Summary
Renal pericytes have been neglected for many years, but recently they have become an intensively studied cell population in renal biology and pathophysiology. Pericytes are stromal cells that support vasculature, and a subset of pericytes are mesenchymal stem cells. In kidney, pericytes have been reported to play critical roles in angiogenesis, regulation of renal medullary and cortical blood flow, and serve as progenitors of interstitial myofibroblasts in renal fibrogenesis. They interact with endothelial cells through distinct signaling pathways and their activation and detachment from capillaries after acute or chronic kidney injury may be critical for driving chronic kidney disease progression. By contrast, during kidney homeostasis it is likely that pericytes serve as a local stem cell population that replenishes differentiated interstitial and vascular cells lost during aging. This review describes both the regenerative properties of pericytes as well as involvement in pathophysiologic conditions such as fibrogenesis.
Keywords: Pericytes, mesenchymal stem cells, kidney fibrosis, capillary rarefaction
Pericytes, also called mural cells or Rouget cells, are extensively branched mesenchymal cells that surround endothelial cells in the capillary bed and postcapillary venules.1,2 Embedded in the capillary basement membrane, pericytes form an extensive network around microvasculature throughout the body, where they perform a variety of homeostatic functions related in particular to angiogenesis, vessel maturation, and response to injury.
During kidney development, pericytes and other stromal cells derive from odd-skipped-related 1 (Osr1)+ intermediate mesoderm.3 In other major organs such as heart, liver, and gut, pericytes derive from the mesothelium.2 Osr1+ intermediate mesoderm gives rise both to the Sine oculis homeobox homolog 2 (Six2)+ nephron progenitor population as well as the Forkhead box D1 (FoxD1)+ stromal progenitor population. These FoxD1+ cells surround the cap mesenchyme and differentiate into pericytes, resident fibroblasts, mesangial cells, and vascular smooth muscle cells.4,5 According to the accepted definition, pericytes are distinguished from resident fibroblasts because they are completely or partially embedded within the capillary vascular basement membrane.2,5 This definition is somewhat impractical because it relies on electron microscopy, therefore pericytes also are defined using a combination of other criteria such as gene expression, surface markers, location, and morphology.2 Complicating the field, however, is the absence of any single marker that identifies pericytes directly and distinguishes them from other mesenchymal cells.2 Table 1 summarizes currently used markers to identify renal pericytes.
Table 1.
Renal Pericyte Markers
| Pericyte Marker | Comment |
|---|---|
| PDGFR-β | Receptor tyrosine kinase that is expressed strongly in various other cell types including mesangial cells and vascular smooth muscle cells, expression persists in all kidney myofibroblasts2,7 |
| CD73 (ecto-5′-nucleotidase) | Mesenchymal stem cell marker,79 which also is expressed in T lymphocytes,80 marks cortical stroma but not medullary; in our hands not a reliable marker of kidney pericytes |
| CD248 (endosialin) | C-type lectin transmembrane receptor, also expressed on vascular smooth muscle cells, fibroblasts, myofibroblasts, and T cells2 |
| α-SMA | Intracellular protein linked to cell contractility expressed by smooth muscle cells and myofibroblasts, and not expressed by quiescent pericytes81 |
| NG2 (chondroitin sulfate proteoglycan 4) | Proteoglycan expressed by pericytes during early angiogenesis,1 vascular smooth muscle cells, adipocytes, and neuronal cells2,81 |
| Desmin | Type III intermediate filament, also expressed by mesangial cells, cardiomyocytes, and skeletal muscle cells2,82 |
| p75 (low-affinity nerve growth factor receptor) | Tumor necrosis factor–receptor superfamily member expressed on hepatic stellate cells and adult pericytes1,81 |
Interest in renal pericyte biology has increased recently because of evidence linking it to kidney disease processes. Kidney pericytes regulate renal blood flow6 and are emerging key players in the development of renal fibrosis and chronic kidney disease (CKD). Pericytes contribute to the renal myofibroblast pool in renal fibrogenesis via pericyte–myofibroblast trans-differentiation, and their detachment of capillaries might trigger capillary rarefaction, one of the hallmarks of acute kidney injury to CKD progression.4,7–9 Pericytes recently were linked to mesenchymal stem cells (MSCs) and a critical new question is whether a subset of renal pericytes are MSCs, and contribute to kidney regeneration.10 In this review, we discuss the function of renal pericytes during homeostasis, disease, and regeneration.
PHYSIOLOGIC ROLE OF PERICYTES
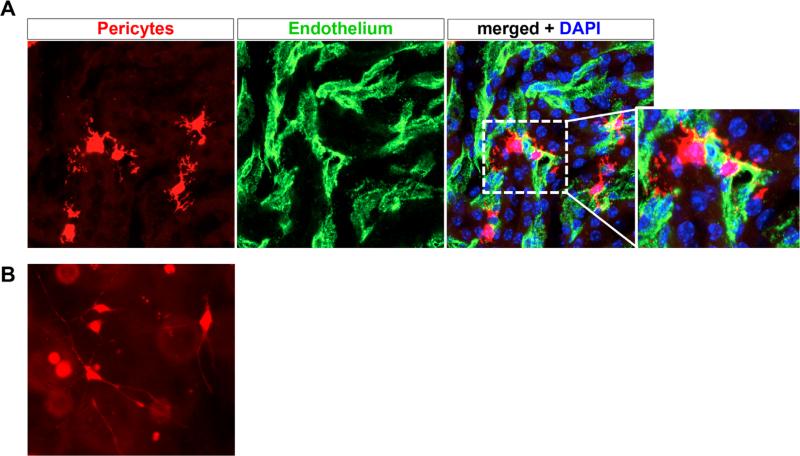
The density of pericytes from different tissues varies, with a relatively high number of pericytes in the kidney.6 Because they are linked so closely with endothelial cells, one way to measure pericyte density is by comparing their frequency with that of the endothelial cells they appose. The pericyte:endothelial cell ratio varies considerably between organs, with ratios of 1:1 to 1:3 in brain, but ratios as low as 1:10 in other organs.11 There is regional heterogeneity of pericyte frequency within the kidney as well. Pericyte distribution peaks in the outer medulla, for example, where regulation of blood flow to match reabsorptive demands is greatest.6,12 Figure 1 shows pericytes in the outer medulla and in the culture dish, illustrating their extensively branched morphology.
Figure 1.
Renal pericytes associate closely with vasculature. (A) Col1α1-CreERt2 mice were crossed against R26RtdTomato reporter mice, and pulsed with tamoxifen to induce recombination in medullary pericytes. A high-power view of tdTomato+ pericytes is shown, note the extensive branching processes emanating from the cell body. Endothelial cells were co-stained green using fluorescein isothiocyanate FITC-CD31 antibodies, and the merged image shows how tightly the pericytes associate with and wraparound peritubular capillaries. Genetically tagged renal pericytes (left panel) surrounding peritubular capillaries (middle panel, CD31). (B) Genetically labeled pericytes were dissociated from kidney by enzymatic dissociation and purified by fluorescence activated cell sorting. Subsequently, they were plated in a three-dimensional collagen gel, where long delicate processes extend from the cell body, similar to the in vivo situation. DAPI, 4',6-diamidino-2-phenylindole.
One of the first functions suggested for pericytes was constriction of capillaries.2 Indeed, studies have linked the renal pericyte as a regulator of renal medullary blood flow (recently reviewed by Kennedy-Lydon et al6). Pericytes of the vasa recta have been shown to regulate both vasoconstriction and dilation in the isolated perfused vasa recta model.6,13,14 The free radical nitric oxide has been proposed as one important regulator of pericyte-mediated vasoconstriction in the kidney.6,12 Another mechanism regulating the pericyte-mediated vascular tone might be the release of neuro-transmitters such as noradrenaline or acetylcholine by medullary sympathetic nerve terminals.6 Finally, pericytes may regulate blood flow and permeability by varying the fraction of abluminal endothelial cell surface covered by pericyte processes. Pericyte defficiency was shown to increase brain-vessel permeability, therefore, by extension, pericytes in the kidney may be important for peritubular capillary permeability.15,16 Intriguingly, there is increased pericyte coverage of abluminal endothelial surface area in lower parts of the body, implying a role in controlling orthostatic blood pressure and reducing dependent edema.1 However, most studies reporting the role of pericytes in regulating renal blood flow have been performed in vitro and convincing in vivo evidence still is lacking. Elucidating pericyte-dependent regulation of renal blood flow is an important area for future research.
Additional critical functions for pericytes include regulating microvessel stability, endothelial survival, and immunologic surveillance. Pericytes contribute to the synthesis of the vascular basement membrane by secreting collagen IV, fibronectin, and laminin.17 Pericytes regulate angiogenesis and thus might be interesting targets for anti-angiogenic therapies. Readers are directed to recent excellent reviews discussing the role of pericytes in angiogenesis and as promising targets in angiogenic therapies.1,18 Macrophage-like properties with phagocytosis in vitro and in vivo have been described for a subset of pericytes and it has been hypothesized that this functionally defined pericyte subset may originate from bone marrow mesenchymal progenitors.19 Another possible role for pericytes in the immune defense system might be antigen presentation to T lymphocytes.1,20 For human brain pericytes, a possible role in the extrinsic pathway of blood coagulation by expression of procoagulant enzyme complexes has been reported.21 However, it is unclear whether renal pericytes have similar functions in immunologic defense or activation of blood coagulation.
ARE PERICYTES THE RENAL ERYTHROPOIETIN-PRODUCING CELLS?
The kidney produces approximately 90% of the body's total erythropoietin (EPO), with liver contributing a minor amount.22 In response to anemia and hypoxia, EPO synthesis is increased markedly, primarily via increased gene expression. Patients with advanced CKD and end-stage renal disease suffer from a normocytic, normochromic, hypoproliferative anemia caused predominantly by the reduced renal EPO production.23 One reason for this reduced EPO synthesis might be a dysfunction of renal EPO-producing cells. Several observations have indicated that EPO-producing cells are mesenchymal in origin, therefore it is reasonable to postulate that during CKD progression, kidney pericytes become activated, detach from the microvasculature, expand, and differentiate into myofibroblasts. During this process they may lose their capability to produce EPO in response to increased levels of hypoxia-inducible factor 2 α.
Several recent observations point toward pericytes as possible renal EPO-producing cells. Obara et al22 induced anemia in EPO–green fluorescent protein (GFP) mice via bleeding and studied the renal GFP-expressing cells. They observed EPO-GFP+ cells primarily at the medullary cortical border and described these cells as stellar or arboroid-shaped. These EPO-GFP+ cells expressed neuronal markers such as microtubule-associated protein 2 and neuro-filament light polypeptide, and co-stained positive for the pericyte/mesenchymal stem cell marker CD73. Furthermore, the investigators showed that EPO-GFP+ cells were located in close association to CD31+ endothelial cells but were negative for CD31 itself, and they also did not express the macrophage marker Mac1. This indirectly implicates a stromal cell as the EPO-producing cell. Of note, several groups in the early 1990s also described that EPO+ cells co-stain for CD73, using either immunostaining combined with in situ hybridization in rat kidneys or a transgenic mouse model.24,25
Two recent publications provided novel insight into the dynamics of renal EPO-producing cells during fibrogenesis. First, Asada et al26 showed in fate-tracing experiments that the majority of renal EPO-producing cells arise from myelin protein zero-Cre (P0-Cre+) cells. During development, these P0-Cre+ cells first were detected in embryonic day 13.5 (E13.5) in metanephric kidneys and located in the stroma around the cap mesenchyme. Although the investigators described that these cells are neural crest–derived of extrarenal origin, the localization and expression pattern (platelet-derived growth factor receptor [PDGFR]-β+) of these cells suggests that they may in fact be identical to FoxD1+ progenitor cells. The investigators convincingly showed that these P0-Cre+ cells express EPO using transgenic P0-Cre;R26tdred fluorescent protein (RFP);Epo-GFP mice and that they differentiate into α-smooth muscle actin (α-SMA)+ myofibroblasts after unilateral ureteral obstruction (UUO) surgery. Further experiments in Epo-GFP mice suggested that myofibroblast differentiation reduced the expression of EPO whereas α-SMA+ myofibroblasts still possess a reduced capability to increase EPO production upon anemic stimuli.26
Souma et al27 recently reported related experiments using the inherited super anemic mouse (a homozygous EPO–GFP knock-in resulting in EPO knockout, with lethality rescued by an Epo-expressing transgene) in which EPO-producing renal cells become myofibro-blasts after UUO while reducing EPO messenger RNA expression. Interestingly, the investigators reported that the loss of EPO production by myofibroblasts was reversed by removing the profibrotic stimuli (ie, ureteral ligation). These findings strongly suggest that EPO-producing cells are critically involved in kidney fibrosis and that their differentiation toward myofibro-blasts might reduce EPO production, causing anemia during CKD progression. Importantly, however, it remains unclear whether the EPO-producing cells are interstitial fibroblasts or pericytes. Further studies are needed to solve this fundamental question.
PERICYTES AS MESENCHYMAL STEM/ STROMAL CELLS
In 1990, a report was published regarding the osteogenic potential of bovine retinal pericytes.28 In the following years it became apparent that pericytes also possess chondrogenic and adipogenic differentiation capabilities, suggesting that pericytes fulfill the trilineage differentiation criteria of mesenchymal stem/stromal cells (MSCs).29 The chondrogenic and osteogenic differentiation capacity of pericytes points toward a possible role in vascular diseases such as atherosclerosis and vascular calcification. Indeed, it has been reported that pericyte-like α-SMA and 3G5-positive cells produce bone morphogenic protein 2a and calcify.30,31 However, the precise role of pericytes in vascular disease remains undefined. For example, in arteries, CD34+, Sca1+ MSC-like progenitors that are distinct from pericytes of the vasa vasorum have been reported in the adventitia.32,33 Interestingly, in culture, these adventitial MSCs can acquire a pericyte-like phenotype.34 Crisan et al35 reported that pericytes (NG2+, CD146+, PDGFRβ+) purified from human placenta, skeletal muscle, pancreas, and adipose tissue showed an osteogenic, chondrogenic, adipogenic, and myogenic potential and showed expression of typical MSC surface markers such as CD90, CD73, CD105, and CD44. Whether perivascular MSC might serve as a local stem cell pool for pericytes is an open question, as discussed later.
Possible links between kidney MSCs and pericytes have been suggested indirectly by several reports over the past decade. Plotkin and Goligorsky36 isolated a fibroblast-like cell clone (4E) from adult mouse kidney using a tyrosine kinase, endothelial (Tie-2)–GFP mouse. These Sca1+, CD44+, vimentin+, nestin+, CD45-, ckit- cells were able to differentiate into osteoblasts, adipocytes, and myofibroblast (α-SMA+) upon short-term treatment with transforming growth factor-β. Long-term treatment with transforming growth factor-β resulted in a smooth muscle phenotype with expression of SM22-α and calponin. Interestingly, the investigators also observed a macrophage-like differentiation (Monocyte/Macrophage antibody, MOMA-2+) and an endothelial cell differentiation (Tie-2+, vascular endothelial growth factor receptor [VEGFR]2+).36 Under hypoxic (2%) culture conditions a vimentin+, α-SMA-subset of 4E cells expressed EPO whereas non–EPO-expressing cells showed characteristics of myofibro-blasts (vimentin+, α-SMA+).36 However, despite being from one clone, the investigators clearly discuss that the 4E cell cultures are a heterogenous mixture of cells and no single marker can identify these cells clearly in vivo. Chen et al37 also reported that 4E cells, when cultured in Matrigel (Sigma-Aldrich, St. Louis, MO), form capillary networks and partly differentiate toward the endothelial lineage (14.8% CD31+, Tie-2–GFP+). Mice intravenously injected with 4E cells 24 hours after ischemia reperfusion injury (IRI) showed reduced plasma creatinine levels at 2 to 3 days after ischemic injury, with significantly increased tubular epithelial cell proliferation and a significantly reduced degree of microvascular capillary rarefaction.37 Interestingly, at 30 days after injection, the investigators described that fluorescent dye-labeled 4E cells predominantly differentiated toward a myofibroblast phenotype (α-SMA+, 45%), whereas a minority of 4E cells showed expression of Tie-2–GFP (9%).37
These interesting findings are hypothesis-generating and lead to three important questions: (1) are renal MSC pericytes or a subset of pericytes, or do they serve as a pool of pericyte progenitors? (2) Do renal MSCs have an endothelial differentiation capacity in vivo? (3) Are renal MSCs myofibroblast progenitors? Although clearly more studies are needed to answer these questions, it is important to note that Tie-2 is not a specific marker of endothelial cells. De Palma et al38 showed that endothelial cells, hematopoietic cells, and mesenchymal pericyte precursors express Tie-2 in mammary tumors. Adding to the controversy, Medici et al39 performed lineage tracing using Tie-2 as an endothelial marker and reported that endothelial cells differentiate into mesenchymal stem cell–like cells with trilineage differentiation capacity. The fact that intravenously injected 4E cells ameliorate acute kidney injury (AKI) might be explained by their paracrine effect, however, based on the studies from the Goligorsky group, we also may speculate that the injected 4E cells stabilize the renal microvasculature through pericyte-like functions.36,37
The notion that pericytes may harbor an MSC population suggests that this cellular niche may play a much more important role in tissue homeostasis than previously suspected. A very recent publication from Zhao et al40 showed a previously unappreciated lineage hierarchy linking MSC and pericytes. These investigators determined that Gli1 expression defined an MSC population in the mouse incisor. These cells were slow cycling and localized in the perivascular space, a niche previously hypothesized to harbor an odontoblast stem cell population. In genetic fate mapping experiments, they showed conclusively that these Gli1+ MSCs support both homeostatic replacement and regeneration of mouse incisor mesenchyme after injury. Moreover, Gli1+ cells gave rise to Ng2+ and CD146+ pericytes, indicating that these MSCs repre-sent a pericyte stem cell pool. One important implication from this study is that Gli1 is a marker for MSCs in vivo. Traditionally, MSCs have been defined by a loose set of functional and phenotypic criteria.41 Indeed, if this observation holds in other organs, it should open up entirely new avenues of exploration into the roles of MSC in vivo, areas that could not be investigated previously because of the lack of such a marker. In addition, these findings define a novel lineage relationship between MSC and pericytes, at least in mouse incisor. It will be important to investigate whether such a relationship also exists in other solid organs.
PERICYTES AS EMERGING KEY PLAYERS IN KIDNEY FIBROSIS
It has been widely accepted that myofibroblasts are the scar-forming, extracellular matrix–producing cells that drive fibrogenesis. However, the major source of these cells remains controversial. In the past decade, the work of several groups has pointed toward pericytes as key myofibroblast progenitors, however, this recently has been questioned as well.4,42,43 Faulkner et al44 reported that renal myofibroblasts might expand from a perivascular location in an angiotensin 2–induced kidney fibrosis model. The study by Lin et al45 showed that collagen1α1-GFP+ pericytes co-express NG2 and PDGFRβ and become α-SMA+ myofibroblasts after unilateral ureteral obstruction.
Our own genetic lineage tracing studies provide strong evidence for pericytes as the major myofibro-blast progenitors in the kidney. We used an inducible CreERt2 driven by FoxD1 to genetically tag interstitial pericytes. During kidney development, FoxD1 is expressed by the stromal cell population surrounding the cap mesenchyme, these stromal FoxD1+ cells give rise to perivascular fibroblasts, pericytes, mesangial cells, and vascular smooth muscle cells (Fig. 2).46,47 Our studies showed that during kidney fibrosis, genetically tagged pericytes and perivascular fibroblasts gain α-SMA and become myofibroblasts.4,7,48 However, it remains unclear whether all FoxD1+ cells are pericytes or a mixture of pericytes and resident fibroblasts. There is strong evidence that pericytes are a major source of myofibroblasts in fibrogenesis of other major solid organs. It has been reported that stellate cells, resident pericytes of the liver, are the major source of myofibroblasts after carbon tetrachloride injection.49 Hung et al50 recently reported that FoxD1+ pericytes are a major source of myofibroblasts in bleomycin-induced pulmonary fibrosis. Interestingly, these investigators also reported that a collagen1α1-expressing mesenchymal population of distinct lineage with features of resident fibroblasts also contributes to the myofibro-blast pool in lung fibrosis. After spinal cord injury, it has been shown that glutamate-aspartate transporter (Glast)-expressing pericytes expand, gain α-SMA, and secrete extracellular matrix and PDGFRα+, and ADAM12+ pericytes have been reported to be a major source of myofibroblasts in fibrosis of muscle and dermis.51,52
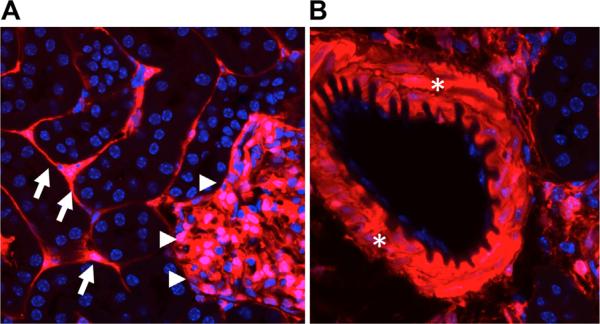
Figure 2.
Fate of FoxD1+ cells in kidney development. FoxD1-Cre mice were crossed against the R26RtdTomato reporter line to genetically label FoxD1+ cells and all of their descendants. (A) cells include thin branched stromal cells located in the interstitium (arrows). These cells represent pericytes and perivascular fibro-blasts. In addition, the glomerular staining indicates that both mesangial cells and podocytes express FoxD1 (arrowheads). However, podocytes acquire FoxD1 expression later in development, so only mesangial cells have a lineage relationship with FoxD1 precursors in development.83 (B) FoxD1+ lineage cells also generate vascular smooth cells that line arteries and kidney arterioles (asterisks).
Lebleu et al43 recently questioned the contribution of pericytes to the myofibroblast pool in kidney fibrosis. These investigators performed lineage tracing studies using NG2-yellow fluorescent protein (YFP) and PDGRβ-RFP mice and showed that NG2+ and PDGFRβ+ cells expand after UUO. The investigators generated mice that express the viral thymidine kinase under the control of a NG2 or PDGFRβ promoter fragment, allowing ablation of proliferating NG2- or PDGFRβ-expressing cells after ganciclovir injection. Ganciclovir injection after UUO resulted in a signifi-cant reduction of PDGFRβ+ and NG2+ cells, however, the severity of fibrosis remained unchanged, prompting the investigators to conclude that pericytes do not contribute to renal fibrosis.43 Although the investigators were able to show that ablation of proliferating α-SMA+ cells ameliorates fibrosis, their results regarding the contribution of PDGFRβ+ cells are difficult to reconcile with other studies clearly showing that PDGFRβ+ cells contribute to the renal myofibroblast pool.4,43,45,53,54 In particular, the fact that Lebleu et al43 reported that only 6% of PDGFRβ+ cells co-express α-SMA after UUO raises questions about the efficiency of transgene expression in their random-integration promoter fragment mouse model. In our own experiments the vast majority of renal α-SMA+ myofibroblasts co-express PDGFRβ.7 In addition, it recently was reported that extrarenal myelin protein zero-cre (P0-Cre) become myofibroblasts in the kidney after UUO and co-label with α-SMA and PDGFRβ.26
Given the current lack of a pericyte-specific marker to perform lineage-tracing studies and the ongoing controversy in the field, more studies clearly will be required to better define the role of renal pericytes in kidney fibrosis.
PERICYTES AND CAPILLARY RAREFACTION AFTER KIDNEY INJURY
Loss of the renal microvasculature is a key component in acute kidney injury to CKD progression and renal fibrosis.8,55,56 Capillary rarefaction after kidney injury has been described in a variety of experimental animal models, and in human beings with CKD the decrease of renal capillary density correlates with the severity of fibrosis (Fig. 3).56–62 Basile et al55 previously used silicone rubber injection to show that rats develop a signi-ficant reduction of peritubular capillary density in kidney (cortex, -25%-30%; inner stripe outer medulla, -35%-40%) at 4 to 40 weeks after IRI. Horbelt et al58 subsequently identified endothelium by immunostaining postischemic mice and also reported a 45% decrease of microvascular density at 30 days after IRI. We investigated capillary dynamics in response to AKI using fluorescence microangiography and found a previously unappreciated reduction not only in number but also the caliber of surviving peritubular capillaries.8 In this study, the initial severity of AKI was a strong predictor of the future development of peritubular capillary loss and CKD, likely reflecting the absence of a significant regenerative response in endothelium after severe damage.
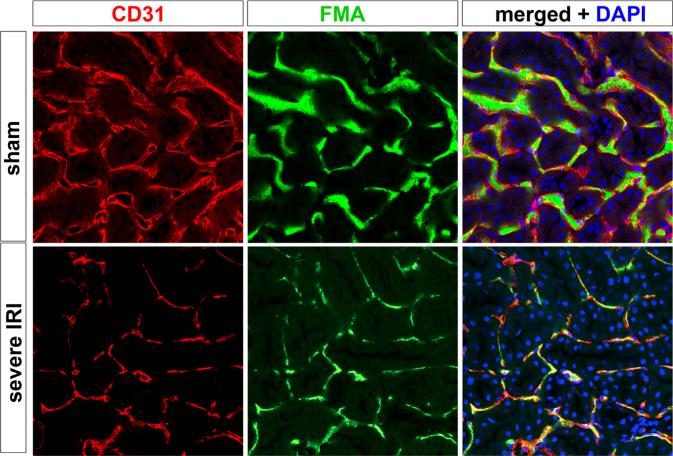
Figure 3.
Capillary rarefaction after kidney injury. Sham kidneys were co-stained for peritubular capillaries with CD31 antibodies (left panels). Before death, mice were perfused with FITC-conjugated microbeads in agarose to generate a fluorescence microangiogram (FMA, middle panels). The merged image illustrates luminal filling of CD31-positive capillaries with green microbeads, providing a readout of vascular perfusion. In the lower panels, mice were subject to severe AKI and allowed to recover for 8 weeks. They were subject to the same protocol as the sham mice. The results show substantially reduced perfusion of peritubular capillaries, as well as endothelial cell density. This capillary rarefaction strongly correlated with the degree of kidney injury at day 1 after AKI, suggesting an inability of damaged capillaries to repair.8 DAPI, 4',6-diamidino-2-phenylindole.
The cellular basis for the link between AKI and future peritubular capillary loss is a subject of intense investigation. Activation of pericytes after injury is likely to be a key component leading to capillary destabilization and rarefaction.63 Activated pericytes migrate into the renal interstitium, proliferate, gain α-SMA, and transdifferentiate into myofibroblasts, thus leaving the endothelium of the renal peritubular capillary bed unstable. The toxic renal microenvironment after AKI or during CKD progression promotes cellular death of the unstable endothelium, with subsequent capillary rarefaction.54,63–65
Pericytes and endothelial cells undergo continuous cross-talk, both through direct interaction and via paracrine factors. Although a basal membrane separates both cell types, pericyte processes protrude through the basement membrane, allowing direct contact through distinct types of cell–cell contact.2 More than 1,000 contacts between one pericyte and a single endothelial cell have been reported.2 These contacts allow direct communication between both cell types. Elegant co-culture experiments have identified some of the factors regulating pericyte stabilization of endothelium. Endothelial recruitment of pericytes is mediated in part by endothelial secretion of platelet-derived growth factor (PDGF)-BB.66 Subsequent pericyte contact with endothelial cells induces expression of endothelial adhesion molecules, which regulate contact between the two cell types. Integrin α4β1 vascular cell adhesion molecule has been implicated in this kind of EC–pericyte interaction, and is up-regulated with vascular stabilization.67 Pericytes also induce expression of prosurvival factors in endothelial cells. For example, autocrine VEGF-A is expressed by endothelium in coculture with pericytes. This in turn induces expression of anti-apoptotic Bcl-w, promoting endothelial survival.68
Bidirectional signaling between endothelial cells and pericytes after injury also has been described, including their involvement in capillary rarefaction after AKI. A study by Lin et al54 showed that blocking PDGFRβ on pericytes or VEGFR2 on endothelial cells by circulating receptor ectodomains ameliorated perivascular rarefaction and renal fibrosis after UUO. Interestingly, the investigators also reported that kidney injury resulted in a switch of secreted VEGF-A isomers with reduced secretion of angiogenic VEGF164 and increased dys-angiogenic VEGF iso-forms (VEGF 120/188).54 Such a switch would be expected to cause capillary destabilization.
Schrimpf et al65 reported that kidney pericytes are able to stabilize an in vitro capillary tube network of human umbilical cord endothelial cells whereas myofibroblasts from fibrotic kidneys lack this stabilizing function after exposure to the vascular serine protease kallikrein. After injury, kidney pericytes up-regulate the expression of a disintegrin and metalloprotease with thrombospondin motifs-1 (ADAMTS1) and down-regulate its inhibitor (tissue inhibitor of metalloproteinase 3).65 Tissue inhibitor of metalloproteinase 3 was able to stabilize the three-dimensional human umbilical cord endothelial cell network, whereas ADAMTS1 destabilized it. Interestingly, the knockout of tissue inhibitor of metalloproteinase 3 in mice resulted in a spontaneous kidney phenotype with decreased capillary density and interstitial fibrosis.65 It recently was reported that renal ischemia reperfusion injury leads to pericyte proliferation and transdifferentiation with decreased pericyte expression of angiopoietin-1 and increased endothelial expression of angiopoietin-2.69 Angiopoietin-1 is a strong agonist of the Tie-2 receptor, which is responsible for suppressing vascular leakage and maintaining survival of the endothelium, whereas angiopoietin 2 antagonizes angiopoietin-1.69
PERICYTE TRANSCRIPTOME AFTER INJURY
A powerful approach toward unraveling pericyte biology is to define transcriptomic changes in pericytes and perivascular fibroblasts as they transition to activated myofibroblasts. Few reports have tackled this question to date. Complicating this task, standard approaches for isolation of kidney RNA are compromised by the low abundance of pericytes and stroma in health, meaning that most RNA isolated from whole kidney derives from other cell types. This limitation is exacerbated in disease states in which inflammatory cells invade and distort normal architecture. Fluorescence-activated cell sorting is one approach to solve this problem, but it requires suitable antibodies and the process of kidney disaggregation itself rapidly induces transcriptional stress responses confounding analysis. Laser capture microdissection of frozen sections cannot resolve interstitial cell types that are intermixed and closely apposed to noninterstitial cell types.
We recently reported a comprehensive gene expression analysis of pericyte-to-myofibroblast transition in medullary kidney pericytes during fibrosis.70 To overcome the challenges in isolating pericyte and myofibroblast-specific RNA, we applied a unique approach called translating ribosome affinity purification.71 This involves the cell-specific expression of the ribosomal subunit protein L10a fused in-frame to enhanced green fluorescent protein (eGFP). The eGFP-L10a fusion allows affinity purification of polysomal messenger RNA, which is bound to ribosomes by anti-GFP antibody affinity purification. This occurs in a single-step procedure from whole-kidney lysate. To isolate pericyte-specific polysomes, we created a transgenic mouse with heterologous expression of eGFP-L10a under control of the collagen1α1 promoter. Col1α1-eGFP-L10a mice express the fusion protein in podocytes in kidney cortex, and in pericytes in kidney medulla.45,72 During fibrosis, eGFP-L10a is expressed specifically in α-SMA–positive interstitial myofibro-blasts. In this way we could isolate pericyte-specific RNA during homeostasis and at several time points in experimental fibrotic disease.
Gene ontology analysis of early expression changes in pericytes after injury showed highly significant up-regulated terms including cell proliferation, extracellular matrix binding, and microtubule motor activity. These terms imply a proliferative pericyte response characterized by cell shape change and matrix interactions. Similarly, top down-regulated genes were characterized by the gene ontology terms cell junction and anchoring junction, implying a loss of cell–cell contacts as pericytes detach from endothelial cells. A number of novel secreted proteins may be useful as noninvasive biomarkers of renal fibrosis.70 The signaling pathways most notably up-regulated include integrin PDGF signaling, and up-regulated gene families include snail homologs as well as the Wnt modulating gene family, secreted frizzled related proteins. These pathways comprise known important regulators of myofibroblast function and kidney fibrosis, providing further internal validation of this approach.
This analysis also identified several genes that were specifically and strongly up-regulated in pericytes and myofibroblasts in fibrotic injury. Thrombospondin-2 is produced by the THBS2 gene and it is an extracellular glycoprotein with antiangiogenic properties. THBS2 is expressed during kidney development but is not expressed in adult kidney. It is up-regulated in kidney interstitium in mouse models of glomerulonephritis and knock-out studies have established that it regulates angiogenesis and inflammation in this context.73 We noted a 26-fold up-regulation of THBS2 in myofibro-blasts during UUO, and circulating THBS2 has been measured in human plasma, in which patients with the highest quartile of serum levels have increased cardiovascular mortality.74 The SerpinF1 gene produces the pigment epithelium-derived factor (PEDF), a secreted multifunctional protease inhibitor that regulates stem cell fate.75,76 In kidney, PEDF serves as a human urinary biomarker for diabetic nephropathy and exogenous PEDF ameliorates glomerular disease.77,78
CONCLUSIONS
Accumulating evidence indicates that renal pericytes play a critical role in kidney homeostasis, injury, and repair. Kidney pericytes are major contributors to the myofibroblast pool after injury and their detachment from endothelial cells is a key event in the pathophysiology of capillary rarefaction and chronic kidney disease progression. It will be important to better define the lineage hierarchies of pericytes during kidney homeostasis and aging to better inform regenerative medicine approaches to combat loss of kidney function through aging or disease.
Acknowledgments
Financial support: Supported by National Institutes of Health grant DK088923 (B.D.H.), by an Established Investigator Award of the American Heart Association (B.D.H.), and by a fellowship from the Deutsche Forschungsgemeinschaft (KR 4073/1-1 to R.K.).
Footnotes
Conflict of interest statement: none.
REFERENCES
- 1.Diaz-Flores L, Gutierrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–69. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 2.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Mugford JW, Sipila P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphreys BD. Targeting pericyte differentiation as a strategy to modulate kidney fibrosis in diabetic nephropathy. Semin Nephrol. 2012;32:463–70. doi: 10.1016/j.semnephrol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy-Lydon TM, Crawford C, Wildman SS, Peppiatt-Wildman CM. Renal pericytes: regulators of medullary blood flow. Acta Physiol (Oxf) 2013;207:212–25. doi: 10.1111/apha.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramann R, Dirocco DP, Humphreys BD. Understanding the origin, activation and regulation of matrix-producing myofibro-blasts for treatment of fibrotic disease. J Pathol. 2013;231:273–89. doi: 10.1002/path.4253. [DOI] [PubMed] [Google Scholar]
- 8.Kramann R, Tanaka M, Humphreys BD. Fluorescence micro-angiography for quantitative assessment of peritubular capillary changes after acute kidney injury in mouse. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013101121. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramann R, Dirocco DP, Maarouf OH, Humphreys BD. Matrix producing cells in chronic kidney disease: origin, regulation, and activation. Curr Pathobiol Rep. 2013:301–11. doi: 10.1007/s40139-013-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Sims DE. The pericyte—a review. Tissue Cell. 1986;18:153–74. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 12.Crawford C, Kennedy-Lydon T, Sprott C, et al. An intact kidney slice model to investigate vasa recta properties and function in situ. Nephron Physiol. 2012;120:17–31. doi: 10.1159/000339110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pallone TL. Vasoconstriction of outer medullary vasa recta by angiotensin II is modulated by prostaglandin E2. Am J Physiol. 1994;266:F850–7. doi: 10.1152/ajprenal.1994.266.6.F850. [DOI] [PubMed] [Google Scholar]
- 14.Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Exp Nephrol. 2001;9:165–70. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- 15.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 17.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–30. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly-Goss MR, Sweat RS, Stapor PC, Peirce SM, Murfee WL. Targeting pericytes for angiogenic therapies. Microcirculation. 2014;21:345–57. doi: 10.1111/micc.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Flores L, Jr, Gutierrez R, Madrid JF, et al. Adult stem cells and repair through granulation tissue. Front Biosci (Landmark Ed) 2009;14:1433–70. doi: 10.2741/3317. [DOI] [PubMed] [Google Scholar]
- 20.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identi-fication. J Leukoc Biol. 2004;75:388–97. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 21.Bouchard BA, Shatos MA, Tracy PB. Human brain pericytes differentially regulate expression of procoagulant enzyme complexes comprising the extrinsic pathway of blood coagulation. Arterioscler Thromb Vasc Biol. 1997;17:1–9. doi: 10.1161/01.atv.17.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Obara N, Suzuki N, Kim K, et al. Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood. 2008;111:5223–32. doi: 10.1182/blood-2007-10-115857. [DOI] [PubMed] [Google Scholar]
- 23.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–4. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maxwell PH, Osmond MK, Pugh CW, et al. Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int. 1993;44:1149–62. doi: 10.1038/ki.1993.362. [DOI] [PubMed] [Google Scholar]
- 25.Bachmann S, Le Hir M, Eckardt KU. Co-localization of erythropoietin mRNA and ecto-5’-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem. 1993;41:335–41. doi: 10.1177/41.3.8429197. [DOI] [PubMed] [Google Scholar]
- 26.Asada N, Takase M, Nakamura J, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121:3981–90. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souma T, Yamazaki S, Moriguchi T, et al. Plasticity of renal erythropoietin-producing cells governs fibrosis. J Am Soc Nephrol. 2013;24:1599–616. doi: 10.1681/ASN.2013010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schor AM, Allen TD, Canfield AE, Sloan P, Schor SL. Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci. 1990;97:449–61. doi: 10.1242/jcs.97.3.449. [DOI] [PubMed] [Google Scholar]
- 29.Farrington-Rock C, Crofts NJ, Doherty MJ, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–32. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 30.Bostrom K, Watson KE, Horn S, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–9. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–36. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CS, Lue TF. Defining vascular stem cells. Stem Cells Dev. 2013;22:1018–26. doi: 10.1089/scd.2012.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenmark KR, Yeager ME, El Kasmi KC, et al. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol. 2013;75:23–47. doi: 10.1146/annurev-physiol-030212-183802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corselli M, Chen CW, Sun B, et al. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299–308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crisan M, Chen CW, Corselli M, et al. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118–23. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 36.Plotkin MD, Goligorsky MS. Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibro-blasts. Am J Physiol Renal Physiol. 2006;291:F902–12. doi: 10.1152/ajprenal.00396.2005. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Park HC, Addabbo F, et al. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74:879–89. doi: 10.1038/ki.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Medici D, Shore EM, Lounev VY, et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–6. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao H, Feng J, Seidel K, et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14:160–73. doi: 10.1016/j.stem.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cyto-therapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 42.Greenhalgh SN, Iredale JP, Henderson NC. Origins of fibrosis: pericytes take centre stage. F1000Prime Rep. 2013;5:37. doi: 10.12703/P5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebleu VS, Taduri G, O'Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–53. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faulkner JL, Szcykalski LM, Springer F, Barnes JL. Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am J Pathol. 2005;167:1193–205. doi: 10.1016/S0002-9440(10)61208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–27. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levinson RS, Batourina E, Choi C, et al. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–39. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 47.Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–78. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 48.Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–83. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kisseleva T, Cong M, Paik Y, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–53. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hung C, Linn G, Chow YH, et al. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:820–30. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–70. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 52.Goritz C, Dias DO, Tomilin N, et al. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–42. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 53.Chen YT, Chang FC, Wu CF, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–81. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 54.Lin SL, Chang FC, Schrimpf C, et al. Targeting endotheliumpericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–23. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–99. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 57.Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol. 2003;163:2289–301. doi: 10.1016/s0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horbelt M, Lee SY, Mang HE, et al. Acute and chronic micro-vascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2007;293:F688–95. doi: 10.1152/ajprenal.00452.2006. [DOI] [PubMed] [Google Scholar]
- 59.Kang DH, Joly AH, Oh SW, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–47. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 60.Choi YJ, Chakraborty S, Nguyen V, et al. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum Pathol. 2000;31:1491–7. doi: 10.1053/hupa.2000.20373. [DOI] [PubMed] [Google Scholar]
- 61.Seron D, Alexopoulos E, Raftery MJ, Hartley B, Cameron JS. Number of interstitial capillary cross-sections assessed by monoclonal antibodies: relation to interstitial damage. Nephrol Dial Transplant. 1990;5:889–93. doi: 10.1093/ndt/5.10.889. [DOI] [PubMed] [Google Scholar]
- 62.Bohle A, Mackensen-Haen S, Wehrmann M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res. 1996;19:191–5. doi: 10.1159/000174072. [DOI] [PubMed] [Google Scholar]
- 63.Fligny C, Duffield JS. Activation of pericytes: recent insights into kidney fibrosis and microvascular rarefaction. Curr Opin Rheumatol. 2013;25:78–86. doi: 10.1097/BOR.0b013e32835b656b. [DOI] [PubMed] [Google Scholar]
- 64.Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol. 2013;24:559–72. doi: 10.1681/ASN.2012080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schrimpf C, Xin C, Campanholle G, et al. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol. 2012;23:868–83. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–51. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garmy-Susini B, Jin H, Zhu Y, et al. Integrin alpha4beta1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest. 2005;115:1542–51. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–17. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khairoun M, van der Pol P, de Vries DK, et al. Renal ischemiareperfusion induces a dysbalance of angiopoietins, accompanied by proliferation of pericytes and fibrosis. Am J Physiol Renal Physiol. 2013;305:F901–10. doi: 10.1152/ajprenal.00542.2012. [DOI] [PubMed] [Google Scholar]
- 70.Grgic I, Krautzberger AM, Hofmeister A, et al. Translational profiles of medullary myofibroblasts during kidney fibrosis. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013101143. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heiman M, Schaefer A, Gong S, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–48. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grgic I, Brooks CR, Hofmeister AF, et al. Imaging of podocyte foot processes by fluorescence microscopy. J Am Soc Nephrol. 2012;23:785–91. doi: 10.1681/ASN.2011100988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daniel C, Amann K, Hohenstein B, Bornstein P, Hugo C. Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in experimental glomerulonephritis in mice. J Am Soc Nephrol. 2007;18:788–98. doi: 10.1681/ASN.2006080873. [DOI] [PubMed] [Google Scholar]
- 74.Golledge J, Clancy P, Hankey GJ, Norman PE. Relation between serum thrombospondin-2 and cardiovascular mortality in older men screened for abdominal aortic aneurysm. Am J Cardiol. 2013;111:1800–4. doi: 10.1016/j.amjcard.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 75.Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–9. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez R, Jennings LL, Knuth M, et al. Screening the mammalian extracellular proteome for regulators of embryonic human stem cell pluripotency. Proc Natl Acad Sci U S A. 2010;107:3552–7. doi: 10.1073/pnas.0914019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen H, Zheng Z, Li R, et al. Urinary pigment epithelium-derived factor as a marker of diabetic nephropathy. Am J Nephrol. 2010;32:47–56. doi: 10.1159/000314326. [DOI] [PubMed] [Google Scholar]
- 78.Fujimura T, Yamagishi S, Ueda S, et al. Administration of pigment epithelium-derived factor (PEDF) reduces proteinuria by suppressing decreased nephrin and increased VEGF expression in the glomeruli of adriamycin-injected rats. Nephrol Dial Transplant. 2009;24:1397–406. doi: 10.1093/ndt/gfn659. [DOI] [PubMed] [Google Scholar]
- 79.Boxall SA, Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012;2012:975871. doi: 10.1155/2012/975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cobbold SP, Adams E, Nolan KF, Regateiro FS, Waldmann H. Connecting the mechanisms of T-cell regulation: dendritic cells as the missing link. Immunol Rev. 2010;236:203–18. doi: 10.1111/j.1600-065X.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- 81.Smith SW, Schrimpf C, Parekh DJ, Venkatachalam M, Duffield JS. Kidney pericytes: a novel therapeutic target in interstitial fibrosis. Histol Histopathol. 2012;27:1503–14. doi: 10.14670/HH-27.1503. [DOI] [PubMed] [Google Scholar]
- 82.Nehls V, Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry. 1993;99:1–12. doi: 10.1007/BF00268014. [DOI] [PubMed] [Google Scholar]
- 83.Brunskill EW, Georgas K, Rumballe B, Little MH, Potter SS. Defining the molecular character of the developing and adult kidney podocyte. PLoS One. 2011;6:e24640. doi: 10.1371/journal.pone.0024640. [DOI] [PMC free article] [PubMed] [Google Scholar]