Abstract
The very limited ability of adult podocytes to proliferate in vivo is clinically significant because: podocytes form a vascular barrier which is functionally critical to the nephron; podocyte hypoplasia is a characteristic of disease; and inadequate regeneration of podocytes is a major cause of persistent podocyte hypoplasia. Excessive podocyte loss or inadequate replacement leads to glomerulosclerosis in many progressive kidney diseases. Thus, restoration of podocyte cell density is almost certainly reliant on regeneration by podocyte progenitors. However such putative progenitors have remained elusive until recently. In this review we describe the developmental processes leading to podocyte and parietal epithelial cell (PEC) formation during glomerulogenesis. We compare evidence that in normal human kidneys PECs expressing ‘progenitor’ markers CD133 and CD24 can differentiate into podocytes in vitro and in vivo with evidence from animal models suggesting a more limited role of PEC-capacity to serve as podocyte progenitors in adults. We will highlight tantalizing new evidence that specialized vascular wall cells of afferent arterioles including those which produce renin in healthy kidney, provide a novel local progenitor source of new PECs and podocytes in response to podocyte hypoplasia in the adult, and draw comparisons with glomerulogenesis.
Keywords: glomerulus, parietal epithelial cells, WT-1, proteinuria, glomerulosclerosis, cells of renin lineage
Importance of podocyte number in glomerular diseases
When podocytes are injured in glomerular diseases, apoptosis,1, 2 necrosis,3 altered autophagy,4 and detachment5 can cause an acute and/or chronic reduction in podocyte density. Because proliferation of existing cells is inadequate to replace those depleted 6 (see below), podocyte number decreases progressively with time in progressive glomerular diseases,7 and hypertrophy of persisting podocytes ensues.8 Several studies have examined the consequences of a decrease in podocyte number, the details of which are beyond the scope of this review. Briefly, seminal studies by the laboratories of Kriz,9 Ichikawa10 and Wiggins11 have all show that the depletion in podocyte number precedes the development of glomerulosclerosis, and the lower the number, the more severe is the scarring. These data provide a compelling therapeutic rationale that every effort needs to be made to not only limit podocyte depletion, but to augment glomerular repair by restoring podocyte number above the critical threshold that causes undesirable consequences.
Limited proliferative capacity of adult podocytes in disease
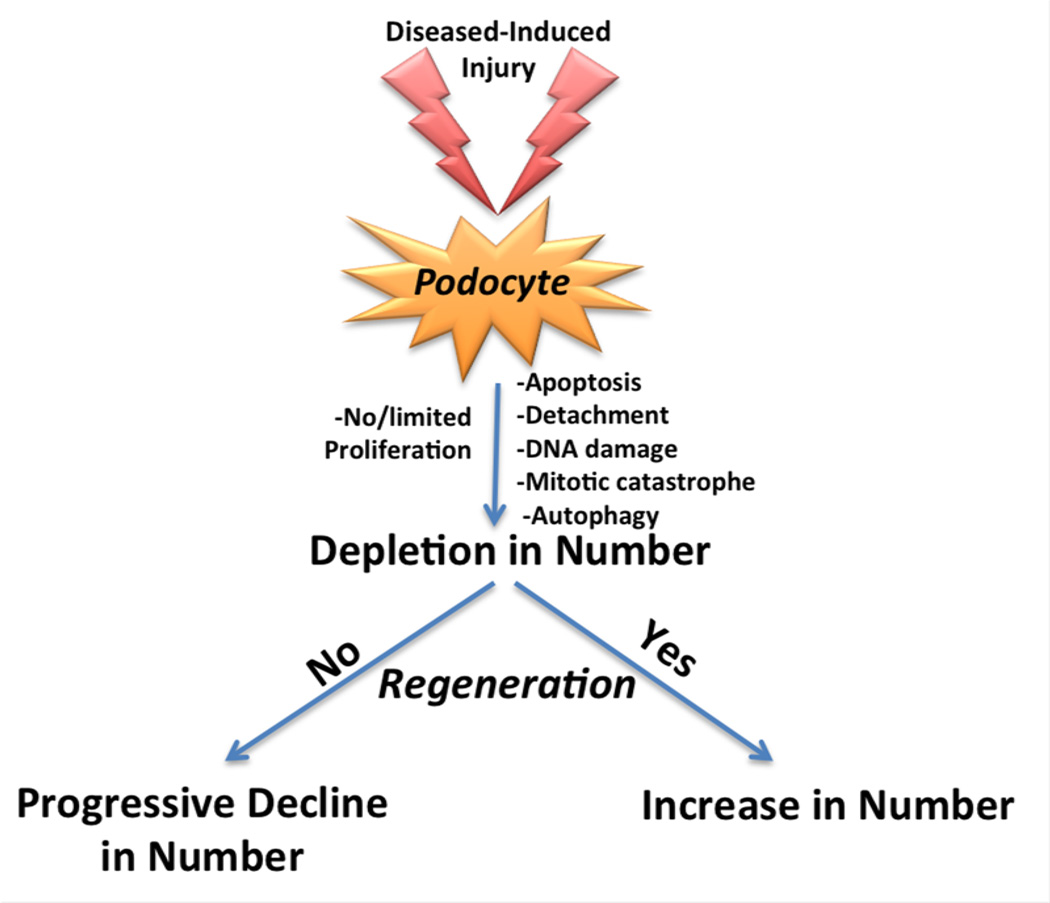
In the majority of glomerular diseases characterized by primary or secondary podocyte injury, studies show that podocyte proliferation is very unusual, and is clearly inadequate to replace acute and chronic depletion of podocytes.6 Several lines of evidence might explain the inadequate proliferation in these terminally differentiated cells. First, although cell cycle proteins required for DNA synthesis such as cyclin E and cyclin A are occasionally increased in podocytes in experimental glomerular disease,12, 13 the cell cycle inhibitors p21 and p27 are also increased.14, 15 p21 and p27 inhibit the activity of cyclin-cdk complexes required for DNA synthesis. When p21 and/or p27 are genetically deleted in null mice, podocytes readily proliferate following disease-induced injury.14, 15 A few podocytes do express the mitotic-phase cyclins B on occasion.16 However, rather than complete mitosis, two fates are more common that do not lead to cell proliferation. These are mitotic catastrophe associated with cell death,17 or an inability to exit cell cycle, leading to DNA accumulation and polyploidy (Figure 1). Finally, injury-induced DNA damage limits proliferation by causing cell cycle arrest.18 Although genetically altering certain pathways in adult podocytes can enhance their proliferative capacity, this likely does not occur in human glomerular diseases. Taken together, surviving adult podocytes are unable to replace those depleted in disease, which leads to progressive glomerulosclerosis. Thus, restoration of podocyte density will require regeneration by alternate cells such as a podocyte progenitor.
Figure 1. Schema showing the fate of podocytes in response to injury.
Podocyte number can increase following depletion despite absence of proliferation
Recent experimental studies support a previously unrecognized paradigm: following podocyte depletion as a consequence of injury, podocyte number can be partially, or completely replenished under certain circumstances. Most importantly, this occurs in the absence of podocyte proliferation. Benigni and colleagues showed that ACE-inhibition restored podocyte number in experimental FSGS.19 Alpers showed that improving the diabetic milieu reverses podocyte depletion,20 and in mice with experimental FSGS, administration of retinoids21 or corticosteroids22 in the course of established disease when podocyte hypoplasia is established, results in greater restoration of podocyte density compared to control mice given vehicle, in the absence of proliferation. Such reports suggest in order for podocyte number to increase following depletion in the absence of proliferation, regeneration must be taking place from another cell source. Although reports have proposed a circulating progenitor from the bone marrow, under careful scrutiny these have not been reproduced in animal models nor substantiated in human disease. Therefore, local kidney sources of progenitors are more likely to explain adult podocyte regeneration in the absence of proliferation.
To put these in to context, we will begin by discussing the developmental programs that give rise to glomerular podocytes and glomerular parietal epithelial cells during glomerulogenesis, and compare mechanisms of maintenance of tubular structures in other organs by progenitors. We will then discuss two leading candidates progenitor sources that have emerged as likely adult podocyte progenitors: glomerular parietal epithelial cells (abbreviated as PECs), and arteriolar vascular wall cells of renin lineage (abbreviated as CoRL). The merits of each will be discussed below.
Comparisons between the Nephron and other Epithelial Structures
Epithelia of the gut, skin and mammary gland have a population of progenitor cells that lie at the base of epithelial cell-lined tubular structures which supply new epithelial cells to these structures.23,24,25 The progenitors supply phenotypically-distinct types of epithelial cells, and as these epithelial cells mature they are eventually shed from the lumen and replaced. In the mammary gland a single progenitor can give rise to myoepithelial cells which surround the mammary ducts as well as lining epithelial cells of the alveoli and ducts and have contractile functions.26 Stratified and pseudo-stratified epithelia in structures such as the bronchial tree and skin have a basal population of progenitors that supplies the different types of mature epithelial cells of that epithelium.27 The nephron is also a blind ending epithelial tube with structural similarities to the mammary gland, skin, hair follicle and crypts of the intestine. Unlike these structures however, epithelial cells of the nephron are remarkably stable, and are not continually shed or turned over; unlike the mammary gland, the nephron is not subjected to hormonally regulated growth and regression. The mammalian kidney does however appear to regress slowly but steadily over the adult life span. In animal models designed to mimic human disease, injury to the adult mouse tubule stimulates loss of epithelium followed by complete regeneration of the epithelium.28 This model has provided no clear evidence of an epithelial progenitor cell lying within the tubule or outside of the tubule, but indicates that tubular epithelium regenerates by successful proliferation of surviving epithelial cells. Exhaustive searches for latent progenitors in the adult tubule have borne no fruit.29 The glomerulus, however lies at the base of the epithelial tube and has, unique cell populations and a number of features that might suggest latent progenitors exist and can be activated.
Developmental Origins of Podocytes and Parietal Epithelial cells
The kidney arises from a population of OSR1+ mesenchymal progenitors in the intermediate mesoderm. These progenitors differentiate into four distinct progenitors, which give rise to the epithelium, ureteric epithelium, stroma and microvascular endothelium of the kidney.30 The epithelial progenitors, located in cap mesenchyme, retain mesenchymal characteristics and express critical transcription factors including WT1, PAX2 and SIX2, which regulate progenitor pool maintenance and differentiation to epithelium.31–33 A single progenitor gives rise to multiple types of epithelium within a single nephron. The nephron develops from the distal end first, and the blind ending tubule, which becomes the glomerulus, develops last. This process of transition from a mesenchymal cell in the cap mesenchyme of the nephrogenic kidney to an epithelial cell requires activation of a number of well-described critical transcription factors including PAX2 and WT1. Various signals from neighboring structures regulate this process including WNT9b, GDNF, FGF9 released from the ureteric bud tip are critical.34
Similar to tubule epithelial cells, podocytes and parietal epithelial cells (PECs) arise from cap mesenchyme progenitors and require activation of SIX2, PAX2 and WT1. Thus, these cells undergo a mesenchymal to epithelial transition during early nephrogenesis. These primitive epithelial cells in the embryonic kidney exhibit typical morphology of cuboidal epithelium. Mature functioning podocytes and PECs bear little resemblance to epithelial cells however, and whereas the epithelial cells that become tubules silence WT1 expression, podocytes are characterized by persistent expression of WT1.35 Although podocytes and PECs express tight junction proteins 36 and therefore form a barrier, they lack polarity, and lack secretory or obvious transporting functions, typical of epithelium.
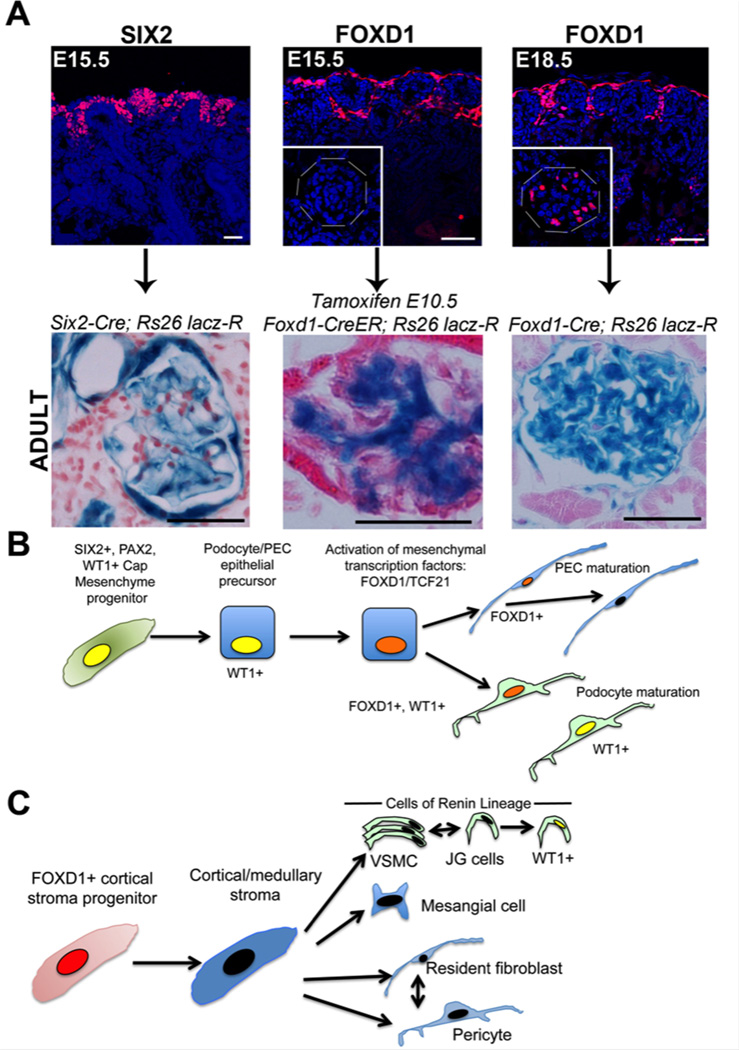
Mature podocytes exhibit many similarities to mural cells or pericytes. These include the production of angioregulatory factors such as VEGF and ANGPT1, ephrins, and the synthesis of high levels of capillary basement membrane proteins including COL1 protein.37 Like pericytes, they also express many genes restricted to neurons or associated glial cells, which may regulate the highly branched cytoplasmic structure.38 PECs also express many similar genes to podocytes. At later time points in nephrogenesis (16.5dpc onward in mice), podocytes and PECs activate the transcription factors FOXD1 and TCF21, and the cyclin dependent kinase p57Kip2. 20, 39 FOXD1 and TCF21 are critical transcription factors expressed in the progenitor cells which overlie the cap mesenchyme and give rise to the stroma of the kidney.40,41,39 These FOXD1+ nephrogenic progenitors arise much earlier in nephrogenesis (9.5dpc in mice) and give rise to all the kidney stromal cells, including vascular smooth muscle or arterioles and pericytes (known as collectively as mural cells), including vascular wall cells which express Renin. They also give rise to mesangial cells, and fibroblasts (FIGURE 2).
Figure 2. Podocytes and PECs derive from SIX2+ epithelial progenitor cells in cap mesenchyme but late in nephrogenesis activate FOXD1, a transcription factor critical in formation of mural cells in the kidney.
(A) Images (upper) showing two discrete progenitor populations in the nephrogenic outer zone of the developing kidney at E15.5 defined by expression of SIX2 and FOXD1 respectively. Below shows results of fate mapping of these progenitors using Cre/LoxP transgenic reporter mice, indicating in adult mice they given rise to podocytes/PECs and mesangial cells respectively. At E18.5 FOXD1 is now active additionally in podocytes of primitive glomeruli (inset), and mapping of FOXD1 expression shows that all podocytes and PECs have expressed FOXD1 at this late time in nephrogenesis. (B) Schema showing the results of fate mapping of SIX2+ epithelial progenitors which co-express WT1 and PAX2 in development. These progenitors give rise to precursor cells for podocytes and PECs which have a cuboidal appearance. Late in development they activate FOXD1 and TCF21 which appear to regulate the final differentiation into mature cells, and they persistently express WT1. (C) Schema showing the results of fate mapping of FOXD1+ stromal progenitors which give rise to mesangial cells, fibroblasts, pericytes and vascular smooth muscle including JG cells. In glomerular disease, JG cells activate WT1.
FOXD1 regulates activation of genes associated with mural cell development including COL2, COLXI and CHTRC1 and UNC5 whereas TCF21 regulates genes involved with membrane binding and regulation of the WNT pathway (FIGURE 2). Studies in which FOXD1 has been mutated show severe developmental defects as a result of severely disrupted stromal development, but they also show a persistence of cuboidal epithelial morphology in podocytes precursors in developing glomeruli.39, 42 Similar findings have recently been reported when TCF21 is disrupted in podocytes late in development.43 Together these findings suggest that from 15.5dpc onward, podocyte and PEC epithelial precursors undergo transcriptional reprogramming to acquire characteristics of mural cells.
A lineage boundary normally exists between SIX2+ cap mesenchyme derived epithelium, and FOXD1+ progenitor-derived kidney stroma,31 and appears to be maintained in adult disease states. That is to say epithelium remains as epithelium and stroma remains as stroma. However, podocytes and PECs have broken this lineage boundary, by first undergoing a mesenchymal to epithelial transition, then later activated the critical mesenchymal transcription factors.
The sequential activation of SIX2 and later FOXD1 and TCF21 in podocytes and PECs is exemplified in mice used to trace the lineage the two kidney progenitor populations (FIGURE 2). SIX2+ cap mesenchyme gives rise to tubule epithelium except collecting duct and gives rise to podocytes and PECs. Labeling the fate of the separate population of FOXD1 progenitors at 10.5dpc shows that in the glomerulus only the mesangium is populated at this time point by FOXD1 derived cells, whereas no podocytes or PECs are labeled. By contrast, in adult mice which map all cells that have activated FOXD1 in development, podocytes and PECs, in addition to mesangium and mural cells, have activated this transcription factor. The fact that all mesangium is labeled by 12.5dpc but no podocytes or PECs were labeled at this time indicates that podocytes and PECs activate FOXD1 transcription factor after the glomerulus has formed. This makes sense developmentally, since the glomerulus forms at the end of a blind epithelial tube when podocytes and PECs resemble a simple cuboidal epithelium. Only once the glomerulus has formed and has blood flow, are the mesenchymal transcription factors activated (FIGURE 2). These developmental insights provide us with two critical pieces of information. Firstly, podocytes and PECs share transcriptional programming during development, and are distinct from other epithelia of the kidney. Secondly, podocytes and PECs exhibit hybrid transcriptional characteristics between epithelium and mural cells.
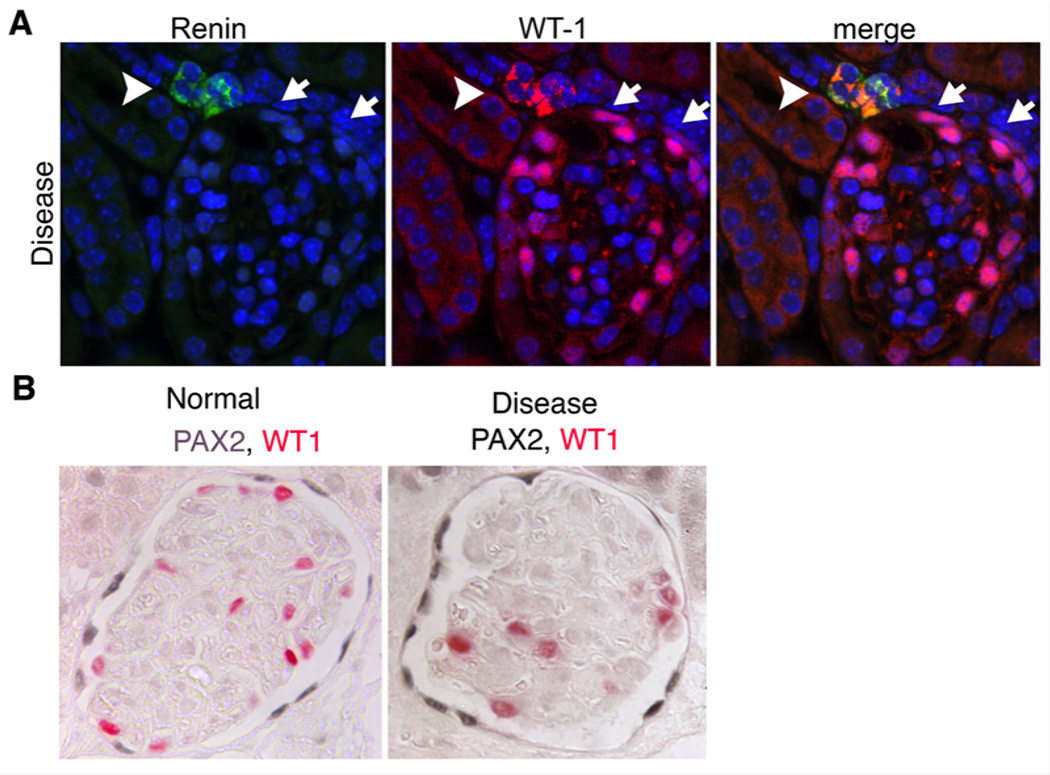
In the setting of adult glomerular disease sufficient to result in podocyte depletion due to cell death, two striking observations can be made. Firstly vascular wall cells at the distal tip of the afferent arteriole activate WT1 (FIGURE 3). Secondly PECs in affected glomeruli up-regulate expression of PAX2. As stated earlier, both of these transcription factors are critical in epithelial cell formation. The cells lining the tip of distal afferent arteriole are modified smooth muscle cells derived from FOXD1+ progenitors (FIGURE 3). They produce Renin and release it in response to factors including stretch. These modified VSMCs show plasticity in the adult kidney and may be in flux with other more proximal VSMCs.44 The de-novo activation of WT1 might suggest activation of a transcriptional program similar to that which occurs in cap mesenchyme during development (FIGURE 2). Upregulation of PAX2 by PECs might also suggest recapitulation of this same developmental program (FIGURE 2).
Figure 3. Renin producing vascular smooth muscle cells activate WT1 and PECs upregulate PAX2 in adult glomerular disease with podocyte loss.
(A) Split panel images of a glomerulus following administration of anti-podocyte antibodies showing de novo expression of WT1 in the renin producing cells of the JGA (arrowhead). In addition to expression of WT1 in podocytes attached to the tuft, WT1 can also be seen in some PECs (arrows). (B) Images of normal and diseased glomeruli following anti-podocyte antibody administration. Note increased expression intensity of PAX2 in PECs
New studies using genetic fate-mapping strategies provide tantalizing evidence that in adult disease settings or in post natal glomerular growth, renin producing VSMCs and PECs have the capacity to undergo transcriptional reprogramming, migrate to the glomerular capillary tuft, integrate into the tuft and acquire many characteristics of podocytes. In the following sections we will review the current knowledge of these processes derived from fate-mapping studies in animal models of glomerular disease and comparative studies in human disease.
Glomerular parietal epithelial cells (PECs) as adult podocyte progenitors
Three observations support the hypothesis that PECs possess biological programs necessary to enable them differentiate into adult podocytes. First, as stated earlier, because PECs and podocytes arise from a common restricted pool of mesenchymal cells, it is appealing to propose that PECs might simply revert to a fetal developmental reprogramming and become podocytes.45 Second, in normal adult human, and rodent kidneys, a subpopulation of PECs lining Bowman’s capsule typically at the vascular pole of the glomerulus co-express both PEC and podocyte proteins, and even show ultra-structural features of podocytes.46 These cells typically located at the vascular pole are have been named ‘glomerular epithelial transitional cells’, When they only express proteins normally restricted to podocytes, despite PEC location, have been referred to as ‘ectopic podocytes’,47 and when they express WT-1, typical of adult podocytes, they have been called ‘parietal podocytes.’47 Other investigators have also described another subpopulation PECs called, defined as cells at the tubular pole that in addition to expressing PEC proteins, co-express markers CD133 and CD24.48 These rare PECs cells have been proposed to have multi-progenitor potential, although definitive studies are lacking.
Because of the common origin of podocytes and PECs during development (discussed earlier), and the presence of different subpopulations of PECs in normal glomeruli, many investigators have focused on PECs as potential progenitors for podocytes. We will discuss data supporting and not supporting this paradigm, and highlight different experimental systems, species, disease models and other factors that might explain these differences (see Table 1).
Table 1.
Summary of published studies on parietal epithelial cells as adult podocyte progenitors
| Model System | Supporting Data | Reference | |
|---|---|---|---|
| Studies strongly supporting PECs as podocyte progenitors | PEC reporter mouse |
|
49 51 |
| Human kidney |
|
54 48 53 |
|
| Studies suggesting, but not definitively proving, that a subset of PECs are adult podocyte progenitors | Rat model |
|
19 |
| Diabetic mouse |
|
55 |
Studies supporting a role for PECs as adult podocyte progenitors
The field of glomerular epithelial cell regeneration has been markedly enhanced by the generation of a transgenic mouse model expressing the tetracycline trans-activator protein rtTA, under regulation of a hybrid 3Kb fragment of the human and rabbit Podocalyxin promoter by Dr. Moeller’s laboratory that affords specific permanent labeling of PECs in vivo. A seminal study by Appel showed that permanently-labeled PECs migrate to the glomerular tuft during mouse adolescence, and begin to express podocyte proteins, and take on their ultrastructural shape.49 However, repeated studies by Moeller’s group,50 and recently by Huber’s laboratory,51 using similar methods indicated that PECs do not serve as podocyte progenitors in adult mice. Thus, in this reporter mouse, PECs serve as juvenile, but not adult podocyte progenitors, an observation that probably reflects glomerular growth during preadult development.
Using normal human kidneys, Romagnani’s laboratory isolated cells from Bowman’s capsule expressing PEC proteins and CD133/CD24, and propagated them ex vivo in a cell culture system.48 These cells can be expanded under cell culture conditions (i.e. have self-renewal potential), and can differentiate into podocytes and tubular cells. 52, 53 When administered intravenously to mice with Adriamycin nephropathy, these cells populated the glomerulus (and tubules), began to express podocyte proteins, and also acquired some ultra-structural features characteristic of podocytes. Disease outcomes were significantly improved. Collectively these results support a biological role for this PEC sub-population as adult podocyte progenitors in experimental glomerular disease. When these cells were cultured under different conditions, they began to de novo express several podocyte proteins, which required a decrease in Notch signaling.54 Anders and colleagues showed that blockade of the chemokine stromal-derived factor (SFD/CXCL12) enhanced the differentiation of renal progenitors towards a podocyte phenotype.55
More recently, several studies have highlighted factors that inhibit PEC progenitors. First, Peirid showed that the subpopulation of PECs expressing CD133/CD24 requires retinoids for normal survival and function.56 In albuminuric states, the filtered albumin in the urinary space binds to retinoic acid in the urinary space, thereby limiting the exogenous pool of retinoids available to PECs. Moreover, when albumin was taken up by PECs, a phenomenon that has been shown previously,36 endogenous retinoid synthesis was impaired. 56 Importantly, the decrease in the exogenous and endogenous retinoids limited the capacity of adult human parietal epithelial multipotent progenitors to perform their normal progenitor function, which might explain in part why podocyte regeneration is limited in albuminuric states. Second, Rizzo recently showed that a subpopulation of PECs co-expressing the differentiation marker NCAM also express the angiotensin 1 receptor, and that proliferation of these cells could be reduced by giving rats an AT1R blocker.57 Third, studies from the Anders laboratory showed that Interferons alpha and beta reduced the capacity of PEC progenitors to induce nephrin mRNA expression suggesting these agents may limit the capacity of these progenitors to become podocytes.58
Studies lacking a supporting a role for PECs as adult podocyte progenitors
Studies have shown that in several states of podocyte depletion, the number of glomerular epithelial transition cells (defined as cells co-expressing PEC and podocyte proteins) are increased both along Bowman’s capsule, and in the glomerular tuft.59, 60 In addition, the number of transition cells can be increased by administration of retinoids,21 corticosteroids22 and ACE-inhibition,(in press) as well as an improvement in the diabetic milieu.20 However, these studies are all observational and none has provided functional evidence that transition cells differentiate into podocytes. Guhr showed that the de novo expression of podocyte proteins in PECs was due to reduced ubiquitin-mediated degradation.61 More recently, studies using reporter mice have suggested a very different paradigm. These studies reported that following podocyte injury, a subset of labeled podocytes could be detected having moved from the glomerular tuft and now lining Bowman’s capsule.62–65 In this location in some instances, the labeled podocytes co-expressed PEC proteins in addition to podocyte proteins. These data suggest that one explanation for cells co-expressing PEC and podocyte proteins along Bowman’s capsule is that they derive from migrating cells of podocyte origin, and not from PEC origin. The biological significance of these findings remains to be determined.
Taken together, there are compelling data that support a biological role for PECs as adolescent as well as adult podocyte progenitors. Yet, there is not consistency across all the models and marker systems. We need to consider several variables that might explain these differences such as species and the types of experimental and human glomerular diseases studied. We also need to be cautious. While a PEC might well differentiate into an adult podocyte, the magnitude of “regeneration” that results from this may be inadequate to fully replace the number of podocytes depleted, and the precise cues and mechanisms underlying such events are not yet well delineated. Finally, we need to keep an open mind that there may well be bi-directional repair/regenerative process ongoing, where PECs differentiate into podocytes, and podocytes differentiate into PECs. In addition we must be cautious that the animal models of PEC fate mapping have not been reproduced in independent studies, and that use of temporally expressed markers in tissue sections is not a surrogate for fate mapping and result at best in suggestive observations.
Cells of Renin Lineage as adult podocyte progenitors
Studies in the past few years have shown that vascular smooth muscle cells which produce, or have produced renin (known as Cells of Renin Lineage [CoRL]), exhibit marked plasticity for a variety of cell-types. These cells restricted to the juxta-glomerular compartment in adults and best known for their synthesis and secretion of renin, comprise 0.01% of the total kidney number. They derive from FoxD1-positive stromal cells.66 They participate in kidney vasculature assembly and branching, and thereafter are normally distributed along large intrarenal arteries. Studies led by Gomez shows that CoRL can differentiate into smooth muscle cells, mesangial cells, and maybe pericytes too.67, 68 Studied by Kurtz and colleagues show that when VHL is deleted, CoRL can differentiate in to erythropoietin-producing cells.69 Adult CoRL themselves can be regenerated by adult renal mesenchymal-like cells that express CD44, c-Kit and/or CD105 markers, events that recapitulate developmental processes.70 Taken together, these studies show that under different conditions, adult CoRL are multipotent, serving as progenitors for several kidney cell types, and that they themselves can be replaced by a progenitor too.
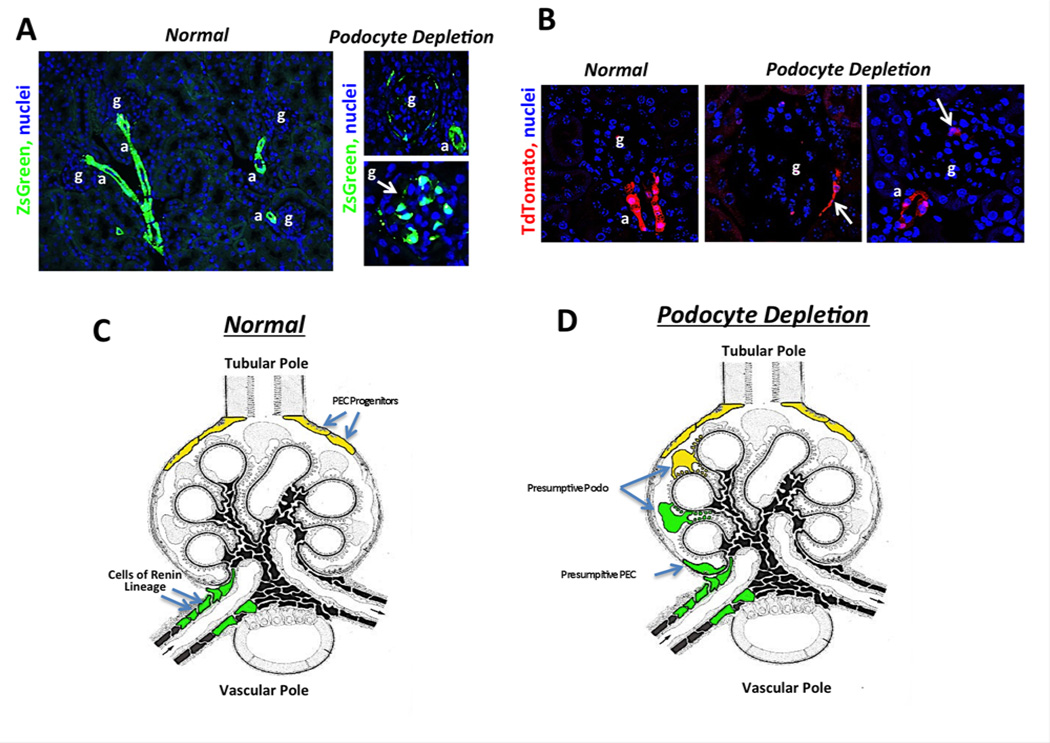
In order to determine whether CoRL also can serve as progenitors for adult glomerular epithelial cells in disease, reporter mice were developed that permanently tag Renin producing cells using Cre/ loxP somatic DNA recombination such that fluorescent proteins are permanently activated in those cells and any daughters. Two reporter mouse lines that permanently label CoRL constitutively (Ren1c-Cre; zsGreenR and Ren1d-Cre; Rs26-GFP-R), and one reporter that only labels CoRLs in an inducible manner following the administration of tamoxifen (Ren1c-CreER; tdTomatoR), were used for study.71 As expected, administering tamoxifen labeled CoRL only in Ren1c-CreER; tdTomatoR , and labeled cells were confined to the juxta-glomerular compartment. The majority co-expressed renin, and none were detected in glomeruli.71 In a model of classic Focal Segmental Glomerulosclerosis (FSGS) in reporter mice induced by cytotoxic anti-podocyte antibodies, a 30–40% decrease in overall podocyte number was observed, followed by proteinuria, increased serum creatinine and segmental glomerular scarring in a focal distribution.71 The first major finding was that labeled CoRL were detected in a subpopulation of injured glomeruli (FIGURE 4). In the majority of glomeruli containing labeled CoRL, cells were distributed either within the glomerular tuft adherent to the GBM, or along Bowman capsule. Such de novo appearance of permanently tagged cells within the glomerulus can only be explained by migration from the juxta-glomerular apparatus since in mice that were not exposed to tamoxifen and did not undergo genetic recombination in the Renin producing cells prior to disease, no cells were detected either in the juxta- or intra glomerular compartments.71 The degree of scarring within individual glomeruli was significantly lower in glomeruli that contained labeled CoRL. These data support the notion that following an abrupt decline in podocyte number, labeled CoRL migrate from the juxta- to the intra-glomerular location.
Figure 4. Images showing the fate of Cells of Renin Lineage in glomerular regeneration and Schema showing proposed dual role for PECs and CoRL as adult podocyte progenitors in glomerular disease.
(A) Images from normal adult kidney and following podocytes loss during kidney disease in RenCre;ZsGreen reporter mice. Note all of the vascular arteriolar wall is labeled due to Renin expression during development. Note that the adjacent glomerulus is devoid of cells of renin lineage but following podocyte loss cells can be seen along Bowman’s capsule and in the glomerular tuft. (B) Images from RenCreER;tdTomato reporter mice that received tamoxifen to induce recombination to activate tdTomato expression in renin producing cells at 6 weeks of age. Only cells in the JGA at the tip of the afferent arteriole are permanently labeled. Note that following podocytes loss during kidney disease, there is migration of renin labeled cells from the JGA to Bowman’s capsule and the glomerular tuft. (C-D) Schema showing the fate of juxtaglomerular cells of renin lineage and tubular pole PECs in the setting of kidney disease. Both of these cells from fate mapping experiments have the capacity to acquire podocytes like qualities.
Second, several lines of evidence show that the appearance of labeled CoRL in disease was not simply due to (re)expression of renin in diseased glomeruli.71 First, in the inducible mouse, CoRL were labeled with tamoxifen, prior to disease. Several weeks later a survival biopsy confirmed labeling restricted to the JG location. Only then was disease induced, and because tamoxifen was not given subsequently, no additional cells could be labeled. Second, we generated a mouse that expressed GFP only when renin was transcribed. As expected, renin mRNA was confined to cells in the JG location in normal and diseased states. Finally, renin staining was never detected in glomeruli of diseased mice.
Third, fate-mapped CoRL that were detected in the glomerulus expressed glomerular epithelial cell markers in disease (FIGURE 4).71 A subpopulation of intra-glomerular CoRL co-expressed one of the following podocyte proteins: WT-1, nephrin, podocin or synaptopodin. Additionally, a subpopulation of labeled CoRL lining Bowman’s capsule co-expressed one of the PEC restricted proteins PAX2 or claudin-1. CoRL did not express nephrin, podocin, synaptopodin, PAX2 or claudin1 when in a juxta-glomerular location in normal or diseased states. In this model of FSGS, intra-glomerular labeled CoRL did not co-express markers of mesangial or endothelial cells. Some mapped CoRL cells that had moved in to diseased glomeruli did not co-express markers of podocytes or PECs and therefore had not obviously differentiated into a resident glomerular cell type at the time of study. Their subsequent fate was not determined.
Having shown that a subpopulation of CoRL move from the juxta-glomerulus to the glomerulus following abrupt podocyte depletion, where they begin to co-express either podocyte and/or PEC proteins, we next asked if these events could be replicated in a model of chronic FSGS characterized by progressive podocyte depletion. Accordingly, CoRL were labeled with a pulse of tamoxifen in inducible reporter mice, and the tamoxifen was then withdrawn, followed by a kidney biopsy several weeks later to obtain baseline tissue. Labeled CoRL were confined to the JG as expected. The 5/6 nephrectomy remnant kidney model was then induced surgically, and individual mice were followed over time. Mice developed mild FSGS. However, in a subpopulation of glomeruli that did not have obvious segmental scarring, labeled CoRL could be detected. Moreover, a subset of these cells co-expressed several podocyte proteins (manuscript submitted). Glomerulosclerosis was substantially reduced in glomeruli with labeled CoRL. The results suggested that CoRL might be serving as adult podocyte progenitors during chronic depletion. However, the ability of CoRL to fully replace or normalize podocyte number was not sufficient to limit overall glomerulosclerosis.
Aging nephropahy is characterized by a progressive segmental and global glomerulosclerosis, accompanied by, and largely likely to a slow decline in podocyte number with age. In CoRL reporter mice aged 1 year, the decrease in podocyte number was oftentimes accompanied by the presence of CoRL in the intra-glomerular compartment, and many of these cells co-expressed podocyte proteins.72 Noteworthy is that when CoRL entered aged glomerulus, electron microscopy studies showed that a subset displayed characteristic ultra-morphological features of podocytes, namely foot processes and slit diaphragms.72 With increasing age, the density of labeled CoRL decreased significantly compared to younger ages. These results suggest that the reservoir of CoRL in the juxta-glomerulus decreases in number with progressive aging
Taken together, in three settings of podocyte depletion (acute FSGS, chronic FSGS, and aging), labeled CoRL were detected in glomeruli, where a subset co-expressed podocyte and PEC proteins, and some acquired morphological features of adult podocytes. These findings support the notion that CoRL might serve as adult podocyte progenitors in states of podocytes hypoplasia or depletion.
The mechanisms underlying CoRL transdifferentiation to adult podocytes, and the cues that initiate and maintain this process, are not known to date. Noteworthy was the observation (FIGURE 2) that WT-1, is readily detected in renin-positive cells in the juxta-glomerular location in acute FSGS, and also in the passive Heymann nephritis model of membranous nephropathy (unpublished). Many of these WT-1 stained cells co-express the CoRL reporter in reporter mice with FSGS. It is tempting to speculate that the de novo expression of this transcription factor might play an important role in regulating the fate of CoRL. Consistent with this notion is that WT-1 suppresses activity of the renin promoter, and might also explain why renin mRNA and protein are no longer detected when cells move in to the glomerulus to become podocytes expressing WT-1.73 Further studies are required to validate these observations. One fact is becoming increasingly clear regarding WT-1: it is likely not a marker specific to podocytes, but rather a transcription factor that is expressed by podocytes, PECs and CoRL in the kidney under different conditions, as it is in the epicardium and lung by progenitor cells of these organs.
Finally, the observation that when labeled CoRL are in the glomerulus following a model of acute podocyte depletion they express both podocyte and PEC proteins raises the yet unanswered question if CoRL provide a source for the subset of PECs that might ultimately differentiate into podocytes.
Summary and future directions needed to fill in missing pieces
It is critical that podocyte number be maintained in order to limit the development of proteinuria and glomerulosclerosis in glomerular diseases. Once podocyte depletion has occurred due to injury-induced apoptosis, detachment, dysregulated autophagy and other causes, regeneration is dependent on progenitors due to the inability of adult podocytes to proliferate. Circulating and bone marrow derived progenitors have not been well demonstrated to serve this role. Within the kidney, parietal epithelial cells (PECs) and specialized vascular smooth muscle cells of the arteriolar wall known as cells of renin lineage (CoRL) are two candidates that may well fulfill an adult podocyte progenitor role in states of podocyte depletion in glomerular diseases. Not all agree that PECs serve this role. We propose a model that they provide a dual role, where PEC progenitors localize predominantly to the tubular pole of the glomerulus, and CoRL to the vascular pole (FIGURE 4). This does not imply that their roles are restricted to these anatomic locations. However, the fact that podocyte number decreases in disease implies that the capacity of these putative podocyte progenitors to adequately restore podocyte number is limited.
Although the field is advancing, several critical questions remain unanswered:
Demonstration that adult podocyte progenitors prevent the development of glomerulosclerosis.
Understanding the cues that signal to local progenitors to serve their role.
Understanding the molecular mechanisms regulating progenitor differentiation and migration.
Understanding whether progenitors are replenished by asymmetric cell division or by repopulation from the vascular wall, or whether there is a limited supply of progenitors.
Do currently used therapeutics improve disease outcomes by augmenting progenitor differentiation.
What new therapeutic targets might there be to improve the outcomes of patients with glomerular diseases?
Acknowledgments
Financial Support: SJS was supported by the NIH (DK056799-10); JSD was supported by the following NIH grants: DK93493, DK94768, DK87389, AHA grant 12040023.
Abbreviations
- CoRL
cells of renin lineage
- FOXD1
forkhead box D1
- PAX2
paired box gene 2
- PECs
parietal epithelial cells
- WT-1
wilms tumor suppressor protein
- FSGS
focal segmental glomerulosclerosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: JSD is employed by Biogen Idec.; SJS and JWP have no conflicts of interest
REFERENCES
- 1.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233. [PubMed] [Google Scholar]
- 2.Brinkkoetter PT, Olivier P, Wu JS, Henderson S, Krofft RD, Pippin JW, et al. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. The Journal of clinical investigation. 2009 doi: 10.1172/JCI37978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cybulsky AV, Takano T, Papillon J, Kitzler TM, Bijian K. Endoplasmic reticulum stress in glomerular epithelial cell injury. Am J Physiol Renal Physiol. 2011;301(3):F496–F508. doi: 10.1152/ajprenal.00728.2010. [DOI] [PubMed] [Google Scholar]
- 4.Hartleben B, Wanner N, Huber TB. Autophagy in glomerular health and disease. Semin Nephrol. 2014;34(1):42–52. doi: 10.1016/j.semnephrol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Petermann AT, Pippin J, Krofft R, Blonski M, Griffin S, Durvasula R, et al. Viable podocytes detach in experimental diabetic nephropathy: potential mechanism underlying glomerulosclerosis. Nephron Experimental nephrology. 2004;98(4):e114–e123. doi: 10.1159/000081555. [DOI] [PubMed] [Google Scholar]
- 6.Kriz W. Progressive renal failure--inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1996;11(9):1738–1742. [PubMed] [Google Scholar]
- 7.Wiggins The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney International. 2007;71(12):1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 8.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, et al. Podocyte hypertrophy, "adaptation," and "decompensation" associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16(10):2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 9.Kriz W. The pathogenesis of 'classic' focal segmental glomerulosclerosis-lessons from rat models. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18(Suppl 6):vi39–vi44. doi: 10.1093/ndt/gfg1064. [DOI] [PubMed] [Google Scholar]
- 10.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16(4):1013–1023. doi: 10.1681/ASN.2004080720. [DOI] [PubMed] [Google Scholar]
- 11.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16(10):2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 12.Shankland SJ, Pippin JW, Couser WG. Complement (C5b-9) induces glomerular epithelial cell DNA synthesis but not proliferation in vitro. Kidney International. 1999;56(2):538–548. doi: 10.1046/j.1523-1755.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 13.Shankland SJ, Floege J, Thomas SE, Nangaku M, Hugo C, Pippin J, et al. Cyclin kinase inhibitors are increased during experimental membranous nephropathy: potential role in limiting glomerular epithelial cell proliferation in vivo. Kidney Int. 1997;52(2):404–413. doi: 10.1038/ki.1997.347. [DOI] [PubMed] [Google Scholar]
- 14.Kim YG, Alpers CE, Brugarolas J, Johnson RJ, Couser WG, Shankland SJ. The cyclin kinase inhibitor p21CIP1/WAF1 limits glomerular epithelial cell proliferation in experimental glomerulonephritis. Kidney International. 1999;55(6):2349–2361. doi: 10.1046/j.1523-1755.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 15.Ophascharoensuk V, Fero ML, Hughes J, Roberts JM, Shankland SJ. The cyclin-dependent kinase inhibitor p27Kip1 safeguards against inflammatory injury. Nature Medicine. 1998;4(5):575–580. doi: 10.1038/nm0598-575. [DOI] [PubMed] [Google Scholar]
- 16.Petermann AT, Pippin J, Hiromura K, Monkawa T, Durvasula R, Couser WG, et al. Mitotic cell cycle proteins increase in podocytes despite lack of proliferation. Kidney International. 2003;63(1):113–122. doi: 10.1046/j.1523-1755.2003.00723.x. [DOI] [PubMed] [Google Scholar]
- 17.Lasagni L, Lazzeri E, Shankland SJ, Anders HJ, Romagnani P. Podocyte mitosis - a catastrophe. Curr Mol Med. 2012 doi: 10.2174/1566524011307010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao L, Haas M, Pippin J, Wang Y, Miwa T, Chang A, et al. Focal and segmental glomerulosclerosis induced in mice lacking decay-accelerating factor in T cells. The Journal of clinical investigation. 2009;119(5):1264–1274. doi: 10.1172/JCI36000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, et al. Inhibiting angiotensinconverting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. American Journal Of Pathology. 2011;179(2):628–638. doi: 10.1016/j.ajpath.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pichaiwong W, Hudkins KL, Wietecha T, Nguyen TQ, Tachaudomdach C, Li W, et al. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol. 2013;24(7):1088–1102. doi: 10.1681/ASN.2012050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, et al. Retinoids Augment the Expression of Podocyte Proteins by Glomerular Parietal Epithelial Cells in Experimental Glomerular Disease. Nephron Exp Nephrol. 2012;121(1–2):e23–e37. doi: 10.1159/000342808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Pippin JW, Krofft RD, Naito S, Liu Z, Shankland SJ. Podocyte Repopulation by Renal Progenitor Cells Following Glucocorticoids Treatment in Experimental FSGS. Am J Physiol Renal Physiol. 2013;304(11):F1375–F1389. doi: 10.1152/ajprenal.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476(7360):293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 24.Stingl J. Detection and analysis of mammary gland stem cells. J Pathol. 2009;217(2):229–241. doi: 10.1002/path.2457. [DOI] [PubMed] [Google Scholar]
- 25.Kinzel B, Pikiolek M, Orsini V, Sprunger J, Isken A, Zietzling S, et al. Functional roles of Lgr4 and Lgr5 in embryonic gut, kidney and skin development in mice. Dev Biol. 2014;390(2):181–190. doi: 10.1016/j.ydbio.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Eirew P, Stingl J, Raouf A, Turashvili G, Aparicio S, Emerman JT, et al. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med. 2008;14(12):1384–1389. doi: 10.1038/nm.1791. [DOI] [PubMed] [Google Scholar]
- 27.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108(52):E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, et al. Intrinsic epithelial cells repair the kidney after injury. Cell stem cell. 2008;2(3):284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A. 2011;108(22):9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mugford JW, Sipila P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol. 2008;324(1):88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell stem cell. 2008;3(2):169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartwig S, Ho J, Pandey P, Macisaac K, Taglienti M, Xiang M, et al. Genomic characterization of Wilms' tumor suppressor 1 targets in nephron progenitor cells during kidney development. Development (Cambridge, England) 2010;137(7):1189–1203. doi: 10.1242/dev.045732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saifudeen Z, Liu J, Dipp S, Yao X, Li Y, McLaughlin N, et al. A p53-Pax2 pathway in kidney development: implications for nephrogenesis. PLoS One. 2012;7(9):e44869. doi: 10.1371/journal.pone.0044869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9(2):283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B. Nuclear localization of the protein encoded by the Wilms' tumor gene WT1 in embryonic and adult tissues. Development. 1993;119(4):1329–1341. doi: 10.1242/dev.119.4.1329. [DOI] [PubMed] [Google Scholar]
- 36.Ohse T, Chang AM, Pippin JW, Jarad G, Hudkins KL, Alpers CE, et al. A new function for parietal epithelial cells: a second glomerular barrier. American Journal of Physiology-Renal Physiology. 2009;297(6):F1566–F1574. doi: 10.1152/ajprenal.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358(11):1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139(1):193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa N, Roach A, Gomez I, Kobayashi A, Duffield J. MicroRNAs are critical regulators of FOXD1 progenitors and kidney stroma during nephrogenesis. J Am Soc Nephrol. 2014;24(184A) In Press. [Google Scholar]
- 40.Duffield JS, Humphreys BD. Origin of new cells in the adult kidney: results from genetic labeling techniques. Kidney International. 2011;79(5):494–501. doi: 10.1038/ki.2010.338. [DOI] [PubMed] [Google Scholar]
- 41.Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. American Journal Of Pathology. 2010;176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132(3):529–539. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 43.Maezawa Y, Onay T, Scott RP, Keir LS, Dimke H, Li C, et al. The bHLH transcription factor Tcf21 is required for differentiation and maintenance of podocytes in development, health and disease. J Am Soc Nephrol. 2014;24(184A) In Press. [Google Scholar]
- 44.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, et al. Genes that confer the identity of the renin cell. J Am Soc Nephrol. 2011;22(12):2213–2225. doi: 10.1681/ASN.2011040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol. 2013;9(3):137–146. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- 46.Bariety J, Mandet C, Hill GS, Bruneval P. Parietal podocytes in normal human glomeruli. J Am Soc Nephrol. 2006;17(10):2770–2780. doi: 10.1681/ASN.2006040325. [DOI] [PubMed] [Google Scholar]
- 47.Shankland SJ, Smeets B, Pippin JW, Moeller MJ. The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol. 2014 doi: 10.1038/nrneph.2014.1. [DOI] [PubMed] [Google Scholar]
- 48.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20(2):322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, et al. Recruitment of podocytes from glomerular parietal epithelial cells. Journal of the American Society of Nephrology : JASN. 2009;20(2):333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berger K, Schulte K, Boor P, Kuppe C, van Kuppevelt TH, Floege J, et al. The regenerative potential of parietal epithelial cells in adult mice. J Am Soc Nephrol. 2014;25(4):693–705. doi: 10.1681/ASN.2013050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, et al. Unraveling the Role of Podocyte Turnover in Glomerular Aging and Injury. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 2012;30(8):1714–1725. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 53.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, et al. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. Journal of the American Society of Nephrology : JASN. 2006;17(9):2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 54.Lasagni L, Ballerini L, Angelotti ML, Parente E, Sagrinati C, Mazzinghi B, et al. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells. 2010;28(9):1674–1685. doi: 10.1002/stem.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darisipudi MN, Kulkarni OP, Sayyed SG, Ryu M, Migliorini A, Sagrinati C, et al. Dual blockade of the homeostatic chemokine CXCL12 and the proinflammatory chemokine CCL2 has additive protective effects on diabetic kidney disease. Am J Pathol. 2011;179(1):116–124. doi: 10.1016/j.ajpath.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peired A, Angelotti ML, Ronconi E, la Marca G, Mazzinghi B, Sisti A, et al. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J Am Soc Nephrol. 2013;24(11):1756–1768. doi: 10.1681/ASN.2012090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizzo P, Perico N, Gagliardini E, Novelli R, Alison MR, Remuzzi G, et al. Nature and Mediators of Parietal Epithelial Cell Activation in Glomerulonephritides of Human and Rat. Am J Pathol. 2013;183(6):1769–1778. doi: 10.1016/j.ajpath.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Migliorini A, Angelotti ML, Mulay SR, Kulkarni OO, Demleitner J, Dietrich A, et al. The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. American Journal Of Pathology. 2013;183(2):431–440. doi: 10.1016/j.ajpath.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Hansen KM, Pippin JW, Chang AM, Taniguchi Y, Krofft RD, et al. De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol. 2012;302(5):F571–F580. doi: 10.1152/ajprenal.00516.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, et al. De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. American Journal of Physiology-Renal Physiology. 2010;298(3):F702–F711. doi: 10.1152/ajprenal.00428.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guhr SS, Sachs M, Wegner A, Becker JU, Meyer TN, Kietzmann L, et al. The expression of podocyte-specific proteins in parietal epithelial cells is regulated by protein degradation. Kidney Int. 2013;84(3):532–544. doi: 10.1038/ki.2013.115. [DOI] [PubMed] [Google Scholar]
- 62.Schulte K, Berger K, Boor P, Jirak P, Gelman IH, Arkill KP, et al. Origin of parietal podocytes in atubular glomeruli mapped by lineage tracing. J Am Soc Nephrol. 2014;25(1):129–141. doi: 10.1681/ASN.2013040376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hackl MJ, Burford JL, Villanueva K, Lam L, Susztak K, Schermer B, et al. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med. 2013;19(12):1661–1666. doi: 10.1038/nm.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakamoto K, Ueno T, Kobayashi N, Hara S, Takashima Y, Pastan I, et al. The direction and role of phenotypic transition between podocytes and parietal epithelial cells in focal segmental glomerulosclerosis. Am J Physiol Renal Physiol. 2014;306(1):F98–F104. doi: 10.1152/ajprenal.00228.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyazaki Y, Shimizu A, Ichikawa I, Hosoya T, Pastan I, Matsusaka T. Mice are unable to endogenously regenerate podocytes during the repair of immunotoxin-induced glomerular injury. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft413. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22(12):2156–2165. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6(5):719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 68.Gomez RA, Belyea B, Medrano S, Pentz ES, Sequeira-Lopez ML. Fate and plasticity of renin precursors in development and disease. Pediatr Nephrol. 2013 doi: 10.1007/s00467-013-2688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurt B, Paliege A, Willam C, Schwarzensteiner I, Schucht K, Neymeyer H, et al. Deletion of von Hippel-Lindau protein converts renin-producing cells into erythropoietin-producing cells. J Am Soc Nephrol. 2013;24(3):433–444. doi: 10.1681/ASN.2012080791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Gomez JA, Klein S, Zhang Z, Seidler B, Yang Y, et al. Adult renal mesenchymal stem cell-like cells contribute to juxtaglomerular cell recruitment. J Am Soc Nephrol. 2013;24(8):1263–1273. doi: 10.1681/ASN.2012060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, et al. Cells of Renin Lineage Are Progenitors of Podocytes and Parietal Epithelial Cells in Experimental Glomerular Disease. Am J Pathol. 2013;183(2):542–557. doi: 10.1016/j.ajpath.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pippin JW, Glenn ST, Krofft RD, Rusiniak ME, Alpers CE, Hudkins KL, et al. Cells of renin lineage take on a podocyte phenotype in aging nephropathy. Am J Physiol Renal Physiol. 2014 doi: 10.1152/ajprenal.00699.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steege A, Fahling M, Paliege A, Bondke A, Kirschner KM, Martinka P, et al. Wilms' tumor protein (-KTS) modulates renin gene transcription. Kidney Int. 2008;74(4):458–466. doi: 10.1038/ki.2008.194. [DOI] [PubMed] [Google Scholar]