Abstract
Objective
Suicide is a psychiatric emergency. Currently, there are no approved pharmacologic treatments for suicidal ideation. Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist that rapidly reduces suicidal ideation as well as depression and anxiety, but the dynamic between these symptoms is not known. The aim of this analysis was to evaluate whether ketamine has an impact on suicidal thoughts, independent of depressive and anxiety symptoms.
Methods
133 patients with treatment-resistant depression (major depressive disorder or bipolar I/II disorder) received a single subanesthetic infusion of ketamine (0.5mg/kg over 40 minutes). Post-hoc correlations and linear mixed models evaluated the relationship between suicidal ideation and depression and anxiety symptoms using the Hamilton Depression Rating Scale (HAMD), Scale for Suicidal Ideation (SSI), Beck Depression Inventory (BDI), and Hamilton Anxiety Rating Scale (HAMA) focusing on 230 minutes post-infusion.
Results
At 230 minutes post-infusion, correlations between changes in suicidal ideation and depression ranged from 0.23 to 0.44 (p <. 05), accounting for up to 19% in the variance of ideation change. Correlations with anxiety ranged from 0.23 to 0.40 (p < .05), accounting for similar levels of variance. Ketamine infusion was associated with significant reductions in suicidal ideation compared to placebo, when controlling for the effects of ketamine on depression (F(1,587)= 10.31, p = .001) and anxiety (F(1,567)= 8.54, p = .004).
Conclusions
Improvements in suicidal ideation after ketamine infusion are related to, but not completely driven by, improvements in depression and anxiety. Investigation of the specific effects of ketamine on suicidal thoughts is warranted.
Keywords: Ketamine, Suicide, Depression, Suicidal Ideation
Introduction
Suicidal ideation is a frequent and potentially life-threatening indication for psychiatric emergency services (Ting et al., 2012). Over 38,000 Americans died by suicide in 2010 (bCenters for Disease Control and Prevention, 2013b), and it is estimated that many of these individuals were currently depressed or had a history of an affective disorder (aCenters for Disease Control and Prevention, 2013a; Conwell et al., 1996). Due to the known relationship between suicidal ideation and attempts/death (Baca-Garcia et al., 2011), effective interventions are a priority, as highlighted by the 2012 U.S. National Strategy for Suicide Prevention (2012). Unfortunately, few psychiatric medications have been shown to reduce suicidal ideation as compared to the anti-aggressive and anti-impulsive effects of lithium and clozapine (Frogley et al., 2012; Hennen and Baldessarini, 2005; Quiroz et al., 2010), which may be more suited to reducing suicide attempts than suicidal ideation. Additionally, available antidepressants may take weeks-to-months to achieve the desired effects. As a consequence, the 400,000 individuals who seek emergency treatment for suicidal thoughts and behavior each year often do not receive timely relief (Ting et al., 2012).
Novel, rapid-acting antidepressants hold great potential in the emergent treatment of suicidal patients. Ketamine is a glutamatergic, N-methyl-D-aspartate (NMDA) receptor antagonist that has rapid-acting antidepressant effects (Berman et al., 2000; Diazgranados et al., 2010b; Ibrahim et al., 2012; Zarate et al., 2006) in both unipolar and bipolar depressed patients. A single subanesthetic dose (0.5mg/kg over 40 minutes) of ketamine has antidepressant efficacy within hours-to-days with concomitant reductions in suicidal ideation on a similar time frame. In an open label trial of patients with Major Depressive Disorder (MDD), symptoms including suicidal ideation, anxiety and hopeless thoughts dissipated after a single infusion (DiazGranados et al., 2010a). Ketamine has also been shown to reduce implicit measures of suicidal cognition in both single and repeated-dose paradigms (Price et al., 2014; Price et al. 2009). Naturalistic studies and case reports in emergency department settings have also demonstrated reduced suicidal ideation after ketamine infusion (Larkin and Beautrais, 2011; Zigman and Blier, 2013).
As the evidence base for ketamine’s anti-suicidal properties continues to grow, a critical question emerges: how does ketamine reduce suicidal thoughts? Suicidal ideation has a demonstrated relationship to both depression and anxiety symptoms and diagnoses (Nock et al., 2010; Rappaport et al., 2013; Sareen et al., 2005). Since ketamine has been shown to reduce both depression and anxiety (Diazgranados et al., 2010b; Ibrahim et al., 2012; Irwin et al., 2013; Zarate et al., 2006), a simple explanation may be that ketamine reduces suicidal thoughts through its impact on these symptoms. In support of this perspective, a re-analysis of 9185 patients across several randomized clinical trials of fluoxetine and venlafaxine found that depression mediated the reductions in suicidal thoughts and behaviors in adults, but not adolescents (Gibbons et al., 2012). Similarly, a recent analysis of ketamine in patients with treatment-resistant depression found that reductions in depressive symptoms mediated the reductions in suicide ideation after infusion (Price et al., 2014).
However, not all suicidal ideation and behavior are explained by mood and anxiety symptoms alone. Many individuals think about, attempt and die by suicide outside the context of a depressive or anxious episode (aCenters for Disease Control and Prevention, 2013a; Conwell et al., 1996). Furthermore, a meta-analyses of psychotherapy for depression has found inconclusive effects of these therapies on suicidal thoughts and behaviors (Cuijpers et al., 2013), leading to the development of suicide-focused psychotherapies, such as Cognitive Behavioral Therapy for Suicidal Patients (Brown et al., 2005b). It is possible that ketamine decreases suicidal thoughts independently from depression and anxiety. If ketamine is found to impact suicidal thoughts directly, this would have important implications for both the neurobiological profile of suicidal thoughts and the treatment of suicidal patients.
This analysis takes advantage of the history of ketamine research conducted by our group to evaluate the relationship between suicidal ideation, depression and anxiety in a sample of depressed patients. We hypothesized that reductions in suicidal ideation post-infusion would be independent of reductions in depression and anxiety symptoms. To this end, we evaluated changes in ideation post-infusion. We also evaluated the effect of ketamine on suicidal thoughts when controlling for depressive symptoms and anxiety symptoms. Lastly, we identified whether ketamine had an impact on two cognitive aspects of suicidal ideation: the wish to live and the wish to die.
Methods
Patient-level data was obtained from four independent, previously-published clinical trials, including one open-label trial and one ongoing mechanism of action clinical trial, investigating the use of ketamine in treatment-resistant MDD and bipolar disorder (BD) depression without psychotic features (Diazgranados et al, 2010b, Ibrahim et al., 2012; Zarate et al., 2012; Zarate et al., 2006). Patients 18–65 years old were admitted to the inpatient unit at the NIMH Mood and Anxiety Disorders Research Program in Bethesda, MD, USA. All participants provided written informed consent as approved by the NIH Combined Central Nervous System (CNS) Institutional Review Board. Patients were experiencing a major depressive episode of at least moderate severity [objectively defined as ≥ 18 on the 21-item Hamilton Depression Rating Scale (HAMD) (Zarate et al., 2006), or ≥ 20 or ≥ 22 on the Montgomery-Asberg Depression Rating Scale (MADRS) (Diazgranados et al., 2010b; Zarate et al., 2012)] at screening and at the start of each infusion. In addition, all patients met DSM-IV criteria for either MDD or BD in a depressive episode based on clinical assessment and the Structured Clinical Interview for Axis I Diagnostic and Statistical Manual (DSM)–IV Disorders, patient version (SCID-P) (First et al., 2001). No active substance use disorder diagnoses (except nicotine and caffeine) were permitted in the three months prior to screening. Patients were in generally good physical health as determined by clinical history, physical examination and/or laboratory assessments.
A single subanesthetic (0.5mg/kg) dose of ketamine hydrochloride was administered intravenously over 40 minutes. Patients were assessed on a variety of clinician-administered and self-reported psychiatric measures, as described below, at 60 minutes before and at 40, 80, 120, 230 minutes and one, two and three days following the ketamine infusion.
Measures
The Hamilton Rating Scale for Depression (HAMD) is a 17-item clinician-administered scale used to assess depression severity (Hamilton, 1960). Ratings are made on the scale from 0–4; there is one item that specifically assesses suicidal ideation and propensity for suicidal behavior. This suicide item was used as the criterion in this analysis as it was administered to all patients in this sample (n = 133) and has been correlated with other measures of suicidal thoughts (Desseilles et al., 2012). The Anxiety Somatization Factor Score is a sub-score of the HAMD that is used to assess anxious features of depression via the following six items: psychic anxiety, somatic anxiety, general somatic symptoms, gastrointestinal symptoms, hypochondriasis, and insight. This score was previously derived from a factor analysis of the HAMD (Cleary and Guy, 1977), and has been deemed useful for studying anxiety within depression for both research and clinical purposes (McClintock et al., 2011). Patients scoring ≥7 on this subscale have been found to have higher levels of suicidal thoughts and behaviors (Fava et al., 2006).
The Scale for Suicide Ideation (SSI) is a 19-item, clinician-administered measure of current suicidal thinking (Beck et al., 1979). Patients are administered the first 5 items; if they endorse any desire to make a suicide attempt or passive suicidal ideation, they are administered the remaining 14 items. Scores from the SSI reported in this analysis are limited to the first two items, the “Wish to Live” and “Wish To Die” items, which have been found to predict later suicide (Brown et al., 2005a). The SSI was administered to a subset (n = 106) of the participating patients.
The Beck Depression Inventory (BDI) is a 19-item self-reported measure of depression severity (Beck et al., 1961). Each item is measured on a scale of 0–3 and there was one item assessing suicidal thoughts which was excluded from total score for the purposes of this analysis.
The Hamilton Psychiatric Rating Scale for Anxiety (HAMA) is a 14-item clinician-administered measure of anxiety severity (Hamilton, 1959). Each item is rated on a scale of 0 to 4.
Statistical Analysis
To evaluate the relationship between changes in ideation, depression and anxiety, a series of Pearson correlations evaluated absolute change in symptoms at 230 minutes post-infusion. This time point was chosen as previous analyses have demonstrated that suicidal ideation decreases in the first four hours after ketamine infusion (DiazGranados et al., 2010a). Suicidal ideation, depression and anxiety were each evaluated across two scales to address potential differences in symptom change across assessment type. When correlating ideation to either of the depression rating scales (HAMD or BDI), suicide items were removed from the total score to reduce redundancy. Also, because the Anxiety Somatization Factor Score was created from the HAMD, these items were removed when assessing for depression.
A series of linear mixed models were conducted in a subset of patients (n = 57), who participated in double-blind, placebo-controlled, crossover studies of ketamine and had any suicidal thoughts at baseline (DiazGranados et al., 2010a; Zarate et al., 2012; Zarate et al., 2006). Time and intervention status were included as fixed within-subjects factors. A fixed intercept and the interaction between drug and time were also included. A compound symmetry covariance structure was used as prior analyses suggested this had the best fit according to Schwarz’s Bayesian criteria. The model was run to evaluate the effects of ketamine on suicide ideation alone controlling for baseline suicidal ideation and then a separate model controlling for ketamine’s effects on depression over time by including the three-way interaction of ketamine, depression and time. If the three-way interaction was not significant, it was excluded from the model and the two way interaction between depression and ketamine was included. A similar set of models were run with anxiety. As a final analysis, two linear mixed models were conducted to evaluate the effect of ketamine on “Wish to Live” and “Wish to Die” as assessed by the first two items on the SSI in patients reporting any suicidal thoughts from the SSI (n = 39). All statistics were conducted using SPSS version 21. Significance was considered at p<.05, two-tailed.
Results
A total of 133 patients were included in this analysis; 35 with Bipolar Disorder (19 Bipolar I and 16 Bipolar II in a depressive episode) and 98 with Major Depressive Disorder (MDD). 51% of the sample was male (n = 68), and mean age was 47.8 (SD = 12.3). At baseline (60 minutes before infusion), 61% of the sample (n = 81) reported any type of suicidal ideation (measured by a score > 0 on HAMD suicide item) and 40% (n = 53) reported a history of suicide attempts. Ideation at baseline was not associated with gender (χ2 = 0.32, p = .57), age (t(131) = −0.24, p = .81) or age of MDD/BD onset (t(124) = −0.05, p = .96) but was associated with suicide attempt history (χ2 = 8.31, df=124, p = .004).
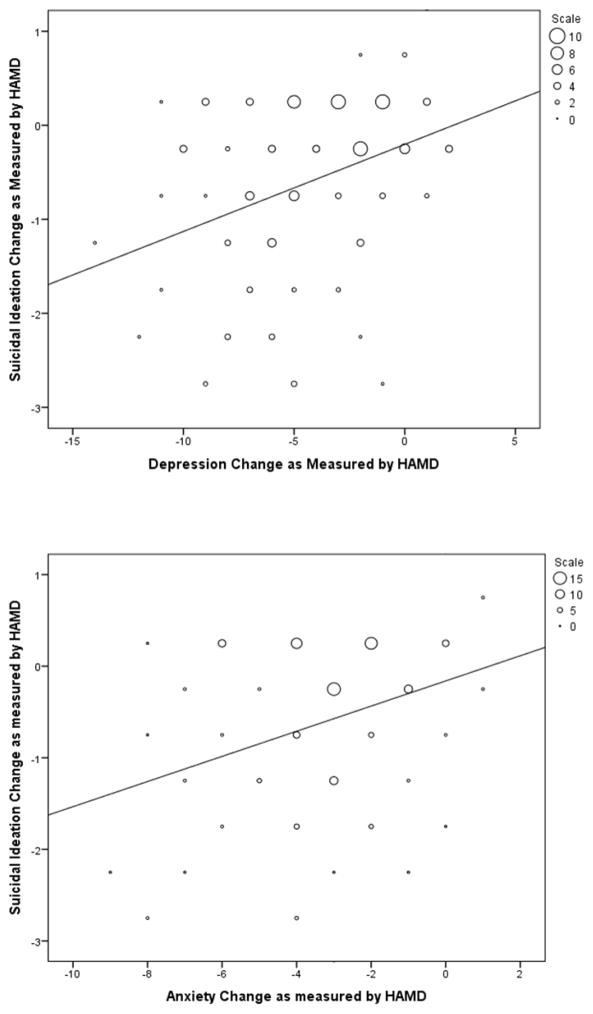
Correlations between changes in suicidal ideation, anxiety and depressive symptoms at 230 minutes post-ketamine infusion are presented in Table 1. The patients received ketamine in both randomized and open label studies. Changes in suicidal ideation were significantly associated with changes in depression with correlations ranging from .31 to .44. The strongest correlation of .44 (between the HAMD depression cluster and the SSI Wish To Die item) indicated that only 19% of the variance in ideation can be accounted for by depression scores. Changes in ideation were also significantly associated with anxiety with correlations ranging from .27 to .40, accounting for at most 16% of the variance. Changes in ideation in relation to changes in depression and anxiety are presented in Figure 1. In order to examine whether depression or anxiety were independently related to ideation, the HAMD anxiety cluster and the remaining HAMD items were entered into the same linear regression model to predict ideation change. The HAMD depression cluster was significantly associated with change in suicidal ideation, standardized β = .24, p = .03, while HAMD anxiety was not, standardized β = .16, p = .13.
Table 1.
Pearson Correlations between Change in Suicidal Ideation, Anxiety and Depressive Symptomatology at 230 minutes Post-Ketamine Infusion
| Change in Suicide Ideation
|
|||||||
|---|---|---|---|---|---|---|---|
| HAMD Suicide Item | SSI Reduced Wish to Live | SSI Wish to Die | |||||
|
| |||||||
| r | p | r | p | r | p | ||
| Change in Depression | HAMD without Anxiety Somatization Factor Score or suicide item | .35 | <.001 | .23 | .02 | .44 | <.001 |
| BDI without suicide item | .31 | .001 | .27 | .006 | .39 | <.001 | |
| Change in Anxiety | HAMA | .27 | .02 | .23 | .04 | .30 | .008 |
| HAMD-Anxiety Somatization Factor Score | 33 | <.001 | .29 | .003 | .40 | <.001 | |
BDI: Beck Depression Inventory; HAMA: Hamilton Anxiety Rating Scale; HAMD: Hamilton Rating Scale for Depression; SSI: Scale for Suicide Ideation
Figure 1.
Figure 1a and 1b. Change in ideation at 230 minutes after ketamine infusion compared to changes in depression and anxiety.
Note: Markers in graphs represent frequencies; larger markers represent more patients scoring at that value.
Linear Mixed Models
From patients who participated in a placebo-controlled crossover study and had suicidal ideation at baseline as measured by the HAMD (n = 57), there was a significant reduction in suicidal ideation following ketamine compared to placebo infusion, F1,586= 42.84, p < .001, but the intervention by time interaction did not reach significance, F76,546= 2.12, p = .050. When controlling for the impact of ketamine on depression (HAMD without suicide items or Anxiety Somatization Score) over time in a three-way interaction, the effect of ketamine on suicidal ideation was significant, F1,587= 10.31, p = .001, and the time by intervention interaction became significant, F6,534= 2.39, p = .03. On post-hoc analysis, suicidal ideation significantly differed in ketamine compared to placebo at the 40 minute timepoint (p < .001). In a separate model, when controlling for the impact of ketamine on anxiety (HAMD Anxiety Somatization Factor Score) over time in another three way interaction, ketamine still had a significant impact on suicidal ideation, F1,567= 8.54, p = .004, but the time by intervention interaction was not significant, F6,535= 1.02, p = .41.
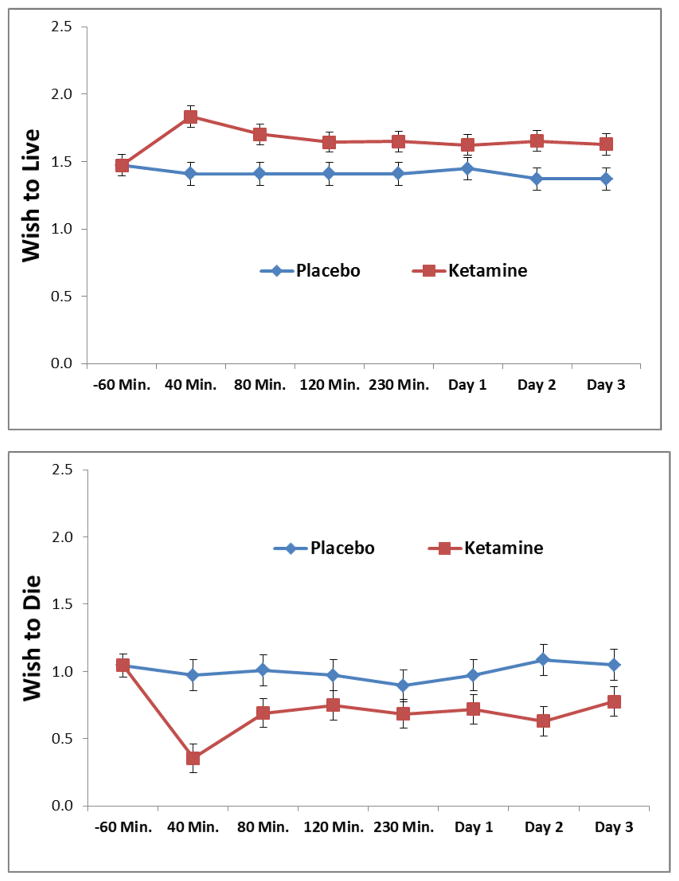
The effect of ketamine on “Wish to Live” and “Wish to Die,” as measured by the SSI, is presented in Figures 2a and 2b. This linear mixed model was limited to participants in a placebo-controlled trial and had suicidal ideation at baseline as measured by the SSI (Total SSI score > 0, n = 39). Ketamine infusion was associated with increased wish to live, F1,393= 53.05, p < .001, and decreased wish to die, F1,389= 48.53, p < .001. Neither time by intervention interaction was significant (p > .09). On further post-hoc analysis, the relationship of ketamine to increased wish to live and decreased wish to die persisted when controlling for depressive (p < .01) and anxiety symptoms (p < .001) across all time points.
Figure 2.
Figures 2a and 2b. Reductions in cognitions related to suicidal ideation as measured by the SSI in placebo-controlled trials of ketamine, limited to patients any suicidal thoughts at baseline.
2a. Wish to Live
2b. Wish to Die
Discussion
In this post-hoc analysis of 133 patients in a depressive episode, ketamine was associated with reductions in suicidal ideation independent of reductions in depressive and anxiety symptoms. Furthermore, ketamine had an impact on increased wish to live and decreased wish to die, two cognitive aspects of suicidal ideation which have been shown to predict later death by suicide (Brown et al., 2005a). While changes in suicidal ideation, depression and anxiety were significantly correlated, the combination of depression and anxiety symptoms only accounted for up to 19% of the variance in suicidal ideation change.
These results extend previous analyses from our group demonstrating ketamine’s ability to reduce suicidal thoughts (DiazGranados et al., 2010a; Zarate et al., 2012) and highlight the potential role of glutamatergic neurotransmission in the neurobiology of suicidal thinking. As interest in ketamine as a rapid antidepressant has grown, potential treatment response biomarkers, including PET neuroimaging (Carlson et al., 2013) and baseline clinical predictors (Ionescu et al., In Press; Niciu et al., 2014), have been evaluated to better understand its clinical and mechanistic implications (Niciu et al., 2013). With an understanding that ketamine impacts suicidal thoughts independent of improvement in depression or anxiety, comparable research can investigate biomarkers of ideation response to ketamine may illuminate potential neurobiological pathways. In preclinical studies, ketamine’s antidepressant effects are critically dependent on enhanced synaptic plasticity, which is believed to occur in response to NMDA receptor-mediated release of inhibition and concomitant “glutamate surge” from projection neurons in the prefrontal cortex and hippocampus (Duman and Aghajanian, 2012). Kavalali and Monteggia have also proposed that NMDA receptor antagonism deactivates eukaryotic elongation factor 2 (eEF2) kinase, leading to reduced eEF2 phosphorylation and increased translation of brain-derived neurotrophic factor (BDNF) (Kavalali and Monteggia, 2012). While these mechanisms remain speculative in clinical populations, we hypothesize that ketamine reduces suicidal thinking by enhancing neuroplasticity (Cornwell et al., 2012; Duncan et al., 2013).
These findings also emphasize the role of anxiety in suicidal thoughts. While the relationship between depression and suicidal thoughts is well-established, reductions in anxiety accounted for comparable reductions in ideation as depression. Results underscore the importance of evaluating both anxiety and depression when assessing suicide risk. Elevated anxiety and a history of anxiety disorder in the context of an affective disorder have been associated with imminent and long-term risk for suicidal behavior (Fawcett et al., 1990; Goldberg and Fawcett, 2012). In fact, disorders characterized by anxiety and agitation, rather than depression, have been associated with the transition from suicidal thoughts to suicidal behavior (Nock et al., 2010). Therefore, our findings reinforce the need for further investigation into the role of comorbid anxiety and depression in the development of suicidal thoughts. As outcomes in these trials were measured via self- and clinical assessments, additional psychophysiological-based measures of anxiety, including fear and anxiety-potentiated startle paradigms (Ballard et al., 2014; Schmitz and Grillon, 2012) may also be able to elucidate the relationship between suicidal thoughts and anxiety after a ketamine infusion.
There are some limitations of this study. First, this was a secondary data analysis and does not permit evaluation of causality. Second, the patients in our sample were specifically selected for current major depression, not suicidal ideation; therefore, the sample does not include acutely suicidal patients who may be seen in emergency settings. Replication of these results in a patient sample selected for higher ideation severity outside the context of a psychiatric diagnosis such as MDD or BP, e.g. a score of 4 or more on MADRS item 10 or moderate-to-high total baseline score on the Columbia Suicide Severity Rating Scale (C-SSRS), is necessary. Such trials examining ketamine for acute suicidal ideation are currently underway (ClinicalTrials.gov identifiers: NCT01700829 and NCT01887990). Third, while patients were administered the SSI, which assesses suicidal thoughts using several items, other cognitive constructs, such as hopelessness, have been demonstrated to predict future suicide (Beck et al., 1985; Hawton et al., 2013) and may have a significant association with suicidal thoughts. A more comprehensive measure of hopelessness, such as the Beck Hopelessness Scale (Beck et al., 1974), may help elucidate how pessimism and negative perceptions for the future are affected by ketamine at antidepressant doses. Lastly, it is important to note that ketamine’s antisuicidal effects were most robust at 40 minutes after ketamine infusion, the time point associated with greatest psychotomimetic symptoms, dissociative side effects and hemodynamic changes (Luckenbaugh et al., 2014).
Conclusion
Reductions in suicidal ideation following ketamine infusion are related to, but also independent of, reductions in depression and anxiety. As interventions for suicidal ideation are critically needed in psychiatry, findings suggest that evaluations of ketamine can be used to further investigate the neurobiology and best treatments for suicidal individuals.
Highlights.
We investigated the effects of ketamine on suicidal ideation across clinical trials.
Suicidal ideation, as independent from depression or anxiety, was evaluated.
Depression accounted a limited amount of variance of change in suicidal thoughts.
Ketamine had an effect on suicidal thoughts independent of depression and anxiety.
Anxiety may be an important treatment target for suicide risk.
Acknowledgments
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH) (NCT00088699 and 04- M-0222) by a NARSAD Independent Investigator and by the Brain & Behavior Mood Disorders Research Award to CAZ
Footnotes
Presented as a Poster at the American Psychopathological Association, New York City, March 6–8, 2014
Conflict of Interest
Dr. Furey is listed as a co-inventor on a patent application for the use of scopolamine in major depression and Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. Drs. Furey and Zarate have assigned their rights in the patent to the U.S. Government but will share a percentage of any royalties that may be received by the Government. The remaining authors have no conflicts of interest to disclose, financial or otherwise.
Contributors: All authors contributed to manuscript writing or data analysis, and agreed to submit the final version for publication.
Dr. Ballard conceptualized the study design, completed and interpreted the statistical analysis, drafted the manuscript and edited the manuscript for intellectual content. She approved the final manuscript before submission.
Dr. Ionescu assisted in the conceptualization of the study design, assisted in the interpretation of the statistical analysis and edited the manuscript for intellectual content. She approved the final manuscript before submission.
Dr. Vande Voort assisted in the conceptualization of the study design, assisted in the interpretation of the statistical analysis and edited the manuscript for intellectual content. She approved the final manuscript before submission.
Dr. Niciu assisted in the conceptualization of the study design, assisted in the interpretation of the statistical analysis and edited the manuscript for intellectual content. He approved the final manuscript before submission.
Dr. Richards assisted in the conceptualization of the study design, assisted in the interpretation of the statistical analysis and edited the manuscript for intellectual content. She approved the final manuscript before submission.
Mr. Luckenbaugh assisted in the statistical design, analysis and interpretation and edited the manuscript for intellectual content. He approved the final manuscript before submission.
Ms. Brutsche assisted in the interpretation of the statistical analysis and edited the manuscript for intellectual content. She approved the final manuscript before submission.
Dr. Ameli assisted in the interpretation of the statistical analysis and edited the manuscript for intellectual content. She approved the final manuscript before submission.
Dr. Furey assisted in the interpretation of the statistical analysis and edited the manuscript for intellectual content. She approved the final manuscript before submission.
Dr. Zarate provided research supervision, assisted in the conceptualization of the study design, assisted in the interpretation of the statistical analysis and edited the manuscript for intellectual content. He approved the final manuscript before submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- U.S. Department of Health and Human Services (HHS) Office of the Surgeon General and National Action Alliance for Suicide Prevention. Washington, DC: HHS; 2012. 2012 National Strategy for Suicide Prevention: Goals and Objectives for Action. [PubMed] [Google Scholar]
- Baca-Garcia E, Perez-Rodriguez MM, Oquendo MA, Keyes KM, Hasin DS, Grant BF, et al. Estimating risk for suicide attempt: are we asking the right questions? Passive suicidal ideation as a marker for suicidal behavior. J Affect Disord. 2011;134:327–32. doi: 10.1016/j.jad.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Ionescu DF, Voort JL, Slonena EE, Franco-Chaves JA, Zarate CA, Jr, et al. Increased fear-potentiated startle in major depressive disorder patients with lifetime history of suicide attempt. J Affect Disord. 2014;162:34–8. doi: 10.1016/j.jad.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343–52. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: a 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry. 1985;142:559–63. doi: 10.1176/ajp.142.5.559. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol. 1974;42:861–5. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Brown GK, Steer RA, Henriques GR, Beck AT. The internal struggle between the wish to die and the wish to live: a risk factor for suicide. Am J Psychiatry. 2005a;162:1977–9. doi: 10.1176/appi.ajp.162.10.1977. [DOI] [PubMed] [Google Scholar]
- Brown GK, Ten Have T, Henriques GR, Xie SX, Hollander JE, Beck AT. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005b;294:563–70. doi: 10.1001/jama.294.5.563. [DOI] [PubMed] [Google Scholar]
- Carlson PJ, Diazgranados N, Nugent AC, Ibrahim L, Luckenbaugh DA, Brutsche N, et al. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol Psychiatry. 2013;73:1213–21. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. National Violent Death Reporting System. 2013a [cited July 7, 2014]. Available from URL: www.cdc.gov/ncipc/wisqars.
- Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) 2013b [cited July 7, 2014]. Available from URL: www.cdc.gov/ncipc/wisqars/nvdrs.
- Cleary P, Guy W. Factor analysis of Hamilton depression scale. Drugs Exp Clin Res. 1977:115–20. [Google Scholar]
- Conwell Y, Duberstein PR, Cox C, Herrmann JH, Forbes NT, Caine ED. Relationships of age and axis I diagnoses in victims of completed suicide: a psychological autopsy study. Am J Psychiatry. 1996;153:1001–8. doi: 10.1176/ajp.153.8.1001. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, et al. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012;72:555–61. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, de Beurs DP, van Spijker BA, Berking M, Andersson G, Kerkhof AJ. The effects of psychotherapy for adult depression on suicidality and hopelessness: a systematic review and meta-analysis. J Affect Disord. 2013;144:183–90. doi: 10.1016/j.jad.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Desseilles M, Perroud N, Guillaume S, Jaussent I, Genty C, Malafosse A, et al. Is it valid to measure suicidal ideation by depression rating scales? J Affect Disord. 2012;136:398–404. doi: 10.1016/j.jad.2011.11.013. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim L, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010a;71:1605–11. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Archives of general psychiatry. 2010b;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2013;16:301–11. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Carmin CN, Balasubramani GK, Wisniewski SR, et al. What clinical and symptom features and comorbid disorders characterize outpatients with anxious major depressive disorder: a replication and extension. Can J Psychiat. 2006;51:823–35. doi: 10.1177/070674370605101304. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Scheftner WA, Fogg L, Clark DC, Young MA, Hedeker D, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147:1189–94. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition. New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- Frogley C, Taylor D, Dickens G, Picchioni M. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15:1351–71. doi: 10.1017/S146114571100201X. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Brown CH, Hur K, Davis J, Mann JJ. Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69:580–7. doi: 10.1001/archgenpsychiatry.2011.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D, Fawcett J. The importance of anxiety in both major depression and bipolar disorder. Depress Anxiety. 2012;29:471–8. doi: 10.1002/da.21939. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Brit J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawton K, Casanas ICC, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147:17–28. doi: 10.1016/j.jad.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Hennen J, Baldessarini RJ. Suicidal risk during treatment with clozapine: a meta-analysis. Schizophren Res. 2005;73:139–45. doi: 10.1016/j.schres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacol. 2012;37:1526–33. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Slonena EE, Vande Voort JL, et al. Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. J Clin Psychiatry. doi: 10.4088/JCP.14m09049. In Press. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Iglewicz A, Nelesen RA, Lo JY, Carr CH, Romero SD, et al. Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: a 28-day open-label proof-of-concept trial. J Palliat Med. 2013;16:958–65. doi: 10.1089/jpm.2012.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. Synaptic Mechanisms Underlying Rapid Antidepressant Action of Ketamine. Am J Psychiatry. 2012;3:12040531. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–31. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, et al. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Bernstein IH, Wisniewski SR, Trivedi MH, Morris D, et al. Assessing anxious features in depressed outpatients. Int J Method Psych. 2011;20:e69–82. doi: 10.1002/mpr.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, et al. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2014;75:e417–23. doi: 10.4088/JCP.13m08698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Matthews DC, Nugent AC, Ionescu DF, Furey ML, Richards EM, et al. Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants. Depress Anxiety. 2014;31:297–307. doi: 10.1002/da.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Hwang I, Sampson NA, Kessler RC. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:868–76. doi: 10.1038/mp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014;31:335–43. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–6. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz JA, Machado-Vieira R, Zarate CA, Jr, Manji HK. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62:50–60. doi: 10.1159/000314310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport LM, Moskowitz DS, Galynker I, Yaseen ZS. Panic symptom clusters differentially predict suicide ideation and attempt. Compr Psychiat. 2014;55:762–9. doi: 10.1016/j.comppsych.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Afifi TO, de Graaf R, Asmundson GJ, ten Have M, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62:1249–57. doi: 10.1001/archpsyc.62.11.1249. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nat Protoc. 2012;7:527–32. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting SA, Sullivan AF, Boudreaux ED, Miller I, Camargo CA., Jr Trends in US emergency department visits for attempted suicide and self-inflicted injury, 1993–2008. Gen Hosp Psychiatry. 2012;34:557–65. doi: 10.1016/j.genhosppsych.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–46. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews DC, Furey ML. Human biomarkers of rapid antidepressant effects. Biol Psychiatry. 2013;73:1142–55. doi: 10.1016/j.biopsych.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zigman D, Blier P. Urgent ketamine infusion rapidly eliminated suicidal ideation for a patient with major depressive disorder: a case report. J Clin Psychopharmacol. 2013;33:270–2. doi: 10.1097/JCP.0b013e3182856865. [DOI] [PubMed] [Google Scholar]