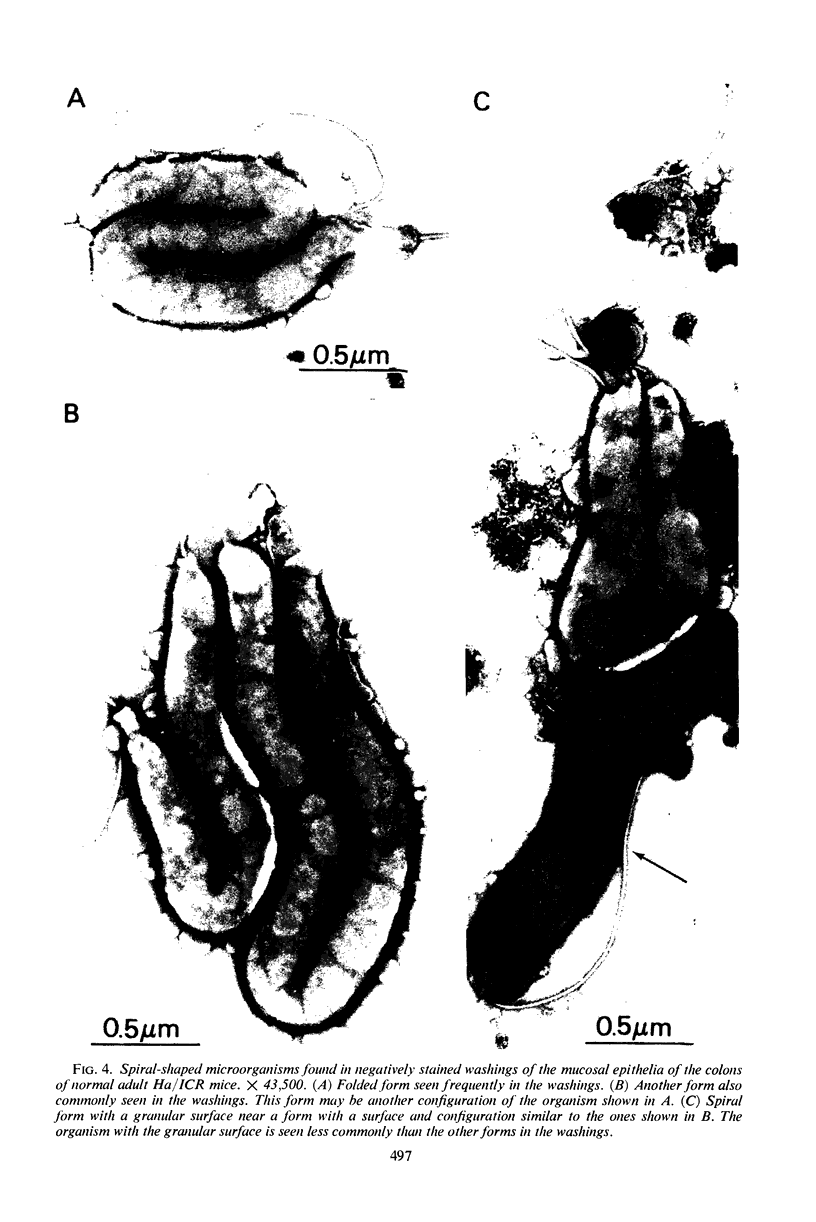
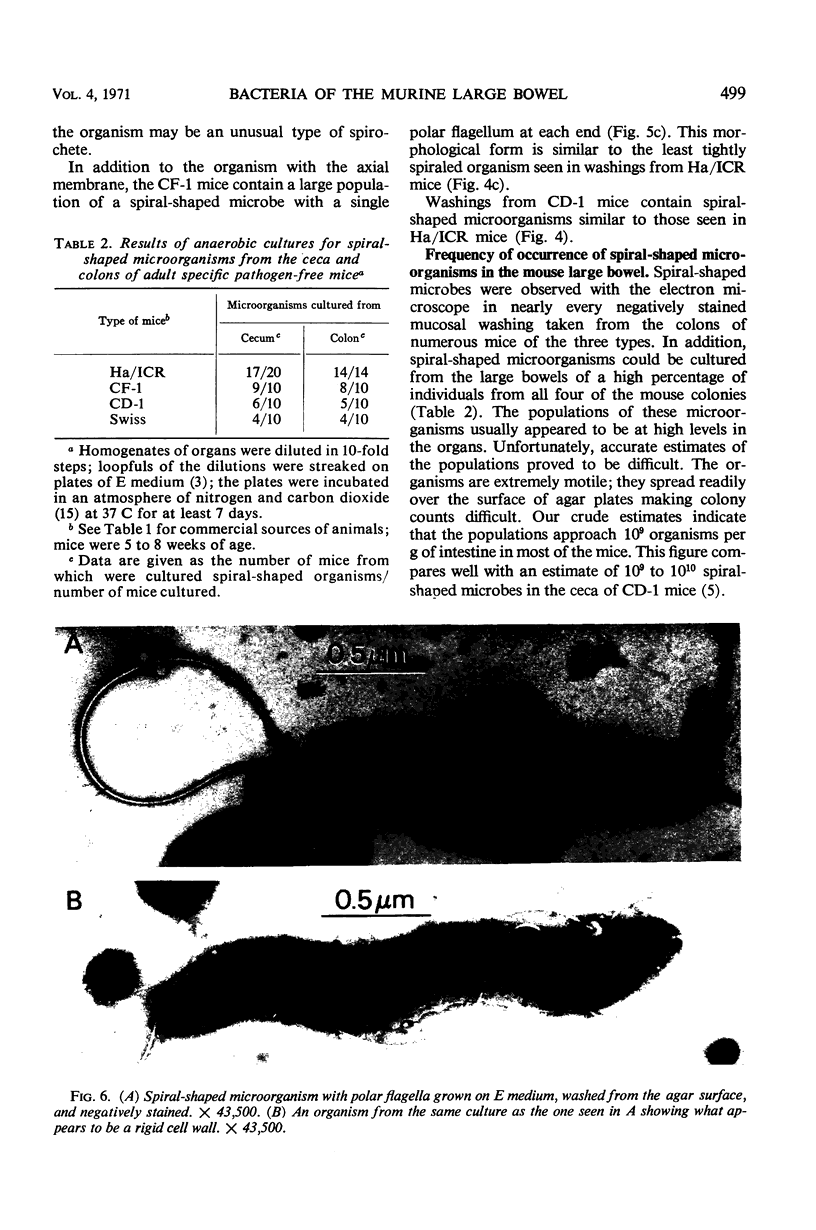
Abstract
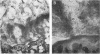
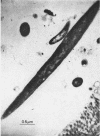
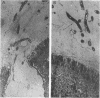
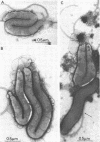
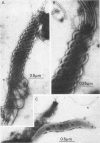
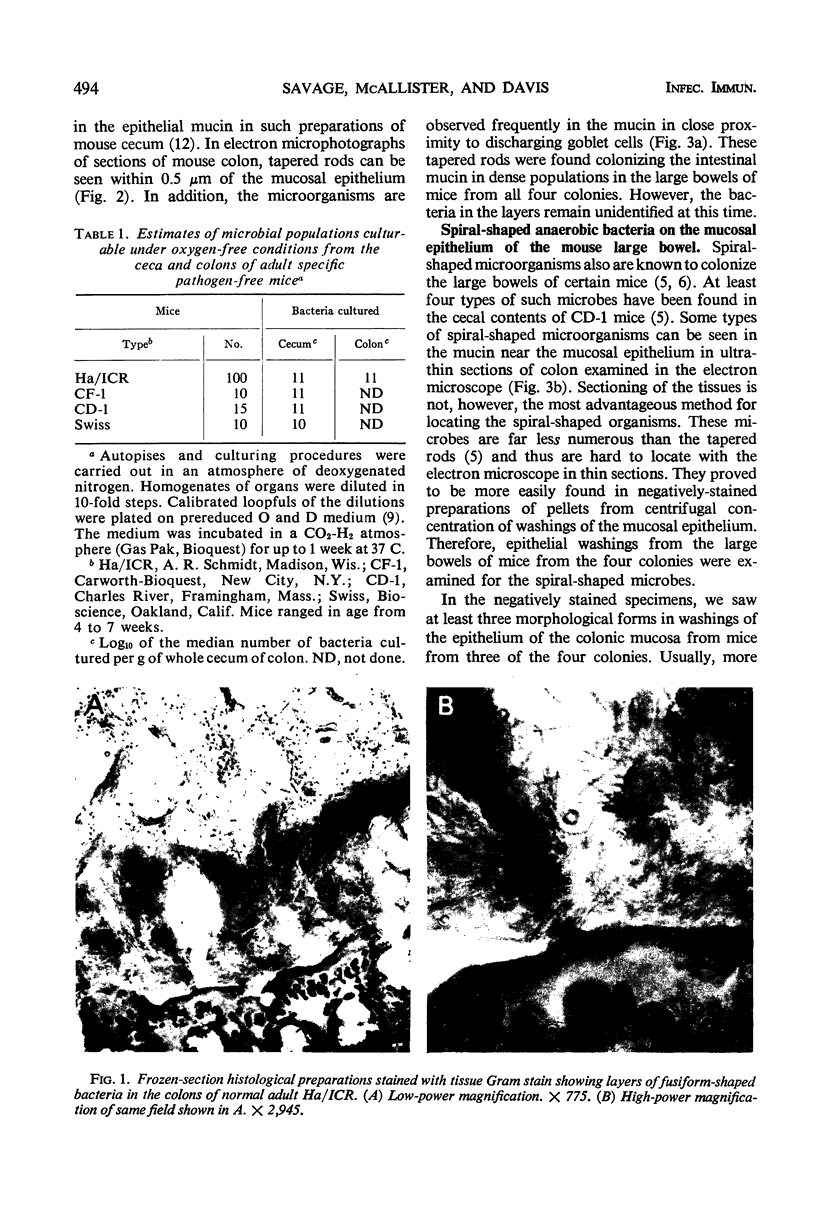
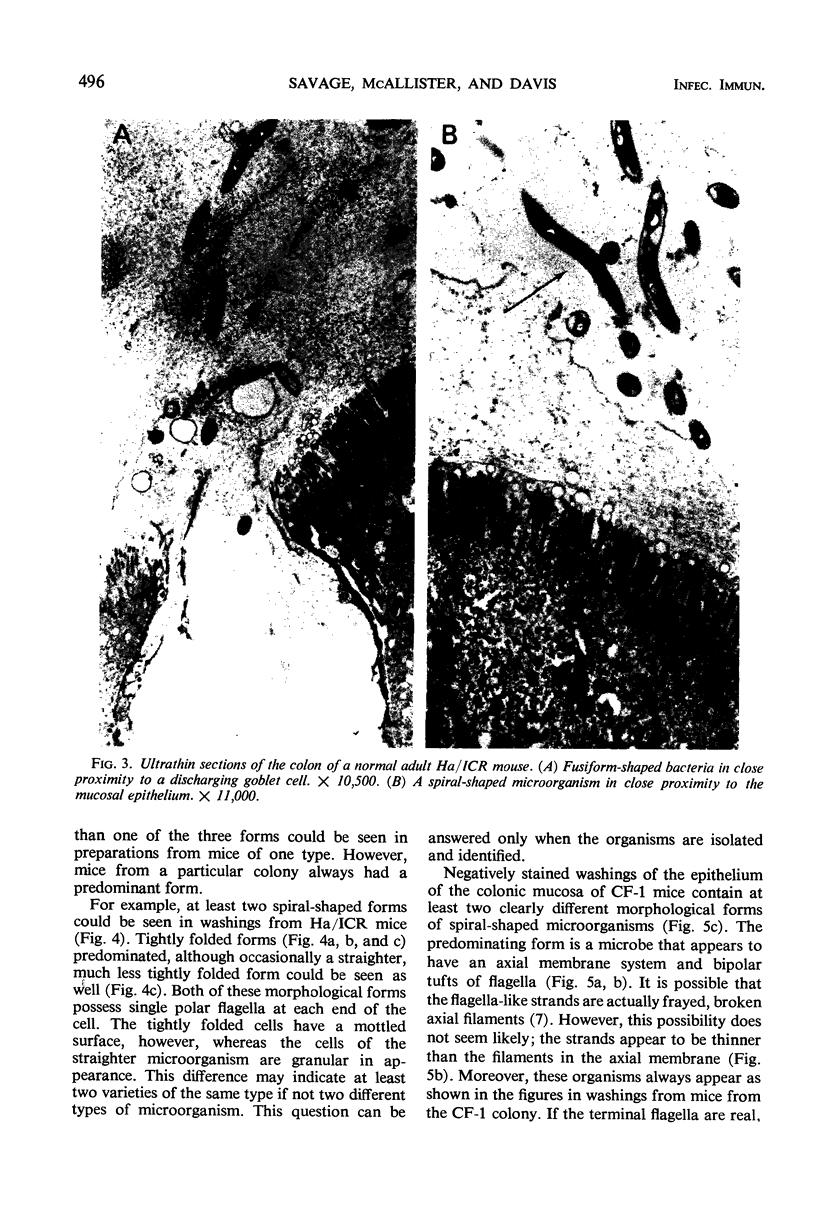
Anaerobic bacteria can be detected at population levels of 1011 organisms per g of cecum or colon in adult mice from four different colonies widely spaced in the United States. Most of these microorganisms are oxygen-intolerant fusiform-shaped bacteria. At least one type of these tapered, rod-shaped bacteria can be seen in layers in the epithelial mucin in frozen-section histological preparations of the large bowels of mice. In addition, such microorganisms can be seen within 0.5 μm of the epithelium in ultrathin sections of colon or cecum examined in an electron microscope. These fusiform-shaped bacteria predominate in the mucin layers. However, spiral-shaped microorganisms can be found as well near the mucosal epithelia in ultrathin sections of colon. Also, such organisms can be seen in negatively-stained preparations of washings of the colonic mucosal epithelia examined in an electron microscope. At least three types of spiral-shaped organisms, including both spiral-shaped bacteria and spirochetes, can be found in preparations from mice from three of the four colonies. Such spiral-shaped microorganisms can be detected at population levels as great as 109 organisms per g of cecum or colon in anaerobic cultures of the large bowels of mice from all four colonies. One anaerobic spiral bacterium was isolated in pure culture. This particular organism was found by immunofluorescence to be intermingled with the fusiform-shaped bacteria in the mucin on the mucosal epithelium in the mouse large bowel.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISRAELY M. N., OMATA R. R. A selective medium for oral fusobacteria. J Bacteriol. 1956 Nov;72(5):677–680. doi: 10.1128/jb.72.5.677-680.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R., SCHAEDLER R. W., COSTELLO R., HOET P. INDIGENOUS, NORMAL, AND AUTOCHTHONOUS FLORA OF THE GASTROINTESTINAL TRACT. J Exp Med. 1965 Jul 1;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerth A. H., Gagnon B. H. The Bacteroides of Human Feces. J Bacteriol. 1933 Apr;25(4):389–413. doi: 10.1128/jb.25.4.389-413.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. H., Dubos R. The anaerobic bacterial flora of the mouse cecum. J Exp Med. 1970 Aug 1;132(2):251–260. doi: 10.1084/jem.132.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY OF AXIAL FIBRILS, OUTER ENVELOPE, AND CELL DIVISION OF CERTAIN ORAL SPIROCHETES. J Bacteriol. 1964 Oct;88:1087–1103. doi: 10.1128/jb.88.4.1087-1103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Gordon J., Dubos R. Enumeration of the oxygen sensitive bacteria usually present in the intestine of healthy mice. Nature. 1968 Dec 14;220(5172):1137–1139. doi: 10.1038/2201137a0. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., MILLER F., BARRNETT R. J. ALDEHYDE FIXATION FOR MORPHOLOGICAL AND ENZYME HISTOCHEMICAL STUDIES WITH THE ELECTRON MICROSCOPE. J Histochem Cytochem. 1964 Feb;12:57–71. doi: 10.1177/12.2.57. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med. 1962 Jun 1;115:1149–1160. doi: 10.1084/jem.115.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Associations of indigenous microorganisms with gastrointestinal mucosal epithelia. Am J Clin Nutr. 1970 Nov;23(11):1495–1501. doi: 10.1093/ajcn/23.11.1495. [DOI] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., McAllister J. S. Cecal enlargement and microbial flora in suckling mice given antibacterial drugs. Infect Immun. 1971 Feb;3(2):342–349. doi: 10.1128/iai.3.2.342-349.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]