Abstract
During the course of normal aging, biological changes occur in the brain that are associated with changes in cognitive ability. This review presents data from neuroimaging studies of primarily “normal” or healthy brain aging. As such, we focus on research in unimpaired or nondemented older adults, but also include findings from lifespan studies that include younger and middle aged individuals as well as from populations with prodromal or clinically symptomatic disease such as cerebrovascular or Alzheimer’s disease. This review predominantly addresses structural MRI biomarkers, such as volumetric or thickness measures from anatomical images, and measures of white matter injury and integrity respectively from FLAIR or DTI, and includes complementary data from PET and cognitive or clinical testing as appropriate. The findings reveal highly consistent age-related differences in brain structure, particularly frontal lobe and medial temporal regions that are also accompanied by age-related differences in frontal and medial temporal lobe mediated cognitive abilities. Newer findings also suggest that degeneration of specific white matter tracts such as those passing through the genu and splenium of the corpus callosum may also be related to age-related differences in cognitive performance. Interpretation of these findings, however, must be tempered by the fact that comorbid diseases such as cerebrovascular and Alzheimer’s disease also increase in prevalence with advancing age. As such, this review discusses challenges related to interpretation of current theories of cognitive aging in light of the common occurrence of these later-life diseases. Understanding the differences between “Normal” and “Healthy” brain aging and identifying potential modifiable risk factors for brain aging is critical to inform potential treatments to stall or reverse the effects of brain aging and possibly extend cognitive health for our aging society.
Keywords: Aging, Brain imaging, Cognition
Introduction
Advancing age is associated with consistent differences in brain structure (DeCarli et al. 2005b) and varying trajectories of cognitive performance (Wilson et al. 1999, 2000) including transition to dementia. Advancing age is also associated with a host of concomitant medical and neurological disorders (Fig. 1), particularly hypertension(Vasan et al. 2002), which adds to the complexity of understanding “healthy” brain aging as distinguished from “normal” brain aging in the presence of concurrent disease. Both Alzheimer’s disease (AD) and cerebrovascular disease (CVD) are known to be present years before clinical symptoms (Gorelick et al. 2011; Mayda and DeCarli 2009; Sperling et al. 2011). CVD, in particular, is thought to contribute to, and possibly confound, the study of normal brain aging (Lockhart et al. 2014; Mayda et al. 2011; Nordahl et al. 2006). The effects of clinically asymptomatic CVD on brain aging are particularly difficult to separate from normal brain aging as CVD and cardiovascular risk factors (CVRFs) are very common (DeCarli et al. 2005b; Wolf et al. 1991), and CVRF-related brain structural differences such as cerebral atrophy, white matter injury and MRI infarction are observable as early as mid-life (Maillard et al. 2012b). Moreover, available treatments and awareness of the health consequences of CVRFs has resulted in significant reductions in population level control of common vascular risk factors such as hypertension (Burt et al. 1995; Jones and Hall 2004).(Draganski et al. 2013) The result has been a substantial decline in death due to stroke, coronary artery disease and heart failure (Chobanian et al. 2003). In fact, even small changes in systolic blood pressure (SBP) are associated with measureable reductions in mortality (Whelton et al. 2002). Surprisingly, however, increased treatment has not reduced stroke incidence or hospitalizations, which may actually be increasing (Goldstein et al. 2011), and evidence from the cardiovascular literature suggests that suboptimal treatment is common (Vasan et al. 2001). As a consequence, people who would have otherwise died from stroke are now suffering with chronic cerebrovascular disease. These data suggest that clinically silent cerebrovascular disease is common among cognitively normal older individuals, and this hypothesis has been supported in community based studies (Aggarwal et al. 2010; Brickman et al. 2008; DeCarli et al. 2005b).
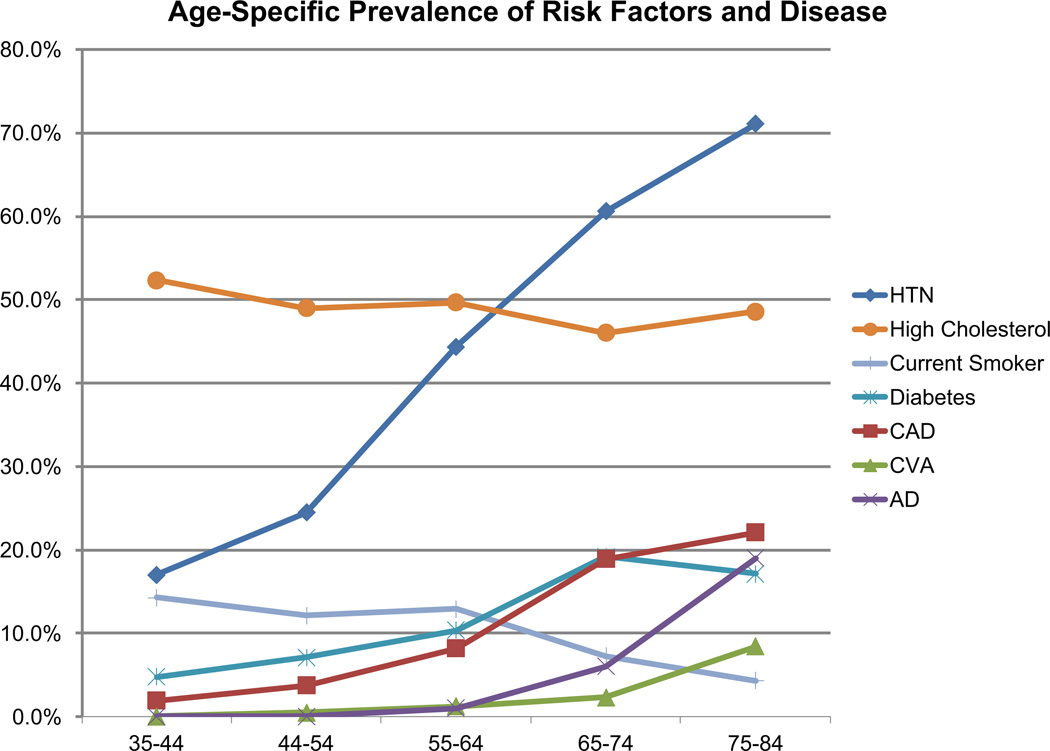
Fig. 1.
Age specific prevalance of vascular risk factors and disease among the Framingham Offspring during 2004–2009. HTN hypertension, CAD coronary artery disease, CVA clinical stroke or TIA, AD Alzheimer’d disease
The older adult population in the United States is predicted to grow considerably over the next few decades (Ortman and Guarneri 2009). This rapid growth will drive demand for biomedical research in diseases common to advanced age, such as AD and CVD, and in age- and disease-related brain changes and symptoms of cognitive impairment. MRI is a commonly available and useful neuroimaging tool that enables neuroscience researchers to observe and measure the effects of brain aging. The study of associated structural differences with advancing age is also critical to the successful prediction of late-life cognitive change and the heterogeneity of cognitive trajectories, which are likely related to heterogeneity of underlying neuropathological processes (Buckner 2004). Even in cognitively normal brains, a variety of underlying neurodegenerative processes may be present (Wilson et al. 1999), which may cause detectable differences on MRI that suggest increased risk of subsequent neurodegeneration and cognitive decline (Mungas et al. 2002; Rusinek et al. 2003). Therefore, detecting brain structural differences and relating them to cognitive function during the aging process will allow us to understand the biological basis of cognitive differences in normal aging. As the brain provides the substrate for cognition, brain aging and cognitive aging are closely linked (Grady 2008).
Functional brain differences, however, begin at a time when the prevalence of concomitant disease is low (Park and Reuter-Lorenz 2009; Salthouse 2009) suggesting that age-related declines in cognitive processes begin early in life and are independent of age-associated diseases. The etiology for these cognitive differences is uncertain, but may reflect age-related differences in neurotransmitter concentrations, particularly dopamine (Klostermann et al. 2012) or may result from alterations in synapse integrity and dendritic spine remodeling in the absence of reduced neuronal cell count (Dickstein et al. 2007; Scheibel et al. 1975). More sophisticated brain imaging techniques, however, could examine subtle differences in brain structure, such as measures of neural systems connectivity (Greicius et al. 2004; Seeley et al. 2009) that might add further knowledge to the understanding of even these earliest differences in cognitive performance.
Finally, despite consistent differences in brain structure and function with advancing age, some individuals remain above average in their cognitive performance and even improve over time, despite advanced age (Wilson et al. 1999). Similarly, there are some older individuals, particularly women, for whom brain volume is retained even beyond age 80 (DeCarli et al. 2005b). As a consequence, age related differences are sometimes discussed in the context of normal brain aging versus healthy brain aging. Normal brain aging usually refers to signs of age-related differences in brain structure and function in the absence of clinically significant impairment among the general population. By contrast, healthy brain aging is the apparent structural and functional preservation of the brain with advancing age and is considered less common. In this regard, the impact of age on brain structure and function can be viewed in context of the balance between risk and protective factors (Mattson and Magnus 2006) as well as possible biological mechanisms or genetic influences that may “protect” the brain against injury or cognitive mechanisms that enable preserved intellectual function despite evidence of brain injury (Reed et al. 2010; Satz 1993; Stern 2003). Use of sophisticated neuroimaging tools may allow for delineation of the various pathologies leading to brain injury (Reed et al. 2010) in relation to cognitive reserve thereby leading to new discoveries related to aging resilience.
Neuroimaging Tools
The primary neuroimaging tools for studying brain aging, as mentioned above, includes various MRI measures. While a full review of these methods is beyond the scope of this manuscript, comprehensive reviews are available to the interested reader (Draganski et al. 2013; Jagust and D’ Eposito 2009; Sullivan and Pfefferbaum 2006, 2007; Yoshita et al. 2005). The following is a brief introduction.
The most historically useful and informative structural MRI methods in the study of brain aging include those used to acquire high-resolution structural anatomical images of the brain, which are usually T1-weighted sequences. These T1-weighted sequences generate images that typically maximize the contrast difference between gray matter, white matter, and cerebrospinal fluid (CSF) and are generally used to elucidate the size of various cortical and subcortical structures. Other important structural MRI sequences include FLAIR (fluid-attenuated inversion recovery) MR images. FLAIR images are T2-weighted images in which the CSF signal is suppressed. This MRI type allows visualization of abnormalities of cerebral white matter, generally denoted as white matter hyperintensities (WMH). WMH are clinically silent abnormalities of cerebral white matter associated with advancing age and a variety of risk factors further discussed below. An additional MRI method useful to the study of brain aging is diffusion tensor imaging (DTI). DTI is a type of MRI sequence that measures microscopic diffusion of water in various brain tissues to infer microstructural integrity and will also be discussed. MR gradient echo imaging is sensitive to the mineral content in the brain. This tool has been used to identify microhemorrhages (Cordonnier 2010; Cordonnier et al. 2010) commonly associated with hypertension and amyloid angiopathy. Functional MRI (fMRI) employs a special type of T2-weighted imaging, sensitive to the ratio of oxygenated to deoxygenated hemoglobin in cerebral blood, in order to indirectly measure differences in neural activity related to resting-state or to performance of selected cognitive tasks. Finally, positron emission tomography (PET) enables the study of cerebral blood flow, metabolism, or, using specifically designed radioligands, the presence of certain disease biomarkers (e.g. fibrillar beta amyloid using ligands such as PIB (Klunk et al. 2004)) in brain tissue.
In summary, advancing age is associated with both structural and functional differences in the brain relative to healthy fully developed young adults. While subtle cognitive differences likely precede overt structural changes early in life, application of advanced imaging measures to understanding these differences may result in novel insights related to the heterogeneity of brain aging. As humans reach middle to later life, however, concurrent medical illnesses become more common, making understanding of brain aging in the absence of disease increasingly difficult to disentangle from alterations due to incipient disease. Neuroimaging tools are particularly applicable to the study of human brain aging because they are sufficiently available, measure brain structure and function during life, and can be repeated multiple times. This manuscript reviews current understanding of mostly structural imaging of the brain and the conclusions that shape our concept of brain aging. To achieve this goal, we first outline the results of measures of brain atrophy, then differences in white matter, and finally various forms of pathological injury to brain tissue.
Measures of Brain Atrophy
A large body of research points to cross-sectional differences and longitudinal change in whole and regional brain volumes with advancing age. While almost all studies show smaller cerebral volumes in association with advancing age, there are substantial regional differences.
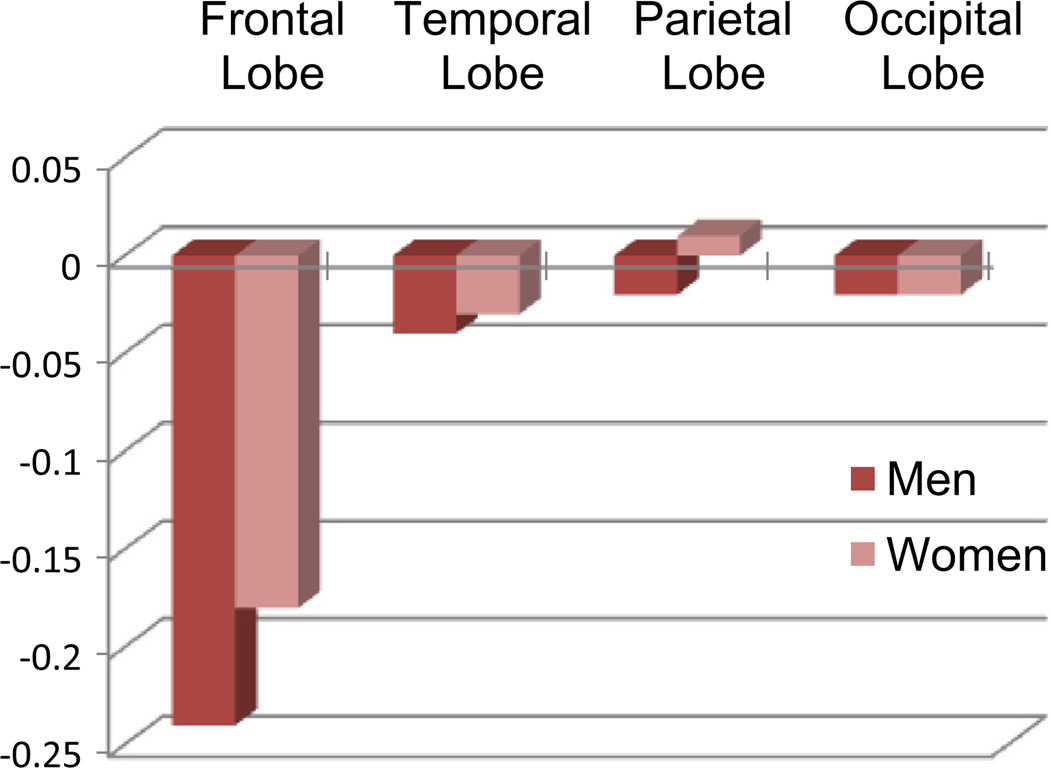
DeCarli et al. (2005b) presented one of the earliest and largest cross-sectional volumetric analyses from the Framingham Heart Study that included 2,200 individuals from 30 to over 90 years of age. Their study, although cross-sectional, demonstrated that advancing age is associated with regionally-specific brain volume differences when controlling for head size. As expected, men had significantly larger brain volumes than women, but this difference was reversed when adjusting for head size such that women have, on average, greater cerebral volume as a percentage of head size throughout the life span. In a multiple regression model that included gender, chronological age explained approximately 50 % of total cerebral brain volume differences. Age-related differences, however, were generally small prior to age 50 with increasing differences found thereafter. Frontal lobe volumes showed the greatest decline with age (approximately 12 % over the age range studied; Fig. 2), whereas smaller differences were found for the temporal lobes (approximately 9 % of the age range studies). Age-related differences in occipital and parietal lobe were modest and generally non-significant. Age-related gender differences were generally small, except for the frontal lobe where men had significantly smaller lobar brain volumes than women throughout the age range studied.
Fig. 2.
Cross-sectional estimates of yearly differences in lobar brain volumes as a percentage of head size for men and woman of the Framingham Heart Study. Significant differences are seen for Frontal and Temporal lobar brain volumes, but not Parietal or Occipital
Age-related differences in lobar brain volumes have been examined in a number of cross-sectional studies (Coffey et al. 1998, 2001, 1999, 1992; DeCarli et al. 1994; Fotenos et al. 2005; Gunning-Dixon et al. 1998; Jernigan et al. 1991, 2001; Murphy et al. 1996, 1992; Pfefferbaum et al. 1994; Raz et al. 2001, 1998; 1997, 1992a, b, 1995; Sullivan et al. 1995, 2001b) with generally comparable results. For example, within a select group of very healthy individuals, Jernigan et al. (Jernigan et al. 1991, 2001) showed results similar to those reported by DeCarli et al. (2005b) with age related differences being largest for total brain, frontal lobe, and temporal brain volumes, whereas age-related differences in parietal and occipital lobe brain volumes were smaller. Age-related differences similar to those reported by DeCarli et al. (2005b) have also been shown for temporal lobe, temporal horn, and ventricular CSF by others (Raz et al. 2005; Sullivan et al. 1995; Sullivan and Pfefferbaum 2007), supporting the consistency of these findings.
Not only do other studies confirm regional differences in brain volume differences with age, but also qualitative differences in the pattern of age-related differences. For example, in the DeCarli et al. study (DeCarli et al. 2005b), advancing age was significantly associated with a non-linear trend in brain volume measures. Conversely, age-related differences in frontal brain volumes showed a more linear decline. The magnitude of change also differed with more frontal than temporal lobe volume loss on a percent basis, again supporting previous reports (Coffey et al. 2001, 1992).
Vascular risk factors have long been recognized as strongly and adversely influence cerebral volumes (Salerno et al. 1992), even when blood pressure is within the higher range of normal (DeCarli et al. 1999; Seshadri et al. 2004). Moreover, differences due to increased blood pressure may be apparent early in life (Maillard et al. 2012b). Furthermore, recent data suggest that increased cerebral atrophy due to vascular risk factors may be more common among cognitively normal individuals than atrophy due to incipient Alzheimer’s disease (Nettiksimmons et al. 2013). Additional studies show that the presence of various vascular risk factors also leads to increasing rates of cerebral atrophy (Debette et al. 2011).
Gray Matter Atrophy
Analyses of tissue-based brain volumes (i.e., volumes of gray matter, white matter, or gray and white matter) also demonstrate cross-sectional and longitudinal associations with advancing age. Analysis of gray matter volumes in particular can elucidate atrophy patterns of cortical and subcortical gray matter structures and provide insight into brain networks undergoing gray matter tissue loss as a result of aging. There is a normal, expected age-related reduction in the gray matter volume of specific brain regions and cortical layers which is thought to represent factors like cortical neuronal degeneration and synaptic density reduction and is generally more prominent in association than in sensory cortices. Conversely, in neurodegenerative diseases there are different, but predictable patterns of atrophy. In AD, for example, regions of significant atrophy include medial temporal, precuneus, and lateral temporoparietal cortex (Seeley et al. 2009).
In one of the earliest and largest studies of tissue volume differences in older age, Fotenos et al. (2005) examined a cohort of 370 adults (aged 18–97 years) including 79 elders, 78 years of age on average followed longitudinally for 1.8 years on average. They directly compared cross-sectional and longitudinal head-size corrected whole brain measures and examined the differences between nondemented aging and mild AD patients in annualized rates of longitudinal rate of brain change. Fotenos and colleagues found that among nondemented adults across the lifespan, whole-brain, gray matter, and white matter volumes all decreased overall with advancing age at about 0.45 % per year, although exact differences varied by brain region (frontal being greatest) and tissue type (Fotenos et al. 2005). Specifically, white matter volumes tended to increase until around age 40 before decreasing with an accelerating quadratic trend, while gray matter volume began to decline more linearly from an early age (i.e. before the age range measured in the study). Slowly accelerating whole-brain volume reductions were evident by age 30. In addition, the cross sectional and longitudinal assessments of whole-brain volumes were in agreement for nondemented elders. And in a comparison of normal aging versus degenerative disease, the rate of decline in gray matter was nearly double (0.98 % vs. 0.45 %) if participants had even mild AD.
The Fotenos et al. study (Fotenos et al. 2005) advanced current understanding of differences in the brain with advance age, because not only did it provide measures of brain aging by tissue class, but also it confirmed that volume reductions begin relatively early in adulthood and tend to accelerate with age. Further, it showed that in late life, cross-sectional and longitudinal estimates of rates of decline with age are in agreement, and affirmed that atrophy rates are greater in patients with AD than in cognitively normal elders. However, this study was limited in that it did not examine regionally specific measures of brain volume or include measures of CVD-related brain structural differences. In addition, it only included a relatively small sample of participants, particularly for longitudinal analysis.
Raz et al. (2005), in a longitudinal analysis of 127 participants ranging from 31 to 83 years of age, further investigated regional heterogeneity of gray and white matter differences across the age span. Manual measurements of structures revealed substantial volume declines in caudate nucleus and cerebellum gray matter, moderate declines in hippocampus, lateral prefrontal cortex, orbitofrontal cortex, inferior temporal cortex, and inferior parietal cortex, and smaller but still significant declines in entorhinal cortex. This study found no appreciable age-related differences in primary visual cortex gray matter. Further, volume losses in the hippocampus, entorhinal cortex, and inferior temporal cortex increased with advancing age. Between-subjects differences in volume loss were correlated across multiple brain regions, suggesting that there were common underlying causes to the gray matter volume loss.
In another study that utilized atlas based regional segmentation, Pfefferbaum et al. (2013) found regional differences in rate of gray matter atrophy and CSF expansion, including comparison to a group of young adults. By including a broader age-range of individuals, Pfefferbaum et al. were able to show a more complex relationship between regional gray matter atrophy and age. For example, frontal cortex measures showed two phase rates of change with acceleration between 20 and 40 years of age, followed by a period of lower rate of atrophy from 40+ to 60 years of age, after which the rate of atrophy again accelerated. These findings are consistent with change in cognitive performance, particularly in timed measures (Salthouse 2000). In contrast, the rate of hippocampal atrophy remained essentially stable until after age 60 where the atrophy rate accelerated substantially with advancing age. These findings extend concepts of brain aging as they show significant differences in atrophy at a time with other pathologies are likely to be minimal. If these findings are replicated, it would suggest that brain aging is a dynamic process subject to early and later influences of unclear origin, but relevant to cognitive ability.
White Matter Atrophy
While there has been considerable focus on regional and gray matter differences with age, relatively fewer studies has explored the potential impact of aging on cerebral white matter volumes. Pathological studies suggest that atrophy of cerebral white matter is a complex process resulting in change in myelinated fiber size and increase in perivascular spaces (Meier-Ruge et al. 1992). Given that the volume of the axon, particularly pyramidal cell axons as compared to dendritic and nerve cell volume, age-related differences in cerebral white matter may be expected to be greater than that of gray matter. In fact, changes in ventricular volume which are on the order of 20 % per decade (Kaye et al. 1992) are significantly greater than any measure of gray matter. Despite these obvious differences, quantitative studies of age-related cerebral white matter are relatively limited. Interestingly, one of the earliest studies of cerebral white matter, found slightly increased age-related differences in white matter volume, while gray matter volume declined substantially in a non-linear fashion accelerating above age 40–50 (Pfefferbaum et al. 1994). This initial report, however, was followed by an apparently contradictory report that suggested age-related differences in white matter volumes were greater than gray matter and consistent with differences in CSF volumes (Guttmann et al. 1998), findings that are more consistent with pathological studies (Meier-Ruge et al. 1992). More recent work suggests that both gray and white matter volumes decline with advancing age, but differ in pattern (Ge et al. 2002) where gray matter declines linearly, and white matter differences are non-linear and accelerate after age 50 and generally correlate with a similar pattern of age-related expansion of CSF. These findings were later confirmed in a somewhat larger study (Fotenos et al. 2005). Raz et al. (2005), in their large study described above, also examined cross-sectional differences and longitudinal change in regional white matter volume measures. This elegant study examined longitudinal differences in regional gray and white matter volumes using manual delineation for quantitative assessment. They found significant age-related volume losses in prefrontal and inferior parietal white matter and demonstrated that prefrontal white matter volume loss tended to accelerate with greater age suggesting that this is a region particularly vulnerable to age-related change.
Therefore, like studies of gray matter, white matter atrophy also has regional specificity with particularly greater age-related differences in frontal regions. However, while gray matter volumes tend to decline linearly across the age range, white matter volumes appear to decline more rapidly with advancing and age, a pattern that coincides with similar nonlinear age-related differences in CSF expansion (DeCarli et al.
2005b).
Hippocampal Volume
Given the common reductions in memory performance as part of advancing age (Wilson et al. 1999) as well as the seminal finding that hippocampal volume loss is substantial in the setting of Alzheimer’s disease (Seab et al. 1988), it is not surprising that hippocampal measures are common to numerous studies of “normal” cognitive aging. The first study of this type was performed by Lim et al. (1990) who compared 8 younger (mean age 24) to 7 older (mean age 73) individuals . They showed a trend towards volume loss with age. Since these early studies, numerous, but not all reports (Sullivan et al. 1995) identify age-related declines in hippocampal volume. Longitudinal studies also show increasing rates of hippocampal atrophy with increasing age (Du et al. 2006; Pfefferbaum et al. 2013; Raz et al. 2005). The inconsistencies amongst studies may reflect differences in image type analyzed as well as variability in the analysis protocol (Frisoni et al. 2013) and more recent studies show very consistent findings of age-related atrophy of the hippocampus (Taylor et al. 2014).
While hippocampal injury is known to be involved early in Alzheimer’s disease (Jack et al. 2013), less consistent results have been found in relation to vascular risk factors. In a large epidemiological study of over 500 subjects in Rotterdam, den Hiejer et al. (2012) found a significant association between hippocampal volume, white matter lesions and diastolic blood pressure. This finding, however, has not been confirmed by other studies (Bender and Raz 2012; Gattringer et al. 2012) which also examined blood pressure measures and in at least one study (Bender and Raz 2012) found associations with prefrontal regions, but not hippocampus.
Similar to other structural brain measures, the hippocampus appears to decline in volume with advancing age, even though individuals remain cognitively “normal.” While it is tempting to propose that these age-related differences in hippocampal volume simply reflect pre-clinical Alzheimer’s disease (Jack et al. 2013; Sperling et al. 2011), more recent data suggest that tau pathology in subcortical structures, including hippocampus, may be present years before the amyloid pathology of Alzheimer’s (Braak et al. 2011). The exact relevance of this finding awaits further research (Chien et al. 2013; Xia et al. 2013) but could suggest a more complicated process leading to age-related differences in hippocampal volume. The exact effect of vascular disease on age-related differences in hippocampal volume is similarly unresolved.
Cortical Thickness
An additional method used for the examination of gray matter tissue shrinkage in aging includes the study of cortical thickness and surface area. In a seminal study of 100 cognitively normal individuals ranging in age from 18–90+, Salat et al. (2004) measured global and regional cortical thickness. They showed considerable regional variability with the greatest age-related reductions in gray matter thickness found primarily in gyri of the frontal cortex. In a follow-up, but larger study of 216 participants aged 18–87, Lemaitre et al. (2012) compared cortical thickness, surface area and gray matter volume across various cortical regions and found age-related reductions in almost all measures across the brain. However, all three measures demonstrated common reductions with age in the prefrontal cortex. Conversely, parietal cortex only showed an age-related thickness reduction. A second, much larger epidemiological study of older individuals (60–90+) also found significant age-related differences in cortical thickness, although reductions appeared greatest in the temporal lobes (van Velsen et al. 2013). Interestingly this group also showed a strong sex interaction in differences in age-related thickness of the frontal lobe where men had lower average frontal lobe thickness and greater reductions with age.
Longitudinal change in cortical thickness and the potential impact of incipient Alzheimer’s disease was recently examined in a multi-site experiment (Fjell et al. 2014b). This novel study of 1,100 individuals studied in cross-section and 207 studied longitudinally examined the effect of incipient Alzheimer’s disease on cortical thickness measures by randomly combining a subset of mild Alzheimer’s disease patients into age-related estimates. Also examined was a group of low risk individuals based on longitudinal observation as well at CSF Alzheimer’s disease measures. The authors concluded that cross-sectional estimates suggest that cortical thickness in most areas declines linearly with age, and their longitudinal data confirmed the general trend in age-related differences from the cross-sectional data. Interestingly and somewhat surprisingly, the authors also showed that the inclusion of 96 individuals with mild Alzheimer’s disease did not substantially change the cross-sectional or longitudinal results. Moreover, they conclude with the statement, “Notably, the present results indicate that the brain changes, even in the regions most prone to AD pathology, are not necessarily caused by age-related neurodegenerative conditions such as AD” (Fjell et al. 2014b). This conclusion was based upon the lack of finding significant differences in trajectories amongst individuals with mild Alzheimer’s disease, but also on analysis of a small group of individuals carefully selected to be at low risk for Alzheimer’s disease. In this highly select group, atrophy rates did not differ from the entire older group, particularly in the thickness of the entorhinal cortex which shows minimal change in younger life, but accelerating rates of shrinkage with increasing age after 50 years of life. The authors postulated that at least some of the differences found may be due to incipient vascular disease.
Despite numerous studies of vascular factors on regional brain and gray matter volumes (Debette et al. 2011; DeCarli et al. 1999; Raz et al. 2005,2007; Salerno et al. 1992; Seshadri et al. 2004; Swan et al. 2000, 1998), only limited work has been done on the relationship between vascular risk factors and cortical thickness (Villeneuve et al. 2014). This study consisted of 66 individuals with normal or mildly impaired cognitive ability, but included individuals with hypertension, hypercholesterolemia, diabetes, stroke and myocardial infarction. The extent of vascular risk as defined by the Framingham Cardiac Risk Profile (Wilson et al. 1998) was significantly and inversely associated with frontotemporal cortex thickness independent of Aβ. Cortical thickness in these same regions were also significantly and inversely associated the extent of Aβ deposition which was also associated with additional thinning in parietal cortices. The combined effects of Aβ and vascular risk were found to interact significantly in parietal cortex where the negative influence of Aβ on cortical thickness was greatest.
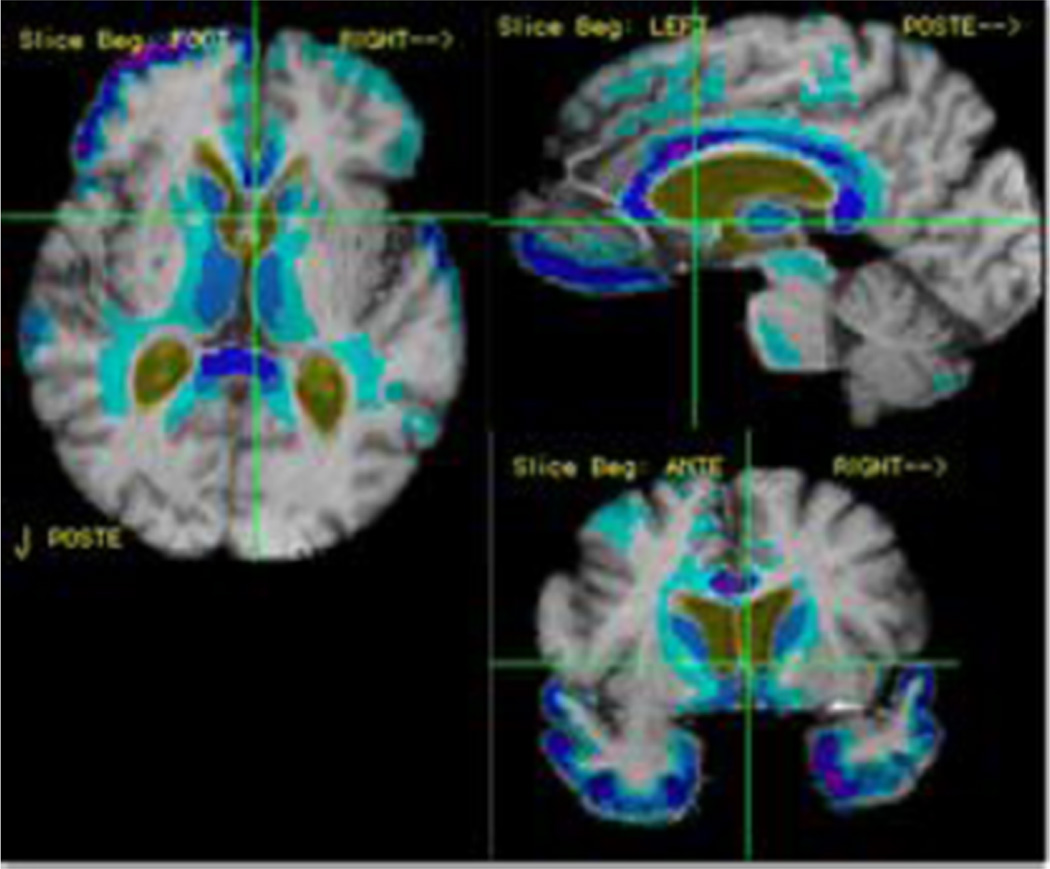
These later findings are potentially important in light of recent study of cortical thickness measures among individuals at very low risk for Alzheimer’s disease (Fjell et al. 2013). This work, which expands on the studies of Fjell and colleagues ((Fjell et al. 2014a) examined longitudinal change in cortical thickness in 132 older individuals who remained free of clinical Alzheimer’s disease for at least 3 years. Moreover, the authors used a number of “healthy aging” definitions, some of which included CSF measures in a subgroup of the subjects. All groups showed significant loss of cortical thickness in anterior and middle temporal cortex and use of various definitions of “healthy aging” did not substantially change the pattern of atrophy. The authors also showed that the rate of cortical atrophy in these areas did not differ substantially between cognitively normal, mildly impaired and diagnosed Alzheimer’s disease. In fact, the low risk group appeared to have greater differences in inferior and infero-lateral frontal cortex than did the other groups. These results are similar to results from the UC Davis, Alzheimer’s Disease Center (UCD ADC) healthy control group of 159 subjects as shown in Figs. 3, 4 and 5. Importantly, this is a diverse group of subjects recruited from the community who were not screened to exclude concomitant vascular risk factors (Hinton et al. 2010). Prominent frontal and anterior temporal atrophy shown in Fig. 3 are remarkably similar cross-sectional cortical thickness patterns seen in amyloid negative group by Villeneuve et al. (2014), suggesting that processes underlying progressive atrophy in this region is likely be unrelated to Alzheimer’s disease pathology and may be related to other factors such as vascular disease or age-related degeneration.
Fig. 3.
Yearly rates of brain change among individuals of the UCD ADC who are cognitively normal. Colored regions reflect areas of significant yearly change. Darker colors reflect atrophy whereas warmer colors reflect expansion (e.g. CSF space enlargement)
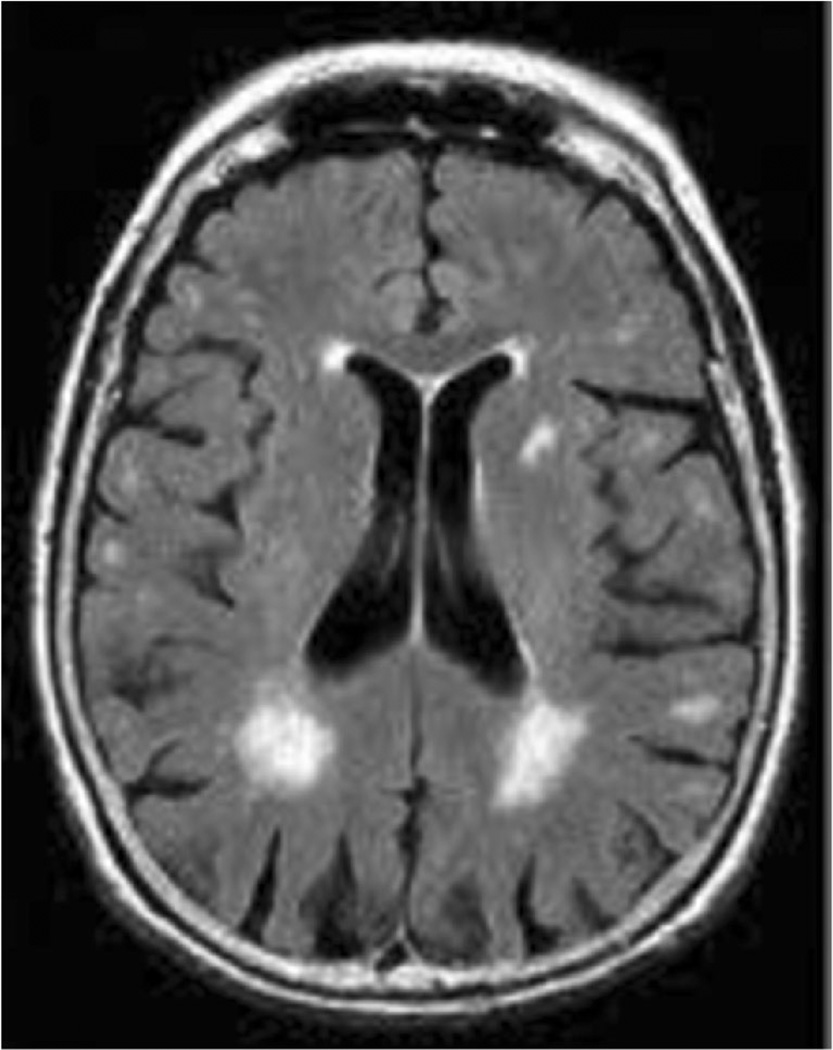
Fig. 4.
One example of white matter hyperintensities as seen on FLAIR imaging
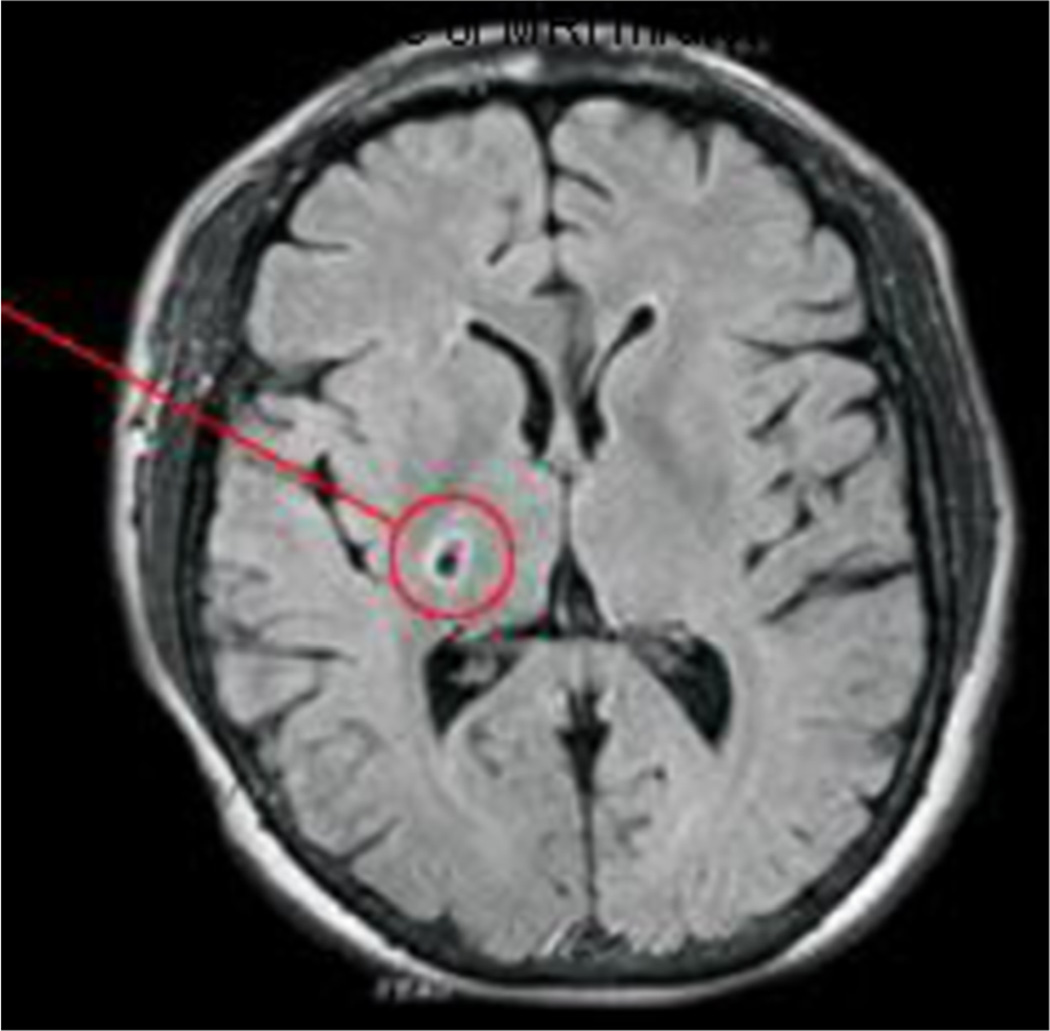
Fig. 5.
One example of an MRI infarct as seen on FLAIR imaging. The infarct is outlined by the circle on the image. The increased intensity surrounding the infarct is believed to reflect gliotic injury
In summary, these results suggest that different cellular changes occur in aging across different areas of gray matter, possibly attributable to different underlying pathologies. As suggested by Buckner (2004) and further elucidated by Fjell et al. (2014a), frontal subcortical systems appear to be more adversely affected by the age process. Injury to these systems is known to cause symptoms of frontal dysfunction commonly associated with advancing age (Cummings 1993, 1995; Mega and Cummings 1994; West 1996). One hypothesis suggests that, because these are the regions late to mature during development (Gogtay et al. 2004), they are more susceptible to later-life degeneration (Fjell et al. 2014a). Alternatively, these are the same regions that undergo evolutionary expansion, but as Fjell et al. note (Fjell et al. 2014a), neither hypothesis fully explains the data. Intriguingly, Fjell et al. suggest that there exists specific vulnerability in cortical systems constituting the default mode network, a hypothesis earlier present by Buckner et al. (Buckner et al. 2009) to explain the predilection of these areas to amyloid deposition. If in fact these areas are particularly susceptible to age- and Alzheimer’s disease related injury, beginning with “… normalcy-pathology homology as starting point, studying the brain regions that are vulnerable to both aging and AD may allow us to disentangle disease-specific mechanisms from normal age-related events (Fjell et al. 2014a)”.
White Matter Integrity and Injury
Research on brain-behavior relationships historically has stressed the role of the cerebral cortex and subcortical gray matter for cognitive processing. Recent evidence, however, indicates that, as an essential component of extended neural networks in the brain, white matter is also critical for many higher order cognitive processes including attention, executive functioning, non-verbal/visual-spatial processing, and generalized processing speed (Gunning-Dixon and Raz 2000). In addition, more recent studies suggest similar associations between specific the fornix (Fletcher et al. 2013; Lee et al. 2012) and cingulum tracts (Zhang et al. 2007) and memory performance. Though the relationship between cerebral white matter and cognition continues to be explored, Geschwind’s seminal 1965 paper linked disconnection of the cerebral white matter to a variety of neurobehavioral disorders over four decades ago (Geschwind 1965a). Clinical evidence of the effects of white matter disconnection can be observed in patients with conduction aphasia, in which a lesion of the arcuate fasciculus uncouples Broca’s area and Wernicke’s area, leading to poor verbal repetition skills (Geschwind 1965a, b). Although data from these patients clearly support white matter disconnection leading to dysfunction, recent evidence suggests that even asymptomatic disruption of the white matter may lead to subtle cognitive impairments. Both white matter injury, which indicates the presence of WMH, and white matter integrity, which refers to subtler changes assessed by DTI, are associated with decreases in speed and cognitive performance, and in some cases have been shown to mediate age-related changes in brain functional connectivity and cognition (Andrews-Hanna et al. 2007; Head et al. 2008; Madden et al. 2004; Mayda et al. 2011; Nordahl et al. 2006; O’Sullivan et al. 2001; Sullivan et al. 2001a; Ziegler et al. 2008).
WMH are clinically silent abnormalities visible on fluid attenuated inversion recovery (FLAIR) images in deep or periventricular white matter regions (DeCarli et al. 2005a). Pathological studies suggest that WMH appear brighter on FLAIR due to increased water content and molecular degeneration in damaged white matter, and that the underlying nonspecific pathology of WMH includes demyelination, gliosis, and axonal atrophy (Bronge 2002; Fazekas et al. 1998; Simpson et al. 2007a, b). The extent of WMH are strongly associated with age (DeCarli et al. 2005b) and a variety of vascular risk factors, such as hypertension, diabetes and smoking, even in the absence of clinically symptomatic cognitive impairment (de Leeuw et al. 1999; Jeerakathil et al. 2004a, b; Wen and Sachdev 2004). There is generally agreement that WMH are primarily related to small vessel ischemic pathology or more general vascular processes (Pantoni 2002). WMH are greater in number and size even amongst individuals with only borderline differences in blood pressure (DeCarli et al. 1995; Longstreth et al. 1996; Swan et al. 1998), suggesting that vascular risk factors influence white matter injury along a continuum. The presence of WMH are also associated with increased prevalence of MRI infarction, brain atrophy and reductions in frontal lobe metabolism (DeCarli et al. 2005b, 1999, 1995), and the effect of WMH on frontal hypometabolism appears to be independent of location (Tullberg et al. 2004). At least two hypotheses explain this finding. The first is that WMH occur preferentially around periventricular locations, extending outward with increasing volume, thereby preferentially affecting axons connecting the frontal lobe to posterior targets (DeCarli et al. 2005a; Mayda et al. 2011; Nordahl et al. 2006). The second hypothesis is that WMH are the expression of a more diffuse and ongoing process (Maillard et al. 2011). Through an elegant series of experiments, Maillard et al. showed that WMH lesions are surrounded by an area of reduced white matter integrity called a WMH “penumbra” (Maillard et al. 2011). This initial report was followed by a second report where Maillard et al. examined longitudinal change in “normal” appearing cerebral white matter. They showed that baseline measures of normalized fractional anisotropy (FA) and normalized FLAIR intensity were strongly predictive of subsequent WMH (Maillard et al. 2013). These data suggest that WMH are the result of continuous and ongoing damage to cerebral white matter and that FA and normalized FLAIR maybe excellent markers of white matter injury and degeneration. A follow-up study extended these findings to describe heterogeneity of the process of white matter degeneration within “normal” appearing white matter and WMH. This study identified three types of WMH: 1) growing (or enlarging) WMH, 2) stagnant WMH (unchanging), and 3) incident WMH. This study made a number of findings. The first was that all white matter shows declining integrity over time as measured by FA. The second finding was that the magnitude of differences increased continuously from “normal” appearing white matter to white matter within enlarging WMH which showed the greatest degree of change with time. Conversely, FLAIR imaging only identified significant change in signal with incident WMH lesions. These results suggest that WMH and even normal appearing white matter undergo continuous changes with time and therefore are subject to potential intervention. Future use of DTI measures in combination with FLAIR imaging are therefore possible bio-markers for early intervention trials aimed at improving cognitive health.
Large epidemiological studies, while sometimes limited in the extent of cognitive testing available, consistently show moderate associations between WMH volumes and diminished cognitive impairment (Breteler 2000; de Groot et al. 1998, 2000; Longstreth et al. 2000, 1996; Ott et al. 1996). A number of smaller, cross-sectional studies consistently demonstrate deficits in tests of attention and mental processing (Boone et al. 1992; Breteler et al. 1994; DeCarli et al. 1995; Schmidt et al. 1995), although impairments in memory and general intelligence are also observed (Breteler et al. 1994; DeCarli et al. 1995). A number of these studies also show a threshold effect where extensive amounts of WMH are necessary before cognitive impairments are detected (Boone et al. 1992; DeCarli et al. 1995; Schmidt et al. 1995). More recent studies show that WMH are also associated with longitudinal change in cognitive performance (Carmichael et al. 2010, 2012; Debette et al. 2011; Maillard et al. 2012a) and substantially increased risk for clinically relevant cognitive impairment such as mild cognitive impairment and dementia (Debette et al. 2010). Other studies also suggest that WMH may be “contaminate” concepts of “normal” cognitive aging. A series of studies show that WMH may mediate age-related differences in cognitive performance in areas of working memory (Nordahl et al. 2006), cognitive control (Mayda et al. 2011), and visual search performance (Lockhart et al. 2014), cognitive domains commonly assumed to be affected by the aging process (Park and Reuter-Lorenz 2009). Moreover, WMH may compromise frontal systems resulting in impaired memory encoding (Lockhart et al. 2012; Nordahl et al. 2005; Parks et al. 2011) and therefore may be further associated with memory complaints common to advancing age.
MR diffusion tensor imaging (DTI) enables examination of white matter tracts and markers of white matter microstructural integrity at macroscopic resolution in living human participants by measuring water diffusion (and indirectly, its local restrictions) in tissue, (Mori et al. 2005). Of particular utility have been measures of microstructural integrity such as fractional anisotropy (FA) and mean diffusivity (MD). FA is a quantitative measure that in many studies is positively associated with white matter tract integrity (Assaf and Pasternak 2008). DTI studies across the age-range show that advancing age is associated with reduction in FA in both normal older adults (Sullivan and Pfefferbaum 2007) and in patients with neurodegenerative and cerebrovascular disease (Chua et al. 2008). DTI studies of normal older adults show reductions in white matter integrity with advancing age in regionally specific areas such as the corpus callosum (O’Sullivan et al. 2001), frontal and parietal white matter (Nusbaum et al. 2001). In addition, age-related reductions in FA are usually greater in anterior white matter than in more posterior brain regions (O’Sullivan et al. 2001; Sullivan et al. 2001a; Sullivan and Pfefferbaum 2006), and greater in the normal appearing white matter of participants with larger volumes of WMH (O’Sullivan et al. 2004).
Other DTI studies find that vascular and degenerative diseases appear to have differential impact on specific white matter pathways (Lee et al. 2009, 2010; Yoshita et al. 2006). Moreover, studies of specific white matter pathways across the spectrum of cognitive ability now suggest that diminished white matter integrity may occur simultaneously to neuronal degeneration in degenerative diseases such as Alzheimer’s disease (Fletcher et al. 2013; Lee et al. 2012; Zhang et al. 2007).
In summary, evidence of white matter pathology as seen as WMH on FLAIR imaging is common to advancing age and is enhanced in the presence of vascular risk factors. Most WMH increase in extent over time although small numbers of incident WMH also occur. The process may be enhanced by the co-occurrence of vascular risk factors as well. During “normal” cognitive aging, WMH are most prevalent in anterior periventricular regions, but the extent and distribution of WMH increase in the setting of mild cognitive impairment and dementia. WMH are not benign processes as the presence of extensive WMH is associated with increased risk for stroke, dementia and even mortality. Measures of white matter integrity by DTI confirm age-related differences in myelination seen pathologically, but also show that these differences have some topographical specificity with particularly large age-related differences found in frontal subcortical white matter. DTI measures also show areas of decreased white matter integrity surrounding WMH (WMH penumbra). These areas are particularly susceptible to future conversion to WMH suggesting that WMH lesions are only one manifestation of a continuous process of white matter pathology. DTI measures, however, also differ in the setting of degenerative diseases suggesting that white matter pathology may be common to multiple injury mechanisms. White matter research is a relatively recent area of expanding interest, but these data reinforce the relevance of white matter pathology to the function of distributed cognitive networks and warrant further research endeavors.
Studies of Evident Cerebral Pathology
Clinically Silent Brain Infarcts
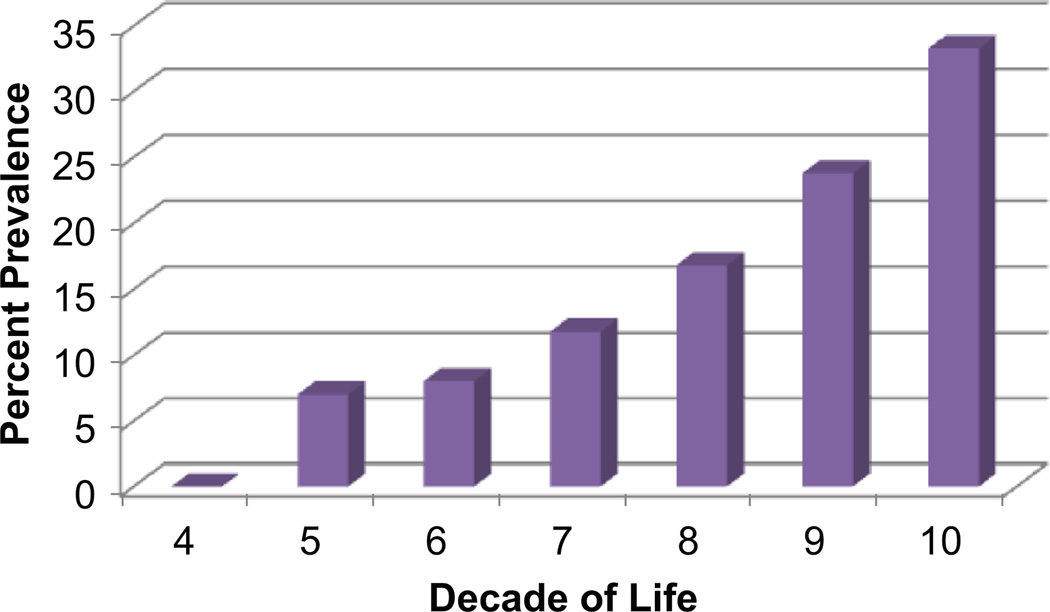
MRI infarcts are parenchymal lesions that have the MRI characteristics of tissue infarction, but generally have not been associated in that individual with clinical signs or symptoms corresponding to a stroke (Hachinski et al. 2006; Wardlaw et al. 2013; Zhu et al. 2011). First described by Fisher (1965) in a cohort of 114 subjects who came to autopsy they were given the term “lacune”. Of this group, 88 brains had at least one lacune in the absence of clinical deficits or a stroke history. With the advent of MRI, the detection of clinically silent MRI infarcts has been extensively studied (Boon et al. 1994; Das et al. 2008; DeCarli et al. 2005b; Longstreth et al. 1998, 2002; Vermeer et al. 2003a, 2002, 2007). Despite variable definitions of MRI infarction (Hachinski et al. 2006; Wardlaw et al. 2013), all studies confirm age-related increases in the prevalence of these lesions [Fig. 6 modified after DeCarli, et al. (DeCarli et al. 2005b)].
Fig. 6.
Prevalence of MRI infarction by decade of life among subjects of the Framingham Heart Study
The presence of MRI infarcts has been associated with a variety of other abnormalities on brain imaging. For example, MRI infarcts commonly co-occur with symptomatic infarcts, are associated with increased extent of WMH and have the same risk factors (Das et al. 2008; DeCarli et al. 2005b; Vermeer et al. 2003b, 2007). In addition, individuals with MRI infarction have greater extent of cerebral atrophy (DeCarli et al. 2005b) and the presence of MRI infarction increases future risk of stroke, dementia and death (Debette et al. 2010; Vermeer et al. 2003a, b).
Cerebral Microbleed Studies
Cerebral microbleeds (CMB) are small, dot-like lesions with low signal intensity in the brain observed on gradient-echo T2*-weighted magnetic resonance imaging (Good et al. 1998). Pathological studies suggest that MR-visible lesions correspond to hemosiderin-laden macrophages in perivascular tissue, consistent with vascular leakage of blood cells (Fazekas et al. 1999; Shoamanesh et al. 2011). Amongst community based studies, the prevalence averages 10 % (Jeerakathil et al. 2004a, b), increasing to about 20 % in AD (Cordonnier et al. 2006), 50 % amongst individuals with ischemic stroke (Takashima et al. 2011) and over 80 % in patients with lobar intracranial hemorrhage (Takashima et al. 2011). In community studies, approximately 70 % of CMBs are located in lobar white or gray matter, while approximately 10 % are located in basal ganglia and the remaining 20 % are located infratentorially (Cordonnier et al. 2006; Sveinbjornsdottir et al. 2008) although different distributions have been noted when comparing CMBs due to vascular disease versus amyloidoses (Lee et al. 2007). The cognitive effects of CMBs have been difficult to fully determine due primarily to the associated brain pathologies that accompany them. In a comprehensive review of the subject, Cordonnier and van der Flier (2011) concluded that while there is sufficient evidence to suggest that CMBs contribute to cognitive impairment in association with cerebrovascular disease, the evidence of an independent effect of CMBs on cognition amongst individuals with AD is less certain (Figs. 7 and 8). One study suggested an independent effect (Goos et al. 2010), whereas another did not (Pettersen et al. 2008). The authors conclude that the evidence may be weak due to the low numbers of individuals in most studies (Cordonnier and van der Flier 2011). In studies of both cerebrovascular disease and AD, however, there has been limited effort to control for the confounding effects of concurrent pathology such as atrophy infarcts or white matter pathology associated with CMBs. A recent study by Werring et al. (2010), which reviewed available information on this topic, concluded that CMBs are best considered another measure of microvascular disease and that while some evidence exists for the cognitive effects of CMBs in cognitively normal populations or populations with cerebrovascular disease, there is a need for studies that adjust for all available measures of brain vascular injury (Fig. 8).
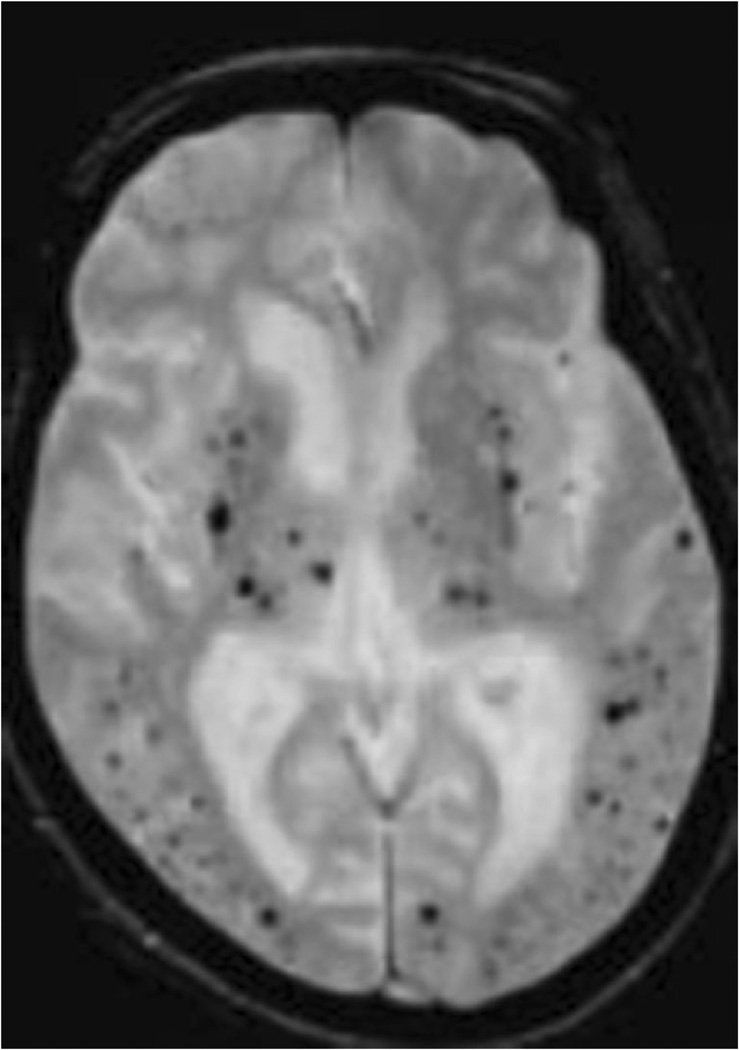
Fig. 7.
One example of extensive cerebral microbleeds as seen on gradient echo imaging
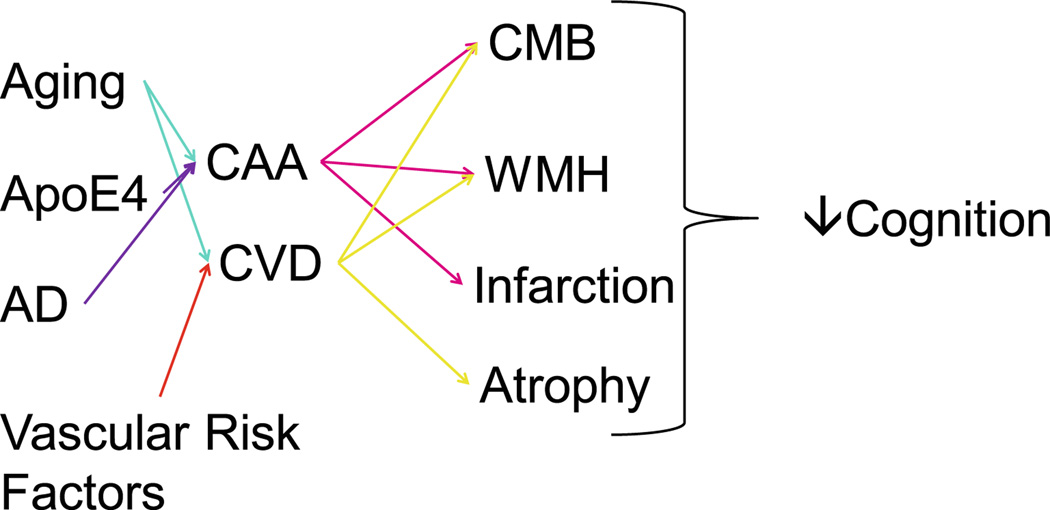
Fig. 8.
Graphic summary of the multi-factorial relationships between age, genetics, vascular risk factors and Alzheimer’s disease that result in cerebral amyloid angiopathy (CAA) or cerebrovascular disease (CVD) that lead to cerebral microbleeds (CMB), white matter hyperintensities (WMH), infarction and brain atrophy all of which contribute to reduced cognitive ability
Summary of Structural Brain Differences During “Normal Aging”
The preceding sections emphasize the consistency of differences and longitudinal change associated with advancing age. Measures of brain gray and white matter volumes show differing regional patterns reflecting differential vulnerability. Whereas cortical thickness and surface area measures differ slightly in magnitude, patterns of age-related differences are remarkably consistent. The causes for this differential vulnerability to age are unclear, but newer evidence suggests homology between age-related and degenerative processes. Moreover, it is apparent that common co-morbid medical illnesses such as hypertension also influence age-related differences and various measures of cerebral pathology such as WMH, infarction and cerebral microbleeds are remarkably common to advancing age. Recent data from amyloid imaging suggest similar increases in the prevalence of extensive amyloid retention with advancing age (Morris et al. 2009). These findings suggest that “healthy brain aging” (i.e., brain aging in the absence of pathology) may be less frequent that previously realized and that our concepts of “normal brain aging” may need to be revised in the setting of potential contamination by common, co-occurring disease (DeCarli 2013; DeCarli et al. 2012). In fact, investigations of older individuals who have intact brain structure may lead to new insights into resilience to pathology, better operational definition of the construct “healthy brain aging” and serve as a goal of cognitive aging research. Of course, it is expected that modifying processes that accelerate brain aging will likely result in improved cognitive health across the lifespan.
Studies of Cognitive Aging in Relation to Brain Aging
Cognitive aging is a gradual, late-life decline in cognitive performance, typically assessed using cognitive task measures, experienced to a degree by most humans who reach old age (Grady 2008). It is both ubiquitous yet difficult to define, and even more challenging to accurately and reliably measure. Decades of research have demonstrated age-correlated deficits both in basic cognitive resources (such as speed of processing) and in higher-order cognitive functions (Park and Reuter-Lorenz 2009; Salthouse 2004; Salthouse 2000, 2009). These complex cognitive functions include episodic memory and executive control. Accumulating evidence shows that while some cognitive functions decline, other functions like semantic memory can remain intact and even improve with age (Grady and Craik 2000; Salthouse 2004, 2009).
Even so, researchers frequently observe small but significant declines in cognitive performance (particularly in episodic memory and executive control) in studies of older adults without clinically symptomatic neurodegenerative or vascular conditions. Such age-associated cognitive declines (in the absence of clinically apparent disease) are often termed “normal” or “healthy cognitive aging,” and these older adults as “cognitively normal” or “cognitively healthy older adults.” However, even though these cognitive declines are minor, they can measurably influence clinical outcomes in older adults. For example, cognitive differences, even among unimpaired, cognitively healthy older adults predict poorer medication adherence (Hayes et al. 2009), and increase the rate of hospitalization (Wilson et al. 2014). Understanding the biological basis of cognitive differences in normal aging has the potential for enhancing quality of life and survival in late life.
Studies of brain structural measures and their relation to concurrent or future cognitive performance have become an important part of developing the ability to understand and predict—and potentially treat—age-related cognitive decline. In a diverse and well-defined older adult sample of 307 participants, Carmichael et al. (2012) examined whether structural brain MRI measures were reliable predictors of longitudinal cognitive function. They specifically assessed domains affected by aging and diseases of aging, including executive function, semantic memory, and episodic memory and found that participants who had larger baseline whole brain volumes and smaller baseline WMH volumes had improved future performance on the included cognitive measures. One potential mechanism underlying these findings is that higher brain volume could enable recruitment of greater compensatory processes in order to allow participants to maintain cognitive performance following structural brain injury (Cabeza et al. 2002; Farias et al. 2012). In addition, MRI measures of brain atrophy, WMH and MRI infarcts have all been associated with increased risk for dementia, stroke and even mortality (Debette et al. 2010; van der Veen et al. 2014) further emphasizing the association between healthy brain aging and longevity.
In another study relating brain aging to cognitive aging, Head et al. (2008) used path analysis to explore the mediating role of differences in brain structure, executive functions, and processing speed on age-related differences in episodic memory. Neuroanatomical variables included volumes of prefrontal gray and white matter, hippocampus, caudate nucleus, as well as primary visual cortex (thought to be largely excluded from brain aging). As expected, age was linked to smaller regional brain volumes and poorer cognitive performance, including episodic memory. Moreover, neural and cognitive factors completely mediated age differences in episodic memory. Thus, there not only exist multiple paths of influence mediating age effects on memory, but brain structural measures are also central in cognitive aging. In addition, this seminal study highlighted the value of multivariate approaches to study brain and cognitive aging.
Disruption of cerebral white matter may be critical to age-related cognitive declines, and Kennedy and Raz (2009) investigated the impact of age-related white matter integrity differences on cognition. They used FA and another DTI parameter, apparent diffusion coefficient (ADC), to examine associations of regional microstructural white matter integrity with performance on age-sensitive cognitive tasks, in 52 healthy adults (aged 19–81 years). Cognitive constructs included processing speed, working memory, inhibition, task switching, and episodic memory. They found that age and regional white matter integrity differentially influenced cognitive performance. Age-related degradation in anterior white matter areas was associated with decreased processing speed and poorer working memory, whereas reduced inhibition and greater task switching costs were linked to posterior white matter decline, and poorer episodic memory was associated with age-related differences in central white matter regions. The observed multiple dissociations among specific age-sensitive cognitive skills and their purported neuroanatomical substrates support the view that age-related cognitive declines are unlikely to stem from a single aspect of brain aging and are consistent with previous findings reported by Ziegler et al. (Ziegler et al. 2008).
Also in the realm of brain aging mediators of cognitive aging is a study from Bucur et al. (2008) investigating regional white matter FA as a mediator of age-related slowing of memory retrieval. Participants performed speed and episodic memory encoding and retrieval tasks to test whether white matter integrity, particularly in prefrontal regions, would mediate the relationship between speed and episodic memory retrieval. Results indicated that lower FA in pericallosal frontal regions and in genu of the corpus callosum, but not other regions, mediated the relationship between speed and episodic retrieval. This relation held, albeit to a different degree, for both hits and correct rejections, consistent with dual-process models of episodic memory (Yonelinas 2002). These findings suggest that prefrontal white matter integrity reduction is one mechanism underlying the relation between age-related individual differences in perceptual speed and episodic retrieval. The authors concluded that deterioration of white matter tracts due to normal brain aging slows the transmission of information across a network of cortical structures necessary for the retrieval of information from episodic long-term memory.
In summary, age-related differences in structural brain measures consistently coincide with age-related differences in cognitive performance across a broad range of cognitive tasks. These associations support the concept that brain behavior relationships actually increase in association with advancing age. As one investigator suggests, the use of “age correction” to investigate brain behavior associations may actually reduce this association (Mungas et al. 2009). Correcting for age while investigating brain behavior relationships could, therefore, potentially obfuscate findings that may be important to developing therapeutics to preserve brain health as a method to maintain life-long cognitive ability.
Conclusions
Normal aging involves multiple overlapping processes occurring in brain structure that can be quantitatively assessed using MRI, PET, and other imaging methods, and can inform the study of cognitive aging when judiciously used in conjunction with careful cognitive testing. More recent multivariate approaches, particularly using regionally specific analyses and longitudinal assessments, have the potential to elucidate critical aspects of brain aging, understand differences between “Normal” and “Healthy” brain aging and to inform potential treatments to stall or reverse the effects of brain aging and extend cognitive health.
Contributor Information
Samuel N. Lockhart, Department of Neurology and Center for Neuroscience, University of California at Davis, Sacramento, CA, USA
Charles DeCarli, Email: cdecarli@ucdavis.edu, Department of Neurology and Center for Neuroscience, University of California at Davis, Sacramento, CA, USA.
References
- Aggarwal NT, Wilson RS, Bienias JL, De Jager PL, Bennett DA, Evans DA, DeCarli C. The association of magnetic resonance imaging measures with cognitive function in a biracial population sample. Archives of Neurology. 2010;67(4):475–482. doi: 10.1001/archneurol.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. Journal of Molecular Neuroscience : MN. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Bender AR, Raz N. Age-related differences in memory and executive functions in healthy APOE varepsilon4 carriers: the contribution of individual differences in prefrontal volumes and systolic blood pressure. Neuropsychologia. 2012;50(5):704–714. doi: 10.1016/j.neuropsychologia.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon A, Lodder J, Heuts-van Raak L, Kessels F. Silent brain infarcts in 755 consecutive patients with a first-ever supratentorial ischemic stroke. Relationship with index-stroke subtype, vascular risk factors, and mortality. Stroke. 1994;25(12):2384–2390. doi: 10.1161/01.str.25.12.2384. [DOI] [PubMed] [Google Scholar]
- Boone KB, Miller BL, Lesser IM, Mehringer CM, Hill-Gutierrez E, Goldberg MA, Berman NG. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect. Archives of Neurology. 1992;49(5):549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of Neuropathology and Experimental Neurology. 2011;70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Breteler MM. Vascular involvement in cognitive decline and dementia. Epidemiologic evidence from the Rotterdam Study and the Rotterdam Scan Study. Annals of the New York Academy of Sciences. 2000;903(11 Suppl 5):457–465. doi: 10.1111/j.1749-6632.2000.tb06399.x. [DOI] [PubMed] [Google Scholar]
- Breteler MM, van Amerongen NM, van Swieten JC, Claus JJ, Grobbee DE, van Gijn J, Hofman A, van Harskamp F. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on MRI: the Rotterdam Study. Stroke. 1994;25:1109–1115. doi: 10.1161/01.str.25.6.1109. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Archives of Neurology. 2008;65(8):1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronge L. Magnetic resonance imaging in dementia. A study of brain white matter changes. Acta Radiologica. Supplementum. 2002;43(428):1–32. doi: 10.1034/j.1600-0455.43.s.428.1.x. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. Journal of Neuroscience. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiology of Aging. 2008;29(7):1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25(3):305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, Mcintosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neurolmage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Carmichael O, Mungas D, Beckett L, Harvey D, Tomaszewski Farias S, Reed B, Decarli C. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiology of Aging. 2012;33(1):83–95. doi: 10.1016/j.neurobiolaging.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L Initiative, t. A. D. N. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimers disease neuroimaging initiative. Archives of Neurology. 2010 doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, Kolb HC. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. Journal of Alzheimer’s Disease. 2013;34(2):457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Current Opinion in Neurology. 2008;21(1):83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Lucke JE, Saxton JA, Ratcliff G, Unitas LJ, Billig B, Bryan RN. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Archives of Neurology. 1998;55(2):169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Ratcliff G, Saxton JA, Bryan RN, Fried LP, Lucke JF. Cognitive correlates of human brain aging: a quantitative magnetic resonance imaging investigation. Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13(4):471–485. doi: 10.1176/jnp.13.4.471. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999;53(1):189–196. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WE, Parashos IA, Soady SA, Sullivan RJ, Patterson LJ, Djang WT. Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology. 1992;42(3 Pt 1):527–536. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- Cordonnier C. Brain microbleeds. Practical Neurology. 2010;10(2):94–100. doi: 10.1136/jnnp.2010.206086. [DOI] [PubMed] [Google Scholar]
- Cordonnier C, Klijn CJ, van Beijnum J, Al-Shahi Salman R. Radiological investigation of spontaneous intracerebral hemorrhage: systematic review and trinational survey. Stroke. 2010;41(4):685–690. doi: 10.1161/STROKEAHA.109.572495. [DOI] [PubMed] [Google Scholar]
- Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer’s disease: innocent observation or key player? Brain. 2011;134(Pt 2):335–344. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66(9):1356–1360. doi: 10.1212/01.wnl.0000210535.20297.ae. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcorical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. In: Grafman J, Holyoak KJ, editors. Annals of the New York Academy of Sciences. Vol. 769. New York: The New York Academy of Sciences; 1995. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Wolf PA. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39(11):2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Breteler MM. Cognitive correlates of cerebral white matter changes. Journal of Neural Transmission Supplementum. 1998;53(1):41–67. doi: 10.1007/978-3-7091-6467-9_5. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study [see comments] Annals of Neurology. 2000;47(2):145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. A follow-up study of blood pressure and cerebral white matter lesions. Annals of Neurology. 1999;46(6):827–833. doi: 10.1002/1531-8249(199912)46:6<827::aid-ana4>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Debette S, Beiser A, Decarli C, Au R, Himali JJ, Kelly-Hayes M, Seshadri S. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality. The Framingham Offspring Study. Stroke. 2010;41(4):600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Decarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C. Clinically asymptomatic vascular brain injury: a potent cause of cognitive impairment among older individuals. Journal of Alzheimer’s Disease. 2013;33(Suppl 1):S417–S426. doi: 10.3233/JAD-2012-129004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005a;36(1):50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Kawas C, Morrison JH, Reuter-Lorenz PA, Sperling RA, Wright CB. Session II: Mechanisms of age-related cognitive change and targets for intervention: neural circuits, networks, and plasticity. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2012;67(7):747–753. doi: 10.1093/gerona/gls111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiology of Aging. 2005b;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;3(3):529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Gillette JA, Haxby JV, Teichberg D, Schapiro MB, Horwitz B. Lack of age-related differences in temporal lobe volume of very healthy adults. AJNR American Journal of Neuroradiology. 1994;15(4):689–696. [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45(11):2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- den Heijer T, van der Lijn F, IKram A, Koudstaal PJ, van der Lugt A, Krestin GP, Breteler MM. Vascular risk factors, apolipoprotein E, and hippocampal decline on magnetic resonance imaging over a 10-year follow-up. Alzheimers Dement. 2012;8(5):417–425. doi: 10.1016/j.jalz.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6(3):275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Lutti A, Kherif F. Impact of brain aging and neurodegeneration on cognition: evidence from MRI. Current Opinion in Neurology. 2013;26(6):640–645. doi: 10.1097/WCO.0000000000000029. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiology of Aging. 2006;27(5):733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed B, Carmichael O, Beckett L, Harvey D, Decarli C. Maximal brain size remains an important predictor of cognition in old age, independent of current brain pathology. Neurobiology of Aging. 2012;33(8):1758–1768. doi: 10.1016/j.neurobiolaging.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, Hartung HP. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR American Journal of Neuroradiology. 1999;20(4):637–642. [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Schmidt R, Scheltens P. Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dementia and Geriatric Cognitive Disorders. 1998;9(Supp 1):2–5. doi: 10.1159/000051182. [DOI] [PubMed] [Google Scholar]
- Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology. 1965;15:774–784. doi: 10.1212/wnl.15.8.774. [DOI] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Alzheimer’s Disease Neuroimaging I. Brain changes in older adults at very low risk for Alzheimer’s disease. Journal of Neuroscience. 2013;33(19):8237–8242. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Alzheimer’s Disease Neuroimaging I. What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Progress in Neurobiology. 2014a;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Alzheimer Disease Neuroimaging I. Accelerating cortical thinning: unique to dementia or universal in aging? Cerebral Cortex. 2014b;24(4):919–934. doi: 10.1093/cercor/bhs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Raman M, Huebner P, Liu A, Mungas D, Carmichael O, DeCarli C. Loss of fornix white matter volume as a predictor of cognitive impairment in cognitively normal elderly individuals. JAMA Neurology. 2013;70(11):1389–1395. doi: 10.1001/jamaneurol.2013.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64(6):1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Bocchetta M, Chetelat G, Rabinovici GD, de Leon MJ, Kaye J for, I. s. N. P. I. A. Imaging markers for Alzheimer disease: which vs how. Neurology. 2013;81(5):487–500. doi: 10.1212/WNL.0b013e31829d86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattringer T, Enzinger C, Ropele S, Gorani F, Petrovic KE, Schmidt R, Fazekas F. Vascular risk factors, white matter hyperintensities and hippocampal volume in normal elderly individuals. Dementia and Geriatric Cognitive Disorders. 2012;33(1):29–34. doi: 10.1159/000336052. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR - American Journal of Neuroradiology. 2002;23(8):1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965a;88(2):237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965b;88(3):585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Outcomes R. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- Good CD, Ng VW, Clifton A, Britton JA, Hart Y, Wilkins P. Amyloid angiopathy causing widespread miliary haemorrhages within the brain evident on MRI. Neuroradiology. 1998;40(5):308–311. doi: 10.1007/s002340050590. [DOI] [PubMed] [Google Scholar]
- Goos JD, Henneman WJ, Sluimer JD, Vrenken H, Sluimer IC, Barkhof E, van der Flier WM. Incidence of cerebral microbleeds: a longitudinal study in a memory clinic population. Neurology. 2010;74(24):1954–1960. doi: 10.1212/WNL.0b013e3181e396ea. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age. Current Opinion in Neurobiology. 2000;10(2):224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Head D, McQuain J, Acker JD, Raz N. Differential aging of the human striatum: a prospective MR imaging study. AJNR - American Journal of Neuroradiology. 1998;19(8):1501–1507. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50(4):972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Hayes TL, Larimer N, Adami A, Kaye JA. Medication adherence in healthy elders: small cognitive changes make a big difference. Journal of Aging and Health. 2009;21(4):567–580. doi: 10.1177/0898264309332836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Rodrigue KM, Kennedy KM, Raz N. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology. 2008;22(4):491–507. doi: 10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton L, Carter K, Reed BR, Beckett L, Lara E, DeCarli C, Mungas D. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Disease and Associated Disorders. 2010;24(3):234–241. doi: 10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurology. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W, D’ Eposito M. In: Imaging the aging brain. Jagust W, D’ Eposito M, editors. New York: Oxford; 2009. [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au Rs, Kase CS, DeCarli C. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004a;35(8):1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke; A Journal of Cerebral Circulation. 2004b;33(8):1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: localization of age-related changes. Biological Psychiatry. 1991;29(1):55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22(4):581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jones DW, Hall JE. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and evidence from new hypertension trials. Hypertension. 2004;43(1):1–3. doi: 10.1161/01.HYP.0000110061.06674.ca. [DOI] [PubMed] [Google Scholar]
- Kaye JA, DeCarli C, Luxenberg JS, Rapoport SI. The significance of age-related enlargement of the cerebral ventricles in healthy men and women measured by quantitative computed X-ray tomography. Journal of the American Geriatrics Society. 1992;40(3):225–231. doi: 10.1111/j.1532-5415.1992.tb02073.x. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47(3):916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann EC, Braskie MN, Landau SM, O’Neil JP, Jagust WJ. Dopamine and frontostriatal networks in cognitive aging. Neurobiology of Aging. 2012;33(3):e615–e624. doi: 10.1016/j.neurobiolaging.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Långström B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of Neurology. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Carmichael OT, Singh B, Mungas D, Reed B, DeCarli C. Sub-Regional Hippocampal Injury is Associated with Fornix Degeneration in Alzheimer’s Disease. Frontiers in Aging Neuroscience, 4. 2012;1 doi: 10.3389/fnagi.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, Decarli C. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009;73(21):1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, DeCarli C. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer disease. Stroke. 2010;41(8):1791–1797. doi: 10.1161/STROKEAHA.110.582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim SM, Kim N, Yoon BW, Roh JK. Cortico-subcortical distribution of microbleeds is different between hypertension and cerebral amyloid angiopathy. Journal of Neurological Sciences. 2007;258(1–2):111–114. doi: 10.1016/j.jns.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology of Aging. 2012;33617(3):e611–e617.e619. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Zipursky RB, Murphy GM, Jr, Pfefferbaum A. In vivo quantification of the limbic system using MRI: effects of normal aging. Psychiatry Research. 1990;35(1):15–26. doi: 10.1016/0925-4927(90)90005-q. [DOI] [PubMed] [Google Scholar]
- Lockhart SN, Mayda AB, Roach AE, Fletcher E, Carmichael O, Maillard P, Decarli C. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Frontiers in Human Neuroscience, 6. 2012;56 doi: 10.3389/fnhum.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SN, Roach AE, Luck SJ, Geng J, Beckett L, Carmichael O, DeCarli C. White matter hyperintensities are associated with visual search behavior independent of generalized slowing in aging. Neuropsychologia. 2014;52:93–101. doi: 10.1016/j.neuropsychologia.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Arnold AM, Manolio TA, Burke GL, Bryan N, Jungreis CA, Fried L. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3,301 elderly people. The Cardiovascular Health Study. Collaborative Research Group. Neuroepidemiology. 2000;19(1):30–42. doi: 10.1159/000026235. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Archives of Neurology. 1998;55(9):1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O’Leary D, Furberg CD. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33(10):2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study [see comments] Stroke. 1996;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neurolmage. 2004;21(3):1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Maillard P, Carmichael O, Fletcher E, Reed B, Mungas D, DeCarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012a;79(5):442–448. doi: 10.1212/WNL.0b013e3182617136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P, Carmichael O, Harvey D, Fletcher E, Reed B, Mungas D, DeCarli C. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR -American Journal of Neuroradiology. 2013;34(1):54–61. doi: 10.3174/ajnr.A3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P, Fletcher E, Harvey D, Carmichael O, Reed B, Mungas D, DeCarli C. White matter hyperintensity penumbra. Stroke. 2011;42(7):1917–1922. doi: 10.1161/STROKEAHA.110.609768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurology. 2012b;11(12):1039–1047. doi: 10.1016/S1474-4422(12)70241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nature Review Neuroscience. 2006;7(4):278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayda AB, Westphal A, Carter CS, DeCarli C. Late life cognitive control deficits are accentuated by white matter disease burden. Brain. 2011;134(Pt 6):1673–1683. doi: 10.1093/brain/awr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayda AV, DeCarli C. Vascular cognitive impairment: Prodrome to VaD? In: Wahlund L-O, Erkinjuntti T, Gauthier S, editors. Vascular cognitive impairment in clinical practice. Cambridge: Cambridge University Press; 2009. pp. 11–31. [Google Scholar]
- Mega MS, Cummings JL. Frontal-subcortical Circuits and Neuropsychiatric Disorders. Neurosciences. 1994;6:358–370. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W, Ulrich J, Bruhlmann M, Meier E. Age-related white matter atrophy in the human brain. Annals of the New York Academy of Sciences. 1992;673:260–269. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI atlas of human white matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Archives of Neurology. 2009;66(12):1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Farias ST, Decarli C. Age and education effects on relationships of cognitive test scores with brain structure in demographically diverse older persons. Psychology and Aging. 2009;24(1):116–128. doi: 10.1037/a0013421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Jagust WJ, DeCarli C, Mack WJ, Kramer JH, Chui HC. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59(6):867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, Mcintosh AR, Daly E, Mentis MJ, Pietrini P, Rapoport SI. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Archives of General Psychiatry. 1996;53(7):585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, Schapiro MB, Rapoport SI, Horwitz B. Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging [published erratum appears in Arch Neurol 1994 Jan;51(l):60] Archives of Neurology. 1992;49(8):839–845. doi: 10.1001/archneur.1992.00530320063013. [DOI] [PubMed] [Google Scholar]
- Nettiksimmons J, Beckett L, Schwarz C, Carmichael O, Fletcher E, Decarli C. Subgroup of ADNI normal controls characterized by atrophy and cognitive decline associated with vascular damage. Psychology and Aging. 2013;28(1):191–201. doi: 10.1037/a0031063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. Journal Cognitive Neuroscience. 2006;18(3):418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Reed BR, Jagust WJ. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia. 2005;43(11):1688–1697. doi: 10.1016/j.neuropsychologia.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum AO, Tang CY, Buchsbaum MS, Wei TC, Atlas SW. Regional and global changes in cerebral diffusion with normal aging. AJNR American Journal of Neuroradiology. 2001;22(1):136–142. [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75(3):441–447. doi: 10.1136/jnnp.2003.014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortman JM, Guarneri CE. United States population projections: 2000 to 2050. United States Census Bureau. 2009 [Google Scholar]
- Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39(11):1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- Pantoni L. Pathophysiology of age-related cerebral white matter changes. Cerebrovascular Diseases (Basel, Switzerland) 2002;13(Suppl 2):7–10. doi: 10.1159/000049143. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CM, Iosif AM, Farias S, Reed B, Mungas D, Decarli C. Executive function mediates effects of white matter hyperintensities on episodic memory. Neuropsychologia. 2011;49(10):2817–2824. doi: 10.1016/j.neuropsychologia.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen JA, Sathiyamoorthy G, Gao FQ, Szilagyi G, Nadkarni NK, George-Hyslop P, St, Black SE. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Archives of Neurology. 2008;65(6):790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neurolmage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR -American Journal of Neuroradiology. 2001;22(6):1161–1167. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21(2):149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Acker JD. Age-related shrinkage of the mamillary bodies: in vivo MRI evidence. Neuroreport. 1992a;3(8):713–716. doi: 10.1097/00001756-199208000-00016. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Acker JD. Age, gender, and hemispheric differences in human striatum: a quantitative review and new data from in vivo MRI morphometry. Neurobiology of Learning and Memory. 1995;63(2):133–142. doi: 10.1006/nlme.1995.1013. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Spencer WD, White K, Acker JD. Age-related regional differences in cerebellar vermis observed in vivo. Archives of Neurology. 1992b;49(4):412–416. doi: 10.1001/archneur.1992.00530280106030. [DOI] [PubMed] [Google Scholar]
- Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, Decarli C. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133(pt8):2196–2209. doi: 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinek H, De Santi S, Frid D, Tsui WH, Tarshish CY, Convit A, de Leon MJ. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229(3):691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salerno JA, Murphy DG, Horwitz B, DeCarli C, Haxby JV, Rapoport SI, Schapiro MB. Brain atrophy in hypertension. A volumetric magnetic resonance imaging study. Hypertension. 1992;20(3):340–348. doi: 10.1161/01.hyp.20.3.340. [DOI] [PubMed] [Google Scholar]
- Salthouse T. What and when of cognitive aging. Current Directions in Psychological Science. 2004;13(4):140–144. doi: 10.1177/0963721414535212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biological Psychology. 2000;54(1–3):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of Aging. 2009;30(4):507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;7(3):273–295. [Google Scholar]
- Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Experimental Neurology. 1975;47(3):392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Koch M, Kapeller P, Augustin M, Offenbacher H, Lechner H. Magnetic resonance imaging cerebral abnormalities and neuropsychologic test performance in elderly hypertensive subjects. A case-control study. Archives of Neurology. 1995;52(9):905–910. doi: 10.1001/archneur.1995.00540330087019. [DOI] [PubMed] [Google Scholar]
- Seab JP, Jagust WJ, Wong STS, et al. Quantitative NMR measurements of hippocampal atrophy in Alzheimer’s disease. Magnetic Resonance in Medicine. 1988;8:200–208. doi: 10.1002/mrm.1910080210. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, DeCarli C. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63(9):1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovascular Diseases. 2011;32(6):528–534. doi: 10.1159/000331466. [DOI] [PubMed] [Google Scholar]
- Simpson JE, Fernando MS, Clark L, Ince PG, Matthews F, Forster G, Wharton SB. White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathology and Applied Neurobiology. 2007a;33(4):410–419. doi: 10.1111/j.1365-2990.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- Simpson JE, Ince PG, Higham CE, Gelsthorpe CH, Fernando MS, Matthews F, Wharton SB. Microglial activation in white matter lesions and nonlesional white matter of ageing brains. Neuropathology and Applied Neurobiology. 2007b;33(6):670–683. doi: 10.1111/j.1365-2990.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. The concept of cognitive reserve: a catalyst for research. Journal of Clinical and Experimental Neuropsychology. 2003;25(5):589–593. doi: 10.1076/jcen.25.5.589.14571. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001a;12(1):99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiology of Aging. 1995;16(4):591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30(6):749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroradiological characterization of normal adult ageing. The British Journal of Radiology. 2007;80(Spec No 2):S99–S108. doi: 10.1259/bjr/22893432. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Desmond JE, Pfefferbaum A. Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiology of Aging. 2001b;22(4):603–611. doi: 10.1016/s0197-4580(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, Launer LJ. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. Journal of Neurology, Neurosurgery and Psychiatry. 2008;79(9):1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Mller BL, Reed T, Wolf PA, Carmelli D. Biobehavioral characteristics of nondemented older adults with subclinical brain atrophy. Neurology. 2000;54(11):2108–2114. doi: 10.1212/wnl.54.11.2108. [DOI] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Mori T, Hashimoto M, Kinukawa N, Uchino A, Yuzuriha T, Yao H. Clinical correlating factors and cognitive function in community-dwelling healthy subjects with cerebral microbleeds. Journal of Stroke and Cerebrovascular Diseases. 2011;20(2):105–110. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Scanlon BK, Farrell M, Hernandez B, Adamson MM, Ashford JW, Weiner MW. APOE-epsilon4 and aging of medial temporal lobe gray matter in healthy adults older than 50 years. Neurobiology of Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, Jagust WJ. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63(2):246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen PH, Muller M, Vincken KL, Mali WP, van der Graaf Y, Geerlings MI, Group SS. Brain volumes and risk of cardiovascular events and mortality. The SMART-MR study. Neurobiology of Aging. 2014;35(7):1624–1631. doi: 10.1016/j.neurobiolaging.2014.02.003. [DOI] [PubMed] [Google Scholar]
- van Velsen EF, Vernooij MW, Vrooman HA, van der Lugt A, Breteler MM, Hofman A, Ikram MA. Brain cortical thickness in the general elderly population: the Rotterdam Scan Study. Neuroscience Letters. 2013;550:189–194. doi: 10.1016/j.neulet.2013.06.063. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287(8):1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. New England Journal of Medicine. 2001;345(18):1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, DenHeijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003a;34(2):392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003b;34(5):1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2002;33(1):21–25. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurology. 2007;6(1):611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- Villeneuve S, Reed BR, Madison CM, Wirth M, Marchant NL, Kriger S, Jagust WJ. Vascular risk and Abeta interact to reduce cortical thickness in AD vulnerable brain regions. Neurology. 2014 doi: 10.1212/WNL.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R nEuroimaging STf. R. V. c. o. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurology. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neurolmage. 2004;22(1):144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. Journal of Neurological Sciences. 2010;299(1–2):131–135. doi: 10.1016/j.jns.2010.08.034. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA National High Blood Pressure Education Program Coordinating, C. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288(15):1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer disease. Archives of Neurology. 1999;56(10):1274–1279. doi: 10.1001/archneur.56.10.1274. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA. Person-specific paths of cognitive decline in Alzheimer’s disease and their relation to age. Psychology and Aging. 2000;15(1):18–28. doi: 10.1037//0882-7974.15.1.18. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Rajan KB, Barnes LL, Hebert LE, Mendes de Leon CF, Evans DA. Cognitive aging and rate of hospitalization in an urban population of older people. Journals of GerontologySeries A, Biological Sciences and Medical Sciences. 2014;69(4):447–454. doi: 10.1093/gerona/glt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke; A Journal of Cerebral Circulation. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, Kolb HC. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013;9(6):666–676. doi: 10.1016/j.jalz.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]
- Yoshita M, Fletcher E, DeCarli C. Current concepts of analysis of cerebral white matter hyperintensities on magnetic resonance imaging. Topics in Magnetic Resonance Imaging. 2005;16(6):399–407. doi: 10.1097/01.rmr.0000245456.98029.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68(1):13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YC, Dufouil C, Tzourio C, Chabriat H. Silent brain infarcts: a review of MRI diagnostic criteria. Stroke. 2011;42(4):1140–1145. doi: 10.1161/STROKEAHA.110.600114. [DOI] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]