Abstract
Purpose
We investigated whether treatment with the selective cannabinoid receptor 2 agonist GP1a would ameliorate the severity of experimental cystitis. We determined the association of referred hyperalgesia and increased urinary frequency after establishing cystitis in mice by intravesical instillation of acrolein.
Materials and Methods
Cystitis was induced by intravesical instillation of acrolein in female C57BL/6NH mice. Mice were treated with GP1a (10 mg/kg intraperitoneally) or vehicle 3.5, 22 and 30 hours after instillation of acrolein. Mice were tested for mechanical sensitivity of hind paws. Short-term voluntary voiding was assessed by quantifying urine spots of freely moving mice. Bladders were collected, weighed and processed for immunohistochemical, histological and immunoblotting analysis.
Results
At 48 hours after acrolein instillation the bladder of all mice showed histological evidence of inflammation. The severity of edema and increase in bladder weight were inhibited in cannabinoid receptor 2 agonist treated animals (p <0.05). Neither cystitis nor treatment with GP1a or AM630 (selective cannabinoid receptor 2 antagonist) plus GP1a appeared to alter cannabinoid receptor 2-like immunoreactivity abundance in urothelium. Mechanical sensitivity was significantly increased after acrolein and the increase was attenuated in cannabinoid receptor 2 agonist treated mice (p <0.05). The number of small diameter urine spots was significantly increased after acrolein and treatment with GP1a attenuated this increase (p <0.05). GP1a effects were prevented by AM630.
Conclusions
Treatment with a selective cannabinoid receptor 2 agonist decreased severity of established acrolein induced cystitis and inhibited bladder inflammation associated increased referred mechanical sensitivity and increased bladder urinary frequency. Our data indicate that cannabinoid receptor 2 is a potential therapeutic target for treatment of painful inflammatory bladder diseases.
Keywords: urinary bladder; cystitis; interstitial; cannabinoid receptor agonists; N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-1,4-dihydro-6-methylindeno(1,2-c)pyrazole-3-carboxamide; mice
Cannabinoids have anti-inflammatory effects. Study design has consistently entailed cannabinoid administration before initiating inflammation.1,2 The effects of cannabinoids are mediated primarily by CB1 and CB2, which are coupled to inhibitory G proteins.1–3 Intravesical administration of a selective CB1 agonist inhibited bladder afferent nerve sensitization induced by bladder inflammation.4 However, Dinis et al reported that treatment with a selective CB1 antagonist did not affect enhanced reflex reactivity associated with bladder inflammation.5 On the other hand, CB2 is present in the bladder of various species, including humans, monkeys and rodents,6–9 particularly in urothelial cells.6–8 Treatment with a selective CB2 agonist increased micturition interval and volume in normal rats9 and improved bladder function in rats after partial urethral obstruction.10 These studies support a role of CB2 in regulating bladder function under physiological and pathophysiological conditions.
IC/PBS is a painful chronic disorder characterized by increased frequency, urgency and bladder pain.11 The etiology and pathogenesis of IC/PBS remain unknown and no treatment or combination of treatments has been consistently effective in alleviating symptoms in patients with IC/PBS.11 We previously reported that pretreatment with the CB2 agonist GP1a decreased the severity of acute cystitis induced by acrolein and associated referred hyperalgesia.12 To further explore the potential of CB2 as a therapeutic target for inflammatory bladder disease we extended our investigation to examine whether treatment with the CB2 agonist GP1a would ameliorate the severity of experimental cystitis and associated referred hyperalgesia after establishing cystitis by intravesical instillation of acrolein. Such studies may have greater clinical relevance since patients typically seek medical treatment of established disease rather than prevention of impending disorders.
MATERIALS AND METHODS
Study Design
Ten to 12-week-old female C57BL/6NH mice were obtained from Harlan®. Experiments were done in accordance with National Institutes of Health Guidelines. All protocols were reviewed and approved by the University of Wisconsin animal care and use committee.
Mice were anesthetized with Avertin® (250 mg/kg) injected intraperitoneally. Cystitis was induced by intravesical instillation of acrolein (0.5 mM and 150 μl total volume, Ultra Scientific, Kingstown, Rhode Island) via a PE10 urethral catheter (BD™) with an inner and outer diameter of 0.28 and 0.61 mm, respectively. Acrolein remained in the bladder for 40 minutes. Control mice received an equivalent volume of intravesical saline (0.9%) instead of acrolein.
The selective CB2 agonist GP1a and antagonist AM630 (Tocris, Bristol, United Kingdom) were dissolved in ethanol as stock solutions and diluted in saline to desired concentrations. GP1a (10 mg/kg) or vehicle was given intraperitoneally 3.5, 22 and 30 hours after acrolein instillation. AM630 (10 mg/kg) or vehicle was given 10 minutes before GP1a injection by subcutaneous injection.
At 48 hours after acrolein or saline instillation the mice were deeply anesthetized with pentobarbital (50 mg/kg intraperitoneally) and perfused with saline through a cannula inserted in the left ventricle. The bladders were removed and weighed. Bladder weight in mg was normalized to body weight in gm. The bladders were then divided into 2 parts. The caudal part, including the neck region, was fixed and tissue sections were made for morphological and immunohistochemical analysis. The urothelium/suburothelium of the remainder of the bladders was mechanically separated from the detrusor and stored at −80C until analysis.12
Histological Analysis and Immunohistochemistry
Histological analysis was performed after hematoxylin and eosin staining. Acrolein induced cystitis was characterized primarily by edema in the submucosal region. An edema score was determined, including 0—no evident edema, 1—mild, 2—moderate or 3—severe edema.13 The increased bladder weight correlated closely with the severity of edema and changes in bladder weight reliably reflect cystitis severity in this model. Therefore, we also measured bladder weight and used these values for semiquantitative analysis of cystitis severity.13 In contrast to cystitis induced by cyclophosphamide/acrolein in rats, infiltration of inflammatory cells is mild and varies considerably in the same treatment group in mice (unpublished data). Also, urothelial ulceration was not consistently observed with the dose of acrolein used, which produced cystitis of moderate severity.
For immunohistochemistry bladder tissue sections were blocked with 10% normal goat serum. A specific CB2 antibody (1:500, Cayman Chemical, Ann Arbor, Michigan) was applied and tissue sections were incubated in a humid chamber overnight at 4C. Staining was revealed using secondary goat anti-rabbit IgG conjugated with fluorescein isothiocyanate (1:1,000, Sigma®). Slides were rinsed and mounted with antifade solution (Vector Laboratories, Burlingame, California). Slides were examined with a Nikon™ E600 microscope and digital images were captured. CB2 antibody specificity is well described. This antibody reveals CB2 expression in various tissues in wild-type but not in CB2 knockout mice.14
Semiquantitative Immunoblot Analysis
The urothelium/suburothelium was homogenized. Protein samples were resolved on 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to nitrocellulose membranes. Membranes were blocked and incubated at 4C overnight with CB2 antibody (1:1,000, Cayman Chemical). Membranes were incubated with secondary antibody conjugated to horseradish peroxidase. Signals were revealed using a chemiluminescent detection reagent (Amersham™). CB2 abundance was estimated by optical density measurement with ImageJ (http://rsb.info.nih.gov/ij/). Values were normalized to the loading control, as determined by glyceraldehyde 3-phosphate dehydrogenase (1:5,000, Abcam®) abundance in the same sample.13
Peripheral Nociception Testing
Hind paw mechanical sensitivity was assessed using von Frey monofilaments and the up-down method.13 Mice were placed individually in a Plexiglas® chamber with a wire mesh floor and allowed to acclimate for at least 30 minutes or until cage exploration stopped. Hind paw sensitivity was assessed with a series of 6 von Frey filaments of increasing stiffness. Stimulus related retraction of the tested paw was considered a withdrawal response. The 50% paw withdrawal threshold was determined by the nonparametric method of Dixon.15 The individual who performed testing was blinded to the mouse treatment group.
Voiding Behavior Analysis
Short-term voluntary voiding was assessed by measuring and quantifying urine spots produced by freely moving mice. Mice were placed individually on filter paper in a standard cage for 30 minutes. Urine spots were photographed under ultraviolet light and the number of urine spots 0.2 cm2 or less in diameter was counted. An increase in the number of small diameter urine spots is considered to suggest irritative voiding and reflect increased urinary frequency.16
Statistical Analysis
Data are shown as the arithmetic mean ± SEM of 6 to 8 preparations per treatment group. Data were analyzed using 1-way ANOVA followed by Bonferroni post hoc comparisons using Prism® with p <0.05 considered significant.
RESULTS
CB2-Like Immunoreactivity in Urothelium
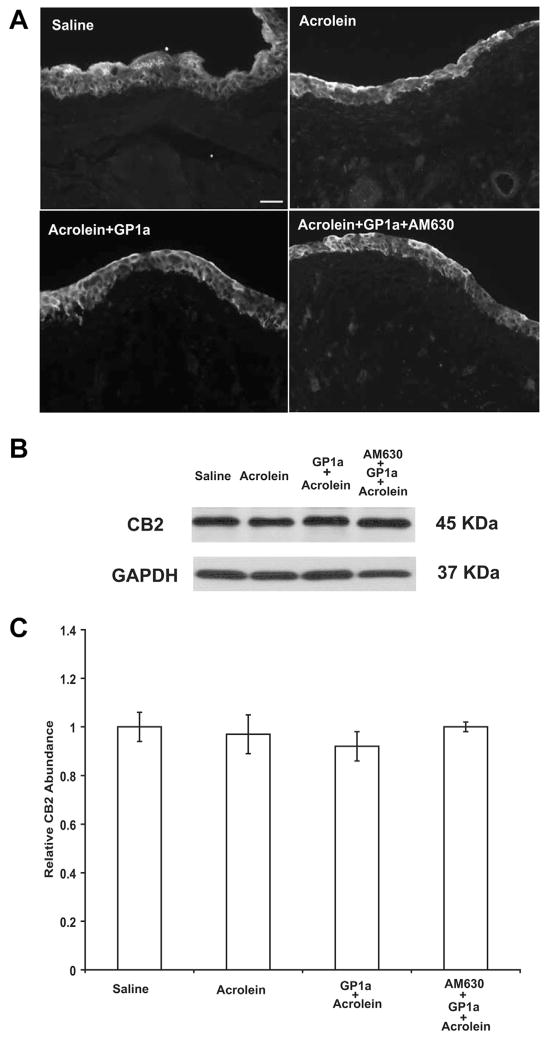
CB2-like immunoreactivity was consistently observed in the urothelium of bladders from all groups (fig. 1, A). Cells were considered to be labeled with the specific antibody when fluorescence intensity was distinctively higher than the background level. Using antibody pre-incubated with the antigen peptide used to generate the antibody resulted in a complete lack of specific staining (data not shown). Immunoblot revealed that neither cystitis nor treatment with GP1a or AM630 plus GP1a appeared to alter CB2-like immunoreactivity abundance in the urothelium (fig. 1, B and C).
Figure 1.
Representative images show immunohistochemical localization of CB2-like immunoreactivity in bladder urothelium of mice treated with saline, acrolein, acrolein plus GP1a and acrolein plus AM630 plus GP1a (A). Scale bar indicates 50 μm. Immunoblot reveals that neither cystitis nor treatment with GP1a or AM630 plus GP1a altered CB2-like immunoreactivity abundance in urothelium (B and C). GAPDH, glyceraldehyde 3-phosphate dehydrogenase (B). Values represent mean ± SEM of 6 saline treated controls and 8 mice with other treatment (C).
GP1a Effects
Cystitis severity
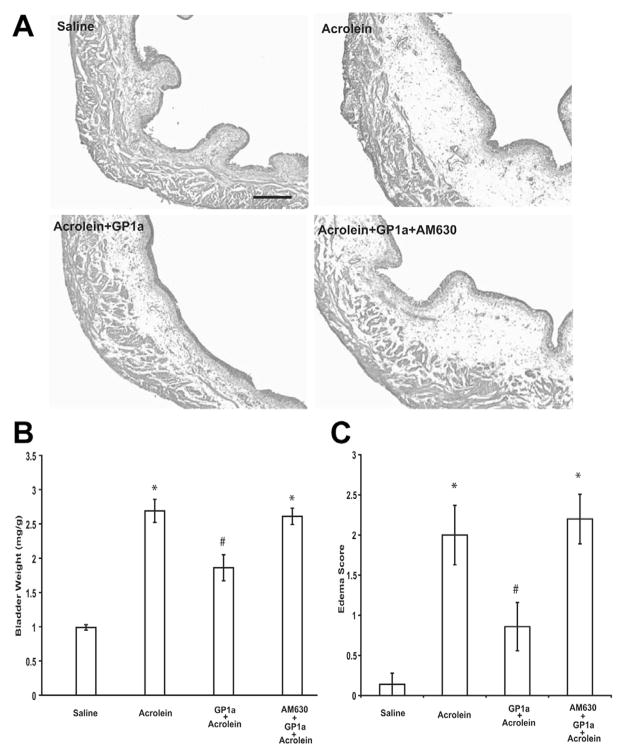
No signs of cystitis were observed in saline treated animals (fig. 2, A). At 48 hours after acrolein administration histological examination of the bladders indicated cystitis, primarily characterized by edema in the submucosal region (fig. 2, A). The weight of acrolein treated bladders was significantly increased relative to that of saline treated controls and the edema score of acrolein treated bladders was also significantly greater (each p <0.01, fig. 2, B and C). GP1a treatment attenuated the increase in bladder weight (vs acrolein each p <0.05, fig. 2, B and C). The severity of edema (vs acrolein p <0.05, fig. 2, C) and the effects of GP1a were prevented by pretreatment with the selective CB2 antagonist AM630 (vs acrolein plus GP1a p <0.05, fig. 2, B and C).
Figure 2.
Representative images show bladder of mice treated with saline, acrolein, acrolein plus GP1a and acrolein plus AM630 plus GP1a (A). Scale bar indicates 100 μm. Acrolein induced cystitis, characterized primarily by edema in submucosal region, and GP1a decreased acrolein induced edema (B) and bladder weight increase (C). GP1a effects were reversed by AM630 pretreatment (B and C). Values represent mean ± SEM of 6 saline treated controls and 8 mice with other treatment. Asterisk indicates p <0.01 vs saline. Pound sign indicates p <0.05 vs acrolein.
Enhanced mechanical sensitivity associated with cystitis
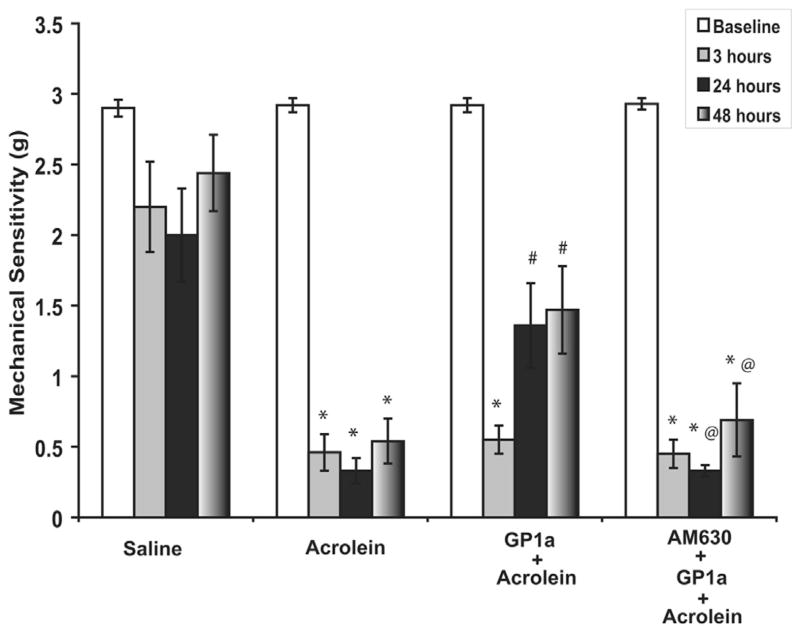
The basal mechanical sensitivity threshold was about 2.8 gm in all groups (fig. 3). Intravesical instillation of saline did not affect peripheral mechanical sensitivity (fig. 3). The mechanical sensitivity threshold was decreased 3, 24 and 48 hours after acrolein instillation (vs saline p <0.01). GP1a treatment attenuated increased mechanical sensitivity at 24 and 48 hours (vs acrolein p <0.05). The effect of GP1a was reversed by the selective CB2 antagonist AM630 (vs acrolein plus GP1a p <0.05).
Figure 3.
Intravesical instillation of saline did not affect peripheral mechanical sensitivity. After acrolein instillation mechanical sensitivity threshold was decreased. GP1a attenuated increased mechanical sensitivity and GP1a effect was reversed by selective CB2 antagonist AM630. Values represent mean ± SEM of 6 saline treated controls and 8 mice with other treatment. Asterisk indicates p <0.01 vs saline. Pound sign indicates p <0.05 vs acrolein. At sign indicates p <0.05 vs acrolein plus GP1a.
Altered bladder function associated with cystitis
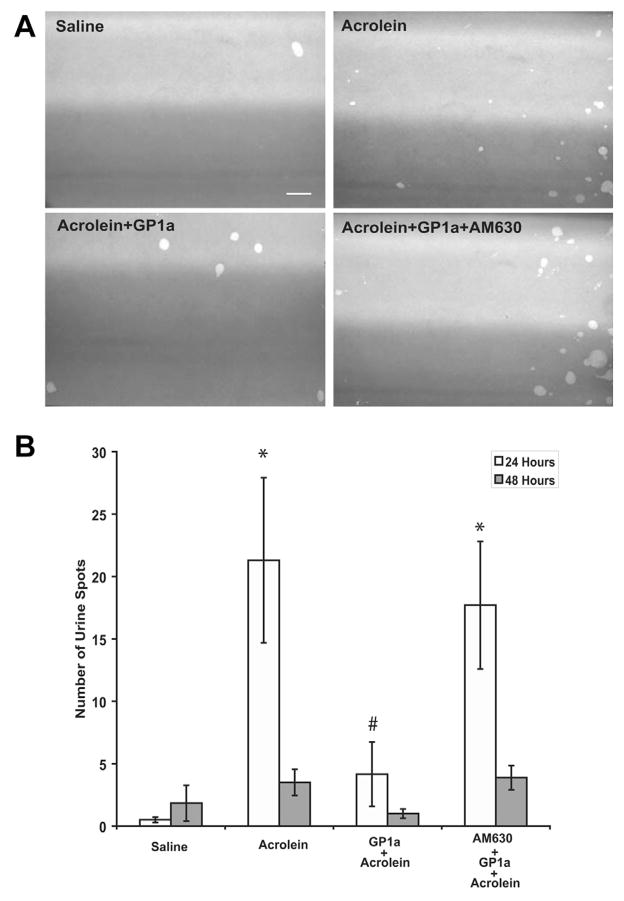
Exposure to acrolein significantly increased the number of small urine spots observed at 24 hours (vs saline p <0.05, fig. 4). GP1a treatment attenuated the increased number of small urine spots vs acrolein treatment (p <0.05, fig. 4). The effect of GP1a was reversed by the selective CB2 antagonist AM630 (vs acrolein plus GP1a p <0.05). At 48 hours the increase in the number of small urine spots was numerically greater in acrolein treated mice but differences did not increase to a significant level in the saline control and acrolein groups (p >0.05, fig. 4).
Figure 4.
Representative images show urine spots of mice treated with saline, acrolein, acrolein plus GP1a and acrolein plus AM630 plus GP1a at 24 hours after saline or acrolein instillation (A). Scale bar indicates 2 cm. Number of spots increased after acrolein intravesical instillation. Acrolein effect was attenuated by GP1a and GP1a effect was reversed by selective antagonist AM630 (B). Values represent mean ± SEM of 6 saline treated controls and 8 mice with other treatment. Asterisk indicates p <0.05 vs saline. Pound sign indicates p <0.05 vs acrolein and vs acrolein plus AM630 plus GP1a.
DISCUSSION
In the current study we found that 1) CB2-like immunoreactivity is present in urothelium, as previously reported, 2) treatment with the selective CB2 agonist GP1a decreased the severity of established acrolein induced cystitis, 3) the CB2 agonist also inhibited increased peripheral sensitivity to mechanical stimuli and increased urinary frequency associated with cystitis, and 4) the effects of GP1a were reversed by the CB2 selective antagonist AM630, confirming GP1a specificity for CB2.
CB2 activation inhibits tissue inflammation.1,17–19 Pretreatment with selective CB2 agonists attenuated carrageenan induced edema in the hind paw of rats and the inhibitory effects were reversed by selective CB2 antagonists.1 Similar treatments also decreased arachidonic acid induced edema in the ear18 and edema of the hind paw elicited by intra-plantar injection of lipopolysaccharide.19 Pretreatment with selective CB2 agonists inhibited the severity of experimental colitis and attenuated inflammatory responses in mice with cardiac and hepatic ischemia/reperfusion injury.20–22 In contrast, a selective CB2 antagonist exacerbated the severity.
Also, the antinociceptive effects of CB2 activation are well documented.23–25 Pretreatment with CB2 agonists decreased the second phase of nocifensive behavior elicited by intraplantar injection of formalin in mice and the allodynia elicited by L5-L6 spinal nerve ligation in rats.26 These effects of CB2 agonists were prevented by selective CB2 antagonists.26 In other studies treatment with CB2 agonists suppressed carrageenan evoked thermal and mechanical hyperalgesia in rodents1,25,27 and cancer induced pain.24 We previously reported that pretreatment with the selective CB2 agonist GP1a decreased the severity of bladder inflammation and inhibited the enhanced peripheral mechanical sensitivity associated with acute cystitis while the inhibitory effects of GP1a were reversed by the selective CB2 antagonist AM630.12 These studies clearly support the anti-inflammatory and anti-nociceptive effects of CB2.
Notably, while bladder inflammation and enhanced mechanical sensitivity persisted 48 hours after acrolein, increased urinary frequency appeared to resolve sooner. We currently lack a satisfactory explanation for this phenomenon. However, inflammation is a complicated process. The factors/mechanisms underlying increased urinary frequency may or may not be identical to those associated with increased referred mechanical sensitivity. Future studies are needed to further investigate the mechanisms underlying bladder inflammation as well as associated increased referred mechanical sensitivity and altered bladder function.
Most studies were designed to evaluate the effects of CB2 agonists/antagonists by administering these compounds before inducing tissue inflammation or applying noxious stimuli. Therefore, the observed effects of these compounds have likely been preventive. On the other hand, patients usually seek treatment of established disease. We found that GP1a treatment in mice with established cystitis decreased the severity of cystitis and reversed the increased urinary frequency and bladder inflammation associated with enhanced peripheral mechanical sensitivity. This demonstrates the potential of using CB2 agonists for the treatment instead of the prevention of inflammatory bladder disease.
Our study and others reveal that CB2 is present in urothelial cells. Urothelial cells have the capacity to secrete various molecules, such as prostaglandin E2, nerve growth factor, nitric oxide and cytokines, which could significantly influence bladder function and participate in bladder inflammation and pain sensation.28 CB2 is coupled to inhibitory G proteins and CB2 activation inhibits adenylyl cyclase,2 and the release of inflammatory cytokines in inflammatory22,29 and tumor24 cells. CB2 activation may possibly inhibit the production and release of inflammatory mediators from urothelial cells, decreasing the local inflammatory response of the bladder. CB2 activation also inhibited the capsaicin induced increase of intracellular calcium in dorsal root ganglia afferent neurons30 and the release of calcitonin gene-related peptide from afferent nerve fibers in bladders7 and spinal cord slices.27 CB2 activation may conceivably suppress the production and release of inflammatory mediators from urothelial cells involved in bladder inflammation and afferent nerve sensitization. Furthermore, CB2 activation may exert a direct inhibitory action on afferent nerves. Therefore, the suppression of bladder inflammation, increased urinary frequency and referred mechanical hyperalgesia by systemic administration of CB2 agonists may result from the combined effects of decreased inflammation and afferent nerve activity inhibition.
CONCLUSIONS
Treatment with a selective CB2 agonist decreased the severity of established cystitis and reversed the increased urinary frequency and referred mechanical hyperalgesia associated with cystitis. Results provide further evidence that CB2 is a potential therapeutic target for painful inflammatory disease.
Acknowledgments
Supported by National Institutes of Health Grants R01 DK 066349 and R01 DK 088806 (DEB).
Abbreviations and Acronyms
- AM630
6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone
- CB
cannabinoid receptor
- GP1a
N-(piperidin-1-yl)-1-(2, 4-dichlorophenyl)-1,4-dihydro-6-methylindeno[1,2-c]pyrazole-3-carboxamide
- IC/PBS
interstitial cystitis/painful bladder syndrome
Footnotes
Study received University of Wisconsin institutional animal care and use committee approval.
References
- 1.Clayton N, Marshall FH, Bountra C, et al. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 2002;96:253. doi: 10.1016/S0304-3959(01)00454-7. [DOI] [PubMed] [Google Scholar]
- 2.Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 3.Elmes SJ, Winyard LA, Medhurst SJ, et al. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 2005;118:327. doi: 10.1016/j.pain.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Walczak JS, Cervero F. Local activation of cannabinoid CB1 receptors in the urinary bladder reduces the inflammation-induced sensitization of bladder afferents. Mol Pain. 2011;7:31. doi: 10.1186/1744-8069-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinis P, Charrua A, Avelino A, et al. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci. 2004;24:11253. doi: 10.1523/JNEUROSCI.2657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gratzke C, Streng T, Park A, et al. Distribution and function of cannabinoid receptors 1 and 2 in the rat, monkey and human bladder. J Urol. 2009;181:1939. doi: 10.1016/j.juro.2008.11.079. [DOI] [PubMed] [Google Scholar]
- 7.Hayn MH, Ballesteros I, de Miguel F, et al. Functional and immunohistochemical characterization of CB1 and CB2 receptors in rat bladder. Urology. 2008;72:1174. doi: 10.1016/j.urology.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Tyagi V, Philips BJ, Su R, et al. Differential expression of functional cannabinoid receptors in human bladder detrusor and urothelium. J Urol. 2009;181:1932. doi: 10.1016/j.juro.2008.11.078. [DOI] [PubMed] [Google Scholar]
- 9.Gratzke C, Streng T, Stief CG, et al. Effects of cannabinor, a novel selective cannabinoid 2 receptor agonist, on bladder function in normal rats. Eur Urol. 2010;57:1093. doi: 10.1016/j.eururo.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Gratzke C, Streng T, Stief CG, et al. Cannabinor, a selective cannabinoid-2 receptor agonist, improves bladder emptying in rats with partial urethral obstruction. J Urol. 2011;185:731. doi: 10.1016/j.juro.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 11.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang ZY, Wang P, Bjorling DE. Activation of cannabinoid receptor 2 inhibits experimental cystitis. Am J Physiol Regul Integr Comp Physiol. 2013;304:R846. doi: 10.1152/ajpregu.00585.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorling DE, Elkahwaji JE, Bushman W, et al. Acute acrolein-induced cystitis in mice. BJU Int. 2007;99:1523. doi: 10.1111/j.1464-410X.2007.06773.x. [DOI] [PubMed] [Google Scholar]
- 14.Ofek O, Karsak M, Leclerc N, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A. 2006;103:696. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1999;53:55. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 16.Cornelissen LL, Misajet B, Brooks DP, et al. Influence of genetic background and gender on bladder function in the mouse. Auton Neurosci. 2008;140:53. doi: 10.1016/j.autneu.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Quartilho A, Mata HP, Ibrahim MM, et al. Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99:955. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- 18.Hanus L, Breuer A, Tchilibon S, et al. HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci U S A. 1999;96:14228. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinsey SG, Mahadevan A, Zhao B, et al. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011;60:244. doi: 10.1016/j.neuropharm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horváth B, Magid L, Mukhopadhyay P, et al. A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br J Pharmacol. 2012;165:2462. doi: 10.1111/j.1476-5381.2011.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montecucco F, Lenglet S, Braunersreuther V, et al. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol. 2009;46:612. doi: 10.1016/j.yjmcc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhyay P, Rajesh M, Horváth B, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med. 2011;50:1368. doi: 10.1016/j.freeradbiomed.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim MM, Deng H, Zvonok A, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100:10529. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozano-Ondoua AN, Wright C, Vardanyan A, et al. A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss. Life Sci. 2010;86:646. doi: 10.1016/j.lfs.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nackley AG, Zvonok AM, Makriyannis A, et al. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004;92:3562. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- 26.Beltramo M, Bernardini N, Bertorelli R, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez T, Farthing JN, Zvonok AM, et al. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol. 2007;150:153. doi: 10.1038/sj.bjp.0706984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93:653. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol. 2003;139:775. doi: 10.1038/sj.bjp.0705304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sagar DR, Kelly S, Millns PJ, et al. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur J Neurosci. 2005;22:371. doi: 10.1111/j.1460-9568.2005.04206.x. [DOI] [PubMed] [Google Scholar]