Healers have been treating heart failure (HF) for millennia, but the central role of neurohormonal abnormalities in its pathogenesis and management was discovered only recently.1 HF previously was understood almost entirely as the result of structural and functional abnormalities of the heart. In the eighteenth century, anatomists described gross enlargement of failing hearts removed at autopsy, and concluded rightly that hypertrophy was central to the pathobiology of HF. Technological advances in the early twentieth century permitted evaluation of the beating heart, and the field of cardiac physiology evolved. As a result, HF came to be conceived in mechanical terms: the fundamental insult in the failing heart was impaired contractility, and this abnormality was either exacerbated or alleviated by alterations in load. Structure and function reconciled well in animal physiology laboratories, because the hypertrophied and failing heart both resulted from and led to altered loading conditions.
The essential role of neurohormonal disturbances in human HF was recognized first in the 1970s and brought to prominence in the 1980s and 1990s.2 In this conception of HF, circulating substances synthesized in the heart, kidneys, adrenal glands, and pituitary glands engendered the characteristic anatomic and physiologic abnormalities described by earlier researchers. HF was no longer simply a disease of the heart.
Increased levels of aldosterone and vasopressin explained the chronically increased preload in the failing heart; norepinephrine and angiotensin (Ang) II induced pathologic hypertrophy and detrimental increases in afterload.
Randomized clinical trials (another important technological advance) reinforced the neurohormonal paradigm. In 1987, the CONSENSUS (Cooperative North Scandinavian Enalapril Survival Study) showed a 31% reduction in 1-year mortality in patients with end-stage HF treated with the angiotensin-converting enzyme (ACE) inhibitor, enalapril, confirming the importance of Ang II in the progression of HF.3 The use of beta-adrenergic receptor blockers (β-blockers) in HF was described first in 1981,4 although the first large mortality trial of β-blockers in HF was the MDC (Metoprolol in Dilated Cardiomyopathy) trial, published in 1993.5 MDC was followed in the next decade by the MERIT-HF (Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure), the US Carvedilol HF trials, CIBIS (Cardiac Insufficiency Bisoprolol Study) I and II, and COPERNICUS (Carvedilol Prospective Randomized Cumulative Survival) trial, collectively proving that β-blockers improve survival in HF (reviewed in Ref.6).
In many respects, clinical trial data have provided the strongest endorsement of the neurohormonal paradigm. Drugs that alter hemodynamic parameters without blocking neurohormonal activation, including digoxin,7 non–potassium-sparing diuretics,8 and positive inotropes,9 have either neutral or negative effects on survival. In this respect, the contemporary use of neurohormonal modulators for HF pharmacotherapy offers an excellent example of reciprocity in translational science: elucidation of basic pathophysiology directs therapeutic targeting, and clinical trial results further inform the understanding of drug mechanism. This article discusses mechanisms of action for neurohormonal antagonists, with attention to both fundamental physiology and clinical trial outcomes.
THE SYMPATHETIC NERVOUS SYSTEM AND CARDIOVASCULAR PHYSIOLOGY
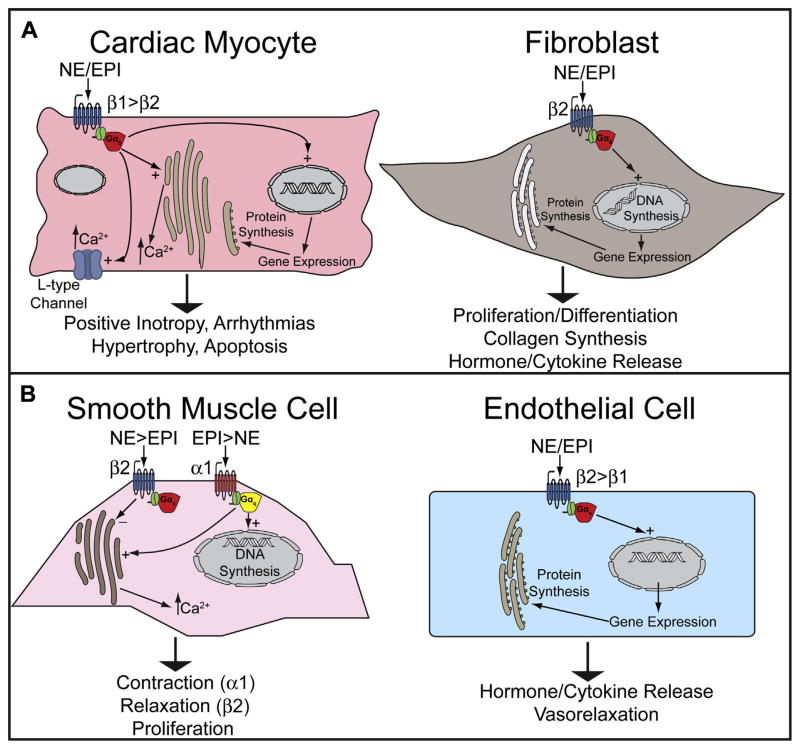
The sympathetic nervous system (SNS) is activated via arterial and venous baroreceptors and arterial chemoreceptors in response to decreases in perfusion pressure or oxygen delivery. In response, efferent fibers increase the release of norepinephrine (NE) (80%) or epinephrine (EPI) (20%) from synaptic varicosities in the myocardium and blood vessels, and stimulate the adrenal medulla to release NE (20%) and EPI (80%) into the blood. These hormones bind at least 9 different subtypes of adrenergic receptors (ARs) (3 beta-ARs [β1, β2, β3], 3 alpha-1 ARs [α1A, α1B, α1D], and 3 alpha-2 ARs [α2A, α2B, α2C]) that are expressed variably by most cell types in the cardiovascular system and function primarily through G protein–coupled signaling cascades (Fig. 1).10
Fig. 1.
SNS effector hormones and adrenergic receptor subtypes in cells of the (A) heart and (B) peripheral vasculature.
β1-ARs predominate in the myocardium (70%–80% of total β-ARs), whereas β2-ARs and β3-ARs are less abundant (15%–18% and 2%–3% respectively) (see Fig. 1A).11 The predominant β-AR in vascular tissue is β2-AR, which mediates vasorelaxation (see Fig. 1B). Stimulation of β1-ARs on cardiomyocytes activates stimulatory G protein (Gs) and protein kinase A (PKA), leading to increased contractility (via activation of L-type calcium channels and ryanodine receptors); heart rate (via stimulation of L-type calcium channels and hyperpolarization-activated cyclic nucleotide-gated [HCN] channels); and rate of relaxation (via indirect stimulation of sarcoplasmic/endoplasmic reticulum calcium ATPase [SERCA] and Na/K-ATPase). Cardiomyocyte β2-AR activation also increases inotropy, although these receptors are less abundant and have a lower affinity for NE. The β2 is the predominant AR on cardiac fibroblasts, in which it likely plays important roles in HF pathobiology. β3-ARs exert an exclusively negative inotropic effect through activation of nitric oxide.12
α1-ARs are best known for their effects in vascular smooth muscle, where they promote vasoconstriction through activation of Gq, although myocardial α1-ARs mediate broadly beneficial effects, including positive inotropy, physiologic cardiomyocyte hypertrophy, and protection from cell death.13 α2-ARs are predominantly found in presynaptic terminals of adrenergic neurons and adrenal chromaffin cells, where they inhibit NE/EPI release via Gi-related signaling cascades that inhibit PKA activation.11,14 In this respect, α2-ARs negatively regulate excess NE/EPI release and spillover in both central and peripheral adrenergic synapses.
THE SNS AND HF PATHOPHYSIOLOGY
Chronic catecholamine excess is central to the pathobiology of HF, and the degree of activation is directly proportional to disease severity.15,16 SNS upregulation also extends to the central nervous system, where NE spillover and turnover is increased.17,18 In the periphery, SNS upregulation is organ specific: it is preferentially activated in cardiac tissue in mild to moderate HF, and only becomes activated in the kidney and other organ systems in severe HF.19,20
Chronic activation of cardiac β-ARs leads to pathologic cardiac hypertrophy and fibrosis: the hallmarks of ventricular remodeling. Increased levels of both local and circulating catecholamines lead to cardiac hypertrophy by acting directly on the cardiomyocyte β1-ARs21 or by stimulating the paracrine release of other hormones such as Ang II and endothelin-1 (ET-1).22 SNS activation also leads to direct stimulation of β2-ARs on cardiac fibroblasts, leading to fibroblast proliferation and increased release of cytokines such as interleukin-6, and hormones such as Ang II and ET-1. These factors in turn lead to increased collagen deposition, fibrosis, pathologic differentiation of fibroblasts into myofibroblasts, and cardiomyocyte hypertrophy.23 Furthermore, chronic β1-AR hyperstimulation in animal models leads to necrotic and apoptotic cardiomyocyte death, implicating sustained SNS activity in another important cellular mechanism of HF.24
Upregulation of the SNS can also cause ventricular arrhythmias25 via direct effects on cardiomyocyte calcium handling mediated in part by catecholamine-induced ryanodine receptor dysfunction.26 Chronic catecholamine surge can also promote both atrial and ventricular arrhythmias in HF indirectly through increased fibrosis and remodeling.27
Chronic myocardial β1-AR activation ultimately results in the depletion of NE from cardiac nerve terminals, and downregulation of myocardial β-ARs.28 The desensitization and inactivation of membrane-bound β-ARs is performed by G protein–coupled receptor kinases (GRKs) that phosphorylate ARs, facilitating binding to beta-arrestins that uncouple the receptor from G proteins and target it for internalization.10 The downregulation of both myocardial and presynaptic ARs results in decreased cardiac inotropic reserve, further disabling the failing heart. Inhibition of β1-AR downregulation by blocking GRK2 activity improves cardiac function and myocyte survival,29 providing further evidence that the diminution of β-AR signaling is at least partially responsible for the pathogenesis of HF.
Inhibitors of the SNS
The success of β-blocker therapy offers perhaps the clearest example of the critical role of neurohormones in HF. Physiologic studies in animals and humans conclusively show negative inotropy resulting from acute β-AR antagonism,30,31 and clinical guidelines historically contained a contra-indication for β-blocker use in patients with HF.32 Nevertheless, studies in cells and animals established the fundamental role of chronic NE exposure in the pathophysiology of HF (reviewed in Ref.33), leading to the incremental translation to clinical trials and practice.
Three β-blockers currently are approved for use in HF: metoprolol succinate, carvedilol, and bisoprolol. These drugs were selected from randomized clinical trial evidence, although debate exists about whether the specific pharmacology of these agents confers superiority or whether β-blocker benefits arise purely from antagonism of the β1-AR.34,35 No trial has convincingly tested head-to-head efficacy of multiple β-blockers in HF.36
β-Blockers can be classified broadly based on selectivity for AR subtypes, vasodilating effects, and intrinsic sympathomimetic or sympatholytic properties (Table 1). First-generation β-blockers are not as well-tolerated in patients with HF, possibly as a result of blockade of vascular β2 receptors, which may shunt catecholamines to α1 receptors and cause vasoconstriction.37 Second-generation β-blockers are considered cardioselective because of their selectivity for β1-ARs.
Table 1.
Classification and effects of β-blockers used in HF clinical trials
| β-Blocker | β1 Block | β2 Block | α1 Block | Vascular Effects | Survival Benefit |
|---|---|---|---|---|---|
| First Generation | |||||
| Propranolol | + | + | − | None or vasoconstriction37 | No |
| Second Generation | |||||
| Bisoprolol | + | − | − | None | Yes38 |
| Metoprolol | + | − | − | None | Yes39 |
| Third Generation | |||||
| Carvedilol | + | + | + | Acute: vasodilation40 Chronic: none41 |
Yes42 |
| Nebivolol | + | − | − | Vasodilation | No43 |
| Bucindolol | + | + | + (weak) | Vasodilation | No44 |
Third-generation β-blockers generally are distinguished from first-generation agents by their vaso-dilating effects. Nebivolol causes vasodilation by stimulating nitric oxide release, possibly through β3-AR activation. Bucindolol is a nonselective β-blocker with intrinsic sympatholytic activity and weak α1-blocking properties. Carvedilol blocks β1-ARs, β2-ARs, and α1-ARs, and is the most widely studied of these agents. Although the benefits of carvedilol have been attributed widely to afterload reduction resulting from α1-AR antagonism, this effect dissipates within weeks.41 Thus, carvedilol’s beneficial effects likely are caused primarily by β1-blockade, although it also has adaptive effects on cellular metabolism, oxidative stress reduction, and protection from apoptotic cell death.45,46
Mechanisms of β-Blocker Benefit in the Human Heart
Underlying mechanisms for the benefits of β-blockers in HF have not been elucidated completely, but likely are complex and multifactorial given the broadly pathologic effects of chronic catecholamine surge (Table 2).
Table 2.
Beneficial effects of neurohormonal antagonists in clinical trials of HF pharmacotherapy
| Drug Class | Hemodynamic Effects | Remodeling Effects | Vascular Effects | Antiarrhythmic Effects |
|---|---|---|---|---|
| β-Blocker | ↑EF25,34 ↓Heart rate47 |
↓Volume48–51 | Minimal | ↓Arrhythmias ↓SCD52 ↓ICD shocks53,54 |
| ACE inhibitor or ARB | ↑EF55 ↓Afterload56 ↓Preload56 |
↓Volume55,57 ↓Hypertrophy58 |
↓Atheroma59 ↓ACS60 ↑Compliance |
None |
| Aldosterone receptor antagonist | ↑EF | ± Volume61 ↓Fibrosis62,63 |
↑Endothelial function | ↓Arrhythmias64,65 ↓SCD64,65 |
Abbreviations: ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; EF, ejection fraction; ICD, implantable cardioverter defibrillator; SCD, sudden cardiac death.
Hemodynamics
Acute blockade of myocardial β1-ARs has negative inotropic and chronotropic effects. Chronic β-blocker use improves cardiac performance in patients with HF, possibly because negative chronotropy increases filling time.66 Heart rate reduction has been used as an index of β-blocker efficacy and a meta-analysis of 23 randomized clinical trials indicates that heart rate reduction is a more powerful predictor of benefit than β-blocker dose. For every heart rate decrease of 5 beats per minute in the pooled β-blocker groups there was an 18% reduction in risk of death.47 The early success of the specific HCN channel blocker ivabradine,67 which slows heart rate without modulating the SNS, may corroborate the primary importance of negative chronotropy in HF therapy.
Chronic β-blocker use does not decrease contractile function in patients with HF. Invasive hemodynamic studies show improved stroke volume and cardiac index, at rest and peak exercise, after chronic carvedilol treatment.68 A meta-analysis of 21 randomized clinical trials found an absolute increase in ejection fraction of 4% in patients with HF treated with β-blocker relative to placebo,34 and a separate analysis of 18 trials reported a 29% relative increase in ejection fraction.25 Ex vivo experiments on failing human heart tissue suggest that β-blocker use improves inotropic response to β-AR agonists and restores aspects of physiologic cardiomyocyte calcium handling69 and responsiveness.70 β-Blockers also improve the diastolic performance of the hypertrophied human heart.71
Reverse remodeling
Numerous clinical trials show the favorable effect of chronic β-blocker use on ventricular remodeling. In a MERIT-HF substudy, left ventricular (LV) end-diastolic volume index decreased by 17% and LV mass index decreased by almost 10% after 6 months of metoprolol.48 Metoprolol also decreased LV end-diastolic index by 10% to 15% in patients with asymptomatic LV dysfunction in the REVERT (Reversal of Ventricular Remodeling with Toprol-XL) study.49 Both CAPRICORN50 and the Australia–New Zealand HF Research Collaborative Group51 showed similar improvements with carvedilol.
β-Blockers decrease fibrosis in animal models of HF72,73 and reduce circulating markers of fibrosis in humans,74 although direct effects are not readily demonstrable in human hearts, perhaps because the β2-AR is the predominant AR on cardiac fibroblasts.
Antiarrhythmic effects
Sudden cardiac death is the primary cause of mortality in patients with New York Heart Association class I - III HF, and the well-established antiarrhythmic effects of β-blockers also contribute to their survival benefit. Although sudden cardiac death was not reduced in all trials of β-blockade in HF, a reduction was seen in the BHAT (Beta-Blocker Heart Attack Trial),75 CAPRICORN,76 CI-BIS II,38 and MERIT-HF.39 A recent meta-analysis of 30 trials (24,779 patients) of β-blockers in HF found a 31% reduction in the risk of sudden cardiac death (odds ratio, 0.69; 95% confidence interval, 0.62–0.77) with a number needed to treat of 43 patients to prevent 1 sudden cardiac death per year.52 β-Blocker use also substantially decreases the risk of both appropriate53 and inappropriate defibrillator therapies.54
Molecular changes in human heart
Chronic β-blocker use in HF mitigates the characteristic decrease in myocardial β-AR abundance, although it is unclear whether this effect is essential for clinical or physiologic benefit.66,77 β-Blocker use also abrogates the pathologic changes in gene expression in the failing heart: α-myosin heavy chain abundance increases, β-myosin heavy chain decreases, and sarcoplasmic reticulum Ca2+ ATPase levels are restored.77
Digoxin and the SNS
In the past, the usefulness of digoxin in HF has been attributed to its positive inotropic effects. However, these effects are only present at high serum digoxin concentrations (>1 ng/mL), at which an increased risk of mortality has also been observed.78 It has been proposed that the benefits of digoxin at lower concentrations result in part from neurohormonal modulation. Among its many pharmacologic actions, digoxin decreases circulating norepinephrine and renin levels79,80 and has a favorable impact on natriuretic peptide release.81
Risks of sympatholysis in HF
Although the essential role of catecholamine excess in the pathophysiology of HF is beyond dispute, direct sympatholytic therapies have been associated with poorer outcomes. In a study of patients with chronic HF, a sustained-release preparation of moxonidine, an imidazoline receptor agonist that reduces sympathetic outflow, improved ventricular performance but led to an increase in serious adverse events.82 These risks were confirmed in a larger trial, which was terminated early because of a nearly 2-fold increase in death among those randomized to moxonidine.83 The intrinsic sympatholytic properties of bucindolol may help explain why outcomes in the BEST (Beta-Blocker Evaluation in Survival Trial) were less favorable than those of other β-blocker clinical trials.44
One conceivable explanation for the apparent risk associated with sympatholysis is the abrogation of adaptive effects of myocardial α1-AR activation. Evidence from human studies suggests that the relative increase in α1-AR expression observed in advanced HF may be a compensatory response to preserve myocardial function in the setting of β1-AR downregulation and dysfunction.84 These cardioprotective effects may explain why therapies that inhibit α1-ARs have been linked to adverse outcomes in patients with HF. An arm of ALLHAT that randomized patients to the α1-blocker doxazosin was stopped early for a 2-fold increase in incident HF.85
THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM AND CARDIOVASCULAR PHYSIOLOGY
The renin-angiotensin-aldosterone system (RAAS) consists of a protease cascade that is activated by renin release from the juxtaglomerular cells of renal afferent arterioles. Renin is secreted in response to decreased renal perfusion pressure, decreased salt delivery to the distal convoluted tubule, increased renal sympathetic nerve activity, or changes in circulating natriuretic peptides. Renin catalyzes the cleavage of angiotensinogen, a circulating protein produced by the liver. The resulting peptide, angiotensin I (Ang I), is then cleaved by ACE, to generate Ang II, which is among the most potent endogenous vasoconstrictors. Ang II binds to 2 G protein–coupled receptors, angiotensin II receptor, type I (AT1) and AT2. AT1 is the primary receptor expressed on vascular smooth muscle, endothelium, myocardium, neurons, and fibroblasts, whereas AT2 is primarily expressed early in development and its effects are less well understood in adults.86,87
The other potent effector hormone of RAAS, aldosterone, is a steroid hormone released primarily from the adrenal cortex in response to increased Ang II and plasma [K+]. Aldosterone binds to the intracellular mineralocorticoid receptor (MR) leading to increased salt and water reabsorption, increased blood volume, and alterations in ion-channel expression.88
In the past, RAAS-associated hormones were considered to be renally controlled endocrine hormones that exerted effects widely throughout the body. However, it is now well understood that tissues such as the heart, blood vessels, lungs, and brain have an intrinsic RAAS that functions in an autocrine/paracrine manner.89 In the heart, local stress, cellular damage, and stretch can each lead to an upregulation of locally produced RAAS components including ACE, Ang II, and aldosterone.90,91 It is now thought that cardiac-generated RAAS components play a major role in the progression of HF.92
THE RAAS AND HF PATHOPHYSIOLOGY
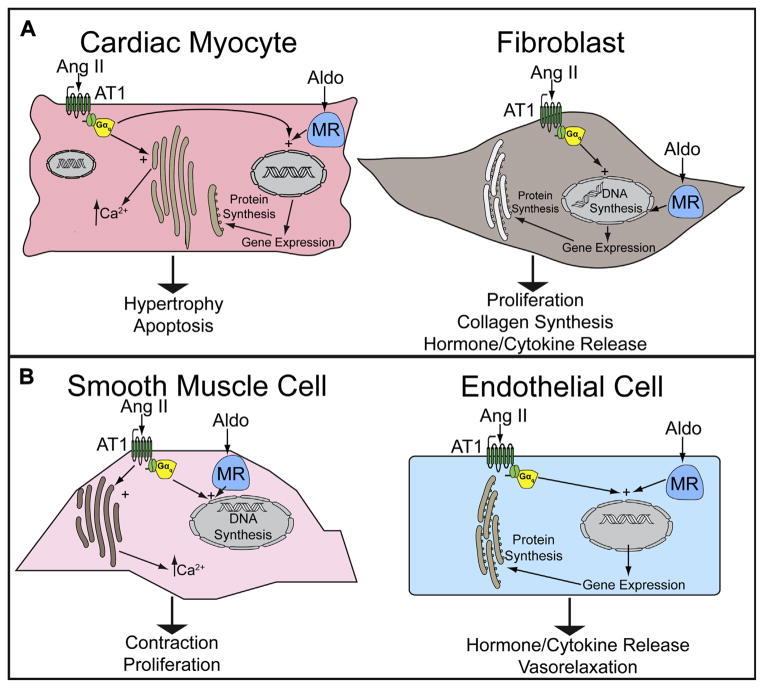
Circulating and intrinsic Ang II and aldosterone are increased in HF, and contribute to HF pathophysiology through both extracardiac and direct cardiac effects.92,93 In vascular tissue, Ang II and aldosterone mediate increased vasoconstriction, unfavorable vascular remodeling, and endothelial dysfunction (Fig. 2B).94,95 Ang II and aldosterone promote sodium and water reabsorption in the proximal and distal convoluted tubules respectively. RAAS hormones also have important direct effects on myocardial cells.96 Ang II induces cardiomyocyte hypertrophy and cardiac fibroblast proliferation through activation of AT1 receptors,97 promoting cardiac hypertrophy independent of effects on blood pressure.98 Aldosterone also promotes cardiac fibrosis through activation of mineralocorticoid receptors on cardiac fibroblasts (see Fig. 2A).
Fig. 2.
RAAS effector hormones and receptors in cells of the (A) heart and (B) peripheral vasculature. Aldo, aldosterone.
In addition to the directly deleterious effects of Ang II and aldosterone, RAAS also interacts with other neurohormonal signals that contribute to the pathobiology of HF. For example, local Ang II production leads to increased NE release from sympathetic nerve terminals in the heart.99 Ang II also has effects on the central nervous system, causing a central activation of sympathetic nerves that target the cardiovascular system.100 Central inhibition of AT1 receptors leads to a decrease in sympathetic nerve activity in the heart.101
In addition to these well-known RAAS constituents, several other RAAS enzymes contribute to cardiovascular regulation. ACE2, neprilysin (also known as neutral endopeptidase),87 prolylendo-peptidases, and prolylcarboxypeptidases break down Ang I and II, ultimately leading to the generation of a peptide known as Ang(1–7).87 Ang(1–7) acts on its own receptor, MasR, counteracting the effects of Ang II by causing vasodilation, decreased fibrosis, decreased oxidative stress, and decreased hypertrophy.102 In addition to cleaving Ang I, ACE also is the main enzyme that breaks down the vasodilator bradykinin. It has been proposed that one of the key mechanisms of ACE inhibitors in the treatment of HF is increasing bradykinin levels, directly leading to vasodilation and decreased afterload.103 However, increased bradykinin is also responsible for several of the side effects of ACE inhibitor treatment such as angioedema and dry cough.104
Inhibitors of the RAAS
Inhibitors of RAAS used in the management of HF include ACE inhibitors, angiotensin receptor blockers (ARBs), and aldosterone receptor antagonists. ACE inhibitors prevent the conversion of Ang I to Ang II, whereas ARBs competitively inhibit the effect of Ang II on AT1 receptors in heart, kidney, and vascular tissue. Aldosterone receptor antagonists competitively inhibit the binding of aldosterone to mineralocorticoid receptors in the heart, kidney, and peripheral vasculature. The benefits of ACE inhibitors, ARBs, and aldosterone receptor antagonists in HF are considered class-wide effects, because clinical trials of multiple agents from each drug class show benefit. Higher doses of ACE inhibitors and ARBs confer greater reductions in hospitalizations compared with lower doses,105,106 but head-to-head comparisons between the two classes have been inconclusive.
Mechanisms of RAAS Blockade Benefit in Human HF
The benefits of RAAS antagonists initially were thought to result from their favorable effects on loading conditions as mediated by activity in the kidney and peripheral vasculature, but salutary direct myocardial effects are now recognized (see Table 2).
Hemodynamics
Decreased activation of vascular AT1 receptors by circulating Ang II produces vasodilation and thus decreases cardiac afterload. In addition, less salt and water are retained as a result of decreased downstream aldosterone release, thereby reducing preload. In one randomized hemodynamic trial, fosinopril decreased pulmonary capillary wedge pressure (preload) and systemic vascular resistance (afterload) acutely. After 10 weeks of therapy, preload and afterload were durably reduced and cardiac index was increased compared with placebo.56 Aldosterone receptor antagonists cause a mild decrease in preload acutely through their potassium-sparing diuretic effects. However, a clinically meaningful diuretic effect is less commonly observed at the low doses used in HF and is unlikely to explain the magnitude of benefit observed in clinical trials.
Reverse remodeling
ACE inhibitors and ARBs have uniformly favorable effects on cardiac remodeling in HF. In the SOLVD (Studies of Left Ventricular Dysfunction) trial, 1 year of enalapril resulted in a 10% decrease in both LV end-diastolic and end-systolic volumes.57 Similar results were reported with post-MI treatment with captopril in SAVE (Survival and Ventricular Enlargement) trial.107 Losartan led to greater regression of LV hypertrophy than atenolol in the LIFE (Losartan Intervention For Endpoint) trial,58 and treatment with valsartan led to decreased ventricular volume and increased ejection fraction in Val-HeFT (Valsartan Heart Failure Trial).55
Aldosterone receptor antagonists reduced circulating markers of collagen turnover in both RALES (Randomized Aldactone Evaluation Study) and EPHESUS (Eplerenone Post-AMI Heart Failure Efficacy and Survival Trial), suggesting that inhibition of cardiac fibrosis contributes to the benefit associated with both spironolactone and eplerenone.62,63 However, aldosterone receptor antagonists do not consistently confer beneficial remodeling in clinical trials.61
Antiarrhythmic effects
In contrast with β-blockers, there is no clear signal that ACE inhibitors or ARBs reduce arrhythmias or sudden cardiac death in patients with HF.108 For example, in CONSENSUS there was a 50% relative risk reduction in death caused by progressive HF, but no change in sudden cardiac death after treatment with enalapril.3 Aldosterone blockade reduces the risk of both atrial and ventricular arrhythmias, which may partly explain the reductions in sudden cardiac death observed in clinical trials.64,65 This antiarrhythmic effect may be caused by maintenance of physiologic serum potassium concentrations in the setting of aggressive loop diuretic therapy or it may be an epiphenomenon of reverse remodeling and decreased fibrosis.
Other effects
Animal studies reveal that ACE inhibitors have antiatherogenic effects resulting from inhibition of vascular smooth muscle cell proliferation and restoration of endothelial function.59 Results from clinical trials substantiate these findings. ACE inhibitors improved post-MI outcomes in both SAVE and SOLVD and a large meta-analysis confirmed a 20% to 25% reduction in risk of acute coronary syndrome in patients with HF who received an ACE inhibitor.60 ACE inhibitors also delay progression of renal dysfunction, which is a harbinger of poor outcome in HF.109
ACE (aldosterone) escape
The efficacy of RAAS antagonists can diminish over time through an effect known as aldosterone or ACE escape, whereby a maladaptive increase in RAAS components is observed after chronic treatment with RAAS antagonists.110 ACE escape occurs in approximately 10% of patients within 6 months and 50% of patients within 12 months of starting treatment.111 There are several potential physiologic explanations for this phenomenon. Prolonged inhibition of ACE or AT1 leads to increased levels of renin and Ang I because of the loss of negative feedback by Ang II. In the setting of ACE inhibition, several other enzymes such as chymase and cathepsin also cleave circulating and local Ang I to Ang II, leading to aldosterone production (escape).104
Further, a receptor for renin and prorenin (inactive renin) has recently been discovered. Binding of (pro)renin to the (pro)renin receptor (PRR) leads to increased fibrosis and release of cytokines and prohypertrophic growth factors. PRR also serves an enzymatic function, generating active renin from inactive (pro)renin.112
Multiple clinical trials have investigated the possibility that dual RAAS antagonist therapy could abrogate ACE escape and improve outcomes in HF. Combined treatment with ACE inhibitor and ARB produced incremental improvements in cardiovascular mortality and hospitalizations but was also associated with an increase in adverse events.113,114 Meta-analysis of 4 published clinical trials of RAAS combination therapy found a 2-fold increased risk of worsening renal function and a nearly 5-fold higher risk of hyperkalemia,115 likely caused by pronounced decreases in circulating aldosterone levels.
The compensatory increase in renin release in the setting of ACE inhibitor and/or ARB therapy has also prompted trials of direct renin inhibitors (DRIs) in patients with HF. It was postulated that renin inhibition would augment downstream RAAS blockade and provide additive benefits when combined with ACE inhibitor or ARB therapy. Although the DRI aliskiren reduces plasma renin activity, there is no evidence of a clinical benefit in HF. In ASTRONAUT (Aliskiren Trial on Acute Heart Failure Outcomes), a large randomized controlled clinical trial of patients on optimal HF therapy (including other RAAS antagonists), aliskiren failed to reduce a composite of cardiovascular death or hospitalizations and was associated with hypotension, renal dysfunction, and hyperkalemia.116 ATMOSPHERE (Aliskiren Trial of Minimizing Outcomes for Patients with Heart Failure), a trial comparing aliskiren with enalapril in patients with HF is underway.117
NATRIURETIC PEPTIDES AND CARDIOVASCULAR PHYSIOLOGY
The natriuretic peptides (atrial natriuretic peptide [ANP], B-type natriuretic peptide [BNP], and C-type natriuretic peptide [CNP]) collectively produce adaptive effects in HF and oppose the actions of the effector hormones of the SNS and RAAS. ANP is released by cells in the atrial wall in response to stretch or increases in plasma Ang II, ET-1, and vasopressin.118 BNP is released primarily from the left ventricle, although atrial cells also release BNP at a much lower concentration than ANP.119 CNP is released by endothelial cells in response to increased cytokines and other hormones such as acetylcholine.120 ANP, BNP, and CNP bind to 2 transmembrane-bound guanylyl cyclases (GCs), GC-A and GC-B, to increase intracellular cyclic guanosine monophosphate (cGMP) and elicit wide-ranging physiologic effects including vasodilation, increased salt and water excretion, decreased renin release, dampened SNS activity, decreased cardiac fibrosis, and blunted cardiomyocyte hypertrophy.121–125
Targeting Natriuretic Peptides for HF Therapy
Nesiritide is a recombinant form of BNP that binds GC receptors on vascular endothelium and in the kidney. Nesiritide mimics the salutary effects of endogenous BNP on cardiovascular hemodynamics and renal physiology, but does not reduce symptoms to a significantly greater extent than diuretics and vasodilators.126 In large clinical trials, nesiritide did not improve survival, although early concerns over increased mortality and worsening renal function have subsided.
BNP has a short plasma half-life because of its removal by cellular reuptake, the natriuretic peptide clearance receptor (GC-C), and breakdown by neprilysin, the same enzyme that generates Ang(1–7) from Ang I.127 Neprilysin inhibitors have been developed in an attempt to increase the half-life of circulating BNP for therapeutic benefit, although they typically also have been designed to antagonize RAAS in order to counter the unfavorable effects of decreased Ang(1–7) levels. Omapatrilat, a vasopeptide inhibitor of both neprilysin and ACE, exerted favorable hemodynamic effects, but was associated with a more than 3-fold increase in angioedema compared with an ACE inhibitor, likely caused by its inhibition of both bradykinin and substance P degradation.128 The focus has since shifted to compounds that both inhibit neprilysin and block Ang II receptors. A study comparing enalapril and LCZ696, a dual neprilysin inhibitor and ARB, in chronic HF is underway.129
VASOPRESSIN IN HF PATHOPHYSIOLOGY AND PHARMACOTHERAPY
Vasopressin (arginine vasopressin [AVP]) is released in response to increased osmolarity, Ang II, or SNS stimulation, and is chronically increased in HF.130 AVP is secreted primarily from the posterior pituitary, but local vasopressin production may also contribute to the progression of HF.131 Vasopressin stimulates the activity of 3 G protein–coupled receptors, V1a, V1b, and V2. V1a, expressed on vascular smooth muscle and ventricular myocardium, couples to Gq, increasing intracellular calcium and causing vasoconstriction, positive inotropy, and hypertrophy.132 The V2 receptor mediates free water reabsorption in the kidney. Excess stimulation can lead to hypervolemic hyponatremia in patients with HF.
Recognition that AVP secretion is upregulated in HF prompted investigations of vasopressin receptor antagonists (VRAs) as another novel therapeutic strategy. Despite aggressive diuretic therapy, many patients with HF continue to retain excess free water and hyponatremia is common. However, whether hyponatremia represents a target of pharmacologic therapy or a surrogate marker for the severity of disease remains an area of controversy. VRAs competitively inhibit V2 receptors in renal collecting ducts, thereby preventing the reabsorption of free water. Tolvaptan, an oral VRA, is selective for V2 receptors, whereas intravenous conivaptan also inhibits V1A receptors. In EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan), tolvaptan conferred improvements in some HF symptoms, but did not improve survival.133 Although tolvaptan partially corrects hyponatremia, the effect is not durable after discontinuation of therapy.134 The reasons for the modest clinical impact of this physiologically rational therapeutic approach are unclear.
SUMMARY
The depth to which disease mechanism is understood is often dictated by extant technologies. In that respect, the conception of HF was informed successively by gross anatomy, organ-level physiology, and cellular physiology. As outlined in this review, recent advances have enabled an expansion in knowledge of the cellular and subcellular mechanisms that underlie the characteristic neurohormonal disturbances in HF. How will today’s emerging technologies influence understanding of HF? Will massively parallel sequencing technologies inform clinicians that HF fundamentally is a disease of genetic and epigenetic modifications?135,136 Will the next generation of HF therapies target DNA methylation? Epigenetic reader proteins?137 Noncoding RNAs or microRNAs?138 As always, only time will tell.
KEY POINTS.
Neurohormonal abnormalities are central to the pathobiology of heart failure and antagonism of their systemic effects is the basis of contemporary heart failure pharmacotherapy.
β-Blockers likely confer benefit through induction of reverse remodeling, reduction of sudden cardiac death, and restoration of adaptive adrenergic signaling.
Antagonists of the renin-angiotensin-aldosterone system have beneficial activities in cells of the heart in addition to their effects in the kidneys and peripheral vasculature.
All agents that improve survival in heart failure target neurohormones, but not all neurohormonal modulators improve survival.
Footnotes
Disclosure: The authors have no relevant financial disclosures.
References
- 1.Katz AM. The “modern” view of heart failure: how did we get here? Circ Heart Fail. 2008;1(1):63–71. doi: 10.1161/CIRCHEARTFAILURE.108.772756. [DOI] [PubMed] [Google Scholar]
- 2.Francis GS, Goldsmith SR, Levine TB, et al. The neurohumoral axis in congestive heart failure. Ann Intern Med. 1984;101(3):370–7. doi: 10.7326/0003-4819-101-3-370. [DOI] [PubMed] [Google Scholar]
- 3.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987;316(23):1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 4.Ikram H, Fitzpatrick D. Double-blind trial of chronic oral beta blockade in congestive cardiomyopathy. Lancet. 1981;2(8245):490–3. doi: 10.1016/s0140-6736(81)90881-3. [DOI] [PubMed] [Google Scholar]
- 5.Waagstein F, Bristow MR, Swedberg K, et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet. 1993;342(8885):1441–6. doi: 10.1016/0140-6736(93)92930-r. [DOI] [PubMed] [Google Scholar]
- 6.Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. JAMA. 2002;287(7):883–9. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 7.Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 8.Domanski M, Norman J, Pitt B, et al. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 2003;42(4):705–8. doi: 10.1016/s0735-1097(03)00765-4. [DOI] [PubMed] [Google Scholar]
- 9.Felker GM, O’Connor CM. Inotropic therapy for heart failure: an evidence-based approach. Am Heart J. 2001;142(3):393–401. doi: 10.1067/mhj.2001.117606. [DOI] [PubMed] [Google Scholar]
- 10.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113(6):739–53. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-trans-membrane-spanning receptors and heart function. Nature. 2002;415(6868):206–12. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier C, Leblais V, Kobzik L, et al. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest. 1998;102(7):1377–84. doi: 10.1172/JCI2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen BC, O’Connell TD, Simpson PC. Alpha-1-adrenergic receptors in heart failure: the adaptive arm of the cardiac response to chronic catecholamine stimulation. J Cardiovasc Pharmacol. 2014;63(4):291–301. doi: 10.1097/FJC.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lymperopoulos A, Rengo G, Koch WJ. Adrenal adrenoceptors in heart failure: fine-tuning cardiac stimulation. Trends Mol Med. 2007;13(12):503–11. doi: 10.1016/j.molmed.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311(13):819–23. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 16.Kaye DM, Lefkovits J, Jennings GL, et al. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26(5):1257–63. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 17.Kaye DM, Lambert GW, Lefkovits J, et al. Neuro-chemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol. 1994;23(3):570–8. doi: 10.1016/0735-1097(94)90738-2. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal A, Esler MD, Lambert GW, et al. Norepinephrine turnover is increased in suprabulbar subcortical brain regions and is related to whole-body sympathetic activity in human heart failure. Circulation. 2002;105(9):1031–3. doi: 10.1161/hc0902.105724. [DOI] [PubMed] [Google Scholar]
- 19.Ramchandra R, Hood SG, Denton DA, et al. Basis for the preferential activation of cardiac sympathetic nerve activity in heart failure. Proc Natl Acad Sci U S A. 2009;106(3):924–8. doi: 10.1073/pnas.0811929106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rundqvist B, Elam M, Bergmann-Sverrisdottir Y, et al. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation. 1997;95(1):169–75. doi: 10.1161/01.cir.95.1.169. [DOI] [PubMed] [Google Scholar]
- 21.Ju H, Zhao S, Tappia PS, et al. Expression of Gq alpha and PLC-beta in scar and border tissue in heart failure due to myocardial infarction. Circulation. 1998;97(9):892–9. doi: 10.1161/01.cir.97.9.892. [DOI] [PubMed] [Google Scholar]
- 22.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–8. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 23.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123(2):255–78. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Singh K, Xiao L, Remondino A, et al. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol. 2001;189(3):257–65. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- 25.Lechat P, Packer M, Chalon S, et al. Clinical effects of beta-adrenergic blockade in chronic heart failure: a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation. 1998;98(12):1184–91. doi: 10.1161/01.cir.98.12.1184. [DOI] [PubMed] [Google Scholar]
- 26.Blayney LM, Lai FA. Ryanodine receptor-mediated arrhythmias and sudden cardiac death. Pharmacol Ther. 2009;123(2):151–77. doi: 10.1016/j.pharmthera.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 28.Bristow MR, Ginsburg R, Minobe W, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307(4):205–11. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 29.Akhter SA, Eckhart AD, Rockman HA, et al. In vivo inhibition of elevated myocardial beta-adrenergic receptor kinase activity in hybrid transgenic mice restores normal beta-adrenergic signaling and function. Circulation. 1999;100(6):648–53. doi: 10.1161/01.cir.100.6.648. [DOI] [PubMed] [Google Scholar]
- 30.Epstein S, Robinson BF, Kahler RL, et al. Effects of beta-adrenergic blockade on the cardiac response to maximal and submaximal exercise in man. J Clin Invest. 1965;44(11):1745–53. doi: 10.1172/JCI105282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nayler WG, Chipperfield D, Lowe TE. The negative inotropic effect of adrenergic betareceptor blocking drugs on human heart muscle. Cardiovasc Res. 1969;3(1):30–6. doi: 10.1093/cvr/3.1.30. [DOI] [PubMed] [Google Scholar]
- 32.Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure) Circulation. 1995;92(9):2764–84. doi: 10.1161/01.cir.92.9.2764. [DOI] [PubMed] [Google Scholar]
- 33.Pool PE, Braunwald E. Fundamental mechanisms in congestive heart failure. Am J Cardiol. 1968;22(1):7–15. doi: 10.1016/0002-9149(68)90241-5. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of beta blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55. doi: 10.1136/bmj.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khazanie P, Newby LK ACP Journal Club. Review: in patients with heart failure, beta-blockers reduce mortality but do not differ from each other. Ann Intern Med. 2013;158(10):JC2–3. doi: 10.7326/0003-4819-158-10-201305210-02002. [DOI] [PubMed] [Google Scholar]
- 36.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362(9377):7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 37.Bristow MR. β-Adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101(5):558–69. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 38.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13. [PubMed] [Google Scholar]
- 39.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353(9169):2001–7. [PubMed] [Google Scholar]
- 40.Yue TL, Cheng HY, Lysko PG, et al. Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. J Pharmacol Exp Ther. 1992;263(1):92–8. [PubMed] [Google Scholar]
- 41.Kubo T, Azevedo ER, Newton GE, et al. Lack of evidence for peripheral alpha(1)-adrenoceptor blockade during long-term treatment of heart failure with carvedilol. J Am Coll Cardiol. 2001;38(5):1463–9. doi: 10.1016/s0735-1097(01)01577-7. [DOI] [PubMed] [Google Scholar]
- 42.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 43.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26(3):215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 44.Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344(22):1659–67. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura K, Kusano K, Nakamura Y, et al. Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation. 2002;105(24):2867–71. doi: 10.1161/01.cir.0000018605.14470.dd. [DOI] [PubMed] [Google Scholar]
- 46.Wang R, Miura T, Harada N, et al. Pleiotropic effects of the beta-adrenoceptor blocker carvedilol on calcium regulation during oxidative stress-induced apoptosis in cardiomyocytes. J Pharmacol Exp Ther. 2006;318(1):45–52. doi: 10.1124/jpet.105.099903. [DOI] [PubMed] [Google Scholar]
- 47.McAlister FA, Wiebe N, Ezekowitz JA, et al. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150(11):784–94. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 48.Groenning BA, Nilsson JC, Sondergaard L, et al. Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol. 2000;36(7):2072–80. doi: 10.1016/s0735-1097(00)01006-8. [DOI] [PubMed] [Google Scholar]
- 49.Colucci WS, Kolias TJ, Adams KF, et al. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the REversal of VEntricular Remodeling with Toprol-XL (REVERT) trial. Circulation. 2007;116(1):49–56. doi: 10.1161/CIRCULATIONAHA.106.666016. [DOI] [PubMed] [Google Scholar]
- 50.Doughty RN, Whalley GA, Walsh HA, et al. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy. Circulation. 2004;109(2):201–6. doi: 10.1161/01.CIR.0000108928.25690.94. [DOI] [PubMed] [Google Scholar]
- 51.Doughty RN, Whalley GA, Gamble G, et al. Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. Australia-New Zealand Heart Failure Research Collaborative Group. J Am Coll Cardiol. 1997;29(5):1060–6. doi: 10.1016/s0735-1097(97)00012-0. [DOI] [PubMed] [Google Scholar]
- 52.Al-Gobari M, El Khatib C, Pillon F, et al. Beta-blockers for the prevention of sudden cardiac death in heart failure patients: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2013;13:52. doi: 10.1186/1471-2261-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedman DJ, Altman RK, Orencole M, et al. Predictors of sustained ventricular arrhythmias in cardiac resynchronization therapy. Circ Arrhyth Electrophysiol. 2012;5(4):762–72. doi: 10.1161/CIRCEP.112.971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruwald MH, Abu-Zeitone A, Jons C, et al. Impact of carvedilol and metoprolol on inappropriate implantable cardioverter-defibrillator therapy: the MADIT-CRT trial (Multicenter Automatic Defibrillator Implantation with Cardiac Resynchronization Therapy) J Am Coll Cardiol. 2013;62(15):1343–50. doi: 10.1016/j.jacc.2013.03.087. [DOI] [PubMed] [Google Scholar]
- 55.Wong M, Staszewsky L, Latini R, et al. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol. 2002;40(5):970–5. doi: 10.1016/s0735-1097(02)02063-6. [DOI] [PubMed] [Google Scholar]
- 56.Sharma S, Deitchman D, Eni JS, et al. The hemodynamic effects of long-term ACE inhibition with fosinopril in patients with heart failure. Fosinopril Hemodynamics Study Group. Am J Ther. 1999;6(4):181–9. doi: 10.1097/00045391-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Konstam MA, Rousseau MF, Kronenberg MW, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. SOLVD Investigators. Circulation. 1992;86(2):431–8. doi: 10.1161/01.cir.86.2.431. [DOI] [PubMed] [Google Scholar]
- 58.Devereux RB, Dahlof B, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110(11):1456–62. doi: 10.1161/01.CIR.0000141573.44737.5A. [DOI] [PubMed] [Google Scholar]
- 59.Pitt B. Potential role of angiotensin converting enzyme inhibitors in treatment of atherosclerosis. Eur Heart J. 1995;16(Suppl K):49–54. doi: 10.1093/eurheartj/16.suppl_k.49. [DOI] [PubMed] [Google Scholar]
- 60.Teo KK, Yusuf S, Pfeffer M, et al. Effects of long-term treatment with angiotensin-converting-enzyme inhibitors in the presence or absence of aspirin: a systematic review. Lancet. 2002;360(9339):1037–43. doi: 10.1016/s0140-6736(02)11138-x. [DOI] [PubMed] [Google Scholar]
- 61.Udelson JE, Feldman AM, Greenberg B, et al. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circ Heart Fail. 2010;3(3):347–53. doi: 10.1161/CIRCHEARTFAILURE.109.906909. [DOI] [PubMed] [Google Scholar]
- 62.Zannad F, Alla F, Dousset B, et al. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the Randomized Aldactone Evaluation Study (RALES) Rales Investigators. Circulation. 2000;102(22):2700–6. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 63.Iraqi W, Rossignol P, Angioi M, et al. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation. 2009;119(18):2471–9. doi: 10.1161/CIRCULATIONAHA.108.809194. [DOI] [PubMed] [Google Scholar]
- 64.Shah NC, Pringle SD, Donnan PT, et al. Spironolactone has antiarrhythmic activity in ischaemic cardiac patients without cardiac failure. J Hypertens. 2007;25(11):2345–51. doi: 10.1097/HJH.0b013e3282e9a72d. [DOI] [PubMed] [Google Scholar]
- 65.Swedberg K, Zannad F, McMurray JJ, et al. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59(18):1598–603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 66.Gilbert EM, Abraham WT, Olsen S, et al. Comparative hemodynamic, left ventricular functional, and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation. 1996;94(11):2817–25. doi: 10.1161/01.cir.94.11.2817. [DOI] [PubMed] [Google Scholar]
- 67.Fox K, Komajda M, Ford I, et al. Effect of ivabradine in patients with left-ventricular systolic dysfunction: a pooled analysis of individual patient data from the BEAUTIFUL and SHIFT trials. Eur Heart J. 2013;34(29):2263–70. doi: 10.1093/eurheartj/eht101. [DOI] [PubMed] [Google Scholar]
- 68.Metra M, Nardi M, Giubbini R, et al. Effects of short-and long-term carvedilol administration on rest and exercise hemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1994;24(7):1678–87. doi: 10.1016/0735-1097(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 69.Reiken S, Wehrens XH, Vest JA, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107(19):2459–66. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 70.Brixius K, Lu R, Boelck B, et al. Chronic treatment with carvedilol improves Ca(2+)-dependent ATP consumption in triton X-skinned fiber preparations of human myocardium. J Pharmacol Exp Ther. 2007;322(1):222–7. doi: 10.1124/jpet.106.116798. [DOI] [PubMed] [Google Scholar]
- 71.Tamaki S, Sakata Y, Mano T, et al. Long-term beta-blocker therapy improves diastolic function even without the therapeutic effect on systolic function in patients with reduced ejection fraction. J Cardiol. 2010;56(2):176–82. doi: 10.1016/j.jjcc.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Morita H, Suzuki G, Mishima T, et al. Effects of long-term monotherapy with metoprolol CR/XL on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Cardiovasc Drugs Ther. 2002;16(5):443–9. doi: 10.1023/a:1022142620189. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi M, Machida N, Mitsuishi M, et al. Beta-blocker improves survival, left ventricular function, and myocardial remodeling in hypertensive rats with diastolic heart failure. Am J Hypertens. 2004;17(12 Pt 1):1112–9. doi: 10.1016/j.amjhyper.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Muller-Brunotte R, Kahan T, Lopez B, et al. Myocardial fibrosis and diastolic dysfunction in patients with hypertension: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) J Hypertens. 2007;25(9):1958–66. doi: 10.1097/HJH.0b013e3282170ada. [DOI] [PubMed] [Google Scholar]
- 75.The Beta-Blocker Heart Attack Trial. Beta-Blocker Heart Attack Study Group. JAMA. 1981;246(18):2073–4. [PubMed] [Google Scholar]
- 76.McMurray J, Kober L, Robertson M, et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol. 2005;45(4):525–30. doi: 10.1016/j.jacc.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 77.Lowes BD, Gilbert EM, Abraham WT, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346(18):1357–65. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- 78.Rathore SS, Curtis JP, Wang Y, et al. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289(7):871–8. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 79.Ribner HS, Plucinski DA, Hsieh AM, et al. Acute effects of digoxin on total systemic vascular resistance in congestive heart failure due to dilated cardiomyopathy: a hemodynamic-hormonal study. Am J Cardiol. 1985;56(13):896–904. doi: 10.1016/0002-9149(85)90778-7. [DOI] [PubMed] [Google Scholar]
- 80.Gheorghiade M. Digoxin therapy in chronic heart failure. Cardiovasc Drugs Ther. 1997;11(Suppl 1):279–83. doi: 10.1023/a:1007743930938. [DOI] [PubMed] [Google Scholar]
- 81.Gheorghiade M, Ferguson D. Digoxin. A neurohormonal modulator in heart failure? Circulation. 1991;84(5):2181–6. doi: 10.1161/01.cir.84.5.2181. [DOI] [PubMed] [Google Scholar]
- 82.Swedberg K, Bristow MR, Cohn JN, et al. Effects of sustained-release moxonidine, an imidazoline agonist, on plasma norepinephrine in patients with chronic heart failure. Circulation. 2002;105(15):1797–803. doi: 10.1161/01.cir.0000014212.04920.62. [DOI] [PubMed] [Google Scholar]
- 83.Cohn JN, Pfeffer MA, Rouleau J, et al. Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON) Eur J Heart Fail. 2003;5(5):659–67. doi: 10.1016/s1388-9842(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 84.Skomedal T, Borthne K, Aass H, et al. Comparison between alpha-1 adrenoceptor-mediated and beta adrenoceptor-mediated inotropic components elicited by norepinephrine in failing human ventricular muscle. J Pharmacol Exp Ther. 1997;280(2):721–9. [PubMed] [Google Scholar]
- 85.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 86.Zhou J, Xu X, Liu JJ, et al. Angiotensin II receptors subtypes mediate diverse gene expression profile in adult hypertrophic cardiomyocytes. Clin Exp Pharmacol Physiol. 2007;34(11):1191–8. doi: 10.1111/j.1440-1681.2007.04694.x. [DOI] [PubMed] [Google Scholar]
- 87.von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail. 2013;6(3):594–605. doi: 10.1161/CIRCHEARTFAILURE.112.000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wehling M. Effects of aldosterone and mineralocorticoid receptor blockade on intracellular electrolytes. Heart Fail Rev. 2005;10(1):39–46. doi: 10.1007/s10741-005-2347-z. [DOI] [PubMed] [Google Scholar]
- 89.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009;81(3):482–90. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lindpaintner K, Jin MW, Niedermaier N, et al. Cardiac angiotensinogen and its local activation in the isolated perfused beating heart. Circ Res. 1990;67(3):564–73. doi: 10.1161/01.res.67.3.564. [DOI] [PubMed] [Google Scholar]
- 91.Re R, Fallon JT, Dzau V, et al. Renin synthesis by canine aortic smooth muscle cells in culture. Life Sci. 1982;30(1):99–106. doi: 10.1016/0024-3205(82)90641-5. [DOI] [PubMed] [Google Scholar]
- 92.Serneri GG, Boddi M, Cecioni I, et al. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res. 2001;88(9):961–8. doi: 10.1161/hh0901.089882. [DOI] [PubMed] [Google Scholar]
- 93.Dzau VJ. Implications of local angiotensin production in cardiovascular physiology and pharmacology. Am J Cardiol. 1987;59(2):59A–65A. doi: 10.1016/0002-9149(87)90178-0. [DOI] [PubMed] [Google Scholar]
- 94.Kato H, Suzuki H, Tajima S, et al. Angiotensin II stimulates collagen synthesis in cultured vascular smooth muscle cells. J Hypertens. 1991;9(1):17–22. [PubMed] [Google Scholar]
- 95.Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci. 2002;103(4):425–31. doi: 10.1042/cs1030425. [DOI] [PubMed] [Google Scholar]
- 96.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52(1):11–34. [PubMed] [Google Scholar]
- 97.Sadoshima J, Izumo S. Molecular characterization of angiotensin II–induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73(3):413–23. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 98.Dostal DE, Baker KM. Angiotensin II stimulation of left ventricular hypertrophy in adult rat heart. Mediation by the AT1 receptor. Am J Hypertens. 1992;5(5 Pt 1):276–80. doi: 10.1093/ajh/5.5.276. [DOI] [PubMed] [Google Scholar]
- 99.Maruyama R, Hatta E, Yasuda K, et al. Angiotensin-converting enzyme-independent angiotensin formation in a human model of myocardial ischemia: modulation of norepinephrine release by angiotensin type 1 and angiotensin type 2 receptors. J Pharmacol Exp Ther. 2000;294(1):248–54. [PubMed] [Google Scholar]
- 100.May CN, Yao ST, Booth LC, et al. Cardiac sympathoexcitation in heart failure. Auton Neurosci. 2013;175(1–2):76–84. doi: 10.1016/j.autneu.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 101.Ramchandra R, Hood SG, Watson AM, et al. Central angiotensin type 1 receptor blockade decreases cardiac but not renal sympathetic nerve activity in heart failure. Hypertension. 2012;59(3):634–41. doi: 10.1161/HYPERTENSIONAHA.111.181131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McKinney CA, Fattah C, Loughrey CM, et al. Angiotensin-(1–7) and angiotensin-(1–9): function in cardiac and vascular remodelling. Clin Sci. 2014;126(12):815–27. doi: 10.1042/CS20130436. [DOI] [PubMed] [Google Scholar]
- 103.Regoli D, Plante GE, Gobeil F., Jr Impact of kinins in the treatment of cardiovascular diseases. Pharmacol Ther. 2012;135(1):94–111. doi: 10.1016/j.pharmthera.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 104.Shearer F, Lang CC, Struthers AD. Renin-angiotensin-aldosterone system inhibitors in heart failure. Clin Pharmacol Ther. 2013;94(4):459–67. doi: 10.1038/clpt.2013.135. [DOI] [PubMed] [Google Scholar]
- 105.Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999;100(23):2312–8. doi: 10.1161/01.cir.100.23.2312. [DOI] [PubMed] [Google Scholar]
- 106.Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374(9704):1840–8. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- 107.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669–77. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 108.Naccarella F, Naccarelli GV, Maranga SS, et al. Do ACE inhibitors or angiotensin II antagonists reduce total mortality and arrhythmic mortality? A critical review of controlled clinical trials. Curr Opin Cardiol. 2002;17(1):6–18. doi: 10.1097/00001573-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 109.Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354(9176):359–64. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 110.Roig E, Perez-Villa F, Morales M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21(1):53–7. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- 111.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Prac Nephrol. 2007;3(9):486–92. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 112.Seva Pessoa B, van der Lubbe N, Verdonk K, et al. Key developments in renin-angiotensin-aldosterone system inhibition. Nat Rev Nephrol. 2013;9(1):26–36. doi: 10.1038/nrneph.2012.249. [DOI] [PubMed] [Google Scholar]
- 113.Cohn JN, Tognoni G Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 114.McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767–71. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 115.Phillips CO, Kashani A, Ko DK, et al. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Arch Intern Med. 2007;167(18):1930–6. doi: 10.1001/archinte.167.18.1930. [DOI] [PubMed] [Google Scholar]
- 116.Gheorghiade M, Bohm M, Greene SJ, et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309(11):1125–35. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 117.Krum H, Massie B, Abraham WT, et al. Direct renin inhibition in addition to or as an alternative to angiotensin converting enzyme inhibition in patients with chronic systolic heart failure: rationale and design of the Aliskiren Trial to Minimize OutcomeS in Patients with HEart failuRE (ATMOSPHERE) study. Eur J Heart Fail. 2011;13(1):107–14. doi: 10.1093/eurjhf/hfq212. [DOI] [PubMed] [Google Scholar]
- 118.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27(1):47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 119.Richards AM, Lainchbury JG, Troughton RW, et al. Clinical applications of B-type natriuretic peptides. Trends Endocrinol Metab. 2004;15(4):170–4. doi: 10.1016/j.tem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 120.Del Ry S. C-type natriuretic peptide: a new cardiac mediator. Peptides. 2013;40:93–8. doi: 10.1016/j.peptides.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 121.Wada A, Tsutamoto T, Matsuda Y, et al. Cardiorenal and neurohumoral effects of endogenous atrial natriuretic peptide in dogs with severe congestive heart failure using a specific antagonist for guanylate cyclase-coupled receptors. Circulation. 1994;89(5):2232–40. doi: 10.1161/01.cir.89.5.2232. [DOI] [PubMed] [Google Scholar]
- 122.Kuhn M. Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res. 2003;93(8):700–9. doi: 10.1161/01.RES.0000094745.28948.4D. [DOI] [PubMed] [Google Scholar]
- 123.Tamura N, Ogawa Y, Chusho H, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97(8):4239–44. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kishimoto I, Rossi K, Garbers DL. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci U S A. 2001;98(5):2703–6. doi: 10.1073/pnas.051625598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Soeki T, Kishimoto I, Okumura H, et al. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2005;45(4):608–16. doi: 10.1016/j.jacc.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 126.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 127.Mangiafico S, Costello-Boerrigter LC, Andersen IA, et al. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34(12):886–893c. doi: 10.1093/eurheartj/ehs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kostis JB, Packer M, Black HR, et al. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17(2):103–11. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 129.McMurray JJ, Packer M, Desai AS, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur J Heart Fail. 2013;15(9):1062–73. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goldsmith SR, Francis GS, Cowley AW, Jr, et al. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol. 1983;1(6):1385–90. doi: 10.1016/s0735-1097(83)80040-0. [DOI] [PubMed] [Google Scholar]
- 131.Hupf H, Grimm D, Riegger GA, et al. Evidence for a vasopressin system in the rat heart. Circ Res. 1999;84(3):365–70. doi: 10.1161/01.res.84.3.365. [DOI] [PubMed] [Google Scholar]
- 132.Li X, Chan TO, Myers V, et al. Controlled and cardiac-restricted overexpression of the arginine vasopressin V1A receptor causes reversible left ventricular dysfunction through Galphaq-mediated cell signaling. Circulation. 2011;124(5):572–81. doi: 10.1161/CIRCULATIONAHA.111.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297(12):1332–43. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 134.Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099–112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 135.Movassagh M, Choy MK, Knowles DA, et al. Distinct epigenomic features in end-stage failing human hearts. Circulation. 2011;124(22):2411–22. doi: 10.1161/CIRCULATIONAHA.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Papait R, Greco C, Kunderfranco P, et al. Epigenetics: a new mechanism of regulation of heart failure? Basic Res Cardiol. 2013;108(4):361. doi: 10.1007/s00395-013-0361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anand P, Brown JD, Lin CY, et al. BET bromodomains mediate transcriptional pause release in heart failure. Cell. 2013;154(3):569–82. doi: 10.1016/j.cell.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kumarswamy R, Thum T. Non-coding RNAs in cardiac remodeling and heart failure. Circ Res. 2013;113(6):676–89. doi: 10.1161/CIRCRESAHA.113.300226. [DOI] [PubMed] [Google Scholar]