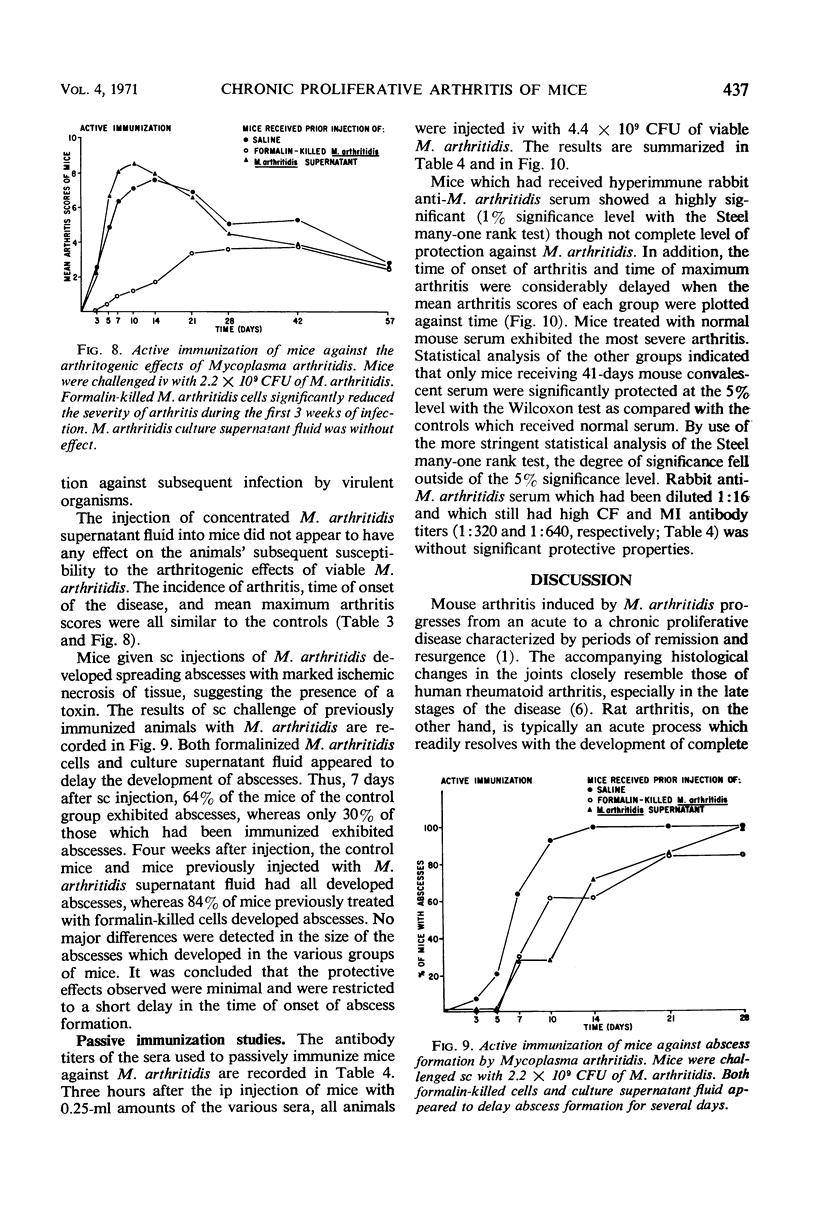
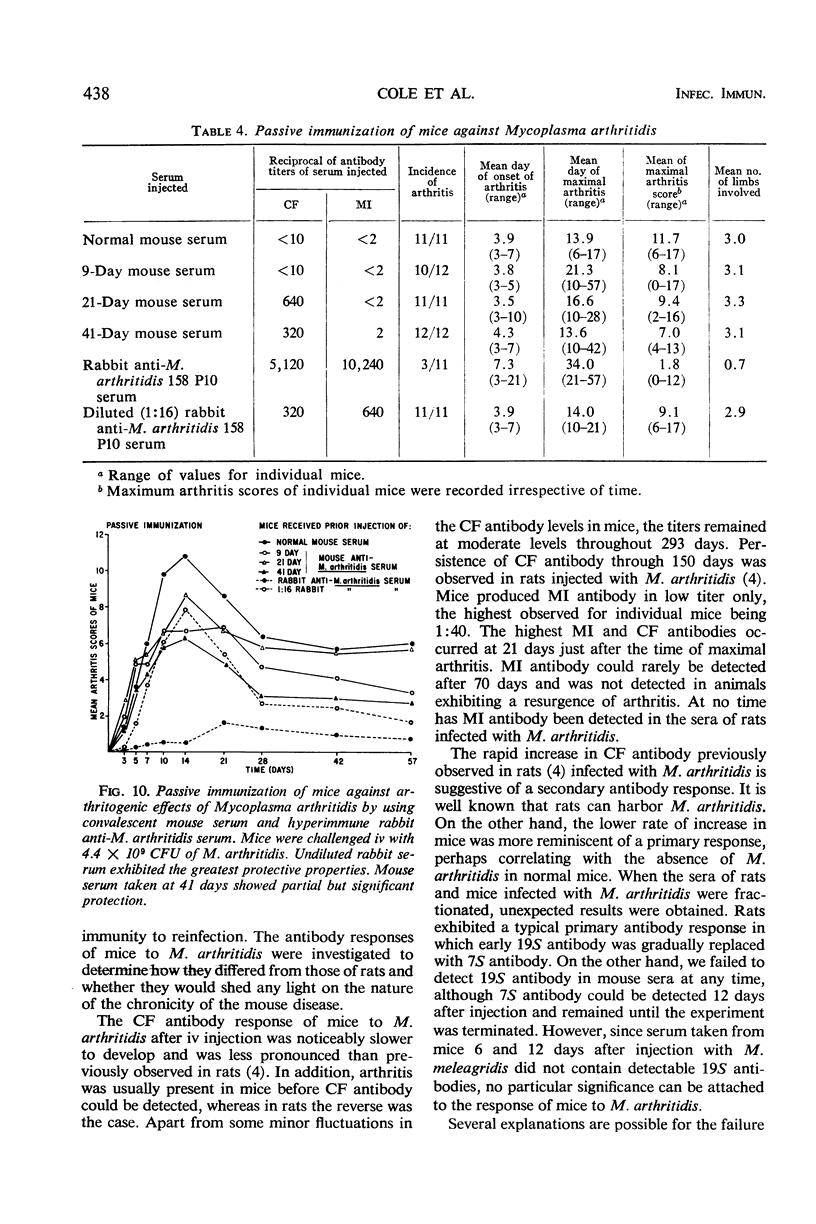
Abstract
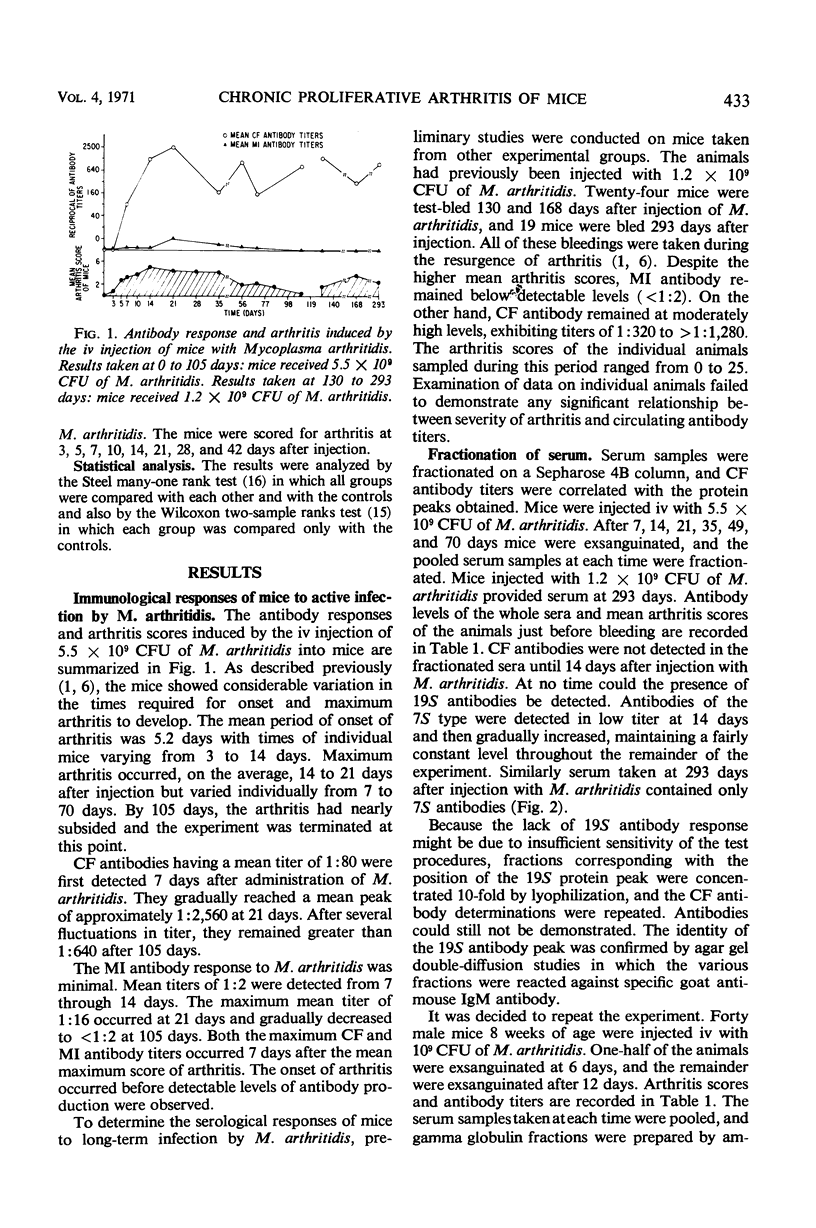
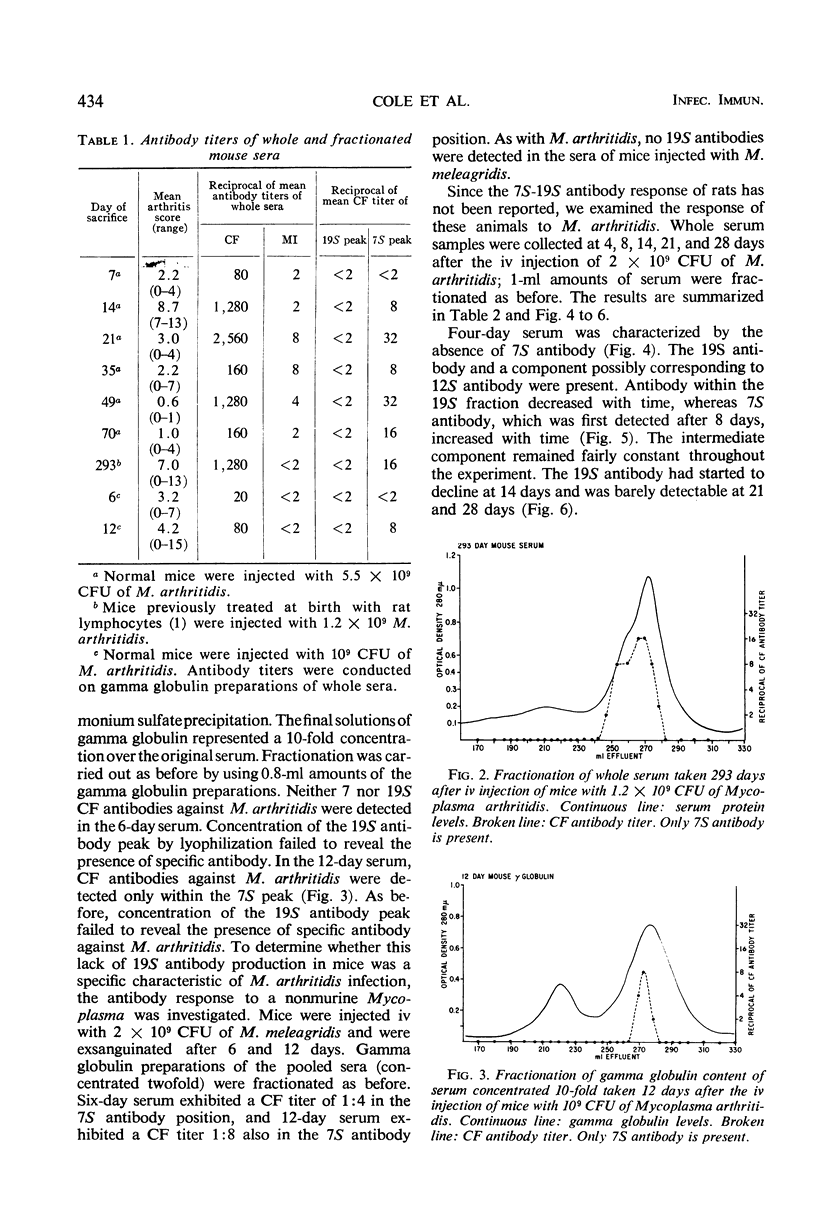
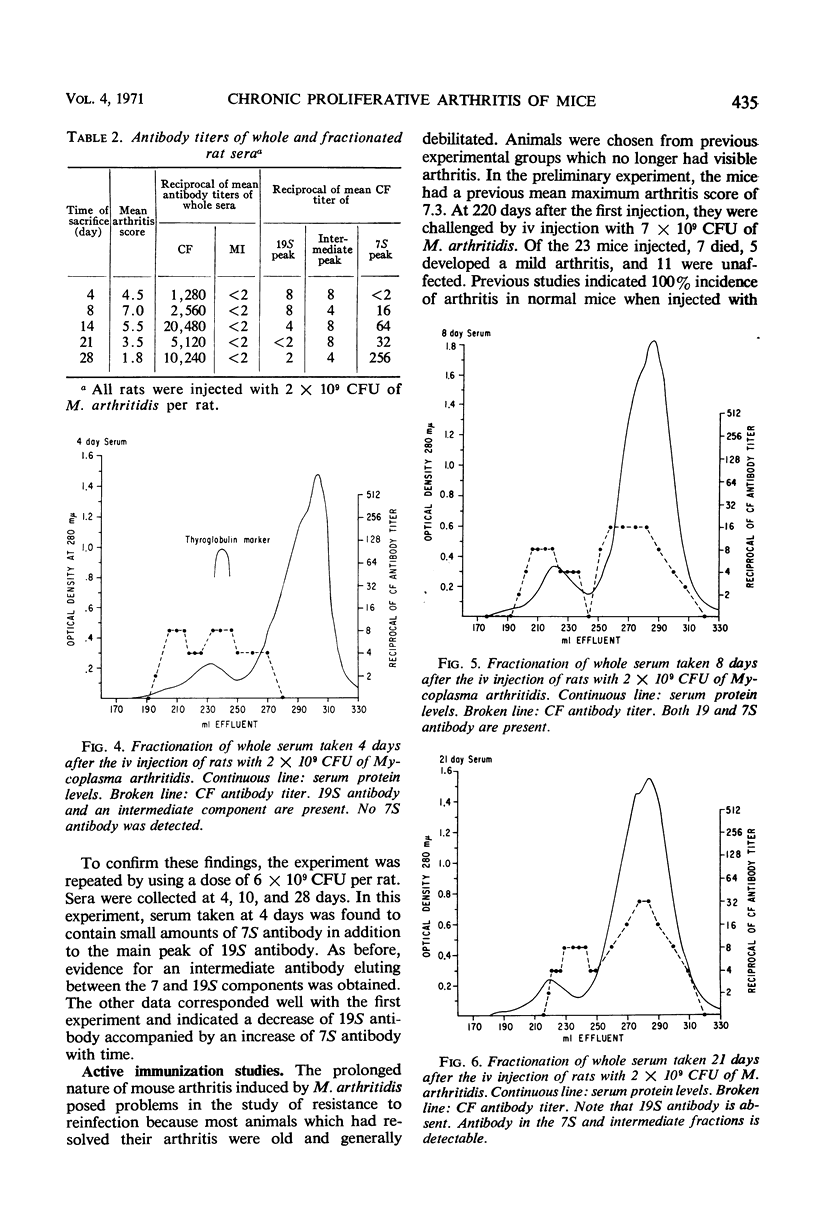
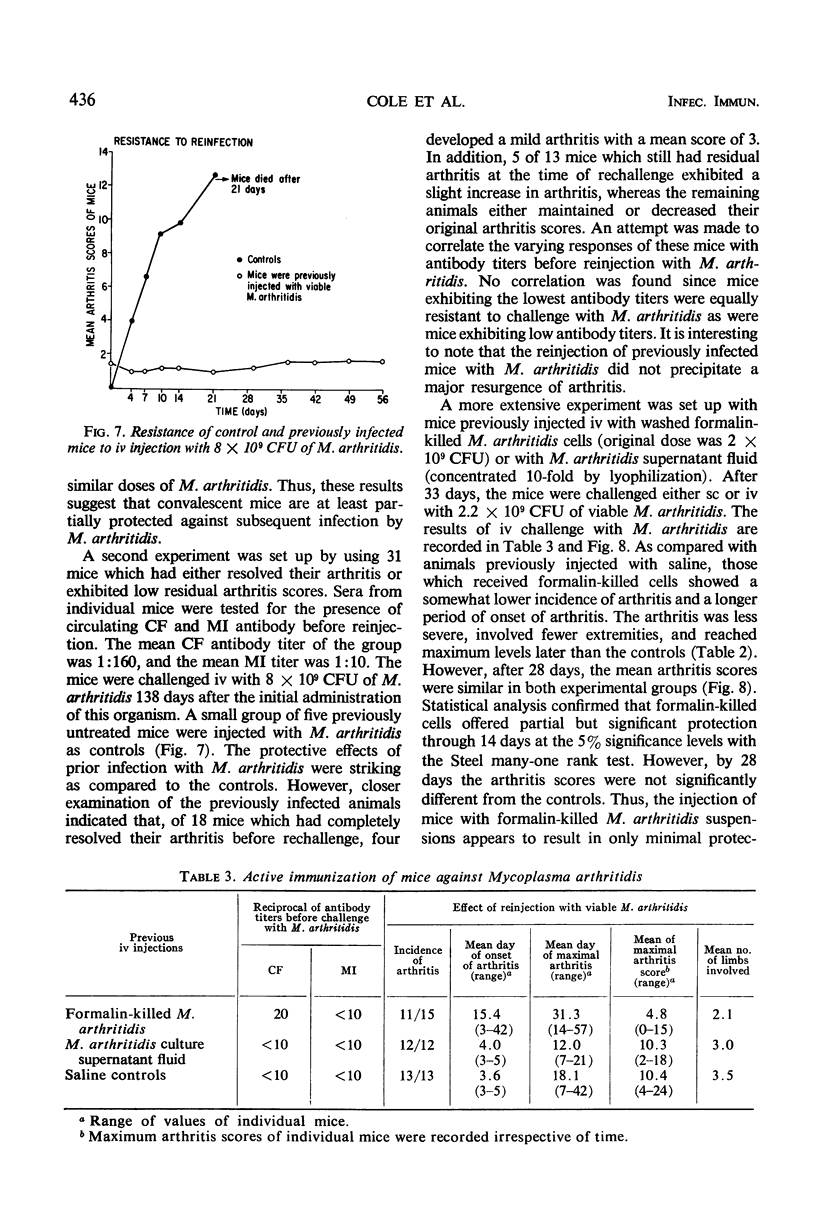
Complement-fixing antibodies were first detected in mice 7 days after intravenous injection with Mycoplasma arthritidis. Peak titers were observed at 21 days, and high levels of antibody persisted through 293 days. The metabolism-inhibiting antibody response was minimal. On fractionation of mouse sera, only 7S antibody was detected which first appeared at 12 days after injection and persisted throughout the experiment. In contrast, serum taken from rats injected with M. arthritidis contained predominantly 19S antibodies in the early stages of the disease which were gradually replaced with 7S antibodies. The intravenous injection of mice with M. arthritidis culture supernatant fluid had no effect upon their subsequent susceptibility to the arthritogenic effects of M. arthritidis, but this procedure appeared to delay the onset of abscess formation after the subcutaneous injection of M. arthritidis. Formalin-killed cells of M. arthritidis partially protected mice against the arthritis induced by M. arthritidis. Previous infections with M. arthritidis conferred partial immunity against the arthritogenic effects of the organism. Serum taken from convalescent mice at 41 days had a partial protective effect when used to immunize passively normal mice against M. arthritidis. However, rabbit anti-M. arthritidis serum which possessed higher complement-fixing and metabolism-inhibiting antibodies was without significant protective properties.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill J. F., Cole B. C., Wiley B. B., Ward J. R. Role of Biological Mimicry in the Pathogenesis of Rat Arthritis Induced by Mycoplasma arthritidis. Infect Immun. 1971 Jan;3(1):24–35. doi: 10.1128/iai.3.1.24-35.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Golightly-Rowland L., Ward J. R., Wiley B. B. Immunological response of rodents to murine mycoplasmas. Infect Immun. 1970 Oct;2(4):419–425. doi: 10.1128/iai.2.4.419-425.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copperman R., Morton H. E. Reversible inhibition of mitosis in lymphocyte cultures by non-viable Mycoplasma. Proc Soc Exp Biol Med. 1966 Dec;123(3):790–795. doi: 10.3181/00379727-123-31605. [DOI] [PubMed] [Google Scholar]
- FREUNDT E. A. Arthritis caused by Streptobacillus moniliformis and pleuropneumonialike organisms in small rodents. Lab Invest. 1959 Nov-Dec;8:1358–1375. [PubMed] [Google Scholar]
- Golightly-Rowland L., Cole B. C., Ward J. R., Wiley B. B. Effect of Animal Passage on Arthritogenic and Biological Properties of Mycoplasma arthritidis. Infect Immun. 1970 Jun;1(6):538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- KAHAN A., AMOR B., DELBARRE F. POLYARTHRITE EXP'ERIMENTALE DU RAT 'A MYCOPLASMA ARTHRITIDIS. IV. PH'ENOM'ENES IMMUNOLOGIQUES (ANIMAUX SOUMIS 'A L'IMMUNISATION ACTIVE) C R Seances Soc Biol Fil. 1964;158:1320–1322. [PubMed] [Google Scholar]
- KILLANDER J., BENGTSSON S., PHILIPSON L. FRACTIONATION OF HUMAN PLASMA MACROGLOBULINS BY GEL FILTRATION ON PEARL-CONDENSED AGAR. Proc Soc Exp Biol Med. 1964 Apr;115:861–865. doi: 10.3181/00379727-115-29058. [DOI] [PubMed] [Google Scholar]
- Purcell R. H., Taylor-Robinson D., Wong D. C., Chanock R. M. A color test for the measurement of antibody to the non-acid-forming human Mycoplasma species. Am J Epidemiol. 1966 Jul;84(1):51–66. doi: 10.1093/oxfordjournals.aje.a120627. [DOI] [PubMed] [Google Scholar]
- Thomas L. Mycoplasmas as pathogens. Yale J Biol Med. 1968 Apr-Jun;40(5-6):444–448. [PMC free article] [PubMed] [Google Scholar]


