Abstract
Quality of cardiopulmonary resuscitation (CPR) improves through the use of CPR feedback devices. Most feedback devices integrate the acceleration twice to estimate compression depth. However, they use additional sensors or processing techniques to compensate for large displacement drifts caused by integration. This study introduces an accelerometer-based method that avoids integration by using spectral techniques on short duration acceleration intervals. We used a manikin placed on a hard surface, a sternal triaxial accelerometer, and a photoelectric distance sensor (gold standard). Twenty volunteers provided 60 s of continuous compressions to test various rates (80–140 min−1), depths (3–5 cm), and accelerometer misalignment conditions. A total of 320 records with 35312 compressions were analysed. The global root-mean-square errors in rate and depth were below 1.5 min−1 and 2 mm for analysis intervals between 2 and 5 s. For 3 s analysis intervals the 95% levels of agreement between the method and the gold standard were within −1.64–1.67 min−1 and −1.69–1.72 mm, respectively. Accurate feedback on chest compression rate and depth is feasible applying spectral techniques to the acceleration. The method avoids additional techniques to compensate for the integration displacement drift, improving accuracy, and simplifying current accelerometer-based devices.
1. Introduction
Chest compressions delivered at an adequate depth and rate, allowing full chest recoil, and with minimal interruptions are key to improve survival from cardiac arrest [1–3]. Current cardiopulmonary resuscitation (CPR) guidelines [4, 5] recommend chest compression depths and rates of at least 5 cm and 100 min−1, respectively. However, out-of-hospital and in-hospital studies on CPR quality show that delivering chest compressions with adequate rate and depth is difficult, even among well-trained responders [6, 7]. The use of real-time CPR feedback devices has contributed to improve the quality of CPR provided by lay people and trained rescuers in both simulated and real life scenarios [8, 9].
The first CPR feedback devices used force/pressure sensors on the assumption of a linear relation between compression force and depth [10–12]. However, the chest has a nonlinear variable stiffness within the compression cycle which varies among individuals [13–16], a fact that has been confirmed on cardiac arrest data with simultaneous force and depth recordings [17]. Consequently, most current CPR feedback devices are based on accelerometers. These devices calculate the instantaneous displacement of the chest, that is, the compression depth (CD) signal, by integrating the acceleration twice [9]. However, noise in the acceleration signal compromises the accuracy of methods based on the double integration. Even a small offset in the acceleration signal produces integration errors that rapidly accumulate, making feedback impossible unless the resulting displacement drift is compensated for every compression [18]. Over the last decade several drift compensation mechanisms have been conceived, giving rise to complex and sometimes bulky devices that incorporate additional sensors [19, 20] and/or use elaborate signal processing techniques [1, 21–23].
Accelerometer-based devices calculate rate and depth values for feedback for each compression [24–26]. Audiovisual feedback to the rescuer is then given at every compression or by averaging these values over the last 3–5 compressions [24, 25]. Feedback on rate and depth at every compression seems excessive and may be ignored by the rescuer [9, 27]. A more sensible approach to feedback would be to average rate and depth over the last compressions, resulting in feedback times somewhere in the 2–5 s range.
This study introduces a new paradigm on accelerometer-based devices. Instead of calculating the CD signal, feedback on the average rate and depth during a short analysis interval is directly computed from the acceleration by means of spectral techniques. Drift compensation or additional sensors would no longer be needed, giving rise to simpler, smaller, and more user-friendly feedback devices.
2. Materials and Methods
2.1. Equipment and Data Collection
A Resusci Anne manikin (Laerdal Medical, Norway) was equipped with a photoelectric sensor (BOD 6K-RA01-C-02, Balluff, USA) to register the actual CD signal, which was used as gold standard. The accelerometer (ADXL330, Analog Devices, USA) was placed in an enclosure which was fixed to the manikin's sternum, and the manikin was placed on the floor, as shown in Figure 1. The three acceleration axes and the CD signal were digitized using an NI-USB6211 (National Instruments) data acquisition card with a sampling rate of 500 Hz and 16-bit resolution.
Figure 1.
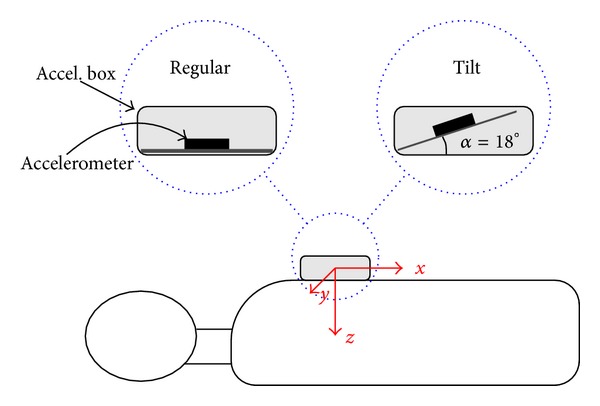
Positioning of the accelerometer within the enclosure for the regular and tilt sessions. During each recording the enclosure was kept fixed to the manikin's chest.
Twenty volunteers received basic compression-only CPR training before participating in two recording sessions: a regular session, in which the vertical axis of the accelerometer was perpendicular to the manikin's chest, and a tilt session, with an 18° misalignment (see Figure 1). These sessions were defined to study situations in which the accelerometer may not be in a fixed position relative to the patient's chest. In each session the volunteers delivered 60 s of uninterrupted compressions eight times, combining different target rates (80, 100, 120, and 140 min−1) and depths (30 mm and 50 mm). A metronome was used to guide compression rate, and a custom-made computer program displayed the CD signal in real-time to guide compression depth.
The recorded signals were preprocessed with a third-order Butterworth low-pass filter (cut-off frequency 15 Hz) to suppress high-frequency noise and resampled to 100 Hz. Compressions were automatically identified in the CD signal using a peak detector with a fixed 15 mm threshold, and the annotations were then manually reviewed.
2.2. Feedback on Rate and Depth
2.2.1. Mathematical Model
Feedback was calculated for short analysis intervals during continuous chest compressions. If the intervals are short, then it is possible to assume that all chest compressions within the analysis interval are very similar. Mathematically this means that acceleration and CD are almost periodic signals, whose fundamental frequency is the mean frequency of the compressions, f cc (Hz). For each analysis interval, their periodic representation, denoted by a(t) for the acceleration and s(t) for the CD signal, is then a good approximation of the real signals. These periodic representations can be modelled using the first N harmonics of their Fourier series decomposition (without DC component):
| (1) |
| (2) |
Since the feedback device records the acceleration the problem is then to obtain s(t) from a(t) knowing that the acceleration and the displacement are related by
| (3) |
which, in the general case, involves a double integration of the acceleration signal. However, for the quasiperiodic approximation, using the Fourier series representation of a(t) and s(t) in (3) yields the following relations between the amplitudes and phases of their harmonics:
| (4) |
These equations can be used to reconstruct s(t) once f cc, A k, and θ k are obtained from the acceleration signal.
2.2.2. Spectral Method for Feedback on Rate and Depth
Spectral analysis was used to estimate the harmonics of a(t) needed to reconstruct s(t). In summary, feedback on the mean rate and depth for each analysis interval were obtained following the steps described in Figure 2. In Step 1, a Hamming window was applied to the acceleration signal to select the analysis interval. Its 2048-point fast Fourier transform (FFT) with zero padding was computed in Step 2. Then, the first three harmonics and their fundamental frequency were estimated (Step 3). Equation (4) was used to compute S k and ϕ k, which were used to reconstruct s(t) from (2) (Step 4). Finally, in Step 5, feedback on rate and depth were obtained using the reconstructed cycle of s(t) as
| (5) |
Figure 2.
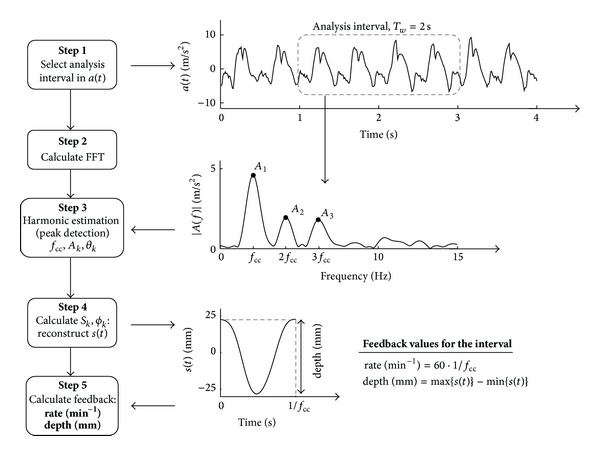
Block diagram of the spectral method for feedback on rate and depth.
Several characteristics of the method such as the Hamming window, the number of harmonics, and the number of points to compute the FFT were selected using signal processing criteria to guarantee a high accuracy.
2.3. Performance Evaluation
To evaluate the accuracy of the method we assumed feedback would be given at the end of each analysis interval; consequently records were divided into nonoverlapping consecutive analysis intervals of duration T w. For each analysis interval, feedback for rate and depth obtained by the method was compared to that obtained from the distance sensor placed inside the manikin.
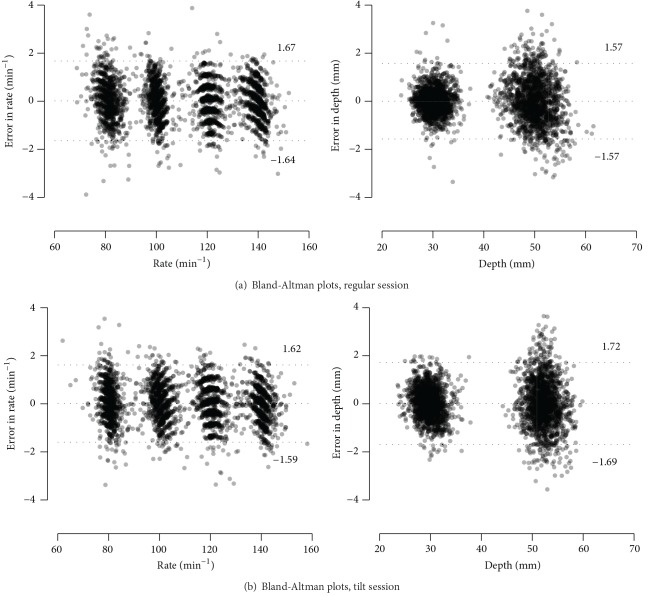
First, the mean rate and depth per record were analysed for the different targeted CPR test conditions. The distributions of the mean rate and depth did not pass the Kolmogorov-Smirnov normality test and are presented as median (5–95 percentiles). The median values obtained from the gold standard and the method were compared using the Mann-Whitney U test, and differences were considered significant for P values under 0.05. Then, errors in rate/depth feedback were obtained for every analysis window. The root-mean-square error (RMSE) of all feedbacks in a session (regular/tilt) was used to measure the global accuracy of the method as a function of the duration of the analysis interval, T w. Finally, a Bland-Altman analysis [28, 29] was conducted for T w = 3 s to assess the agreement on feedback between the gold standard and the method, and the 95% limits of agreement (LOA) were obtained.
3. Results
The dataset comprised 320 60 s records with a total of 35 312 compressions. Table 1 compares the mean rate and depth per episode obtained from the gold standard and the method when T w = 3 s. There was no significant difference between the method and the gold standard for any of the CPR target conditions. Figure 3 shows the RMSE as a function of T w for the tilt and regular sessions. For T w between 2 and 5 s the RMSE for rate and depth were below 1.5 min−1 and 2 mm. Finally, Figure 4 shows the Bland-Altman plots of the difference between the method and the gold standard for T w = 3 s. For the regular session, the differences in feedback for rate and depth showed a 95% LOA of −1.64–1.67 min−1 and −1.57–1.57 mm, respectively. For the tilt session, the differences in feedback for rate and depth showed a 95% LOA of −1.59–1.61 min−1 and −1.69–1.72 mm, respectively.
Table 1.
Median values (5/95 percentile in parenthesis) of the mean rate and depth per record for the regular and tilt sessions with T w = 3 s. The Mann-Whitney U test was used to compare the values obtained from the gold standard (CD signal) and the acceleration (accel. signal). No significant difference was observed for any of the CPR target conditions.
| Target | Regular session | Tilt session | ||
|---|---|---|---|---|
| CD signal | Accel. signal | CD signal | Accel. signal | |
| Ratea | ||||
| 80 min−1 | 80.8 (77.4–84.8) | 80.9 (77.5–84.9) | 80.2 (77.5–83.6) | 80.3 (77.7–83.6) |
| 100 min−1 | 100.1 (97.5–102.2) | 100.0 (97.4–102.3) | 100.3 (96.5–105.1) | 100.1 (96.2–105.3) |
| 120 min−1 | 120.5 (117.4–123.8) | 120.4 (117.6–123.9) | 120.2 (116.9–123.7) | 120.3 (116.9–124.0) |
| 140 min−1 | 140.1 (134.4–142.0) | 140.2 (134.8–142.1) | 140.2 (135.1–143.4) | 140.1 (135.3–143.2) |
| Depthb | ||||
| 30 mm | 30.4 (27.7–33.3) | 30.3 (27.0–33.5) | 29.7 (27.5–32.6) | 29.7 (27.0–33.2) |
| 50 mm | 50.1 (45.4–54.1) | 50.1 (45.6–54.9) | 52.3 (49.1–55.1) | 52.5 (49.1–57.0) |
a40 records per session, b80 records per session.
Figure 3.
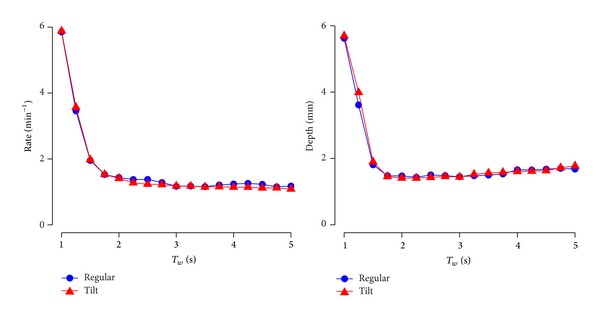
Root-mean-square error (RMSE) in mean rate and depth as a function of the duration of the analysis interval for the regular session and the tilt session.
Figure 4.
Bland-Altman plots of the errors plotted against the gold standard (from the photoelectric sensor), for the regular (a) and tilt (b) sessions. The 95% levels of agreement are indicated in text and by horizontal lines.
4. Discussion
CPR feedback on chest compression rate and depth improves the quality of CPR both during training [25, 30] and in the field [27, 31, 32]. Currently, most real-time devices for CPR feedback are based on the double integration of the acceleration which inevitably requires adding drift compensation techniques [18, 33] that result in bulky devices and/or occasional inaccurate depth feedback [19, 34]. This study presents, to the best of our knowledge, the first accelerometer-based method for feedback on rate and depth of chest compressions that avoids the drift problem. The method is based on simple and optimised spectral techniques making it computationally very efficient. These considerations would utterly simplify current accelerometer-based devices.
The accuracy of the method was tested in a manikin platform. This allowed the recording of the actual instantaneous chest compression depth for use as gold standard but also the testing the algorithm for a wide range of controlled conditions: different rescuers, target depths and rates, and the influence of the relative position of the device and the chest (regular versus tilt). Misalignment between the device and the chest was tested for two reasons. First, although the device is usually in contact with the patient's chest, the sternum may not be completely horizontal due to anatomical considerations, even when the patient is in supine position. Second, other suitable positions of the device could be envisioned, such as on top of the hand or fixed to the wrist. In those situations tilt may vary during chest compressions. In either case, there were no significant differences in rate and depth feedback between the gold standard and the method. Moreover, for all the tested conditions the RMSE for rate and depth were below 1.5 min−1 and 2 mm, respectively, which guarantees a very accurate feedback for analysis intervals in the 2–5 s range. Furthermore, the Bland-Altman analysis revealed that all individual feedbacks were very accurate and that the method is reliable because it did not present outliers.
The method presented in this study directly estimates the mean rate and depth for feedback without the need to obtain the instantaneous CD signal. This avoids the need to integrate the acceleration signal twice. Double integration introduces a large displacement drift [18], which has to be compensated. Over the years several techniques have been developed to correct the displacement drift. Some solutions correct the drift for each compression cycle. This involves the detection of the start of each compression using either additional force sensors [18, 20] or a combined analysis of the CD and the ECG signals [21]. Others compensate the drift adaptively using filters based on additional reference signals such as force, blood pressure, ECG, or thoracic impedance [19]. However, incorporating additional sensors makes the feedback device more complex, and recording the ECG bounds the feedback device to the defibrillator. Alternatively, solutions based exclusively on signal processing techniques have also been developed to minimize or cancel the drift [1, 22, 35]. However, these techniques may introduce errors in depth as large as 6 mm for 95% of the cases [24]. The spectral technique introduced in this paper is more robust to acceleration noise because it only estimates three harmonic components of the acceleration for an accurate feedback.
Improvement of CPR quality relies on two key factors: real-time monitoring of CPR parameters and debriefing [36–38]. Real-time feedback in short time intervals is demonstrated in this study. Debriefing could easily be implemented simply by storing the rate and depth feedback values for each interval. These values could then be used to obtain postresuscitation scorecard with global measures of CPR quality and graphs of the time evolution of rate and depth [39].
The method shares two common limitations of all accelerometer-based devices. First, accurate depth feedback is compromised if there is incomplete chest recoil, that is, rescuer leaning [38]. The actual depth of a compression is the displacement of the patient's sternum from its resting position towards the spine. Accelerometer-based devices are accurate only if the sternum returns to its resting position on every compression [23]. Otherwise, the only solution is to detect incomplete chest recoil and then launch an alarm to correct excessive leaning [38]. Second, the study was conducted for the manikin resting on a hard incompressible surface. On softer surfaces depth is overestimated as the sum of the sternum-spine displacement and mattress compression [40]. This drawback can be corrected by using two aligned accelerometers (chest and back) and processing the difference of the recorded accelerations [26, 35]. Our method can be directly adapted to use the difference of the two accelerometers.
We demonstrated the accuracy of the method during continuous chest compressions. However, a full evaluation of the method using retrospective out-of-hospital cardiac arrest records in which acceleration signal is available is still needed. Such study would serve to evaluate the feasibility and reliability of the method in real resuscitation scenarios, where pauses in chest compressions are frequent and the acceleration patterns may differ from those generated in manikins.
5. Conclusion
This study introduces a new paradigm in accelerometer-based CPR feedback devices because it allows calculating rate and depth values for feedback without reconstructing the instantaneous CD signal. It avoids additional techniques to compensate the drift caused by accelerometer noise and double integration, thus simplifying feedback devices. Feedback is accurate for analysis intervals of a few seconds during continuous chest compressions. Further studies with retrospective episodes would serve to evaluate the feasibility and reliability of the method in a real resuscitation scenario.
Acknowledgments
This work received financial support from the Spanish Government (TEC2012-31144, TEC2012-31928), the Basque Government (Grants BFI-2010-174, BFI-2010-235, and BFI-2011-166), and the University of the Basque Country (unit UFI11/16). The authors would like to thank Dr. Jo Kramer-Johansen (Institute for Experimental Medical Research, Oslo, Norway) for his valuable suggestions on the paper.
Conflict of Interests
The authors Jesus Ruiz, Sofía Ruiz de Gauna, and Unai Irusta have received research support from Bexen Cardio (Ermua, Spain) for studies in resuscitation.
References
- 1.Babbs CF, Kemeny AE, Quan W, Freeman G. A new paradigm for human resuscitation research using intelligent devices. Resuscitation. 2008;77(3):306–315. doi: 10.1016/j.resuscitation.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Vadeboncoeur T, Stolz U, Panchal A, et al. Chest compression depth and survival in out-of-hospital cardiac arrest. Resuscitation. 2014;85(2):182–188. doi: 10.1016/j.resuscitation.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Zhang L, Yang Z, et al Even four minutes of poor quality of cpr compromises outcome in a porcine model of prolonged cardiac arrest. 2013;2013:6 pages. doi: 10.1155/2013/171862.171862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koster RW, Baubin MA, Bossaert LL, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 2. Adult basic life support and use of automated external defibrillators. Resuscitation. 2010;81(10):1277–1292. doi: 10.1016/j.resuscitation.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg RA, Hemphill R, Abella BS, et al. Part 5: adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18) 3:S685–S705. doi: 10.1161/CIRCULATIONAHA.110.970939. [DOI] [PubMed] [Google Scholar]
- 6.Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. Journal of the American Medical Association. 2005;293(3):305–310. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 7.Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. The Journal of the American Medical Association. 2005;293(3):299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 8.Yeung J, Meeks R, Edelson D, Gao F, Soar J, Perkins GD. The use of CPR feedback/prompt devices during training and CPR performance: a systematic review. Resuscitation. 2009;80(7):743–751. doi: 10.1016/j.resuscitation.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Gruber J, Stumpf D, Zapletal B, Neuhold S, Fischer H. Real-time feedback systems in CPR. Trends in Anaesthesia and Critical Care. 2012;2(6):287–294. [Google Scholar]
- 10.Thomas SH, Stone CK, Austin PE, March JA, Brinkley S. Utilization of a pressure-sensing monitor to improve in-flight chest compressions. The American Journal of Emergency Medicine. 1995;13(2):155–157. doi: 10.1016/0735-6757(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 11.Elding C, Baskett P, Hughes A. The study of the effectiveness of chest compressions using the CPR-plus. Resuscitation. 1998;36(3):169–173. doi: 10.1016/s0300-9572(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 12.Boyle AJ, Wilson AM, Connelly K, McGuigan L, Wilson J, Whitbourn R. Improvement in timing and effectiveness of external cardiac compressions with a new non-invasive device: the CPR-Ezy. Resuscitation. 2002;54(1):63–67. doi: 10.1016/s0300-9572(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 13.Tsitlik JE, Weisfeldt ML, Chandra N, Effron MB, Halperin HR, Levin HR. Elastic properties of the human chest during cardiopulmonary resuscitation. Critical Care Medicine. 1983;11(9):685–692. doi: 10.1097/00003246-198309000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Gruben KG, Guerci AD, Halperin HR, Popel AS, Tsitlik JE. Sternal force-displacement relationship during cardiopulmonary resuscitation. Journal of Biomechanical Engineering. 1993;115(2):195–201. doi: 10.1115/1.2894121. [DOI] [PubMed] [Google Scholar]
- 15.Bankman IN, Gruben KG, Halperin HR, Popel AS, Guerci AD, Tsitlik JE. Identification of dynamic mechanical parameters of the human chest during manual cardiopulmonary resuscitation. IEEE Transactions on Biomedical Engineering. 1990;37(2):211–217. doi: 10.1109/10.46262. [DOI] [PubMed] [Google Scholar]
- 16.Nysæther JB, Dorph E, Rafoss I, Steen PA. Manikins with human-like chest properties: a new tool for chest compression research. IEEE Transactions on Biomedical Engineering. 2008;55(11):2643–2650. doi: 10.1109/TBME.2008.2001289. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson AE, Nysaether J, Kramer-Johansen J, Steen PA, Dorph E. Compression force-depth relationship during out-of-hospital cardiopulmonary resuscitation. Resuscitation. 2007;72(3):364–370. doi: 10.1016/j.resuscitation.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Aase SO, Myklebust H. Compression depth estimation for CPR quality assessment using DSP on accelerometer signals. IEEE Transactions on Biomedical Engineering. 2002;49(3):263–268. doi: 10.1109/10.983461. [DOI] [PubMed] [Google Scholar]
- 19.Nysaether J, Eilevstjonn J. Method for accurate determining of CPR chest compression depth in real time. EP Patent App. EP20,080,250,519. 2008
- 20.Myklebust H, Fossan H. System for measuring and using parameters during chest compression for cardio-pulmonary resuscitation or a simulation thereof. EP Patent 1,057,451, 2009.
- 21.Palazzolo JA, Berger RD, Halperin HR, et al. Method of determining depth of compressions during cardio-pulmonary resuscitation. US Patent 6,827,695, 2004.
- 22.Wu Y, Mugler DH. A robust DSP integrator for accelerometer signals. IEEE Transactions on Biomedical Engineering. 2004;51(2):385–389. doi: 10.1109/TBME.2003.820372. [DOI] [PubMed] [Google Scholar]
- 23.Gohier F, Dellimore KH, Scheffer C. Development of a real-time feedback algorithm for chest compression during CPR without assuming full chest decompression. Resuscitation. 2014;85(6):820–825. doi: 10.1016/j.resuscitation.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Malanga B, Geheb FJ. ZOLL Medical Corporation; 2007. Emerging technologies: a primer to real CPR help technology. [Google Scholar]
- 25.Skorning M, Beckers SK, Brokmann JC, et al. New visual feedback device improves performance of chest compressions by professionals in simulated cardiac arrest. Resuscitation. 2010;81(1):53–58. doi: 10.1016/j.resuscitation.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Oh J, Song Y, Kang B, et al. The use of dual accelerometers improves measurement of chest compression depth. Resuscitation. 2012;83(4):500–504. doi: 10.1016/j.resuscitation.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Abella BS, Edelson DP, Kim S, et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73(1):54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. The Statistician. 1983:307–317. [Google Scholar]
- 29.Krouwer JS. Why Bland-Altman plots should use X, not (Y + X)/2 when X is a reference method. Statistics in Medicine. 2008;27(5):778–780. doi: 10.1002/sim.3086. [DOI] [PubMed] [Google Scholar]
- 30.Perkins GD, Augré C, Rogers H, Allan M, Thickett DR. CPREzy: an evaluation during simulated cardiac arrest on a hospital bed. Resuscitation. 2005;64(1):103–108. doi: 10.1016/j.resuscitation.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Kramer-Johansen J, Myklebust H, Wik L, et al. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: a prospective interventional study. Resuscitation. 2006;71(3):283–292. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Hostler D, Everson-Stewart S, Rea TD, et al. Effect of real-time feedback during cardiopulmonary resuscitation outside hospital: prospective, cluster-randomised trial. The British Medical Journal. 2011;342, article d512 doi: 10.1136/bmj.d512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozner CN, Almozlino A, Elmer J, Poole S, McNamara D, Barash D. Cardiopulmonary resuscitation feedback improves the quality of chest compression provided by hospital health care professionals. The American Journal of Emergency Medicine. 2011;29(6):618–625. doi: 10.1016/j.ajem.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Gohier F, Dellimore K, Scheffer C. Development of a smart backboard system for real-time feedback during CPR chest compression on a soft back support surface. Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC ’13); July 2013; Osaka, Japan. pp. 346–349. [DOI] [PubMed] [Google Scholar]
- 35.Song Y, Chee Y. The development of feedback monitoring device for CPR. Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, (EMBS '11); September 2011; pp. 3294–3297. [DOI] [PubMed] [Google Scholar]
- 36.Edelson DP, Litzinger B, Arora V, et al. Improving in-hospital cardiac arrest process and outcomes with performance debriefing. Archives of Internal Medicine. 2008;168(10):1063–1069. doi: 10.1001/archinte.168.10.1063. [DOI] [PubMed] [Google Scholar]
- 37.Seethala RR, Esposito EC, Abella BS. Approaches to improving cardiac arrest resuscitation performance. Current Opinion in Critical Care. 2010;16(3):196–202. doi: 10.1097/MCC.0b013e328338c121. [DOI] [PubMed] [Google Scholar]
- 38.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American heart association. Circulation. 2013;128(4):417–435. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 39.Kramer-Johansen J, Edelson DP, Losert H, Köhler K, Abella BS. Uniform reporting of measured quality of cardiopulmonary resuscitation (CPR) Resuscitation. 2007;74(3):406–417. doi: 10.1016/j.resuscitation.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 40.Perkins GD, Kocierz L, Smith SCL, McCulloch RA, Davies RP. Compression feedback devices over estimate chest compression depth when performed on a bed. Resuscitation. 2009;80(1):79–82. doi: 10.1016/j.resuscitation.2008.08.011. [DOI] [PubMed] [Google Scholar]