Abstract
Although evidence supports the efficacy of early intervention for improving outcomes for children with autism spectrum disorder (ASD), the mechanisms underlying their effectiveness remain poorly understood. This paper reviews the research literature on the neural bases of the early core deficits in ASD and proposes three key features of early intervention related to the neural mechanisms that may contribute to its effectiveness in improving deficit areas. These features include (1) the early onset of intensive intervention which capitalizes on the experience-expectant plasticity of the immature brain, (2) the use of treatment strategies that address core deficits in social motivation through an emphasis on positive social engagement and arousal modulation, and (3) promotion of complex neural networks and connectivity through thematic, multi-sensory and multi-domain teaching approaches. Understanding the mechanisms of effective early intervention will enable us to identify common or foundational active ingredients for promoting optimal outcomes in children with ASD.
Keywords: autism spectrum disorder, early intervention, neural mechanisms
1. Introduction
Collective efforts in the fields of genetics, neurobiology, and psychology are being made to address the etiology, identification, and treatment of autism spectrum disorder (ASD). Studies of early intensive behavioral intervention for young children with ASD embody such integrated approaches and reveal substantial improvements for a large subset of children, particularly when initiated during toddlerhood or preschool age and continued for 2 to 3 years (Wallace & Rogers, 2010). Such behaviorally based interventions are associated with improvements in the domains of cognition, adaptive behavior, and language (Rogers & Vismara, 2008), suggesting a certain level of plasticity in these aspects of development, especially when intervention is started early (Drew et al., 2002; Harris & Handelman, 2000).
Research on early brain development in ASD suggests that the way in which young children with ASD interact with their environment affects brain connections and neural responses, potentially having long-term implications for both behavior and brain development. Early intervention, therefore, is imperative in shaping the brain to be receptive to the social world, and in doing so, preventing or mitigating the symptoms and severity associated with ASD (Dawson, 2008; Redcay & Courchesne, 2005; Sigman et al., 2004; Wallace & Rogers, 2010). What are the mechanisms underlying the effectiveness of early intervention for ASD? The current paper aims to answer this question by proposing three key features of effective early intervention that promote development and address early core deficits by engaging experience-expectant neural mechanisms. The Early Start Denver Model (ESDM) is used as an exemplar to demonstrate the relation between these features of intervention and the mechanisms through which they enhance learning and plasticity. The three key features include (1) the early onset of intensive intervention, which capitalizes on the experience-expectant plasticity of the immature brain, (2) the use of treatment strategies that address core deficits in social motivation through an emphasis on positive social engagement and arousal modulation, and (3) promotion of complex neural networks and connectivity through thematic, multi-sensory and multi-domain teaching approaches. In the next section, we will review progress in the design and implementation of evidence-based interventions with toddlers with ASD, devoting particular attention to components of early intervention design that have been established as essential to its effectiveness.
2. Components of effective early intervention approaches
Studies of early intensive behavioral intervention in ASD reveal substantial improvements for many children with ASD when initiated during toddlerhood or preschool age and continued for at least two years (see Warren et al., 2011 for a review). With advances in the early detection of ASD and the reliable diagnosis in 2 year olds (Chawarska et al., 2007; Turner & Stone, 2007), comprehensive intervention models for children with ASD beginning treatment before 30 months of age now exist and show promising results for improving children's outcomes (Dawson et al., 2010; Landa et al., 2011; McConachie et al., 2005; Vismara et al., 2009; Wetherby & Woods, 2006).
Dawson and Osterling (1997) described common elements of successful early intervention programs for children with ASD, which include (a) curriculum content (attention to social stimuli, imitation, receptive and expressive language, appropriate play, social interaction), (b) highly supportive teaching environments and generalization strategies, (c) predictability and routine, (d) functional approach to problem behaviors, (e) plans for transition from preschool classroom (teaching independent “survival” skills), and (f) family involvement (parents as co-therapists). In 2001, the National Research Council formed the Committee on Educational Interventions for Children with Autism to determine common features of effective early intervention for autism (National Research Council, 2001). Based on the integration of scientific, theoretical, and policy literature, the committee proposed six critical features of early interventions, preschools, and school programs designed for children with ASD from birth to age 8: (a) entry into intervention programs as soon as an autism spectrum diagnosis is seriously considered; (b) active engagement in intensive instructional programming for a minimum of the equivalent of a full school day, 5 days (at least 25 hours) a week, with full year programming varied according to the child's chronological age and developmental level; (c) repeated, planned teaching opportunities generally organized around relatively brief periods of time for the youngest children (e.g., 15–20 minute intervals), including sufficient amounts of adult attention in one-to-one and very small group instruction to meet individualized goals; (d) inclusion of a family component, including parent training; (e) low student/teacher ratios (no more than two young children with autistic spectrum disorders per adult in the classroom); and (f) mechanisms for ongoing program evaluation and assessments of individual children's progress, with results translated into adjustments in programming (National Research Council, 2001).
In recent years, notable progress continues to be made in the area of early intervention for young children with ASD, but adequate evidence pinpointing specific and necessary active ingredients of intervention is still lacking. In an effort to identify evidence-based characteristics on which to build interventions for infants and toddlers with ASD, Wallace and Rogers (2010) conducted a systematic review of 32 controlled, efficacious interventions for infants and toddlers with developmental disorders or developmental risks other than ASD. These target populations included infants born prematurely, those with developmental delays including Down syndrome, and those at risk for intellectual disability due to family characteristics (e.g., poverty or parental intellectual disability). Wallace and Rogers (2010) identified the specific intervention procedures that were used consistently across the studies including: 1) involving parents through ongoing coaching (specifically focusing on parental responsivity and sensitivity to child cues) and teaching parents to provide the interventions, 2) individualizing the intervention with consideration of each infant's developmental profile, 3) targeting a broad rather than narrow range of learning goals, and 4) beginning the intervention as early as the risk is detected and providing intervention that is both intense and longer in duration. In addition to these characteristics of efficacious interventions, Wallace and Rogers (2010) highlight the importance of long-term follow-up in determining the value of an intervention. Overall, the review describes elements that are consistent with those previously proposed by other research groups (Dawson & Osterling, 1997; Landa, 2007; National Research Council, 2001; Woods & Wetherby, 2003) and provides a foundation for designing and evaluating evidence-based interventions for infants and toddlers with ASD.
The importance of early intervention in young children with ASD is underscored by findings that demonstrate the plasticity of the young brain in response to early experience, particularly early intervention or stimulation (e.g., Bates & Roe, 2001; Madjan & Shatz, 2006). Thus, early intervention for young children with ASD likely plays an influential role in the development of synaptic connections as well as the establishment and refinement of brain circuits, particularly those circuits involved in processing social information, having long-term mitigating effects on the symptoms and severity associated with ASD (Dawson, 2008; Wallace & Rogers, 2010).
While essential or critical “components” of early intervention have been well described, the current paper attempts to expand beyond the identification of intervention components to examine ways in which those components are interacting and operating at both a behavioral and a neural level. Research demonstrates significant improvement in toddlers and infants with ASD when the components of parent involvement, individualization, broad developmental targets, and early initiation of treatment are included. How do these components actively influence behavioral and neural development? What are the mechanism of action by which intervention results in positive outcomes for individuals with ASD?
In the next section, we briefly review literature on early brain development in children with ASD. Following this, we will describe three features of early intervention that we propose are closely tied to the neural mechanisms by which early intervention influences the trajectories of brain and behavioral development in children with ASD.
3. Early brain development in ASD: Atypical growth and functional connectivity
Advances in the field of cognitive and developmental neuroscience have shed light on the trajectories of early brain development and function in ASD and their potential relation with the emergence and severity of ASD symptoms (Courchesne et al., 2007; Müller, 2007). MRI studies examining the brain volume of infants and children with ASD demonstrate an unusually rapid rate of brain growth during infancy and toddlerhood followed by an accelerated decline in growth rate (Courchesne et al., 2001; Courchesne et al., 2003; Courchesne et al., 2007; Hazlett et al., 2005; Lainhart et al., 1997; Redcay & Courchesne, 2005). Early neuronal overgrowth is hypothesized to result in both structural abnormalities as well as a disruption in the normal developmental trajectory of anatomical and functional connectivity in the cortex (Courchesne & Pierce, 2005). The period in typical development when synaptogenesis and myelination are at their peak occurs between 2 to 4 years of age (Webb, Monk, & Nelson, 2001). In infancy, there is some evidence of an early excess of structural connectivity followed by a slowing in white matter fiber tract development and a reduction of long-distance connectivity (Wolff et al., 2012), ultimately leading to under-connectivity between distal areas of the brain essential for higher-order social, emotional, and communication functions (Hadders-Algra, 2008; Rippon et al., 2007). Structural and functional evidence of this cortical connectivity deficit in individuals with ASD has been documented using fMRI, EEG, and MEG techniques (e.g., Just et al., 2007; Minshew & Williams, 2007; Murias et al., 2007; Rippon et al., 2007).
The theory of abnormal cortical connectivity has been used to explain the wide range of information-processing deficits seen in ASD. For example, Johnson and colleagues (2002) propose that the development of neural and cognitive processes goes awry in ASD due to a lack of balance between specialization and integration processes. While individuals with ASD show a decrease in global connectivity (i.e., integration processes), they show a normal or increased connectivity within local networks (i.e., specialization processes). The imbalance between distal and local connectivity might contribute to enhancements in some aspects of information-processing, such as visual processing (Mottron et al., 2006), along with deficits in other functions, such as multi-modal integration (Brock et al., 2002; Rippon et al., 2007). Williams and colleagues (2006) describe a “complex information-processing model” of ASD that proposes that individuals with ASD have selective impairments on tasks with high demands for integration of information. According to this model, a breakdown in processing occurs in individuals with ASD when the processing demands exceed capacity or become too complex. According to Williams and colleagues (2006) the concept of complexity has more to do with the effect on the brain's mechanisms during processing information (i.e., processing across domains and sensory modalities) than it does with the type of information (i.e., social or language). Thus, individuals with ASD tend to demonstrate impaired performance on complex or higher order tasks that are well within the capability of individuals of their general ability level, yet they can perform lower order perceptual tasks in the same domains as well as or even exceeding their peers (Williams et al., 2006). This conceptualization is consistent with the idea of sufficient or excess short-distance connectivity in individuals with ASD, but long distance under-connectivity.
Further elaboration of the theory of cortical connectivity differences in ASD is offered by Oberman and Ramachandran (2008). They propose that the social, communicative, and motor symptoms of ASD may be explained by an underlying impairment in multi-sensory integration (MSI) systems. One such system is the mirror neuron system (MNS), which plays a role in both action understanding and imitation (Rizzolatti & Craighero, 2004). Deficits in the MNS would have large implications for ASD, as this system is an integral part of social brain circuitry and is involved in multiple aspects of brain functions including action understanding, imitation, language understanding, empathy, and the development of speech (Oberman & Ramachandran, 2008).
Rubenstein and Merzenich (2003), and others have proposed that ASD is a disorder in the ratio of excitatory and inhibitory activity in cortical networks. Research has identified a reduction in GABAergic signaling as a possible mechanism through which this imbalance occurs (Rubenstein & Merzenich, 2003). Additional advances in understanding how inhibitory signals contribute to the function of individual neurons, circuits, and networks in the cortex (Isaacson & Scanziani, 2011) and improved ability to measure and model neural connectivity (e.g., the ability to track temporal dynamics and signal characteristics using EEG and MEG) will provide new insights into how dysfunction or loss of inhibitory neurons might affect cortical connectivity in ASD (Gogolla et al., 2009).
The disruption of complex information processing and the coordination of high-order brain regions in ASD occur early in life and may lead to impairments in behaviors that require integration among higher-order brain regions. Subsequently, different brain regions are recruited and, through experience, perpetuate ongoing brain changes over time (Bauman & Kemper, 1994; Geschwind & Levitt, 2007). For example, imitation impairments are observed very early in children with ASD and these deficits may disrupt early establishment of “bodily synchrony” (Rogers & Dawson, 2010) and social coordination (Meltzoff & Moore, 1977). Bodily synchrony is the first way in which infants and caregivers attune to each other's feelings and states. For example, shortly after birth, newborns are capable of imitating adult facial expressions and the rate of this facial imitation increases when adults tune into and follow the infant's cues (Meltzoff & Moore, 1977). Impairments in this synchrony may affect the emotional coordination between caregiver and infant, longitudinally impacting development in the areas of early social communication, language, and play (Bono, Daley, & Sigman, 2004; Sigman et al., 1999; Toth, Munson, Meltzoff, & Dawson, 2006).
As discussed above, researchers suggest that the process of cortical specialization and the development of neural networks in individuals with ASD are compromised, resulting in abnormal connectivity and atypical specialization (Johnson et al., 2005). Notable brain regions affected are core areas involved in social and communicative development, often corresponding to observable behaviors such as delays in joint attention, verbal and pre-verbal expressive skills (e.g., vocalizing less than other infants), delays in early language comprehension, passivity, and a tendency to fixate on particular objects in the environment (Zwaigenbaum et al., 2005). For example, in a prospective MRI study of infants at risk for ASD, Elison and colleagues (2013) found that MRI measures taken at 6 months of age of white matter fiber development (fractional anisotropy) reflecting connections between the amygdala and ventral-medial prefrontal cortex and anterior temporal pole predicted individual differences in joint attention skills at 9 months. They posit that the development of core nonverbal social communication skills is dependent on the development of functional connections in frontotemporal brain systems. Such early differences in the development of neural circuitry is likely to lead to a cascade of atypical development in ASD: an infant who does not develop joint attention and who tends to prefer object- rather than people-related events will lack critical opportunities for further stimulation and refinement of brain circuitry necessary for social communication and learning. Instead, as neural networks are stimulated by and become specialized for physical objects, an unusual construction of the world occurs and results in both enhancements in certain aspects of perceptual processing and other skills, along with deficits in abilities typically promoted through social attention and interaction, such as face processing, joint attention, shared emotion, reciprocal engagement, and language development (Mundy, 2003).
To promote social communication and language skills, therefore, the timing and targets of early intervention need to capitalize on the experience-expectant neuroplasticity that occurs within the context of social interactions, with the goal of facilitating cortical specialization for social and linguistic information and neural (Dawson et al., 2005; Johnson & Munakata, 2005; Kuhl, Tsao, & Liu, 2003). Furthermore, intervention strategies (discussed below) should be designed to enhance social engagement and encourage the development of social and linguistic skills that are dependent on long-distance neural connections.
4. Early brain development in ASD: Atypical experience-expectant learning related to social attention
Research indicates that genes and the environment interact continuously to affect the development of the brain, including increased myelination, synaptic growth, neural circuit formation, cell migration, pruning, and metabolic capacity (Shonkoff & Phillips, 2000). Different areas of the brain mature at different rates, reflected in the developmental unfolding of specific behaviors and abilities over time. As neural connections become established, they are strengthened through repeated experiences and interactions with the social and physical worlds (Carter et al., 2005; Kolb, 1999). The literature describing such experience-expectant neuroplasticity demonstrates that experience, especially within social interactions, facilitates cortical specialization (Johnson & Munakata, 2005; Kuhl, Tsao, & Liu, 2003), specifically the fine-tuning of specific perceptual systems as well as cortical connectivity across different brain areas (Leech & Saygin, 2011).
The infant's social environment provides a relational context in which learning takes place in the first months of life (Baillargeon, 1994; Johnson, Grossman, & Farroni, 2008). Infants are primed to orient and attend to social stimuli ensuring that basic needs are fulfilled (e.g., gaining attention of parent when child is hungry) as well as that social and language learning occur (Johnson & Morton, 1991; Farroni et al., 2005). An infant's auditory and visual systems are highly specialized and attuned to social stimuli such as faces and voices, and the development of cortical connectivity between these systems is evident within the first 3 to 7 months of life. For example, 3½ month old infants demonstrate the ability to associate the sound of their mother's voice with her face (Spelke & Owsley, 1979). By 5 to 7 months of age, infants can match voices and faces on the basis of the speaker's age and gender (Walker-Andrews et al., 1991; Bahrick et al., 1998), and by 6 months of age, infants are able to learn the voice-face pairings of same-sex female strangers (Hernandez-Reif et al., 1994).
As these early social behaviors and abilities are emerging, contingent brain activation takes place and shapes neural networks, leading to flexibility, generalization of knowledge, and expertise with continued experience (Eckerman & Didow, 1989; Ross, 1982; Rutter & Durkin, 1987). At the neural level, therefore, brain regions that are activated during these early social encounters are fundamental to the development of social brain circuitry. For example, the areas of an infant's brain that are activated when exposed to faces are similar to those used for face processing by adults (Haxby, Hofman, & Gobbini, 2000). Haxby and colleagues (2000) observed activation in an area of the inferior temporal gyrus in infants, which corresponds with the fusiform face area in adults (Kanwisher, 2000; Kanwisher et al., 1997; Gauthier et al., 1999). The inferior frontal region and superior temporal gyrus are activated in infants during face perception. Both of these areas have been identified as part of the adult language network (Johnson, Grossman, & Farroni, 2008). Tzourio-Mazoyer and colleagues (2002) propose that the co-activation of face and future language networks seen in infants during face perception may be an evolutionary advantage to guide language learning while looking at the speaker's face in the context of social interactions. Additionally, Kuhl and colleagues (2003) demonstrated that exposure to speech is not necessarily sufficient to facilitate the development of speech and language learning. Instead, the key component for the development of typical speech perception is incorporating the infant's experience of language within an interactive, social context (i.e., face to face interactions versus two-dimensional presentation).
Thus, early experiences within a social context have an important effect on concurrent brain development as well as implications for developmental trajectories (Johnson, Grossman, & Farroni, 2008). Children with ASD, however, may have a specific deficit in their early interest in and attention to social stimuli (Dawson et al., 2005; Mundy, 1995; Panksepp, 1979), apparent in the second half of the first year of life (Zwaigenbaum et al., 2005). Deficiencies in social attention have been hypothesized to be related to difficulties in forming representations of the reward value of social stimuli, leading to a social motivational deficit. Evidence for atypical social reward processing in autism comes from studies of pupillary responses to happy faces (Sepeta et al., 2012), event-related potentials measuring reward anticipation to social versus nonsocial stimuli (Stavropoulos and Carver, 2014), and fMRI studies of social reward processing (Delmonte et al., 2012; Dichter et al., 2012; Scott-Van Zeeland et al., 2010; but see Pankert et al., 2014)
Deficiencies in social motivation might be related to dysfunction in the dopaminergic projections to the striatum and frontal cortex, particularly the orbitofrontal cortex, which has been found to mediate the effects of reward on approach behavior. Such representations regarding the anticipate reward value of a stimulus begin to motivate and direct attention by the second half of the first year of life. Importantly, there is evidence that, during the early months of life, infants who later develop autism may initially attend to faces and demonstrate social engagement with others. In a case study of an infant who later developed ASD, it was observed that, during the first 6 months, this infant vocalized and responded socially to others by smiling and cooing. Difficulties in social interaction began to emerge between 6-12 months, including poor eye contact, failure to engage in imitative games, and lack of imitative vocal responses (Dawson et al., 2000). More recently, in a prospective study of infant siblings of children with autism, Jones and Klin (2013) found that infants who later developed ASD showed normal attention to salient social stimuli (eyes of the face) very early in life, but exhibited a decline in attention to eyes from 2-6 months of age. Thus, in the first months of life, the basic mechanism of orienting to social stimuli and social attention appears to be intact, but then declines. By 8-10 months of age, infants who later development ASD fail to orient to their name (Werner et al., 2000). Studies have demonstrated that preschool and older age children with autism fail to orient to social and linguistic stimuli (Dawson et al., 1998, 2004a; Magrelli et al., 2013; Sasson et al., 2007) and orient to non-social contingencies rather than biological motion (Klin et al., 2009). It is unknown to what extent this decline represents (1) fundamental differences in the development of reward circuitry, per se, and/or (2) the development of representations regarding anticipated stimulus reward value, which depends on the more general mechanism of long range functional connectivity (e.g. between the amygdala and frontal regions). It is also possible that the inability to process complex multisensory stimuli, caused by a lack of functional connectivity, makes complex social stimuli (faces talking and expressing emotions simultaneously) difficult to comprehend and, therefore, less rewarding (Stevenson et al., 2014). Thus, it appears that infants who later develop ASD are engaged in normal experience-expectant learning during early infancy (i.e., the first 6 months), in that the brain is primed for “rewiring” through experiences with other people, including their faces, voices, and gestures and the affective experiences that typically accompany those experiences (see Figure 1). However, by the second half of the first year, ASD is associated with atypical experience expectant learning, as a result of biological processes that likely began during the prenatal period.
Figure 1. Atypical brain and behavioral development in ASD.
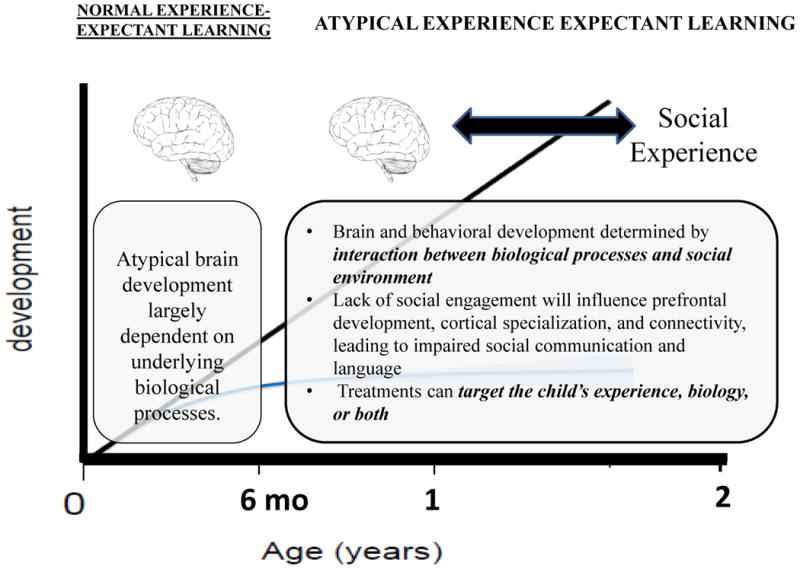
An early lack of social attention and engagement with others may compromise the process of cortical specialization and the development of neural networks, resulting in neuron abundance, abnormal connectivity, and atypical synaptic synchronization (Dinstein et al., 2011; Geschwind & Levitt, 2007; Mundy, 2003). The interplay between the brain and behavior is further described by Geschwind and Levitt (2007), who propose that poor neural synchronization in young children with ASD may be related to the severity of early behavioral symptoms (e.g., joint attention; Geschwind & Levitt, 2007). In a group of 3- and 4-year old children with ASD, Munson and colleagues (2006) reported significant correlations between increased severity of social and communication impairments and enlargement of the right amygdala, an interesting finding given that the amygdala has been associated with social and emotional processing (discussed in more detail below).
In summary, cortical specialization and functional networks involve selective synaptic pruning, based partly on experience, making them more efficient and responsive (Garber, 2007). Much of an infant's early experience occurs within the context of social interactions. It is through these interactions and the simultaneous coordination of communication, behavior regulation, and affective expression, that infants begin to develop joint attention, recognize the similarity between one's own actions and those of others, and form representations about social events and their reward value (Sigman et al., 2004). These abilities are considered foundational skills and precursors to the development of language and the social understanding (Sigman et al., 2004). In young children with ASD, deficits in assigning reward value and attending to social stimuli limit the number of opportunities for learning language and social skills, further compromising their behavioral and neural development .
Hopeful findings in the field of neuroscience reveal the remarkable plasticity of the brain in response to experience, particularly early intervention or stimulation (e.g., Bates & Roe, 2001; Majdan & Shatz, 2006). Thus, early intervention for young children with ASD may be critical for shaping brain structures to be receptive to the social world, thereby preventing or mitigating the symptoms and severity associated with ASD (Wallace & Rogers, 2010). In the following section, we provide the example of one early intervention approach, the Early Start Denver Model (ESDM), and describe features of this intervention, which may shed light on the mechanisms by which early intervention can influence brain function and lead to significant gains in young children with ASD.
5. Key features of early intervention related to mechanisms of change and optimal outcomes
The Early Start Denver Model (EDSM; Rogers & Dawson, 2010) represents a refined and adapted downward extension of the Denver Model (Rogers et al., 1986; 2000; Rogers & Lewis, 1989) and is designed to address the unique needs of toddlers with ASD as young as 12 months old. The foundation of ESDM is a synthesis of several complementary approaches including the Denver Model (Rogers et al., 1986; 2000; Rogers & Lewis, 1989), Roger's and Pennington's (1991) model of interpersonal development in ASD, Dawson and colleagues' (2004a) model of ASD as a disorder of social motivation, Pivotal Response Training (PRT; Koegel et al., 1999; Pierce & Schreibman, 1995, 1997), and general principles of naturalistic applied behavior analysis. Parent and family involvement is an essential component of ESDM. Parents and caregivers are taught specific skills for engaging and interacting with their children throughout the day, and embedding treatment techniques into everyday life (Rogers & Dawson, 2010).
ESDM uses knowledge about typical infant development to promote similar developmental trajectories or prevent negative cascades in young infants and toddlers at risk for or diagnosed with ASD. As a comprehensive intervention, ESDM aims to simultaneously reduce the severity of ASD symptoms as well as accelerate children's developmental rates in all domains, particularly cognitive, social-emotional, and language domains (Charman & Howlin, 2003; Drew et al., 2002; Lord et al., 2005; Rogers & Dawson, 2010). By embedding the delivery of treatment within rich social interactions, ESDM creates a relational context for learning and promotes fine-tuning of specific perceptual systems and cortical connections seen at the level of behavior change (Johnson & Munakata, 2005; Kuhl, Tsao, & Liu, 2003; Leech & Saygin, 2011).
Currently there are ten papers published in peer-reviewed journals that describe the effectiveness of either the Denver Model (Eapen et al., 2013; Fulton et al., 2014; Rogers et al., 1986; 2006; Rogers & DiLalla, 1991; Rogers & Lewis, 1989; Rogers, Lewis, & Reis, 1987) or ESDM (Dawson et al., 2010; Dawson et al., 2012; Vismara & Rogers, 2008), as well as three papers examining parent-coaching interventions based on ESDM (P-ESDM; Estes et al., 2013; Rogers et al., 2012;Vismara, Colombi, & Rogers, 2009). A randomized-controlled trial demonstrated that ESDM is effective for increasing children's cognitive, social, and language abilities, reducing the severity of ASD diagnosis, and improving overall behavior and adaptive skills (Dawson et al., 2010; Dawson et al., 2012). While evidence for the efficacy of comprehensive, intensive ESDM intervention for improving outcomes is relatively strong, there has been inconsistent evidence supporting the efficacy of briefer, solely parent-delivered ESDM intervention. The combination of both therapist- and parent-delivered intervention is still considered best practice (National Research Council, 2001). Although longer-term follow-up studies and replications are necessary to determine the long term benefits of intensive ESDM treatment, the consistency of the evidence across several types of delivery (e.g., classroom and at-home delivery) suggests that ESDM is efficacious in addressing a wide range of early symptoms of ASD and improving child outcomes, at least during the preschool period (Rogers & Dawson, 2010). Additional studies examining the moderators of ESDM effectiveness (i.e., those specific factors and individual child characteristics that determine differential response to treatment) are still greatly needed (Rogers & Dawson, 2010). We next describe the key features of ESDM that we propose are related to neural mechanisms that promote the learning and behavioral improvements associated with this intervention approach.
5.1 Feature 1: Early onset of intervention capitalizes on the experience-expectant plasticity of the immature brain
Evidence suggests that, when intervention is provided early and intensively for at least two years, normalization of brain activity related to social processing is possible. Several studies have demonstrated that young children with ASD show atypical neural responses to faces as compared to objects (Dawson et al., 2002, 2004b; Webb et al., 2006, 2012), which appears to persist into adolescence and adulthood (McPartland et al., 2004). This atypical pattern of brain activity was shown to normalize after early intervention that was started when children were less than 2 ½ years of age. Specifically, it was found that participation in ESDM was associated with normalized patterns of brain activity as measured by event-related brain potentials (ERPs) in response to social (female faces) and nonsocial (toys) stimuli (Dawson et al., 2012). Children with ASD who did versus did not receive ESDM were compared to a group of typically developing 4 year old children. The ESDM group and typical children showed a shorter Nc latency and increased cortical activation (decreased alpha power and increased theta power) when viewing faces, whereas the children who did not receive ESDM showed the opposite pattern (shorter latency ERP and greater cortical activation when viewing objects). Greater cortical activation while viewing faces was correlated with improved social behavior in the ESDM group. The alpha and theta oscillations are generated by an interaction between glutamatergic and g-aminobutyric acidergic (GABA) neurons. Thus, the normalization of alpha and theta brain activity during social processing in the children who received ESDM might index a normalization of the imbalance between excitatory and inhibitory neurons, a hypothesized mechanism explaining neural dysfunction in autism.
In typical development, learning and neural plasticity occur throughout the entire day, with every interaction with the environment representing an opportunity for learning and promoting brain development. As such, enhanced neuroplasticity and experience-expectant learning is promoted not only by providing the intervention intensively (approximately 25 hours a week) by a trained therapist, but also during everyday experiences and routines at home. Thus, the parent-coaching component of ESDM is an essential element for promoting experience-expectant learning opportunities that are comparable to those experienced by a typical infant and toddler. Parents are taught strategies for promoting interaction, play, and communication that can be implemented throughout the day during activities such as meals, bath time, and play, thus increasing the opportunities for learning.
5.2 Feature 2: Strategies that address core deficits in social motivation through an emphasis on positive social engagement and arousal modulation
ESDM builds on evidence indicating that learning in infancy (e.g., development of speech perception) is strongest when it occurs within a social context. For example, in the area of language development, Kuhl and colleagues (2003) demonstrated that typically developing infants between 9 and 10 months of age show phonetic learning from exposure to a foreign language provided in a social and affectively engaged relationship, but not from video recordings of the exact same language stimuli.
In the ESDM model, social attention is explicitly addressed by the use of several strategies that increase the salience of social and linguistic information in the context of the overall environment. For example, therapy is delivered with the therapist or parent situated directly across from the child (referred to as “Stepping into the spotlight”) and preferred objects are positioned close to the adult's face.
Equally important is that the child learns to attach reward value to the adult's actions and their interaction. Evidence from studies of normal brain function have demonstrated that emotionally-salient events are attended to more readily, elicit greater brain activity, and are more likely to be remembered (Markovic et al., 2014). The learning process is enhanced by affectively-rich social interaction. To address deficits in social motivation, several strategies in ESDM are designed to enhance the reward value of social stimuli and social interaction (referred to as “Finding the smile”). An example of a specific interactional strategy is the “sensory social routine”, which focuses explicitly on engaging children in mutually rewarding social exchanges. Sensory social routines are part of every therapy session and parents are taught to use such routines during play at home. Such strategies seek to optimize arousal levels and affective engagement on the part of the child. In some cases, this involves increasing the child's arousal to enhance attention and positive affect during social interaction, whereas in other cases, it involves dampening the child's arousal level. In fact, the therapist's ability to implement therapeutic strategies that regulate the child's arousal level during social interaction, and, thereby, promote social attention and engagement and a positive experience is one of the criteria for fidelity of implementation of ESDM. These techniques, as well as strategies derived from Pivotal Response Training (PRT; Koegel & Koegel, 1999; Koegel, 2000; Schriebman, 1988) are designed to increase the salience and reward value of social stimuli, thus enhancing the child's social attention and motivation for social interaction.
Positive affect and modulation of affective or arousal states directly activates the social brain and its related neurotransmitters, fostering the development of social and communicative behavior (Adolphs, 2003; Morris et al., 1996). ESDM aims to find sources of pleasure for the child with the goal of making social engagement an intrinsic part of the reward. For children with less inherent interest in social engagement, this technique builds reward value through associative learning processes (social experiences paired with nonsocial rewards; Rogers & Dawson, 2010).
ESDM incorporates a number of strategies that enhance motivation and positive affect that were derived from Pivotal Response Training (Cadogan & McCrimmon, 2013), such as the use of child-preferred activities and shared control over the materials and interaction. These strategies were identified as effective for improving motivation and accelerating skill acquisition. In the domain of language, ESDM provides several diverse opportunities for communicating with others and elicits many kinds of communicative behaviors (i.e., verbal and nonverbal) from a child during each intervention session. The power of communication is strongly reinforced by supporting the child's spontaneous communications, reinforcing successful approximations, and demonstrating that through communication, the child exerts a notable amount of control over interactions and activities. Within the context of joint activity routines, the range of communicative, or pragmatic, functions is carefully and intentionally developed so that the child learns not only to request an activity, but also to protest, share attention, greet familiar adults, and comment or narrate during an activity (Rogers & Dawson, 2010).
The objectives of any given teaching episode are carefully chosen to enhance the positive achievement of the objective by the child. Objectives are chosen from a curriculum that is based on research with regard to the sequential acquisition of social, language, cognitive, and adaptive behavior skills. The language of the therapist or parent is only slightly more complex than the child's current skill level (the “One up rule”) and teaching steps for any given objective begin with behaviors that the child is likely to have already achieved. Teaching trials intersperse those in which the child is encouraged to use a behavior that is slightly above his or her current level of ability and those in which the child has already achieved mastery, thereby helping to maintain motivation and a sense of mastery (a strategy promoted by Pivotal Response Training).
ESDM focuses on creating positive emotional states in children during social interactions, with the intention of enhancing the reward value of social interaction and recalibrating sensitivity to social stimuli (i.e., voices, faces, and eyes; Rogers & Dawson, 2010). As mentioned above, teaching episodes are embedded in activities that a child strongly prefers (i.e., sensory social routines or object routines). By creating such positive routines, the child's attention to the social environment increases to support information processing of the social-communicative context (Rogers & Dawson, 2010).
5.3 Feature 3: Promotion of complex neural networks and connectivity through thematic, multi-sensory and multi-domain teaching approaches
A distinguishing feature of ESDM is the simultaneous targeting of multiple teaching objectives within a single teaching trial. In contrast to other autism intervention models, such as Discrete Trial Training, which begin by breaking complex behaviors into individual units (e.g., looking at another's face), the therapeutic approaches used in ESDM encourage a wide range of skills that recruit simultaneous neural activity across brain regions, an approach designed to promote complex neural networks and foster greater connectivity across multiple brain regions (Rogers & Dawson, 2010). Rather than teaching individual behaviors (e.g., raising arms when the therapist requests imitation), ESDM simultaneously targets multiple domains and objectives during a teaching task and fosters affective engagement during the repeated teaching of concepts. For example, in one teaching episode, the therapist simultaneously targets the use of eye contact, vocalizations, and motor imitation. The incorporation of multiple objectives within a teaching activity is one of the criteria by which fidelity of implementation is assessed.
While a given teaching episode revolves around a theme that is varied over time, during each episode, the child is exposed to multiple modalities (voices, faces, touch, movement) involving multiple specialized functions of the brain (face and emotion processing, linguistic processing, mirror neurons, affective responses). These multi-modal experiences are carefully chosen to be developmentally appropriate, based on the ESDM curriculum. The therapist provides these experience in a way that is sensitive to the child's arousal level and associates them with reward. Repetitions and expansions of these experiences and their association with positive affect serve to strengthen connections between brain regions specialized for different sensory modalities and functions.
While the idea that targeting multiple objectives and multiple sensory modalities within a single teaching episode will promote functional connectivity in the brain is appealing, research is needed to validate this notion. For example, neuroimaging studies of children with ASD showed underconnectivity between voice-selective cortex and reward circuitry (Abrams et al., 2013). Children with ASD demonstrated a pattern of underconnectivity between left-hemisphere superior temporal sulcus, a region that mediates processing of speech prosody, and the orbitofrontal cortex and amygdala, regions involved in emotion-related associated learning. In the future, it will be important to assess whether ESDM or other early interventions enhance neural connectivity across brain regions, which can be assessed using fMRI, EEG, or MEG.
6. Conclusion
Multidisciplinary efforts are being made to investigate the etiology, earlier identification, and effective and accessible treatments for ASD. ASD has a genetic, biological basis that results in a cascade of developmental events involving complex interactions between the brain and the social environment that results in deficits across many areas of development.
We used ESDM as an exemplar to hypothesize key features of early interventions that might help identify the potential mechanisms underlying the effectiveness of early interventions for ASD. First, to capitalize on the plasticity of the immature brain, early intensive behavioral intervention helps promote normal early experience-expectant learning for young children with ASD by provide an optimal environment for brain development. In young children with ASD, significant findings from research on high-risk infant siblings indicate that before the first signs and symptoms of ASD, there is evidence of altered connectivity and brain organization (Wolff et al., 2012; Gabard-Durnam et al., 2013). By intervening with toddlers as young as 12 months of age using a developmental approach, ESDM capitalizes on the plasticity of the young brain, aiming to change brain architecture and patterns of functional connectivity during a period of rapid neural development.
Addressing the core impairment in social attention and motivation is another key feature of early intervention, and one that is potentially related to the development and function of social brain circuitry. Research in typical development (Kuhl et al., 2003) as well as in compromised populations (e.g., Bucharest Early Intervention Project, Smyke et al., 2009), indicate the importance of social stimulation. The unique challenge with young children with ASD, however, is that decreased social attention is thought to be related to a lack of sensitivity to the reward value of social stimuli. One of the goals of ESDM, therefore, is to utilize the child's natural environment (especially at home) and his or her existing social relationships to promote a socially rich context for learning. Specifically, family-child and therapist-child interactions are used to help the children attend to key social information in their environment (e.g., faces, actions, emotions), so they can make sense of information that is essential for typical language and social development. A wide range of strategies is used to enhance the reward value of social information, such as optimizing the child's arousal level during social interaction and using child-preferred materials and activities.
Once the stage for intervention is set by starting early and providing a rewarding social context for learning, we hypothesize that the simultaneous targeting of multiple objectives involving multisensory experience helps establish and maintain complex neural networks and long-range functional connectivity.
Understanding the mechanisms of effective early intervention will help us bridge the behavioral and biological approaches to treatment of individuals with ASD and identify common or foundational active ingredients for promoting optimal outcomes. As the field moves forward, larger multi-site randomized controlled trials are needed to gain a better understanding of intervention effectiveness, individual responses, dosing effects, and common ingredients for optimal improvements. The incorporation of brain-based measures in intervention studies will be essential for understanding active ingredients of intervention and the neural mechanisms related to change and outcome. Research on high-risk infant siblings will provide new information about the impact of early intervention that is initiated at the time of symptom onset, before the full syndrome is present. For those high-risk infants who do not go on to receive an ASD diagnosis, identifying protective factors may translate into targets for intervention and opportunities to understand individual differences. Future research contributing to the understanding of which children benefit most from which early intervention approach will have important implications for the individualization of treatment as well as understanding the impact of early intervention on the function and organization of the developing brain.
Highlights.
Early intervention in ASD capitalizes on experience-expectant plasticity of the immature brain
Importance of treatment strategies that address core deficits in social motivation
Use of thematic, multi-sensory and multi-domain teaching approaches to promote neural networks
Acknowledgments
Grant Sponsor: NIMH; Grant Number: U54MH066399
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wendy L. Stone, Email: stonew@uw.edu.
Geraldine Dawson, Email: geraldine.dawson@duke.edu.
References
- Abrams DA, Lynch CJ, Cheng KM, Phillips J, Supekar K, Ryali S, Uddin LQ, Menon V. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proceedings of the National Academy of Sciences USA. 2013;110:12060–5. doi: 10.1073/pnas.1302982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Bahrick LE, Netto D, Hernandez-Reif M. Intermodal perception of adult and child faces and voices by infants. Child Development. 1998;69:1263–1275. [PubMed] [Google Scholar]
- Baillargeon R. How do infants learn about the physical world? Current Directions in Psychological Science. 1994;3:133–140. [Google Scholar]
- Bates E, Roe K. Language development in children with unilateral brain injury. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2001. pp. 281–307. [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomical observation of the brain in autism. In: Bauman ML, Kemper TL, editors. The neurobiology of autism. Baltimore: John Hopkins University Press; 1994. pp. 119–145. [Google Scholar]
- Bono M, Daley T, Sigman M. Relations among joint attention, amount of intervention, and language gain in early autism. Journal of Autism and Developmental Disorders. 2004;34:495–505. doi: 10.1007/s10803-004-2545-x. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Development and Psychopathology. 2002;14:209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Cadogan S, McCrimmon AW. Pivotal response treatment for children with autism spectrum disorder: A systematic review of research quality. Developmental Neurorehabilitation. 2013 doi: 10.3109/17518423.2013.845615. epub. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ahnert L, Grossmann KE, Hrdy SB, Lamb ME, Porges SW, Sachser N. Attachment and bonding: A new synthesis. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Charman T, Howlin P. Research into early intervention for children with autism and related disorders: Methodological and design issues-Report on a workshop funded by the Wellcome Trust, Institute of Child Health, London, UK, November 2001. Autism. 2003;7:217–225. doi: 10.1177/1362361303007002008. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: stability and change in syndrome expression. Journal of Child Psychology and Psychiatry. 2007;48:128–38. doi: 10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. Journal of the American Medical Association. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns C, Davis HR, Ziccardi R, Tigue Z, Pierce K. Unusual growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavior intervention, brain plasticity, and the prevention of autism spectrum disorder. Developmental Psychopathology. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development. 2002;73:700–17. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Jones EJ, Merkle K, Venema K, Lowy R, Faja S, Kamara D, Murias M, Greenson J, Winter J, Smith M, Rogers SJ, Webb SJ. Early behavioral intervention is associated with normalized brain activity in young children with autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:1150–9. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28:479–85. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Osterling J. Early intervention in autism: Effectiveness and common elements of current approaches. In: Guralnick, editor. The effectiveness of early intervention: Second generation research. Baltimore: Brookes; 1997. pp. 307–326. [Google Scholar]
- Dawson G, Osterling J, Meltzoff AN, Kuhl P. Case Study of the Development of an Infant with Autism from Birth to Two Years of Age. Journal of Applied Developmental Psychology. 2000;21:299–313. doi: 10.1016/S0193-3973(99)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Developmental Science. 2004;7:340–59. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27:403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Delmonte S, Balsters JH, McGrath J, Fitzgerald J, Brennan S, Fagan AJ, Gallagher L. Social and monetary reward processing in autism spectrum disorders. Molecular Autism. 2012;3:7. doi: 10.1186/2040-2392-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. Journal of Autism and Developmental Disorders. 2012;42:147–60. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, Courchesne E. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–25. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew A, Baird G, Baron-Cohen S, Cox A, Slonims V, Wheelwright S, Swettenham J, Berry B, Charman T. A pilot randomized control trial of a parent training intervention for pre-school children with autism: Preliminary findings and methodological challenges. European Child & Adolescent Psychiatry. 2002;11:266–272. doi: 10.1007/s00787-002-0299-6. [DOI] [PubMed] [Google Scholar]
- Eapen V, Crnčec R, Walter A. Clinical outcomes of an early intervention program for preschool children with Autism Spectrum Disorder in a community group setting. BMC Pediatrics. 2013;13:3. doi: 10.1186/1471-2431-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerman CO, Didow SM. Toddlers' social coordinations: Changing responses to another's invitation to play. Developmental Psychology. 1989;25:794–804. [Google Scholar]
- Elison JT, Wolff JJ, Heimer DC, Paterson SJ, Gu H, Hazlett HC, Styner M, Gerig G, Piven J IBIS Network. Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Developmental Science. 2013;16:186–97. doi: 10.1111/desc.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A, Vismara L, Mercado C, Fitzpatrick A, Elder L, Greenson J, Lord C, Munson J, Winter J, Young G, Dawson G, Rogers S. The Impact of Parent-Delivered Intervention on Parents of Very Young Children with Autism. Journal of Autism and Developmental Disorders. 2013;44:353–65. doi: 10.1007/s10803-013-1874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni T, Johnson MH, Menon E, Zulian L, Faraguna D, Csibra G. Newborn's preference for face-relevant stimuli: effects of contrast polarity. Proceedings of the National Academy of Sciences USA. 2005;102:17245–17250. doi: 10.1073/pnas.0502205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton E, Eapen V, Crnčec R, Walter A, Rogers S. Reducing maladaptive behaviors in preschool-aged children with autism spectrum disorder using the early start denver model. Frontiers in Pediatrics. 2014 doi: 10.3389/fped.2014.00040. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L, Tierney AL, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Alpha Asymmetry in Infants at Risk for Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1926-4. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. Neuroscience: Autism's cause may reside in abnormalities at the synapse. Science. 2007;317:190–191. doi: 10.1126/science.317.5835.190. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: Developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17:103–11. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. Journal of Neurodevelopmental Disorders. 2009;1:172–81. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadders-Algra M. Reduced variability in motor behavior: An indicator of impaired cerebral connectivity? Early Human Development. 2008;84:787–789. doi: 10.1016/j.earlhumdev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Harris SL, Handleman JS. Age and IQ at intake as predictors of placement for young children with autism: A four- to six-year follow-up. Journal of Autism and Developmental Disorders. 2000;30:137–142. doi: 10.1023/a:1005459606120. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Archives of General Psychiatry. 2005;62:1366–76. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hernandez-Reif M, Cigales M, Lundy B. Memory for arbitrary adult face-voice pairs at six months of age. 1994, June; Paper presented at the International Conference on Infant Studies; Paris. [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–43. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Griffin R, Csibra G, Halit H, Farroni T, de Haan M, Tucker LA, Baron-Cohen S, Richards J. The emergence of the social brain network: evidence from typical and atypical development. Development and Psychopathology. 2005;17:599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Grossmann T, Farroni T. The social cognitive neuroscience of infancy: illuminating the early development of social brain functions. Advances in Child Development and Behavior. 2008;36:331–72. doi: 10.1016/s0065-2407(08)00008-6. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Halit H, Grice SJ, Karmiloff-Smith A. Neuroimaging of typical and atypical development: A perspective from multiple levels of analysis. Developmental Psychopathology. 2002;41:521–536. doi: 10.1017/s0954579402003073. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Morton J. Biology and cognitive development: The case of face recognition. Blackwell; Oxford: 1991. [Google Scholar]
- Johnson MH, Munakata Y. Processes of change in brain and cognitive development. Trends in Cognitive Sciences. 2005;9:152–158. doi: 10.1016/j.tics.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Jones W, Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 2013;504:427–31. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nature Neuroscience. 2000;3:759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun M. The fusiform face area: A module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–61. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel LK. Communication in autism. Journal of Autism and Developmental Disorders. 2000;B30:383–392. doi: 10.1023/a:1005539220932. [DOI] [PubMed] [Google Scholar]
- Koegel LK, Koegel RL. Pivotal response intervention I: Overview of approach. Journal of the Association for Persons With Severe Handicaps. 1999;24:174–185. [Google Scholar]
- Kolb B. Neuroanatomy and development overview. In: Fox NA, Leavitt LA, Warhol JG, editors. The role of early experience in infant development: Summary of a conference held in January 1999. USA: Johnson & Johnson Consumer Companies, Inc.; 1999. pp. 5–14. [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM. Foreign-language experience in infancy: Effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences USA. 2003;100:9096–9101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, Piven P, Wzorek M, Landa R, Santangelo SL, Coon H. Macrocephaly in children and adults with autism. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- Landa R. Early communication development and intervention for children with autism. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:16–25. doi: 10.1002/mrdd.20134. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, O'Neill AH, Stuart EA. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: a randomized controlled trial. Journal of Child Psychology and Psychiatry. 2011;52:13–21. doi: 10.1111/j.1469-7610.2010.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Saygin AP. Distributed processing and cortical specialization for speech and environmental sounds in human temporal cortex. Brain and Language. 2011;116:83–90. doi: 10.1016/j.bandl.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Lord C, Wagner A, Rogers S, Szatmari P, Aman M, Charman T, Dawson G, Durand VM, Grossman L, Guthrie D, Harris S, Kasari C, Marcus L, Murphy S, Odom S, Pickles A, Scahill L, Shaw E, Siegel B, Sigman M, Stone W, Smith T, Yoder P. Journal of Autism and Developmental Disorders. 2005;35:695–708. doi: 10.1007/s10803-005-0017-6. [DOI] [PubMed] [Google Scholar]
- Magrelli S, Jermann P, Noris B, Ansermet F, Hentsch F, Nadel J, Billard A. Social orienting of children with autism to facial expressions and speech: a study with a wearable eye-tracker in naturalistic settings. Frontiers in Psychology. 2013;4:840. doi: 10.3389/fpsyg.2013.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdan M, Shatz CJ. Effects of visual experience on activity-dependent gene regulation in cortex. Nature Neuroscience. 2006;9:650–9. doi: 10.1038/nn1674. [DOI] [PubMed] [Google Scholar]
- Markovic J, Anderson AK, Todd RM. Tuning to the significant: neural and genetic processes underlying affective enhancement of visual perception and memory. Behavioural Brain Research. 2014;259:229–41. doi: 10.1016/j.bbr.2013.11.018. [DOI] [PubMed] [Google Scholar]
- McConachie H, Randle V, Hammal D, Le Couteur A. A controlled trial of a training course for parents of children with suspected autism spectrum disorder. Journal of Pediatrics. 2005;147:335–340. doi: 10.1016/j.jpeds.2005.03.056. [DOI] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45:1235–45. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: Cortex, connectivity, and neuronal organization. Archives of Neurology. 2007;64:945–50. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Müller RA. The study of autism as a distributed disorder. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P. Annotation: The neural basis of social impairments in autism: The role of the dorsal medial-frontal cortex and anterior cingulate system. Journal of Child Psychology and Psychiatry. 2003;44:793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Mundy P. Joint attention and social-emotional approach behavior in children with autism. Development and Psychopathology. 1995;7:63–82. [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry. 2007;62:270–3. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, Shaw D, Artru A, Dager SR. Amygdalar Volume and Behavioral Development in Autism. Archives of General Psychiatry. 2006;63:686–693. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- National Research Council. Educating children with autism. Committee on educational interventions for children with autism. Commission on behavioural and social science and education. Washington, DC: National Academy Press; 2001. [Google Scholar]
- Oberman LM, Ramachandran VS. Preliminary evidence for deficits in multisensory integration in autism spectrum disorders: The mirror neuron hypothesis. Social Neuroscience. 2008;3:348–355. doi: 10.1080/17470910701563681. [DOI] [PubMed] [Google Scholar]
- Pankert A, Pankert K, Herpertz-Dahlmann B, Konrad K, Kohls G. Responsivity to familiar versus unfamiliar social reward in children with autism. Journal of Neural Transmission. 2014 doi: 10.1007/s00702-014-1210-6. epub. [DOI] [PubMed] [Google Scholar]
- Panksepp J. A neurochemical theory of autism. Trends in Neurosciences. 1979;2:174–177. [Google Scholar]
- Pierce K, Schreibman L. Multiple peer use of pivotal response training to increase social behaviors of classmates with autism: Results from trained and untrained peers. Journal of Applied Behavior Analysis. 1997;30:157–160. doi: 10.1901/jaba.1997.30-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Schreibman L. Increasing complex social behaviors in children with autism: Effects of peer-implemented pivotal response training. Journal of Applied Behavior Analysis. 1995;28:285–95. doi: 10.1901/jaba.1995.28-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biological Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: Challenges for the “new psychophysiology”. International Journal of Psychophysiology. 2007;63:164–72. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rogers S, Dawson G. Early Start Denver Model for Young Children with Autism: Promoting Language, Learning, and Engagement. New York, NY: Guilford Press; 2010. [Google Scholar]
- Rogers SJ, DiLalla DL. A comparative study of the effects of a developmentally based instructional model on young children with autism and young children with other disorders of behavior and development. Topics in Early Childhood Special Education. 1991;11:29–47. [Google Scholar]
- Rogers SJ, Estes A, Lord C, Vismara L, Winter J, Fitzpatrick A, Guo M, Dawson G. Effects of a brief Early Start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. Journal of the American Academy of Child Adolescent Psychiatry. 2012;51:1052–65. doi: 10.1016/j.jaac.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Hall T, Osaki D, Reaven J, Herbison J. A comprehensive, integrated educational approach to young children with autism and their families. In: Harris SJ, Handleman J, editors. Preschool Education Programs for Children with Autism. 2nd. Austin, TX: Pro-Ed; 2000. pp. 95–134. [Google Scholar]
- Rogers SJ, Hayden D, Hepburn S, Charlifue-Smith R, Hall T, Hayes A. Teaching young nonverbal children with autism and their families. In: Harris SL, Handleman JS, editors. Preschool education programs for children with autism. 2nd. Austin, TX: Pro-Ed; 2006. pp. 95–134. [DOI] [PubMed] [Google Scholar]
- Rogers S, Herbison J, Lewis H, Pantone J, Reis K. An approach for enhancing the symbolic, communicative, and interpersonal functioning of young children with autism and severe emotional handicaps. Journal of the Division of Early Childhood. 1986;10:135–148. [Google Scholar]
- Rogers S, Lewis H. An effective day treatment model for young children with pervasive developmental disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:207–14. doi: 10.1097/00004583-198903000-00010. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Lewis HC, Reis K. An effective procedure for training early special education teams to implement a model program. Journal of the Division of Early Childhood. 1987;11:180–188. [Google Scholar]
- Rogers SJ, Vismara LA. Evidence-Based Comprehensive Treatments for Early Autism. Journal of Clinical Child and Adolescent Psychology. 2008;37:8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Pennington BF. A theoretical approach to the deficits in infantile autism. Development and Psychopathology. 1991;3:137–162. [Google Scholar]
- Ross HS. Toddler peer relations: Differentiation of games and conflicts. Canadian Journal of Behavioral Science. 1982;14:364–379. [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain, and Behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter DR, Durkin K. Turn-taking in mother-infant interaction: An examination of vocalizations and gaze. Developmental Psychology. 1987;23:54–61. [Google Scholar]
- Sasson N, Tsuchiya N, Hurley R, Couture SM, Penn DL, Adolphs R, Piven J. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia. 2007;45:2580–8. doi: 10.1016/j.neuropsychologia.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibman L. Autism. Newbury Park, CA: Sage Publications; 1988. [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Research. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepeta L, Tsuchiya N, Davies MS, Sigman M, Bookheimer SY, Dapretto M. Abnormal social reward processing in autism as indexed by pupillary responses to happy faces. Journal of Neurodevelopmental Disorders. 2012;4:17. doi: 10.1186/1866-1955-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J, Phillips D. From neurons to neighborhoods. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- Sigman M, Dijamco A, Gratier M, Rozga A. Early detection of core deficits in autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:221–233. doi: 10.1002/mrdd.20046. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ruskin E, Arbeile S, Corona R, Dissanayake C, Espinosa M, Kim N, Lopez A, Zierhut C. Continuity and change in the social competence of children with autism, Down syndrome, and developmental delays. Monographs of the Society for Research in Child Development. 1999;64:1–114. doi: 10.1111/1540-5834.00002. [DOI] [PubMed] [Google Scholar]
- Smyke AT, Zeanah CH, Jr, Fox NA, Nelson CA., 3rd A new model of foster care for young children: the Bucharest early intervention project. Child and Adolescent Psychiatric Clinics of North America. 2009;18:721–34. doi: 10.1016/j.chc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Spelke E, Owsley CJ. Intermodal exploration and perceptual knowledge in infancy. Infant Behavior and Development. 1979;2:13–27. [Google Scholar]
- Stavropoulos KK, Carver LJ. Reward anticipation and processing of social versus nonsocial stimuli in children with and without autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2014 doi: 10.1111/jcpp.12270. epub. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Segers M, Ferber S, Barense MD, Wallace MT. The impact of multisensory integration deficits on speech perception in children with autism spectrum disorders. Frontiers in Psychology. 2014;5:379. doi: 10.3389/fpsyg.2014.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Munson J, Meltzoff AN, Dawson G. Early predictors of communication development in young children with autism spectrum disorder: Joint attention, imitation, and toy play. Journal of Autism and Developmental Disorders. 2006;36:993–1005. doi: 10.1007/s10803-006-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner LM, Stone WL. Variability in outcome for children with an ASD diagnosis at age 2. Journal of Child Psychology and Psychiatry. 2007;48:793–802. doi: 10.1111/j.1469-7610.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, De Schonen S, Crivello F, Reutter B, Aujard Y, Mazoyer B. Neural correlates of woman face processing by 2-month-old infants. Neuroimage. 2002;15:454–461. doi: 10.1006/nimg.2001.0979. [DOI] [PubMed] [Google Scholar]
- Vismara LA, Colombi C, Rogers SJ. Can 1 hour per week of therapy lead to lasting changes in young children with autism? Autism. 2009;13:93–115. doi: 10.1177/1362361307098516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismara L, Rogers SJ. Treating autism in the first year of life: A case study of the Early Start Denver Model. Journal of Intervention. 2008;31:91–108. [Google Scholar]
- Walker-Andrews AS, Bahrick LE, Raglioni SS, Diaz I. Infants' bimodal perception of gender. Ecological Psychology. 1991;3:55–75. [Google Scholar]
- Wallace KS, Rogers SJ. Intervening in infancy: Implications for autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2010;51:1300–1320. doi: 10.1111/j.1469-7610.2010.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren Z, McPheeters ML, Sathe N, Foss-Feig JH, Glasser A, Veenstra-VanderWeele J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics. 2011;127:1303–1311. doi: 10.1542/peds.2011-0426. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Dawson G, Bernier R, Panagiotides H. ERP evidence of atypical face processing in young children with autism. Journal of Autism and Developmental Disorders. 2006;36:881–90. doi: 10.1007/s10803-006-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJ, Merkle K, Venema K, Greenson J, Murias M, Dawson G. Developmental change in the ERP responses to familiar faces in toddlers with autism spectrum disorders versus typical development. Child Development. 2011;82:1868–86. doi: 10.1111/j.1467-8624.2011.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Monk CS, Nelson CA. Mechanisms of Postnatal Neurobiological Development: Implication for Human Development. Developmental Neuropsychology. 2001;19:147–171. doi: 10.1207/S15326942DN1902_2. [DOI] [PubMed] [Google Scholar]
- Werner E, Dawson G, Osterling J, Dinno N. Brief report: Recognition of autism spectrum disorder before one year of age: a retrospective study based on home videotapes. Journal of Autism and Developmental Disorders. 2000;30:157–62. doi: 10.1023/a:1005463707029. [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Woods JJ. Early Social Interaction Project for Children With Autism Spectrum Disorders Beginning in the Second Year of Life: A Preliminary Study. Topics in Early Childhood Special Education. 2006;26:67–82. [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. Neuropsychologic functioning in children with autism: Further evidence for disordered complex information-processing. Child Neuropsychology. 2006;12:279–298. doi: 10.1080/09297040600681190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans AC, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L, Piven J, Network I. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JJ, Wetherby AM. Early identification of and intervention for infants and toddlers who are at risk for autism spectrum disorder. Language, Speech & Hearing Services in Schools. 2003;34:180–193. doi: 10.1044/0161-1461(2003/015). [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–52. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]


