Abstract
Dietary non-adherence to sodium restriction is an important contribution to heart failure (HF) symptom burden, particularly in older adults. While knowledge, skills, and attitudes towards sodium restriction are important, sodium intake is closely linked to the ability to taste salt. The ‘hedonic shift’ occurs when sodium restriction induces changes in an individual’s salt taste that lower subsequent salt affinity. Older adults often have compromised salt taste and higher dietary salt affinity due to age-related changes. Older HF patients may have additional loss of salt taste and elevated salt appetite due to comorbid conditions, medication use, and micronutrient or electrolyte abnormalities, creating a significant barrier to dietary adherence. Induction of the hedonic shift has the potential to improve long-term dietary sodium restriction and significantly impact HF outcomes in older adults.
Introduction
Heart failure (HF) is a significant public health problem affecting over 5 million people in the United States.1 With rates disproportionally affecting older adults, the annual incidence in those above 75 years of age approaches 18 per 1000 persons.2 HF additionally places a tremendous economic burden on the healthcare system, with greater than 1 million hospital admissions yearly and annual inpatient expenditures exceeding $15 billion.3,4
The pursuit of effective therapeutic interventions in older adults has been limited by a difficult-to-treat phenotype of HF, which occurs in the background of normative age related changes in cardiovascular (CV) structure and function and in the setting of multiple comorbidities.5,6 The majority of older adults with HF have preserved ejection fraction with concomitant hypertension (HTN) that contributes to a constellation of abnormalities including left ventricular (LV) hypertrophy, large-arterial stiffness, diastolic, and to a lesser extent systolic LV dysfunction.7 Among the risk factors for HF, HTN has the highest population attributable risk in older adults and is modifiable by dietary interventions.8
Animal models and preliminary human studies suggest that dietary sodium restriction can improve cardiac and vascular function in older HF patients.9,10 Current guidelines promote dietary sodium restriction as an essential element of HF management. Nonetheless, non-adherence to sodium restricted diet remains one of the most important contributions to HF hospitalizations and overall morbidity—particularly in older adults.11–13,14,16 As such, therapeutic approaches that target dietary non-adherence to sodium restriction have the potential for great impact on HF disease burden. In this paper we re-introduce the concept of the hedonic shift—an observation about changes in salt taste affinity after sodium restriction—and make the case for its use as a therapeutic intervention for the treatment of HF in older adults. Of note, the term ‘salt’ will be used in reference to taste thresholds and appetite, whereas ‘sodium’ will be used in reference to dietary characteristics, serum concentrations, and molecular characterizations such as ion channels.
Normal taste changes with age
In addition to enabling humans to recognize the flavor and palatability of a given food, the sense of taste protects the body against rancid food products and toxins, and aids in digestion by triggering gastrointestinal secretions.14 Taste occurs through multiple nervous system pathways responsible for transmitting taste information to the brain from receptors within taste buds on the tongue that can recognize salty, bitter, sweet, sour and umami (a pleasant savory taste characteristic of ripe tomatoes).15 Changes in taste sensation can impact a person’s health through unfavorable food selection or intake, and have been implicated in causing malnutrition, weight loss, impaired immunity and worsening of existing illnesses.16
Important changes occur with normal aging that affect taste perception, but not all of these factors are related to inherent taste sensation (Table 1). The most frequent causes of taste dysfunction in the elderly include deterioration of oral hygiene and subsequent oral and perioral infections,17 increased prevalence of oral appliances such as dentures or prosthetics,18 diminished olfactory sensation,19 consequences of chemical exposures such as prolonged smoking,20 nutritional deficiencies, and medications.
Table 1.
Causes of taste loss in the elderly
| Cause | Example |
|---|---|
| High frequency | |
| Oral hygiene deterioration and infection | Gingivitis, periodontitis, candidiasis, sialadenitis |
| Oral appliances | Dentures, prosthetics, fillings |
| Diminished olfactory sensation | Sinusitis, neurodegenerative disease |
| Chemical exposure | Prolonged smoking |
| Dental procedures | Extractions, root canals |
| Nutritional deficiency | Vitamin B12, Zinc |
| Medications | See Table 2 |
| Moderate frequency | |
| Malignancy | Oral cancer, skull cancers |
| Endocrine disorders | Diabetes mellitus, hypothyroidism, Adrenal insufficiency |
| Head trauma | |
| Radiation to head and neck | |
| Low frequency | |
| Epilepsy | Gustatory aura |
| Migraines | Gustatory aura |
| Sjogren’s syndrome |
With regards to medication use, elderly patients are particularly at risk due to polypharmacy and use of medications with successive taste interactions—including many common classes of drugs such as antibiotics, anti-neoplastic agents, neurologic agents and psychotropics, cardiac medications, and endocrine agents (Table 2).21,22 Head and neck malignancies, radiation, and head trauma are moderately common causes of taste dysfunction in the elderly, as are endocrine disorders such as diabetes mellitus, hypothyroidism, adrenal insufficiency, and Cushing’s syndrome.23 Several less common causes of taste dysfunction in older adults include gustatory auras from epilepsy or migraine disorders, and Sjogren’s syndrome (from reduced secretions that bathe taste buds and are necessary for function).24
Table 2.
Common medications causing taste dysfunction
| Medication Class | Specific Agent |
|---|---|
| Antibiotics | Ampicillin, azithromycin, ciproflozacin, clarithromycin, ethambutol, metronidazole, quinolones, sulfamethoxazole, tetracycline, trimethoprim |
| Antifungals | Griseofulvin, terbinafine |
| Antihistamines | Chlorphenamine, loratadine, pseudoephedrine |
| Anti-inflammatory | Beclometasone, budesonide, olchicine, dexamethasone, fluticasone priprionate, gold, penicillamine |
| Anti-neoplastic | Cisplatin, carboplatin, cyclophosphamide, doxorubicin, fluorouracil, levamisole, methotrexate, tegafur, vincristine |
| Antivirals | Acyclovir, amantadine, gancyclovir, interferon, oseltamivir, zalcitabine |
| Cardiac | Amiodarone, amiloride, captopril, diltiazem, enalapril, hydrochlorothiazide, losartan, nifedipine, nitroglycerin, propagenone, propranolol, spironolocatone, statins |
| Endocrine | Carbimazole, levothyroxine, pancrelipase, propylthiouracil, thiamazole |
| Neurologic | Amphetamine, anticholinergics, baclofen, carbamazepine, dantrolene, dexamphetamine, dihydroergotamine mesilate, levodopa, methylphenidate, phenytoin, sumatriptan topiramate, zolpidem |
| Psychotropic | Alprazolam, amitriptyline, buspirone, clomipramine, clozapine, desipramine, doxepin, flurazepam, imipramine, lithium, nicotine, nortriptyline |
Salt Taste
Sodium is tightly regulated in humans in order to maintain narrow fluid and electrolyte concentration ranges that regulate cellular functionality. This precise sodium homeostasis is mediated through complex and interrelated mechanisms including neuroendocrine, genetic, psychological, and environmental pathways—of which salt taste is critically important. The primary mechanism for transduction of salt taste involves the passage of sodium ions through specific membrane ion channels in the apical region of the taste bud.25 Expression of these ion channels by taste receptor cells mediates salt appetite in response to varying levels of sodium.26 Salt appetite is a motivated behavioral response, triggered through the activation of neuroendocrine pathways, which makes salt taste more rewarding when serum sodium is low.27 The renin-angiotensin-aldosterone system (RAAS) has been implicated in inducing salt appetite via angiotensin II and aldosterone levels in both serum and brain that mediate gustatory changes in response to various sodium concentrations.28 This was demonstrated in Dahl rats using the angiotensin converting enzyme inhibitor captopril (which does not cross the blood brain barrier), in which administration of captopril halted conversion of angiotensin I to II within the systemic circulation but yielded increased angiotensin II within the brain; resulting in higher salt appetite.28–30 In this respect, salt taste is unique—whereas sweet and umami are considered generally attractive tastes, and sour and bitter are generally unpleasant, salt taste can be transformed from attractive to unpleasant based on different levels of sodium concentration.31
The Hedonic Shift
I was at first at a great loss for salt; but custom soon reconciled the want of it; and I am confident that the frequent use of salt among us is an effect of luxury, and was first introduced only as a provocative to drink; except where it is necessary for preserving of flesh in long voyages, or in places remote from great markets. For we observe no animal to be fond of it but man: and as to myself, when I left this country, it was a great while before I could endure the taste of it in anything that I eat.
Gulliver’s Travels, 1726
When dietary sodium restriction alters an individual’s salt taste affinity, the result is an increased acceptance of low-sodium foods. This concept, known as the “Hedonic Shift,” has been observed for centuries, as in Jonathan Swift’s Gulliver’s Travels in which the effect of reduced dietary salt on desire and subsequent tolerance for salt taste is described.32 The scientific community began studying salt restriction more formally in the early 20th century after several early investigations hypothesized that excessive sodium chloride intake played a prominent role in the cause of essential HTN.33–36 Once these observations were confirmed in the 1950s and 1960s,37,38 numerous researchers began examining the trigger for excessive sodium intake—and subsequently showed that alterations in taste acuity may play a role.39,40 These key findings paved the way for several landmark studies of salt taste, demonstrating that hypertensive patients had a greater taste affinity for salt than did normotensive individuals.41
Measuring salt taste characteristics
In the decades that followed, research shifted its focus to understanding and measuring the characteristics of salt taste—including sensitivity, preference, and appetite (Table 3).42 The general term ‘salt affinity’ has since been used to describe an individual’s overall attraction to ingesting salty foods that encompasses the combination of each of these individual salt taste characteristics.
Table 3.
Salt taste characteristics—measurement and implications
| Taste Characteristic | Definition | How it is measured | Clinical/Practical Implications |
|---|---|---|---|
| Sensitivity | Capacity to identify the flavor of salt | Using detection threshold, recognition threshold and suprathresholds as below | Specific and quantifiable features provide metric for evaluating salt taste |
| Detection threshold | Minimum amount of a taste stimulus that can be detected | Applying increasing concentrations of a stimulus to tongue until any taste is recognized | Defines lower threshold of a salt stimulus; can readily be compared between groups |
| Recognition threshold | Minimum amount of a taste stimulus that can be identified as a salty taste (i.e. as opposed to sweet) | Applying increasing concentrations of a stimulus to tongue until salty taste is recognized | Adds salt specificity to detection thresholds in order to isolate this particular sense |
| Suprathreshold sensitivity | Degrees of a taste stimulus above a given threshold level | Assigning ranks or ratings to a given stimulus in proportion to a comparison stimulus | Resembles real-world diets in which salt contents are greater than detection and recognition thresholds; allows for measurement of degrees of saltiness |
| Preference | General inclination toward one taste over another | Rating how frequently one would want to consume a given salt concentration on a regular basis | Accessible for study subjects, but multifactorial etiology makes interpretation difficult |
| Appetite | Drive to consume salt | Quantification of salt consumption in an unrestricted population | Comprised of intrinsic and extrinsic causes, difficult to isolate and study |
Salt taste sensitivity, defined as the capacity for an individual to identify the flavor of salt, is a characteristic measured by two thresholds: detection and recognition. Detection is the minimum amount of a taste stimulus that a subject can detect; recognition is the minimum amount of a taste stimulus that can be identified as a particular taste (i.e. salty versus sweet). Although these thresholds depend on extrinsic conditions such as salivary sodium concentration and mucous membrane dryness, they have been validated in determining differences between individuals in the lower limits of taste and the amounts of a given stimulus needed to identify a particular taste. In a study comparing patients with HTN to those without, for example, detection thresholds were unchanged but recognition thresholds were higher among those with HTN; the investigators concluded that “hypertensives may eat more salt because they cannot taste it as well as can normotensive individuals.”39
Measurement of salt taste sensitivity also includes an assessment of stimuli that are above threshold levels, or suprathreshold sensitivity.42 Suprathreshold sensitivity can be thought of as level of saltiness, and is measured using scales in which an individual ranks a particular stimulus in proportion to a comparison stimulus (i.e. 7/10 salty compared to 3/10 salty). This adds significant information to the evaluation of taste, particularly because evaluation of taste above sensitivity thresholds more closely resemble actual diets that tend to have suprathreshold amounts of salt.43 Although initial studies failed to show differences in suprathreshold salt taste sensitivity according to baseline HTN states, this taste characteristic has proved to be the basis for the hedonic shift in work that will be described further—where levels of saltiness (suprathreshold sensitivity) change in proportion to baseline salt intake.
In contrast to salt taste sensitivity, which has specific and quantifiable features, salt preference represents an individual’s general inclination toward one taste over another. While salt preference is the most accessible salt taste characteristic, and thus readily measured, it is difficult to interpret owing to its multiple contributors. Salt preference may be comprised of a food’s intrinsic qualities including taste, smell, texture, temperature and color, as well as extraneous qualities such as subject’s physical and mental states, dietary history, and genetics.44 Salt preference is measured either by ranking one’s attitude toward a particular stimulus or by indicating how frequently one would like to consume it—the distinction is important because an individual might enjoy an item without wanting to consume it regularly.42 Salt preference has been studied extensively; for example, when patients were offered bottles of either water or saline, hypertensive patients consumed almost 3 times more saline than normotensive patients.45
The last characteristic of salt taste is appetite, defined as the drive to consume salt. Analogous to preference, salt appetite is a complex characteristic that relates to both intrinsic and extrinsic mechanisms, many of which are thought to be biobehavioral in origin and related to development, conditioning, habit, and dietary culture.46 Salt appetite is measured by quantifying salt consumption in an unrestricted population. Several studies have shown that normotensive and hypertensive individuals consumed a similar quantity of salt at baseline. However, when treated with diuretic therapy that results in net sodium loss, HTN patients developed higher salt appetites and ingested higher quantities of salt than non-HTN controls. This suggests that sodium deplete states produce a drive to consume salt which is greater in HTN than controls.42
These observations regarding the salt taste characteristics of sensitivity, preference, and appetite allowed for a formalized approach to examining the relationship between salt taste and CV disease. What followed were a series of studies that refined the concept of the hedonic shift and led to greater insight into its underlying mechanism as well as its potential for use as a therapeutic approach.
Sodium Restriction
An individual’s baseline level of sodium ingestion significantly affects subsequent salt affinity. In subjects with various baseline intake of sodium, for example, those with low sodium intake added less salt to salt-free tomato juice compared with higher sodium intake individuals, suggesting that low-sodium diets reduced affinity for salt.47 Sensitivity to salt taste and preferences for various salt levels are similarly dependent on high versus low levels of salt intake. In one study of 31 subjects, salt taste sensitivity was measured using a 7-category rating scale for intensity of saltiness with 5 concentrations of salt in water, bread and mashed potato. Salt preference was assessed using a 9-category hedonic rating scale with the bread and mashed potato to determine the preferred concentration, and total sodium intake was estimated using urinary sodium excretion over 7 days. Subjects with higher levels of total sodium intake had corresponding higher threshold sensitivity to salt taste and higher salt preferences.48
Yet the breakthrough work came when investigators administered low sodium diets to subjects for prolonged periods of time and studied the effects on their subsequent diets. When 56 young adults (mean age 37 years) were given low-sodium or control diets over 1 year, sodium reduction yielded a preference toward less salt, with significantly lower salt preferences obtained at 3 months (the earliest interval measured).49 Similarly, when 11 subjects were sodium restricted for 9 weeks but given free access to the use of pre-weighed saltshakers throughout the study period, subjects compensated (by increasing saltshaker use) only slightly (~20%) based on the level of reduction of dietary sodium; no changes in salt taste were reported.50 Finally, in a study of subjects placed on self-maintained, low-sodium diets for 5 months, perceived taste responses to salt (measured using 3 types of taste tests in which salt concentration of solutions, soups, and crackers were varied and presented 6 times in irregular order with measurement of intensity and pleasantness using a 9-point category rating scale) were all increased after sodium restriction when compared to controls.51 Although small numbers, healthy populations, and inconsistent methodologies limited these and other studies, the message was clear: reducing sodium intake can lower subsequent salt affinity.
Further insights characterized the nature of the hedonic shift in terms of time course and mechanism (Figure 1). Although most studies required over 2 months of sodium restriction in order for significant differences in salt taste to occur, some reported the hedonic shift after only 2 weeks, and nearly all suggested that before the shift occurred, an initial transient increase in salt affinity took place.49–54 The basic mechanism of the hedonic shift was demonstrated by a series of important studies that used table salt to modify underlying salt taste of foods without significantly altering underlying sodium content. In the first, the salt taste (i.e. suprathreshold sensitivity, modified by lowering added table salt) of a stimulus was reduced while the actual sodium content was kept the same, and the hedonic shift occurred.55,56 In the second, the sodium content was reduced but the salt taste was kept the same—the hedonic shift did not occur.50 Thus, the hedonic shift appears to stem from changes in salt taste rather than purely the sodium content.
Figure 1.
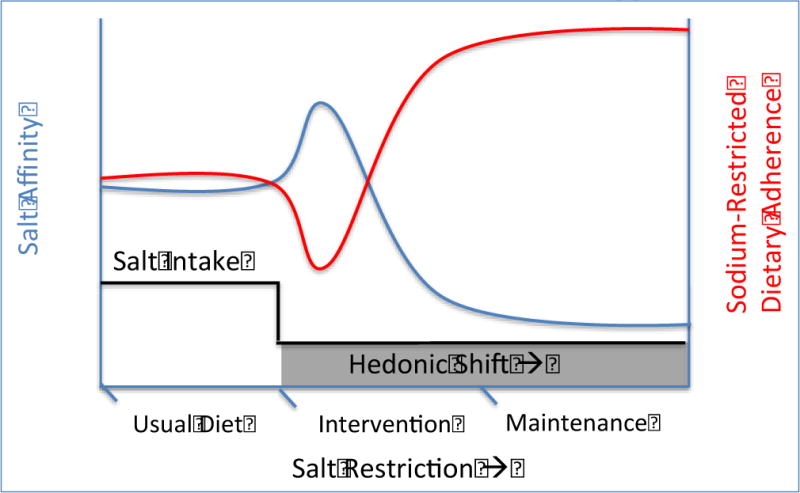
Theoretic representation of Hedonic shift induced changes in salt intake, salt affinity and sodium restricted dietary adherence patterns with salt restriction over time
Salt taste changes with age
Several intrinsic changes to salt taste function occur with aging. Such modulations are related to chemosensory deficits in the elderly that result in taste loss and are primarily responsible for diminished salt taste affinity that leads to increased salt intake. It has been shown both that elderly subjects have a salt detection threshold nearly 12 times higher than a younger cohort,57 and that older adults experience significantly reduced salt taste intensity (i.e. suprathreshold sensitivity) compared to younger controls.58 Chemosensory salt taste loss in older adults was previously thought to be due to a physiological decrease in the density of the taste buds and papillae, however newer studies have suggested that salt taste loss with aging may additionally be due to changes in taste cell membranes that result in impaired functioning of ion channels and receptors rather than reduction in the taste bud quantity.59,60
Zinc levels have additionally been implicated as a major cause of taste dysfunction in older adults. Older adults are prone to low serum zinc as a result of lower nutritional intake, increased diuretic use, and concomitant renal disease, all of which have been shown to decrease zinc in the body.61–63 A population based report of the third National Health and Nutrition Examination Survey (NHANES III) showed that older adults greater than 71 years had among the lowest percentage of adequate zinc intake.64 In one study of hospitalized elderly patients (mean age 84 years), one-third of patients presented with zinc deficiency.65 The mechanism of zinc deficiency and consequent taste dysfunction relates to a protein called gustin (carbonic anhydrase VI), which is essential for generalized taste affinity that encompasses salt taste (via the maintenance of pH homeostasis in saliva that is necessary for taste recognition).66 Zinc is bound to gustin in the blood, and corresponding zinc deficiency leads to gustin deficiency.67 The resulting impairment in taste acuity resulting from zinc deficiency was demonstrated in a study of 29 older adults (mean age 63 years) with chronic kidney disease, in which low serum zinc levels corresponded to reduced salt detection thresholds compared to healthy controls (mean age 38 years).68
Thus, normative changes in salt taste that occur with aging result in higher thresholds of salt sensitivity and salt taste affinity that may predispose older adults to downstream health effects.
Therapeutic implications
In addition to age related changes in salt taste, several features of HF make patients increasingly susceptible to the adverse effects of taste dysfunction. Common HF comorbidities, such as chronic kidney disease and HF, activate neurohormonal pathways through the RAAS that raise salt appetite through low serum sodium states.27,68 Additionally, frequently used HF medications contribute to this mechanism via sodium loss (as with diuretics) or elevated brain angiotensin II levels (as with RAAS pathway inhibitors which raise circulating angiotensin I and subsequent brain angiotensin II).28–30 These effects are exacerbated by zinc- and sodium-deficient states characteristic of the micronutrient and electrolyte environments observed in heart failure patients that elevate sodium appetite.69,70 Each of these qualities along with those that occur with aging make sodium restriction particularly difficult for older adults with HF. As a result, sodium restriction non-adherence is especially prevalent and contributes to a large percentage of HF hospitalizations.12,13
To our knowledge, there has been only one study examining salt taste affinity in HF patients; when 38 clinically stable HF patients (mean age 60 years, average ejection fraction 40%) were given various salt concentrations of bean soup and compared to healthy age-matched controls, the HF patients demonstrated a significantly greater preference for high salt soup.71 Since salt taste loss is pronounced in older adults, and older adults with HF represent a population in which dietary adherence is critical to reduce adverse outcomes, therapeutic interventions that target salt taste affinity in older adults have the potential for tremendous impact in the management of older adult patients with HF. We hypothesize that older adults with HF may benefit from several weeks of sodium restriction that induces a shift in salt taste affinity and yields subsequent dietary adherence (Figure 2).
Figure 2.
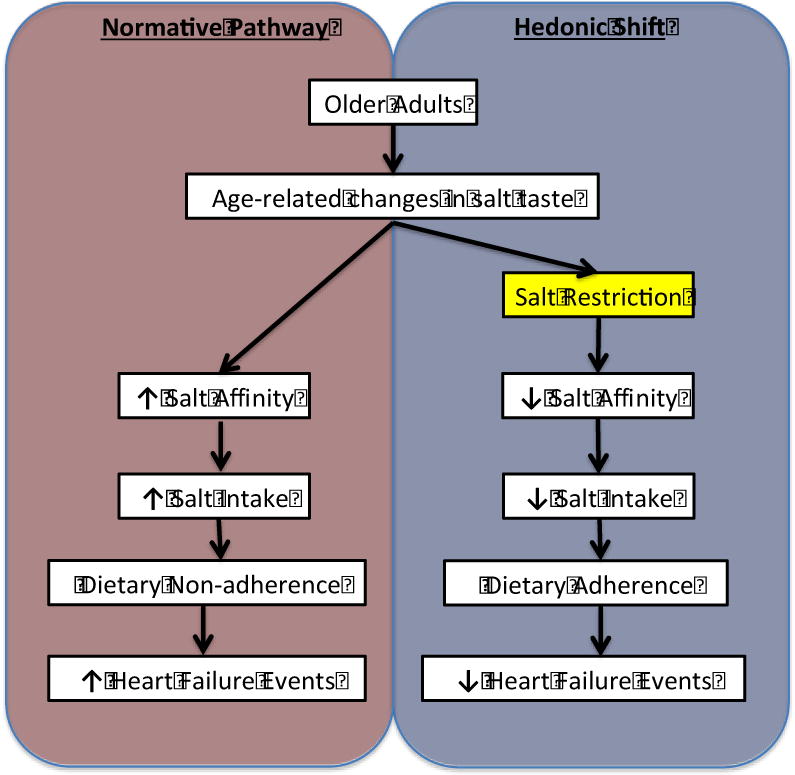
Normative pathway versus Hedonic shift intervention in Older Adults with heart failure
Various approaches to therapeutic sodium restriction have been proposed, including dietary counseling,72 sodium reduction in food supply,73 and labeling food packages,74 but none have focused on stimulating the hedonic shift through prolonged exposure to low sodium diet. The current management of acute decompensated HF involves sodium restriction during hospitalization.75 Yet as we have shown, data suggests that during the initial period of sodium restriction, patients may experience an initial and transient increase in salt taste affinity.49–54 Depending on the length of this period (which varies based on individual) and length of the hospitalization, this may produce a higher salt taste affinity at discharge that places patients at risk for subsequent decompensation due to dietary non-adherence. In the current model, therefore, an approach that targets sodium restriction in the post-discharge period rather than solely in the hospital may achieve greater results at this vulnerable stage.
Future Directions
While the therapeutic utilization of the hedonic shift has great potential for success in older adults with HF, it is a concept that may be widely applicable across populations. Presently, however, more research is needed to confirm the hedonic shift occurs in older adults with HF and further elucidate its mechanistic complexities. Moving forward, we promote the addition of salt taste affinity measurements to future HF and dietary studies as this important and novel field is progressed. Salt taste threshold testing has become relatively simple to measure, and several commercially available test strips and methodologies are now available in which various test strips containing salt concentrations are sequentially placed on a subject’s tongue, with corresponding taste characteristics readily measureable.68
Specifically, we advocate for 5 studies in order to advance the concept of the hedonic shift from theory to practice:
Age-specific study of salt taste in older adults with HF—comparing salt taste characteristics between older adults without HF, younger adults with HF, and older adults with HF (controlling for comorbidities that accompany the HF phenotype).
Evaluating the characteristics of the hedonic shift across age ranges, relevant comorbidities as well as racial and ethnic groups (accounting for cultural food preferences).
Assessing the hedonic shift as a therapeutic intervention in HF—evaluating the effect of sodium restricted diet on hedonic shift and subsequent dietary practices in patients with HF.
Elucidating the mechanisms behind the hedonic shift—animal and in vitro evaluation of taste mechanisms after sodium restriction.
Quantification of hedonic shift characteristics—determining length required for the shift to occur, duration of action, and levels of sodium restriction required to induce the hedonic shift.
Conclusion
In summary, the hedonic shift represents an exciting possibility for a therapeutic dietary intervention in older adults with HF. In the preceding manuscript we have made the case that non-adherence to sodium restricted diet is an important contribution to HF hospitalizations and adverse HF outcomes in older adults, in part owing to compromised salt taste and higher salt affinity that occurs with age related changes. When sodium restriction induces changes in an individual’s salt taste that consequently reduces salt affinity, the result may be improved dietary adherence to sodium restricted diets. Future studies are needed to clarify the characteristics of the hedonic shift, elucidate its mechanism, and evaluate its effectiveness as a successful intervention in older adults with HF.
Abbreviations
- CV
Cardiovascular
- HF
Heart Failure
- HTN
Hypertension
- LV
Left ventricular
- AAS
Renin-angiotensin-aldosterone system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 3.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. The New England journal of medicine. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 4.Fida N, Pina IL. Trends in heart failure hospitalizations. Curr Heart Fail Rep. 2012;9:346–53. doi: 10.1007/s11897-012-0117-5. [DOI] [PubMed] [Google Scholar]
- 5.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 7.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–34. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 8.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. Jama. 1996;275:1557–62. [PubMed] [Google Scholar]
- 9.Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. see comment. Hypertension. 2006;47:901–11. doi: 10.1161/01.HYP.0000215579.81408.8e. [DOI] [PubMed] [Google Scholar]
- 10.Hummel SL, Seymour EM, Brook RD, et al. Low-Sodium Dietary Approaches to Stop Hypertension Diet Reduces Blood Pressure, Arterial Stiffness, and Oxidative Stress in Hypertensive Heart Failure With Preserved Ejection Fraction / Novelty and Significance. Hypertension. 2012;60:1200–6. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinson JM, Rich MW, Sperry JC, Shah AS, McNamara T. Early readmission of elderly patients with congestive heart failure. [See comments] J Am Geriatr Soc. 1990;38:1290–5. doi: 10.1111/j.1532-5415.1990.tb03450.x. [DOI] [PubMed] [Google Scholar]
- 12.Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–54. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 13.Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart. 1998;80:437–41. doi: 10.1136/hrt.80.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bromley SM. Smell and taste disorders: a primary care approach. Am Fam Physician. 2000;61:427–36. 38. [PubMed] [Google Scholar]
- 15.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–94. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 16.Mattes RD, Cowart BJ. Dietary assessment of patients with chemosensory disorders. J Am Diet Assoc. 1994;94:50–6. doi: 10.1016/0002-8223(94)92041-9. [DOI] [PubMed] [Google Scholar]
- 17.Hyde RJ, Feller RP, Sharon IM. Tongue brushing, dentifrice, and age effects on taste and smell. J Dent Res. 1981;60:1730–4. doi: 10.1177/00220345810600100101. [DOI] [PubMed] [Google Scholar]
- 18.Duffy VB, Cain WS, Ferris AM. Measurement of sensitivity to olfactory flavor: application in a study of aging and dentures. Chem Senses. 1999;24:671–7. doi: 10.1093/chemse/24.6.671. [DOI] [PubMed] [Google Scholar]
- 19.Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226:1441–3. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 20.Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. Jama. 1990;263:1233–6. [PubMed] [Google Scholar]
- 21.Schiffman SS. Drugs influencing taste and smell perception. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB, editors. Smell and Taste in Health and Disease. New York: Raven Press; 1991. pp. 845–50. [Google Scholar]
- 22.Doty RL, Shah M, Bromley SM. Drug-induced taste disorders. Drug Saf. 2008;31:199–215. doi: 10.2165/00002018-200831030-00002. [DOI] [PubMed] [Google Scholar]
- 23.Deems DA, Doty RL, Settle RG, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117:519–28. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 24.Negoro A, Umemoto M, Fujii M, et al. Taste function in Sjogren’s syndrome patients with special reference to clinical tests. Auris Nasus Larynx. 2004;31:141–7. doi: 10.1016/j.anl.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–5. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 26.Chandrashekar J, Kuhn C, Oka Y, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagman W. Sodium chloride deprivation: development of sodium chloride as a reinforcement. Science. 1963;140:1403–4. doi: 10.1126/science.140.3574.1403. [DOI] [PubMed] [Google Scholar]
- 28.Tamura R, Norgren R. Intracranial renin alters gustatory neural responses in the nucleus of the solitary tract of rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1108–18. doi: 10.1152/ajpregu.00574.2002. [DOI] [PubMed] [Google Scholar]
- 29.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 30.Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- 31.Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–25. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- 32.Swift J. Gulliver’s Travels. Boston: Houghton Mifflin Company; p. 1726. [Google Scholar]
- 33.Ambard L, Beaujard E. La retention chloruree seche. Sem Med. 1905;25 [Google Scholar]
- 34.Allen FM. Arterial hypertension. Jama. 1920;74:652–5. [Google Scholar]
- 35.Corcoran AC, Taylor RD, Page IH. Controlled observations on the effect of low sodium dietotherapy in essential hypertension. Circulation. 1951;3:1–16. doi: 10.1161/01.cir.3.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Perera GA, Blood DW. The Relationship of Sodium Chloride to Hypertension. J Clin Invest. 1947;26:1109–18. doi: 10.1172/JCI101903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meneely GR, Dahl LK. Electrolytes in hypertension: the effects of sodium chloride. The evidence from animal and human studies. Med Clin North Am. 1961;45:271–83. doi: 10.1016/s0025-7125(16)33891-3. [DOI] [PubMed] [Google Scholar]
- 38.Dahl LK, Love RA. Etiological role of sodium chloride intake in essential hypertension in humans. J Am Med Assoc. 1957;164:397–400. doi: 10.1001/jama.1957.02980040037010. [DOI] [PubMed] [Google Scholar]
- 39.Fallis N, Lasagna L, Tetreault L. Gustatory thresholds in patients with hypertension. Nature. 1962;196:74–5. [Google Scholar]
- 40.Wotman S, Mandel ID, Thompson RH, Jr, Laragh JH. Salivary electrolytes and salt taste thresholds in hypertension. J Chronic Dis. 1967;20:833–40. doi: 10.1016/0021-9681(67)90021-5. [DOI] [PubMed] [Google Scholar]
- 41.Schechter PJ, Horwitz D, Henkin RI. Sodium chloride preference in essential hypertension. Jama. 1973;225:1311–5. [PubMed] [Google Scholar]
- 42.Mattes RD. Salt taste and hypertension: a critical review of the literature. J Chronic Dis. 1984;37:195–208. doi: 10.1016/0021-9681(84)90147-4. [DOI] [PubMed] [Google Scholar]
- 43.Bartoshuk L. Influence of chemoreception and psychologic state on food selection. Int J Obes (Lond) 1980;4:351–5. [PubMed] [Google Scholar]
- 44.Moskowitz HR. Taste and food technology: Acceptability, aethetics and preference. New York: Academic Press; 1978. [Google Scholar]
- 45.Schechter PJ, Horwitz D, Henkin RI. Salt preference in patients with untreated and treated essential hypertension. Am J Med Sci. 1974;267:320–6. doi: 10.1097/00000441-197406000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Leshem M. Biobehavior of the human love of salt. Neurosci Biobehav Rev. 2009;33:1–17. doi: 10.1016/j.neubiorev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Pangborn RM, Pecore SD. Taste perception of sodium chloride in relation to dietary intake of salt. Am J Clin Nutr. 1982;35:510–20. doi: 10.1093/ajcn/35.3.510. [DOI] [PubMed] [Google Scholar]
- 48.Shepherd R, Farleigh CA, Land DG. Preference and sensitivity to salt taste as determinants of salt-intake. Appetite. 1984;5:187–97. doi: 10.1016/s0195-6663(84)80014-8. [DOI] [PubMed] [Google Scholar]
- 49.Blais CA, Pangborn RM, Borhani NO, Ferrell MF, Prineas RJ, Laing B. Effect of dietary sodium restriction on taste responses to sodium chloride: a longitudinal study. Am J Clin Nutr. 1986;44:232–43. doi: 10.1093/ajcn/44.2.232. [DOI] [PubMed] [Google Scholar]
- 50.Beauchamp GK, Bertino M, Engelman K. Failure to compensate decreased dietary sodium with increased table salt usage. Jama. 1987;258:3275–8. [PubMed] [Google Scholar]
- 51.Bertino M, Beauchamp GK, Engelman K. Long-term reduction in dietary sodium alters the taste of salt. Am J Clin Nutr. 1982;36:1134–44. doi: 10.1093/ajcn/36.6.1134. [DOI] [PubMed] [Google Scholar]
- 52.Bertino M, Beauchamp GK, Riskey DR, Engelman K. Taste perception in three individuals on a low sodium diet. Appetite. 1981;2:67–73. doi: 10.1016/s0195-6663(81)80037-2. [DOI] [PubMed] [Google Scholar]
- 53.DiNicolantonio R, Teow BH, Morgan TO. Sodium detection threshold and preference for sodium chloride in humans on high and low sodium diets. Clin Exp Pharmacol Physiol. 1984;11:335–8. doi: 10.1111/j.1440-1681.1984.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 54.McCance RA. Proceedings of the Royal Society of London. Series B–Biological Sciences, Volume 119, 1935–1936: Experimental sodium chloride deficiency in man. Nutr Rev. 1990;48:145–7. doi: 10.1111/j.1753-4887.1990.tb02916.x. [DOI] [PubMed] [Google Scholar]
- 55.Mattes RD. Discretionary salt and compliance with reduced sodium diet. Nutr Res. 1990;10:1337–52. [Google Scholar]
- 56.Mattes RD. The taste for salt in humans. Am J Clin Nutr. 1997;65:692S–7S. doi: 10.1093/ajcn/65.2.692S. [DOI] [PubMed] [Google Scholar]
- 57.Schiffman SS. Perception of taste and smell in elderly persons. Crit Rev Food Sci Nutr. 1993;33:17–26. doi: 10.1080/10408399309527608. [DOI] [PubMed] [Google Scholar]
- 58.Mojet J, Heidema J, Christ-Hazelhof E. Taste perception with age: generic or specific losses in supra-threshold intensities of five taste qualities? Chem Senses. 2003;28:397–413. doi: 10.1093/chemse/28.5.397. [DOI] [PubMed] [Google Scholar]
- 59.Miller IJ., Jr Variation in human taste bud density as a function of age. Ann N Y Acad Sci. 1989;561:307–19. doi: 10.1111/j.1749-6632.1989.tb20991.x. [DOI] [PubMed] [Google Scholar]
- 60.Mistretta CM. Aging effects on anatomy and neurophysiology of taste and smell. Gerodontology. 1984;3:131–6. doi: 10.1111/j.1741-2358.1984.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 61.Wham CA, Teh RO, Robinson M, Kerse NM. What is associated with nutrition risk in very old age? J Nutr Health Aging. 2011;15:247–51. doi: 10.1007/s12603-010-0304-6. [DOI] [PubMed] [Google Scholar]
- 62.Mafra D, Cuppari L, Cozzolino SM. Iron and zinc status of patients with chronic renal failure who are not on dialysis. J Ren Nutr. 2002;12:38–41. doi: 10.1053/jren.2002.29597. [DOI] [PubMed] [Google Scholar]
- 63.Condon CJ, Freeman RM. Zinc metabolism in renal failure. Annals of internal medicine. 1970;73:531–6. doi: 10.7326/0003-4819-73-4-531. [DOI] [PubMed] [Google Scholar]
- 64.Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. Zinc intake of the U.S. population: findings from the third National Health and Nutrition Examination Survey, 1988–1994. J Nutr. 2000;130:1367S–73S. doi: 10.1093/jn/130.5.1367S. [DOI] [PubMed] [Google Scholar]
- 65.Pepersack T, Rotsaert P, Benoit F, et al. Prevalence of zinc deficiency and its clinical relevance among hospitalised elderly. Arch Gerontol Geriatr. 2001;33:243–53. doi: 10.1016/s0167-4943(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 66.Henkin RI, Martin BM, Agarwal RP. Decreased parotid saliva gustin/carbonic anhydrase VI secretion: an enzyme disorder manifested by gustatory and olfactory dysfunction. Am J Med Sci. 1999;318:380–91. doi: 10.1097/00000441-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Shatzman AR, Henkin RI. Gustin concentration changes relative to salivary zinc and taste in humans. Proc Natl Acad Sci U S A. 1981;78:3867–71. doi: 10.1073/pnas.78.6.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kusaba T, Mori Y, Masami O, et al. Sodium restriction improves the gustatory threshold for salty taste in patients with chronic kidney disease. Kidney Int. 2009;76:638–43. doi: 10.1038/ki.2009.214. [DOI] [PubMed] [Google Scholar]
- 69.McKeag NA, McKinley MC, Woodside JV, Harbinson MT, McKeown PP. The role of micronutrients in heart failure. J Acad Nutr Diet. 2012;112:870–86. doi: 10.1016/j.jand.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Oren RM. Hyponatremia in congestive heart failure. The American journal of cardiology. 2005;95:2B–7B. doi: 10.1016/j.amjcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 71.de Souza JT, Matsubara LS, Menani JV, Matsubara BB, Johnson AK, De Gobbi JI. Higher salt preference in heart failure patients. Appetite. 2012;58:418–23. doi: 10.1016/j.appet.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 72.Lennie TA, Chung ML, Moser DK. What should we tell patients with heart failure about sodium restriction and how should we counsel them? Curr Heart Fail Rep. 2013;10:219–26. doi: 10.1007/s11897-013-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacGregor GA, Sever PS. Salt–overwhelming evidence but still no action: can a consensus be reached with the food industry? CASH (Consensus Action on Salt and Hypertension) BMJ. 1996;312:1287–9. doi: 10.1136/bmj.312.7041.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pietinen P, Valsta LM, Hirvonen T, Sinkko H. Labelling the salt content in foods: a useful tool in reducing sodium intake in Finland. Public Health Nutr. 2008;11:335–40. doi: 10.1017/S1368980007000249. [DOI] [PubMed] [Google Scholar]
- 75.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–319. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]


