Abstract
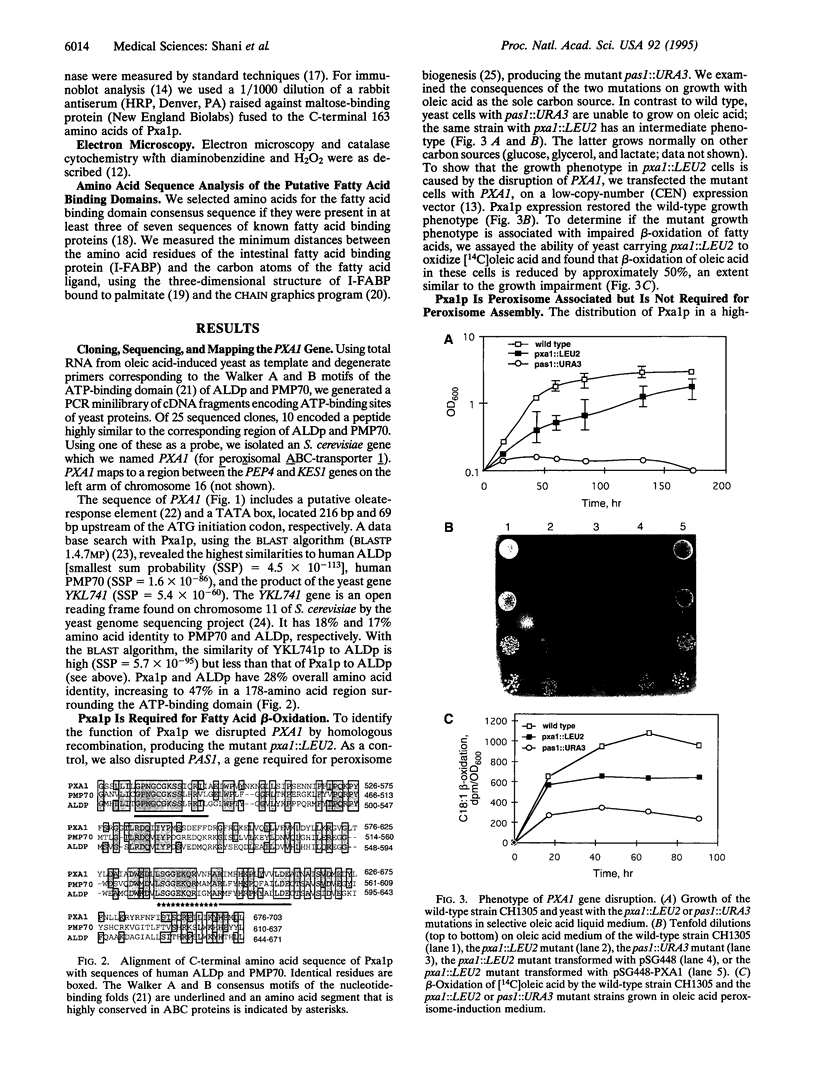
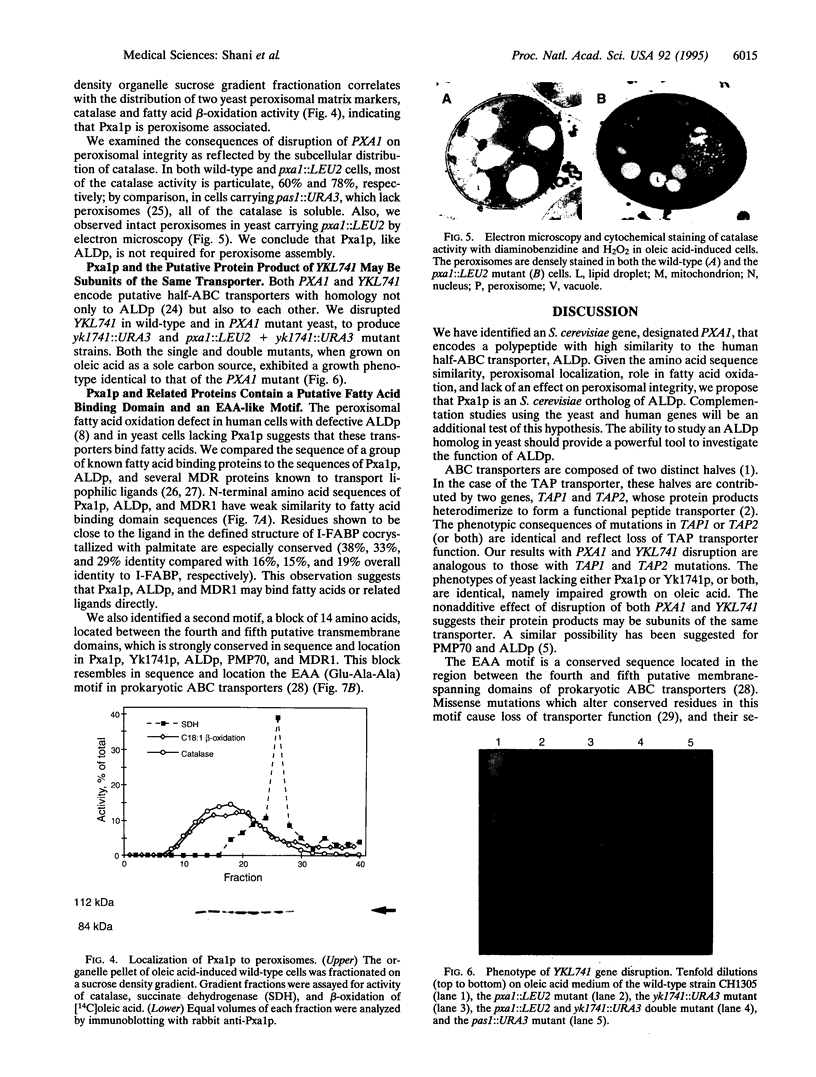
The adrenoleukodystrophy protein (ALDp) is an ATP-binding cassette (ABC) transporter in the human peroxisome membrane. It is defective in X chromosome-linked adrenoleukodystrophy (ALD), a neurodegenerative disorder with impaired peroxisomal oxidation of very long chain fatty acids. We report cloning and characterization of PXA1, a yeast gene encoding a protein (Pxa1p) exhibiting high similarity to ALDp. Disruption of PXA1 results in impaired growth on oleic acid and reduced ability to oxidize oleate. Pxa1p is peroxisome associated; however, in the PXA1 mutant yeast, as in ALD cells, peroxisomes are morphologically intact. Disruption of a second yeast gene, YKL741, which encodes a more distantly related ALDp homolog (Yk174p), in either wild-type or PXA1 mutant yeast, results in a growth phenotype identical to that of the PXA1 mutant. This result suggests that Yk1741p and Pxa1p may be subunits of the same transporter. Sequence analysis of Pxa1p, ALDp, and related ABC transporters reveals a possible fatty acid binding domain and a 14-amino acid EAA-like motif, previously described only in prokaryotes. Because of the similarities in sequence and function, we propose that Pxa1p is the Saccharomyces cerevisiae ortholog of ALDp.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Boguski M. S., Gish W., Wootton J. C. Issues in searching molecular sequence databases. Nat Genet. 1994 Feb;6(2):119–129. doi: 10.1038/ng0294-119. [DOI] [PubMed] [Google Scholar]
- Bossier P., Fernandes L., Vilela C., Rodrigues-Pousada C. The yeast YKL741 gene situated on the left arm of chromosome XI codes for a homologue of the human ALD protein. Yeast. 1994 May;10(5):681–686. doi: 10.1002/yea.320100512. [DOI] [PubMed] [Google Scholar]
- Crane D. I., Kalish J. E., Gould S. J. The Pichia pastoris PAS4 gene encodes a ubiquitin-conjugating enzyme required for peroxisome assembly. J Biol Chem. 1994 Aug 26;269(34):21835–21844. [PubMed] [Google Scholar]
- Einerhand A. W., Kos W. T., Distel B., Tabak H. F. Characterization of a transcriptional control element involved in proliferation of peroxisomes in yeast in response to oleate. Eur J Biochem. 1993 May 15;214(1):323–331. doi: 10.1111/j.1432-1033.1993.tb17927.x. [DOI] [PubMed] [Google Scholar]
- Elgersma Y., van den Berg M., Tabak H. F., Distel B. An efficient positive selection procedure for the isolation of peroxisomal import and peroxisome assembly mutants of Saccharomyces cerevisiae. Genetics. 1993 Nov;135(3):731–740. doi: 10.1093/genetics/135.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Veenhuis M., Mertens D., Kunau W. H. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5419–5423. doi: 10.1073/pnas.86.14.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Wiebel F. F., Flessau A., Rytka J., Beyer A., Fröhlich K. U., Kunau W. H. PAS1, a yeast gene required for peroxisome biogenesis, encodes a member of a novel family of putative ATPases. Cell. 1991 Feb 8;64(3):499–510. doi: 10.1016/0092-8674(91)90234-p. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. Flip-flop: the transmembrane translocation of lipids. Cell. 1994 Nov 4;79(3):393–395. doi: 10.1016/0092-8674(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Kamijo K., Taketani S., Yokota S., Osumi T., Hashimoto T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J Biol Chem. 1990 Mar 15;265(8):4534–4540. [PubMed] [Google Scholar]
- Kelly A., Powis S. H., Kerr L. A., Mockridge I., Elliott T., Bastin J., Uchanska-Ziegler B., Ziegler A., Trowsdale J., Townsend A. Assembly and function of the two ABC transporter proteins encoded in the human major histocompatibility complex. Nature. 1992 Feb 13;355(6361):641–644. doi: 10.1038/355641a0. [DOI] [PubMed] [Google Scholar]
- Kunau W. H., Beyer A., Franken T., Götte K., Marzioch M., Saidowsky J., Skaletz-Rorowski A., Wiebel F. F. Two complementary approaches to study peroxisome biogenesis in Saccharomyces cerevisiae: forward and reversed genetics. Biochimie. 1993;75(3-4):209–224. doi: 10.1016/0300-9084(93)90079-8. [DOI] [PubMed] [Google Scholar]
- Köster W., Böhm B. Point mutations in two conserved glycine residues within the integral membrane protein FhuB affect iron(III) hydroxamate transport. Mol Gen Genet. 1992 Apr;232(3):399–407. doi: 10.1007/BF00266243. [DOI] [PubMed] [Google Scholar]
- Ligtenberg M. J., Kemp S., Sarde C. O., van Geel B. M., Kleijer W. J., Barth P. G., Mandel J. L., van Oost B. A., Bolhuis P. A. Spectrum of mutations in the gene encoding the adrenoleukodystrophy protein. Am J Hum Genet. 1995 Jan;56(1):44–50. [PMC free article] [PubMed] [Google Scholar]
- Mosser J., Douar A. M., Sarde C. O., Kioschis P., Feil R., Moser H., Poustka A. M., Mandel J. L., Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993 Feb 25;361(6414):726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- Mosser J., Lutz Y., Stoeckel M. E., Sarde C. O., Kretz C., Douar A. M., Lopez J., Aubourg P., Mandel J. L. The gene responsible for adrenoleukodystrophy encodes a peroxisomal membrane protein. Hum Mol Genet. 1994 Feb;3(2):265–271. doi: 10.1093/hmg/3.2.265. [DOI] [PubMed] [Google Scholar]
- Petrou S., Ordway R. W., Singer J. J., Walsh J. V., Jr A putative fatty acid-binding domain of the NMDA receptor. Trends Biochem Sci. 1993 Feb;18(2):41–42. doi: 10.1016/0968-0004(93)90050-w. [DOI] [PubMed] [Google Scholar]
- Puziss J. W., Hardy T. A., Johnson R. B., Roach P. J., Hieter P. MDS1, a dosage suppressor of an mck1 mutant, encodes a putative yeast homolog of glycogen synthase kinase 3. Mol Cell Biol. 1994 Jan;14(1):831–839. doi: 10.1128/mcb.14.1.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruetz S., Gros P. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 1994 Jul 1;77(7):1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Saurin W., Köster W., Dassa E. Bacterial binding protein-dependent permeases: characterization of distinctive signatures for functionally related integral cytoplasmic membrane proteins. Mol Microbiol. 1994 Jun;12(6):993–1004. doi: 10.1111/j.1365-2958.1994.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Scapin G., Gordon J. I., Sacchettini J. C. Refinement of the structure of recombinant rat intestinal fatty acid-binding apoprotein at 1.2-A resolution. J Biol Chem. 1992 Feb 25;267(6):4253–4269. doi: 10.2210/pdb1ifc/pdb. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S. Protein import into peroxisomes and biogenesis of the organelle. Annu Rev Cell Biol. 1993;9:445–478. doi: 10.1146/annurev.cb.09.110193.002305. [DOI] [PubMed] [Google Scholar]
- Valle D., Gärtner J. Human genetics. Penetrating the peroxisome. Nature. 1993 Feb 25;361(6414):682–683. doi: 10.1038/361682a0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]









