Abstract
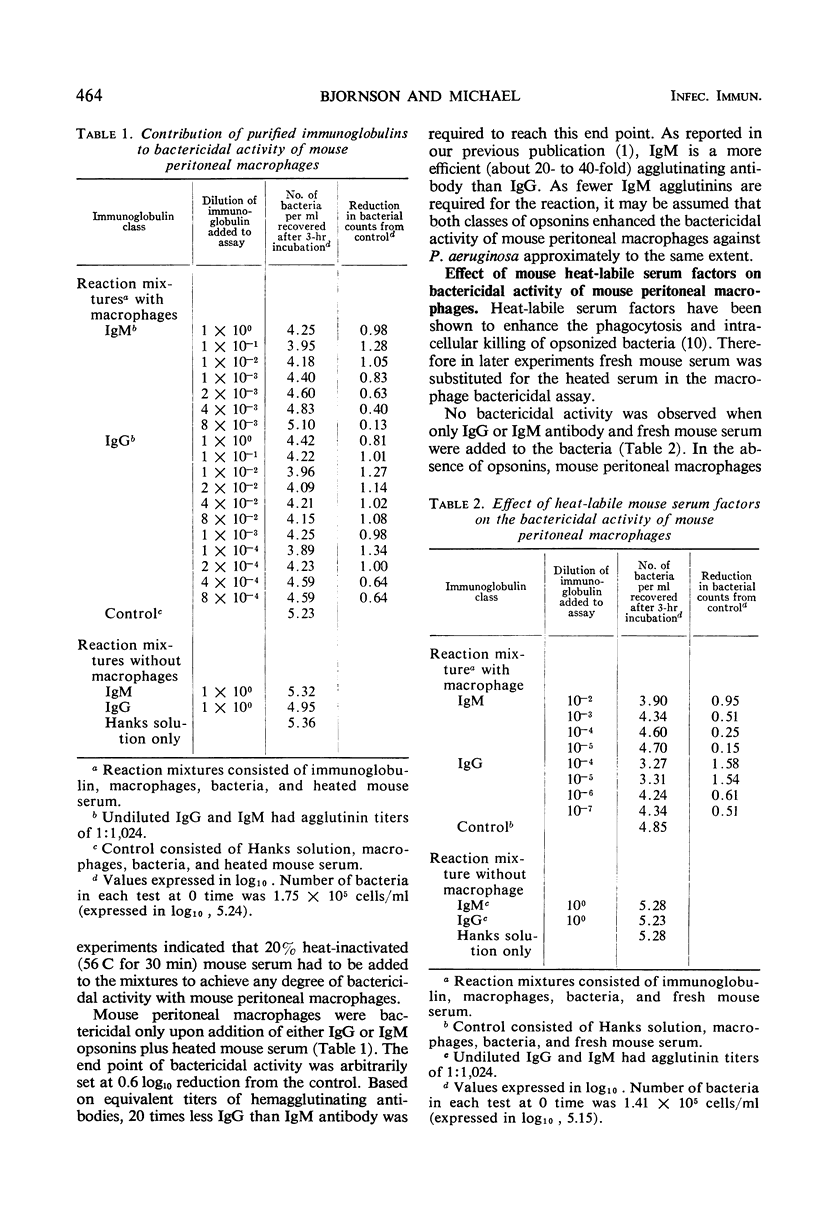
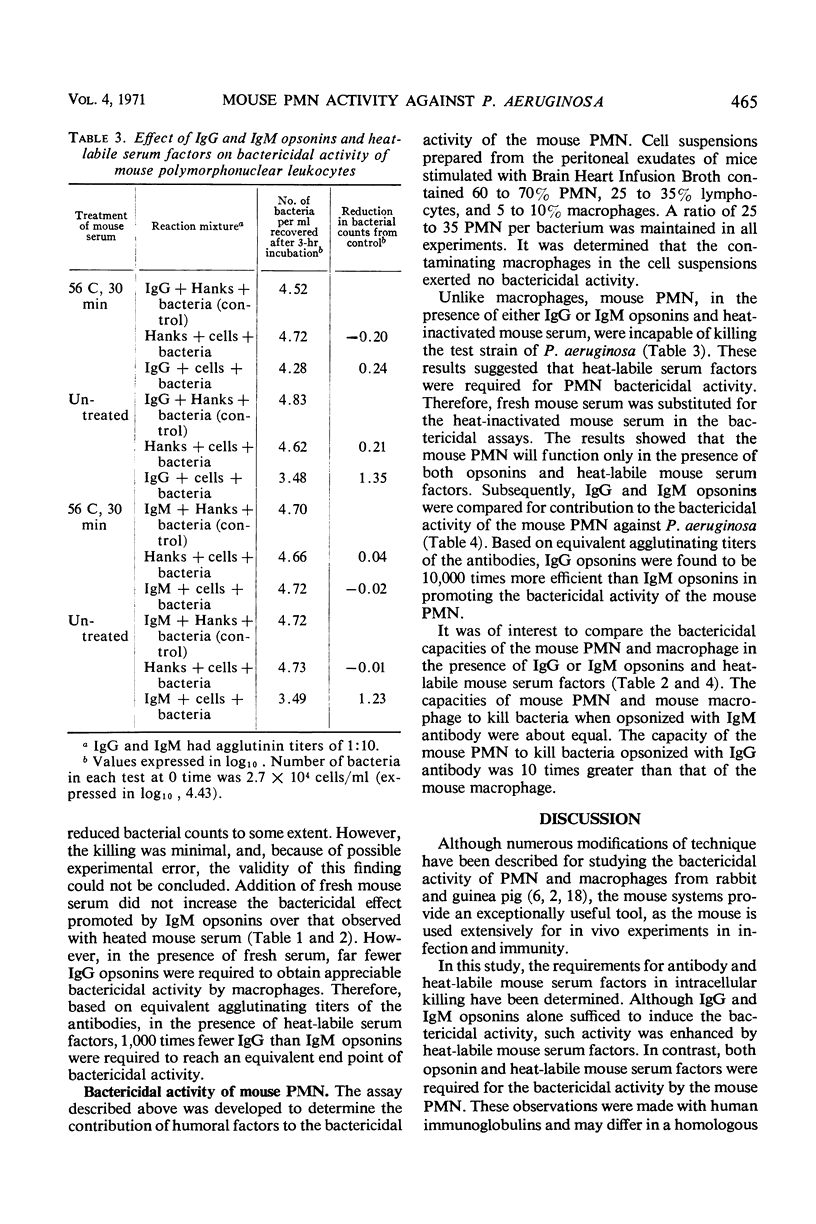
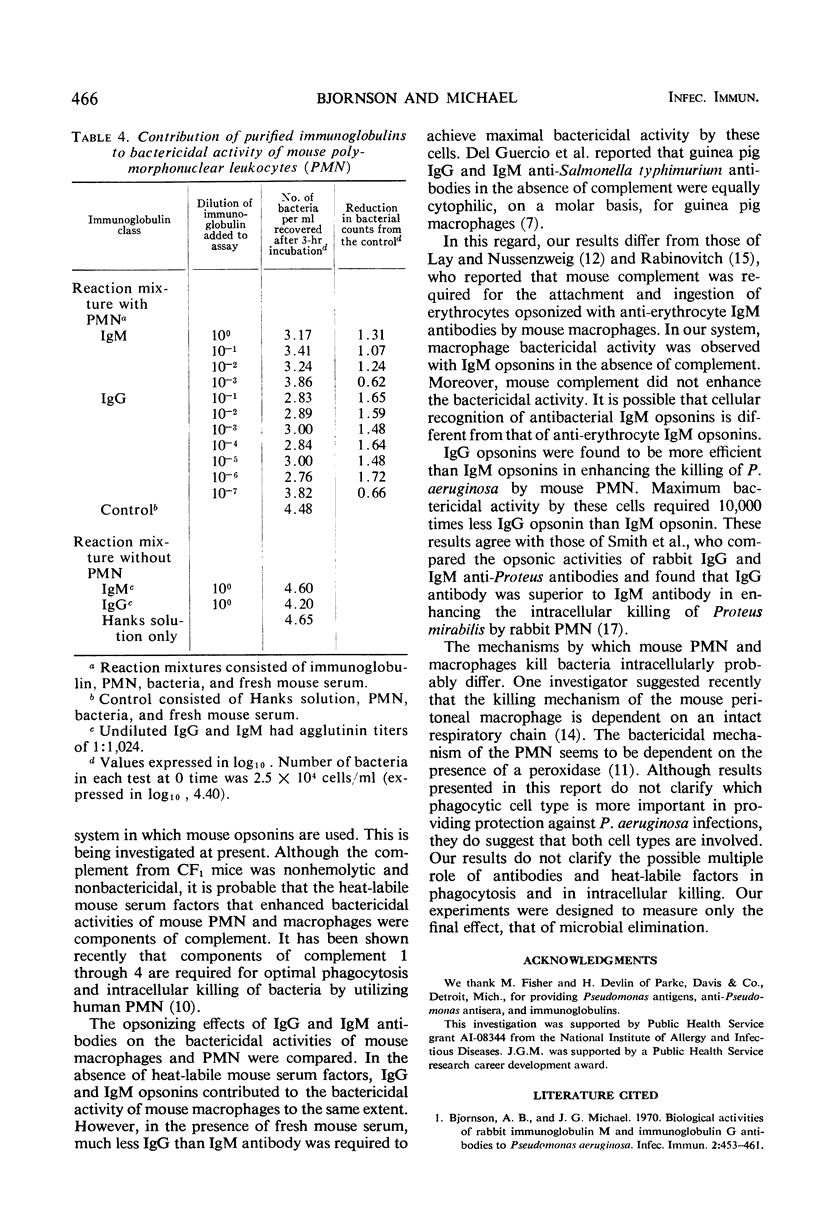
Techniques have been described for the purification of mouse polymorphonuclear leukocytes (PMN) and macrophages and for determining their bactericidal activity against Pseudomonas aeruginosa in a rotating suspension. Requirements for human anti-Pseudomonas opsonins and heat-labile mouse serum factors have been determined. In the macrophage system, although heat-labile mouse serum factors had enhancing properties, both immunoglobulin G (IgG) and immunoglobulin M (IgM) opsonins alone sufficed to induce bactericidal activity. In contrast, both opsonins and heat-labile mouse serum factors were required for bactericidal activity by the mouse PMN. In the absence of heat-labile mouse serum factors, IgG and IgM opsonins contributed to the bactericidal activity of mouse macrophages to the same extent. However, in the presence of these factors, much less IgG than IgM antibody was required to achieve maximum bactericidal activity by these cells. Similarly, IgG opsonins were found to be more efficient than IgM opsonins in enhancing the killing of P. aeruginosa by mouse PMN.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjornson A. B., Michael J. G. Biological Activities of Rabbit Immunoglobulin M and Immunoglobulin G Antibodies to Pseudomonas aeruginosa. Infect Immun. 1970 Oct;2(4):453–461. doi: 10.1128/iai.2.4.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE IN VITRO DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. I. THE INFLUENCE OF INHIBITORS AND THE RESULTS OF AUTORADIOGRAPHY. J Exp Med. 1965 Feb 1;121:279–288. doi: 10.1084/jem.121.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE IN VITRO DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. II. THE INFLUENCE OF SERUM ON GRANULE FORMATION, HYDROLASE PRODUCTION, AND PINOCYTOSIS. J Exp Med. 1965 May 1;121:835–848. doi: 10.1084/jem.121.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Benson B. The in vitro differentiation of mononuclear phagocytes. 3. The reversibility of granule and hydrolytic enzyme formation and the turnover of granule constituents. J Exp Med. 1965 Sep 1;122(3):455–466. doi: 10.1084/jem.122.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Guercio P., Tolone G., Andrade F. B., Biozzi G., Binaghi R. A. Opsonic, cytophilic and agglutinating activity of guinea-pig gamma-2 and gamma-M anti-Salmonella antibodies. Immunology. 1969 Mar;16(3):361–371. [PMC free article] [PubMed] [Google Scholar]
- FLODIN P., KILLANDER J. Fractionation of human-serum proteins by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:402–410. doi: 10.1016/0006-3002(62)90104-x. [DOI] [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Klemperer M. R., Alper C. A., Rosen F. S. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969 Jun 1;129(6):1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. E. Metabolic event involved in the bactericidal activity of normal mouse macrophages. Infect Immun. 1971 Mar;3(3):390–397. doi: 10.1128/iai.3.3.390-397.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M. Studies on the immunoglobulins which stimulate the ingestion of glutaraldehyde-treated red cells attached to macrophages. J Immunol. 1967 Dec;99(6):1115–1120. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SUTER E. Multiplication of tubercle bacilli within mononuclear phagocytes in tissue cultures derived from normal animals and animals vaccinated with BCG. J Exp Med. 1953 Feb 1;97(2):235–245. doi: 10.1084/jem.97.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Barnett J. A., May F. P., Sanford J. P. Comparison of the opsonic activity of gamma-G- and gamma-M-anti-Proteus globulins. J Immunol. 1967 Feb;98(2):336–343. [PubMed] [Google Scholar]



