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 7.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 8.DeLong MR, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism Relat Disord Suppl. 2009;3:S237–40. doi: 10.1016/S1353-8020(09)70822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- 10.Granger CWJ. Investigating causal relations by economic models and cross-spectral methods. Econometrica. 1969;37:424–438. [Google Scholar]
- 11.Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: Multivariate granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry. 2011;16:763–72. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 13.Jahanshani M, Jenkins H, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- 14.Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K. L-dopa modulates functional connectivity in striatal cognitive and motor networks: A double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang AE, Fahn S. Assessment of Parkinson’s disease. In: Munsat TL, editor. Quantification of neurological deficit. Boston: Butterworths; 1989. pp. 285–309. [Google Scholar]
- 16.Menke RA, Jbabdi S, Miller KL, Matthews PM, Zarei M. Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson's disease. NeuroImage. 2010;52:1175–1180. doi: 10.1016/j.neuroimage.2010.05.086. [DOI] [PubMed] [Google Scholar]
- 17.Nagano-Saito A, Liu J, Doyon J, Dagher A. Dopamine modulates default mode network deactivation in elderly individuals during the Tower of London task. Neurosci Lett. 2009;458:1–5. doi: 10.1016/j.neulet.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Nambu A. Seven problems on the basal ganglia. Curr Opin Neurobiol. 2008;18:595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temino B, Mena-Segovia J, Rodriguez M, Olanow CW. The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol. 2008;64(suppl):S30–S46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- 20.Parent A, Hazrati LN. Multiple striatal representation in primate substantia nigra. J Comp Neurol. 1994;344:305–320. doi: 10.1002/cne.903440211. [DOI] [PubMed] [Google Scholar]
- 21.Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology. 2011;76:511–7. doi: 10.1212/WNL.0b013e31820af94e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 24.van Eimeren T, Monchi O, Ballanger B, Strafella AP. Dysfunction of the default mode network in Parkinson disease. A functional magnetic resonance imaging study. Arch Neurol. 2009;66:877–883. doi: 10.1001/archneurol.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson’s disease. NeuroImage. 2010;49:2581–2587. doi: 10.1016/j.neuroimage.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. NeuroImage. 2011;55:204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Depiction of imaging volume used to collect fMRI data with short TR (400 ms).
GCA results from the left SNc during movement performance. Brain regions receiving significant positive (hot color) or negative (cold color) influences from the left SNc in healthy controls (A), and PD patients (B) during movement performance (one-sample t-test, p < 0.05, FDR corrected).
The comparison of GCA results between the movement and resting state. Brain regions receiving significantly increased (hot color) or decreased (cold color) influences from the left SNc in healthy controls (A), and PD patients (B) in the movement state compared to the resting state (paired t-test, p < 0.05, FDR corrected).