Abstract
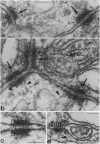
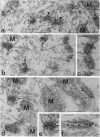
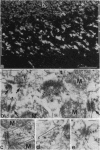
In the glomeruli of the granule cell layer of mammalian cerebellum, neuronal extensions are interconnected by numerous small, nearly isodiametric (diameters up to 0.1 micron), junctions previously classified as puncta adherentia related to the vinculin-containing, actin microfilament-anchoring junctions of the zonula adherens of epithelial and certain other cells. Using immunofluorescence and immunoelectron microscopy, we have found, however, that these junctions are negative for E- and VE-cadherin, for desmosomal cadherins, and also for vinculin, alpha-actinin, and desmoplakin, but they do contain, in addition to the protein plakoglobin common to all forms of adhering junctions, the plaque proteins alpha- and beta-catenin and the transmembrane glycoprotein M-cadherin previously found as a spread--i.e., not junction bound--plasma membrane protein in certain fetal and regenerating muscle cells and in satellite cells of adult skeletal muscle. We conclude that these M-cadherin-containing junctions of the granule cell layer represent a special type of adhering junction, for which we propose the term contactus adherens (from the Latin contactus, for touch, site of bordering upon, also influence), and we discuss the differences between the various adhering junctions on the basis of their molecular constituents.
Full text
PDF

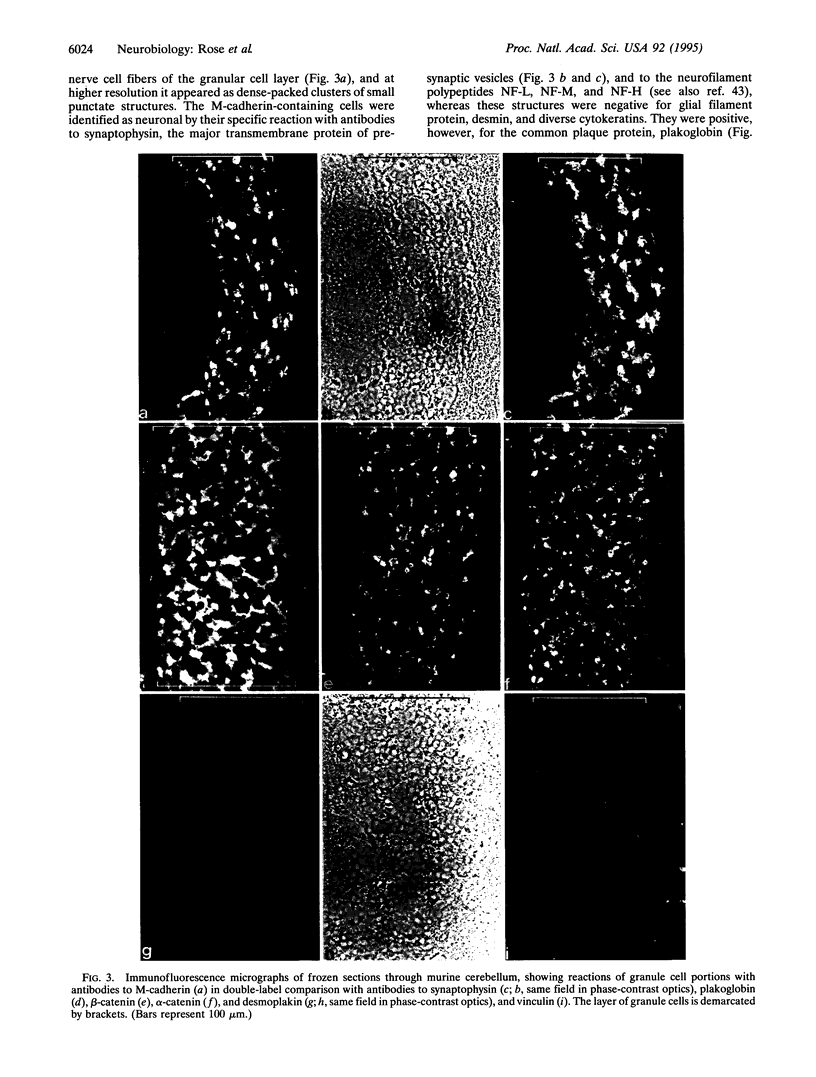


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. S., Blaschuk O. W., Haselton F. R. An N-cadherin-like protein contributes to solute barrier maintenance in cultured endothelium. J Cell Physiol. 1993 Sep;156(3):610–618. doi: 10.1002/jcp.1041560321. [DOI] [PubMed] [Google Scholar]
- Berryman M., Franck Z., Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993 Aug;105(Pt 4):1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- Bornemann A., Schmalbruch H. Immunocytochemistry of M-cadherin in mature and regenerating rat muscle. Anat Rec. 1994 Jun;239(2):119–125. doi: 10.1002/ar.1092390202. [DOI] [PubMed] [Google Scholar]
- Butz S., Stappert J., Weissig H., Kemler R. Plakoglobin and beta-catenin: distinct but closely related. Science. 1992 Aug 21;257(5073):1142–1144. doi: 10.1126/science.257.5073.1142-a. [DOI] [PubMed] [Google Scholar]
- Buxton R. S., Cowin P., Franke W. W., Garrod D. R., Green K. J., King I. A., Koch P. J., Magee A. I., Rees D. A., Stanley J. R. Nomenclature of the desmosomal cadherins. J Cell Biol. 1993 May;121(3):481–483. doi: 10.1083/jcb.121.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P., Kapprell H. P., Franke W. W., Tamkun J., Hynes R. O. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986 Sep 26;46(7):1063–1073. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- Cowin P., Kapprell H. P., Franke W. W. The complement of desmosomal plaque proteins in different cell types. J Cell Biol. 1985 Oct;101(4):1442–1454. doi: 10.1083/jcb.101.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donalies M., Cramer M., Ringwald M., Starzinski-Powitz A. Expression of M-cadherin, a member of the cadherin multigene family, correlates with differentiation of skeletal muscle cells. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8024–8028. doi: 10.1073/pnas.88.18.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Franz H. Identification of actin-, alpha-actinin-, and vinculin-containing plaques at the lateral membrane of epithelial cells. J Cell Biol. 1986 May;102(5):1843–1852. doi: 10.1083/jcb.102.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Goldschmidt M. D., Zimbelmann R., Mueller H. M., Schiller D. L., Cowin P. Molecular cloning and amino acid sequence of human plakoglobin, the common junctional plaque protein. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4027–4031. doi: 10.1073/pnas.86.11.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Osborn M., Weber K. The intermediate-sized filaments in rat kangaroo PtK2 cells. I. Morphology in situ. Cytobiologie. 1978 Aug;17(2):365–391. [PubMed] [Google Scholar]
- Franke W. W., Kapprell H. P., Cowin P. Plakoglobin is a component of the filamentous subplasmalemmal coat of lens cells. Eur J Cell Biol. 1987 Jun;43(3):301–315. [PubMed] [Google Scholar]
- Franke W. W., Moll R., Schiller D. L., Schmid E., Kartenbeck J., Mueller H. Desmoplakins of epithelial and myocardial desmosomes are immunologically and biochemically related. Differentiation. 1982;23(2):115–127. doi: 10.1111/j.1432-0436.1982.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Grund C., Müller H., Engelbrecht I., Moll R., Stadler J., Jarasch E. D. Antibodies to high molecular weight polypeptides of desmosomes: specific localization of a class of junctional proteins in cells and tissue. Differentiation. 1981;20(3):217–241. doi: 10.1111/j.1432-0436.1981.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Herrenknecht K., Ozawa M., Eckerskorn C., Lottspeich F., Lenter M., Kemler R. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A., Zeschnigk M., Starzinski-Powitz A., Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994 Apr;199(4):326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Lampugnani M. G., Resnati M., Raiteri M., Pigott R., Pisacane A., Houen G., Ruco L. P., Dejana E. A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol. 1992 Sep;118(6):1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Löwe A., Laufer J., Franke W. W. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992 Feb;140(2):427–447. [PMC free article] [PubMed] [Google Scholar]
- Moore R., Walsh F. S. The cell adhesion molecule M-cadherin is specifically expressed in developing and regenerating, but not denervated skeletal muscle. Development. 1993 Apr;117(4):1409–1420. doi: 10.1242/dev.117.4.1409. [DOI] [PubMed] [Google Scholar]
- Peifer M., McCrea P. D., Green K. J., Wieschaus E., Gumbiner B. M. The vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biol. 1992 Aug;118(3):681–691. doi: 10.1083/jcb.118.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford L. B., Mayer D. N., Bolin L. M., Rouse R. V. Transiently expressed, neural-specific molecule associated with premigratory granule cells in postnatal mouse cerebellum. J Neurocytol. 1989 Aug;18(4):465–478. doi: 10.1007/BF01474543. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J Comp Neurol. 1973 Nov 15;152(2):133–161. doi: 10.1002/cne.901520203. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973 Nov 15;152(2):103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia. Proc Natl Acad Sci U S A. 1973 Jan;70(1):240–244. doi: 10.1073/pnas.70.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose O., Rohwedel J., Reinhardt S., Bachmann M., Cramer M., Rotter M., Wobus A., Starzinski-Powitz A. Expression of M-cadherin protein in myogenic cells during prenatal mouse development and differentiation of embryonic stem cells in culture. Dev Dyn. 1994 Nov;201(3):245–259. doi: 10.1002/aja.1002010308. [DOI] [PubMed] [Google Scholar]
- Schmelz M., Moll R., Kuhn C., Franke W. W. Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells: II. Different types of lymphatic vessels. Differentiation. 1994 Aug;57(2):97–117. doi: 10.1046/j.1432-0436.1994.5720097.x. [DOI] [PubMed] [Google Scholar]
- Schwarz M. A., Owaribe K., Kartenbeck J., Franke W. W. Desmosomes and hemidesmosomes: constitutive molecular components. Annu Rev Cell Biol. 1990;6:461–491. doi: 10.1146/annurev.cb.06.110190.002333. [DOI] [PubMed] [Google Scholar]
- Schäfer S., Koch P. J., Franke W. W. Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp Cell Res. 1994 Apr;211(2):391–399. doi: 10.1006/excr.1994.1103. [DOI] [PubMed] [Google Scholar]
- Skalli O., Jones J. C., Gagescu R., Goldman R. D. IFAP 300 is common to desmosomes and hemidesmosomes and is a possible linker of intermediate filaments to these junctions. J Cell Biol. 1994 Apr;125(1):159–170. doi: 10.1083/jcb.125.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Itoh M., Nagafuchi A., Yonemura S., Tsukita S. Submembranous junctional plaque proteins include potential tumor suppressor molecules. J Cell Biol. 1993 Dec;123(5):1049–1053. doi: 10.1083/jcb.123.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Tsukita S., Nagafuchi A., Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol. 1992 Oct;4(5):834–839. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]