Abstract
Few randomized controlled trials (RCTs) exist examining the efficacy of behavior therapy (BT) or serotonin reuptake inhibitors (SRIs) for the treatment of trichotillomania (TTM), with no examination of treatment moderators. The present meta-analysis synthesized the treatment effect sizes (ES) of BT and SRI relative to comparison conditions, and examined moderators of treatment. A comprehensive literature search identified 11 RCTs that met inclusion criteria. Clinical characteristics (e.g., age, comorbidity, therapeutic contact hours), outcome measures, treatment subtypes (e.g., SRI subtype, BT subtype), and ES data were extracted. The standardized mean difference of change in hair pulling severity was the outcome measure. A random effects meta-analysis found a large pooled ES for BT (ES= 1.41, p< 0.001). BT trials with greater therapeutic contact hours exhibited larger ES (p= 0.009). Additionally, BT trials that used mood enhanced therapeutic techniques exhibited greater ES relative to trials including only traditional BT components (p= 0.004). For SRI trials, a random effects meta-analysis identified a moderate pooled ES (ES= 0.41, p= 0.02). Although clomipramine exhibited larger ES relative to selective serotonin reuptake inhibitors, the difference was not statistically significant. Publication bias was not identified for either treatment. BT yields large treatment effects for TTM, with further examination needed to disentangle confounded treatment moderators. SRI trials exhibited a moderate pooled ES, with no treatment moderators identified. Sensitivity analyses highlighted the need for further RCTs of SRIs, especially among youth with TTM.
Keywords: Hair pulling disorder, Obsessive-compulsive spectrum disorders, habit reversal training, behavior therapy, selective serotonin reuptake inhibitors, clomipramine
1. Introduction
Hair pulling behaviors are common in the general population (Duke et al., 2009), with between 1–3% of adults reporting clinically significant hair pulling (Christenson et al., 1991b). Hair pulling disorder, commonly referred to as trichotillomania (TTM), is characterized by excessive hair pulling that can be automatic (e.g., outside of awareness) or focused (e.g., consciously pulled) in nature (American Psychiatric Association, 2013; Stein et al., 2010). Individuals with TTM frequently experience co-occurring anxiety disorders, depressive disorders, and other body-focused repetitive behaviors (Duke et al., 2010; Panza et al., 2013). Hair pulling behaviors can result in detrimental physical complications (Bouwer and Stein, 1998), psychosocial impairment (Diefenbach et al., 2005b; Stemberger et al., 2000; Wetterneck et al., 2006; Woods et al., 2006a), and poor quality of life (Diefenbach et al., 2005b; Odlaug et al., 2010). In light of these deleterious physical and psychological consequences, effective and efficient treatments are needed.
Several therapeutic approaches have been investigated to treat TTM symptoms (Franklin et al., 2011b), including behavior therapy (BT) and psychiatric medications (Franklin et al., 2008; Woods et al., 2006a). Behavioral therapies such as habit reversal training (HRT) have demonstrated efficacy reducing hair pulling severity across several randomized controlled trials (RCTs) (Azrin et al., 1980; Diefenbach et al., 2006; Franklin et al., 2011a; Ninan et al., 2000; van Minnen et al., 2003), with more recent BT trials including components of acceptance and commitment therapy (ACT) (Woods et al., 2006b), and dialectical behavior therapy (DBT) (Keuthen et al., 2012). Acute treatment gains obtained from BT have been generally maintained up to six months (Franklin et al., 2011a; Keuthen et al., 2011; Woods et al., 2006b). Indeed, BT is recommended as a first-line treatment for youth and adults with TTM (Flessner et al., 2010). Despite noted efficacy, RCTs of BT have had relatively small sample sizes, with no examination of treatment moderators.
Aside from BT, two types of psychiatric medications (antipsychotics and antidepressants) have demonstrated mixed efficacy in reducing hair pulling. While antipsychotic medications (e.g., olanzapine, aripiprazole) have demonstrated efficacy in open-label trials (Stewart and Nejtek, 2003; White and Koran, 2011), only one RCT has evaluated the efficacy of olanzapine and identified its therapeutic benefit relative to placebo (Van Ameringen et al., 2010). Comparatively, antidepressant medications (e.g., clomipramine, fluoxetine, sertraline) remain the most frequently used treatment for individuals with TTM (Franklin et al., 2008; Woods et al., 2006a), but have mixed evidence across RCTs (Christenson et al., 1991a; Dougherty et al., 2006; Ninan et al., 2000; Streichenwein and Thornby, 1995; Swedo et al., 1989; van Minnen et al., 2003), with some evidence of long-term therapeutic benefit (Swedo et al., 1993). These medications share the commonality of inhibiting the reuptake of serotonin (referred to as serotonin reuptake inhibitors, SRIs) leading to the belief that deficiencies of serotonin may underlie hair pulling behaviors (Ferrão et al., 2009; Mancini et al., 2009). Despite mixed efficacy, side effect profiles, and potential high relapse rates following discontinuation (Iancu et al., 1996; Pollard et al., 1991); SRI medications are frequently used (Franklin et al., 2008; Woods et al., 2006a) and recommended by experts as pharmacological treatment options (Chamberlain et al., 2007).
More recently, N-acetylcysteine (i.e., an over-the-counter amino acid supplement that acts as a glutamate modulator; NAC) has been evaluated in the treatment of individuals with TTM. While demonstrating efficacy in a RCT of adults with TTM (Grant et al., 2009), no significant benefit was found relative to placebo in a sample of youth with TTM (Bloch et al., 2013). Although offering promise for some individuals (Woods, 2013), the small number of RCTs limits inferences about NAC's efficacy for TTM.
When making treatment recommendations, it is important to synthesize empirical evidence to guide clinical decisions (Murad and Montori, 2013). Relative to literature reviews and expert recommendations, meta-analyses provide a quantitative synthesis of treatment trials, and allow for the examination of moderators of treatment effects. Presently, only one meta-analysis has examined the efficacy of behavioral and pharmacological treatments for reducing hair pulling behaviors among individuals with TTM (Bloch et al., 2007). Bloch et al. (2007) found a large treatment effect for HRT [Standardized Mean Difference (SMD)= 1.14, 95% Confidence Interval (CI): −1.89, −0.38] compared to control conditions. Clomipramine (CMI) was found to be superior to comparison conditions (SMD= 0.68, 95% CI: −1.28, −0.07), with no significant effect found for selective serotonin reuptake inhibitor medications (SSRIs) relative to placebo (SMD=0.02, 95% CI: −0.32, 0.35). While beneficial as the first quantitative synthesis of this area, this meta-analysis has several limitations in the present-day context (Bloch et al., 2007). First, since its publication, several additional RCTs of BT for TTM have been published (Diefenbach et al., 2006; Franklin et al., 2011a; Keuthen et al., 2012). Second, this meta-analysis relied upon treatment-blind ratings to limit reporting bias. Although a notable strength, this resulted in the use of some outcome measures that had limited psychometric evaluation (e.g., video-taped hair loss ratings), and interchanged measures that evaluated symptom severity and impairment rather than focusing solely on the construct of symptom severity (Bloch et al., 2007). Finally, this meta-analysis separated treatment effects between CMI and SSRI medications. Although CMI can impact other neurotransmitters (e.g., norepinephrine reuptake inhibitor), it serves as a strong post synaptic serotonin reuptake inhibitor. As CMI and SSRIs both strongly affect serotonin receptors, there is some benefit to examining their collective efficacy, as well as their individual efficacy.
In an effort to address these limitations, this meta-analysis examined the efficacy of evidence-based treatments for individuals with TTM. Although antipsychotics and NAC have emerging empirical support, these treatments were considered too preliminary for inclusion due to the limited number of published RCTs. Thus, this meta-analysis examined the efficacy of BT and SRI treatment to reduce hair pulling severity among individuals with TTM. Additionally, clinically-relevant treatment moderators were examined that included: participant age; percentage of co-occurring anxiety and depressive disorders; outcome measure informant; average number of 1-hour therapy sessions (for BT trials); study methodology; and intervention subtypes.
2. METHOD
2.1 Search Strategy
PubMED (1965- March 2014), PsycInfo, and ProQuest Dissertations and Theses Online were searched using key search terms (i.e., “trichotillomania”, “habit reversal training”, “behavior therapy”, “behavioral intervention”, “competing response training”, “selective serotonin reuptake inhibitor”, or “serotonin reuptake inhibitor”). Identified titles and abstracts were reviewed independently by three raters for appropriateness. The references of eligible treatment trials, and review articles were also searched for published or unpublished research.
Identified abstracts/citations were evaluated for inclusion using the following criteria: (1) a RCT; (2) examined the efficacy of a BT or SRI in treating TTM relative to a non evidence-based comparison condition; (3) available in English; and (4) provided sufficient data to allow calculation of treatment effects. Trials were considered randomized when study authors explicitly represented them as such. Treatments were considered to be BT when they included awareness training and competing response training components. Treatments were considered to be a SRI when they included an antidepressant medication that inhibited the reuptake of serotonin (e.g., clomipramine, fluoxetine, sertraline). When treatment effect data was not sufficiently reported, study investigators were contacted to obtain values.
2.2 Meta-Analytic Procedures
2.2.1 Selection of Outcome Measures
Given that most studies employed multiple measures of TTM severity, a hierarchy of preferred TTM rating scales was established a priori to limit potential reporting bias. Although the Massachusetts General Hospital Hair pulling Scale (MGH-HPS; Keuthen et al., 1995; O'Sullivan et al., 1995) was commonly used, preference was placed on clinician-rated measures due to their standardized administration and objectivity. Three raters reviewed the published psychometric properties of standardized rating scales to determine the preferential order of clinician-rated, parent-report, and self-report ratings (Diefenbach et al., 2005a; McGuire et al., 2012). In order of preference, preferred clinician-rating scales included the National Institute of Mental Health-Trichotillomania Severity Scale (NIMH-TSS; Swedo et al., 1989), Psychiatric Institute Trichotillomania Scale (PITS; Winchel et al., 1992), and the Yale-Brown Obsessive Compulsive Scale modified for Trichotillomania (Y-BOCS-TTM; Stanley et al., 1999). In the absence of clinician ratings, self-report measures of hair pulling severity were preferred, which included the MGH-HPS (Keuthen et al., 1995; O'Sullivan et al., 1995) and the Trichotillomania Scale for Children and Parents (Tolin et al., 2008). When standardized ratings scales were unavailable, self-reported ratings of hair pulling severity were utilized that included weekly ratings of hair pulling severity (Christenson et al., 1991a; Streichenwein and Thornby, 1995), and the number of daily hair pulling episodes (Azrin et al., 1980).
2.2.2 Study Coding
Trials were coded for the following characteristics: (1) comparison condition; (2) mean participants' age; (3) inclusion of youth and/or adults; (4) percentage of co-occurring anxiety and depressive disorders; (5) outcome measure; (6) outcome measure informant; (7) average number of 1-hour therapy sessions (BT only); (8) study methodology; (9) intervention subtypes; and (10) effect size. Comparison interventions were classified as wait-list comparisons (WL), placebo (PLBO), or active comparison (AC; i.e., mass negative practice, supportive psychotherapy, minimal attention control, desipramine). Study methodology was assessed using a 23-item scale (range: 0–46; Moncrieff et al., 2001), with higher values corresponding to greater methodological rigor. For BT trials, intervention subtypes were categorized as using core BT or mood-enhanced BT (BT plus ACT or DBT). Meanwhile for SRI trials, interventions subtypes were categorized as using CMI or a SSRI (i.e., fluoxetine, sertraline). Trials were coded by three raters to ascertain reliability. Rater disagreement was resolved through discussion and consensus.
2.2.3 Effect size (ES) calculation
The primary outcome measure was the mean improvement in trichotillomania symptom severity. The difference between active interventions (BT and SRI) and control conditions was examined by calculating the SMD in Comprehensive Meta-Analysis (CMA) Version 2 (Borenstein et al., 2005). The SMD was chosen as the treatment ES statistic because it facilitated comparison with the prior meta-analysis (Bloch et al., 2007). The mean change in control group from pre-treatment to post-treatment was subtracted from the mean change in the treatment group from pre-treatment to post-treatment and was then divided by the pooled change standard deviation. A moderate-to-large correlation between baseline and post-treatment ratings was assumed for all trials (r= 0.50). Effect sizes were standardized so that a positive result indicated that active intervention performed better than control conditions.
2.3 Statistical Analyses
First, inter-rater agreement of study characteristics and quality ratings was assessed using descriptive statistics and intra-class correlation coefficient (ICC). Second, a random effects model using inverse variance weights examined the SMD for each intervention using CMA (Borenstein et al., 2005). A random effects model was chosen because the true ES were expected to vary across trials due to differences in study characteristics (Borenstein et al., 2009). Heterogeneity of ES was assessed using the forest plot, Q statistic, and I2 statistic. Third, moderator variables were analyzed using either a method-of-moments meta-regression for continuous moderators or an analog to the analysis of variance (ANOVA) for categorical moderators. Moderator analyses were re-examined with only trials that utilized standardized rating scales. Findings were consistent between these two approaches, and thus, only the former is reported as it is more inclusive of the TTM literature. Fourth, publication bias was assessed by visual inspection of the funnel plot and Egger's test for bias. Duval and Tweedie's trim-and-fill method was used to account for potential publication bias, by taking into account unpublished studies within the field, and provided an adjusted summary effect for each intervention (Borenstein et al., 2009). Finally, sensitivity analyses included Rosenthal's Fail-safe N (Rosenthal, 1991), Orwin's Fail-safe N (Orwin, 1983), and a comparison of ES across control conditions. Rosenthal's Fail-safe N determines the number of un-retrieved studies (k) with a mean effect of zero that would be needed to make current findings non-significant. Orwin's Fail-safe N determines the number of un-retrieved studies (k) with a mean effect of zero that would be needed to reduce the summary ES to a trivial effect (ES= 0.30). An analog to ANOVA examined the heterogeneity of ES across control conditions (WL, PLBO, AC) using the Q statistic, with follow-up pair-wise comparisons.
3. RESULTS
3.1 Included Studies and Study Characteristics
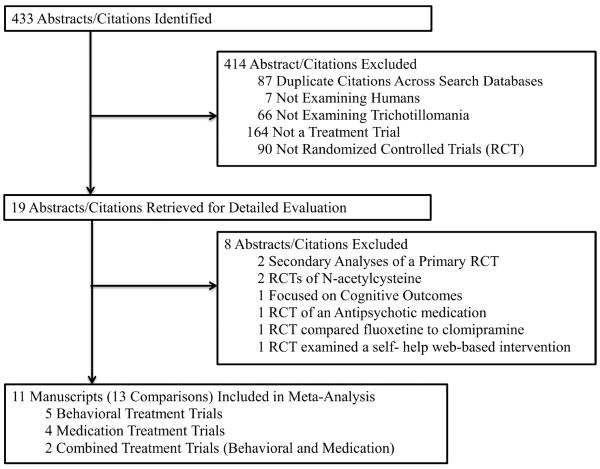
Initial search strategies produced 433 potential abstracts/citations, with 19 abstracts/citations being retrieved for a detailed review (see Figure 1). Table 1 displays the 11 RCTs that met inclusion criteria that allowed for comparisons of seven BT trials (n= 182 participants) and six SRI trials (n= 118 participants).
Figure 1.
Study Selection and Rationale For Inclusion and Exclusion.
Table 1.
Study Characteristics for Trials Included in Meta-analyses
| Study | Active Tx |
Control Condition |
N | Mean Age |
Youth or Adult Sample |
Comorbid Anxiety Disorders |
Comorbid Depressive Disorders |
Outcome Measure | Self v. Clinician Report |
Approx Contact Hours |
Study Quality |
Effect Size |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavior Therapy | ||||||||||||
| Azrin et al., 1980 a | BT | MNP | 32 | 27 | Mixed | NR | NR | number of daily hair pulling episodes | Self-report | 2 | 23 | 0.66 |
| Ninan et al., 2000 b | BT | WL | 10 | 33 | Adult | NR | NR | NIMH-TSS | Clinician-report | 9 | 19 | 1.94 |
| Van Minnen et al., 2003 b | BT | PLBO | 29 | 31 | Mixed | 3% | 17% | MGH-HPS | Self-report | 6 | 36 | 1.28 |
| Diefenbach et al., 2006 | BT | SP | 24 | 40 | Adult | 25% | 21% | MGH-HPS | Self-report | 8 | 30 | 0.56 |
| Woods et al., 2006 | BT | WL | 25 | 35 | Adult | 4% | 32% | MGH-HPS | Self-report | 10 | 28 | 2.14 |
| Franklin et al., 2011 | BT | MAC | 24 | 13 | Youth | 33% | 13% | NIMH-TSS | Clinician-report | 8 | 35 | 1.38 |
| Keuthen et al., 2012 | BT | MAC | 38 | 31 | Adult | 11% | 0% | NIMH-TSS | Clinician-report | 11 | 29 | 2.35 |
| Serotonin Reuptake Inhibitors | ||||||||||||
| Swedo et al., 1989 | CMI | DES | 13 | 32 | Mixed | 8% | 0% | NIMH-TSS | Clinician-report | N/A | 28 | 0.75 |
| Christenson et al., 1991 | FLUX | PLBO | 16 | 32 | Adult | 13% | 19% | weekly rating of hair pulling severity | Self-report | N/A | 25 | 0.14 |
| Streichenwien & Thronby, 1995 | FLUX | PLBO | 16 | 39 | Adult | 31% | 6% | weekly severity of hair pulling episodes | Self-report | N/A | 23 | 0.35 |
| Ninan et al., 2000 b | CMI | PLBO | 11 | 33 | Adult | NR | NR | NIMH-TSS | Clinician-report | N/A | 20 | 0.61 |
| Van Minnen et al., 2003 b | FLUX | PLBO | 26 | 32 | Mixed | 0% | 19% | MGH-HPS | Self-report | N/A | 38 | 0.54 |
| Dougherty et al., 2006 | SERT | PLBO | 36 | 29 | Adult | 30% | 16% | PITS | Clinician-report | N/A | 27 | 0.17 |
Note: CMI = clomipramine, DES = desipramine, FLUX = fluoxetine, SERT = sertraline, PLBO = placebo, BT = behavior therapy, MNP = Mass Negative Practice, WL = waitlist, SP = Supportive psychotherapy, MAC = Minimal Attention Control, NAC = n-acetylcesteine, NR = not reported, NIMH-TSS = National Institute of Mental Health Trichotillomania Severity Scale, MGH-HPS = Massachusetts General Hospital Hair pulling Scale, PITS = Psychiatric Institute Trichotillomania Scale, N/A = not applicable.
used LOCF for participants without post-treatment assessment. Excluded two participants who did not use the same outcome metric.
PLBO group double counted for Van Minnen et al., 2003 and Ninan et al., 2000 due to small sample size of PLBO group
3.2 Reliability of Coding Study Characteristics
There was excellent inter-rater agreement between the three raters on categorical and continuous study characteristics (100% agreement), as well as overall study methodological quality (ICC= 0.99, 95% CI: 0.98, 0.99).
3.3 Treatment Effects of BT
As seen in Figure 2, a random effects meta-analysis identified a large treatment effect of BT compared to control conditions (SMD= 1.41, 95% CI: 0.87, 1.96, z= 5.07, p< 0.001). Visual inspection of the forest plot, Q statistic, and I2 statistic identified the presence of significant heterogeneity among ES across trials [Q(6)= 15.61, p= 0.02, I2= 61.57%]. Given that Azrin et al. (1980) did not use a standardized rating scale, the summary effect was recalculated with this trial excluded to examine the effects of BT for TTM with only standardized rating scales. Results identified a large treatment effect (SMD= 1.56, 95% CI: 0.99, 2.14, z= 5.37, p< 0.001), with less heterogeneity [Q(5)= 11.21, p= 0.05, I2= 55.41%]. Given that Diefenbach et al. (2006) employed a group therapy format, the summary effect was re-calculated with this trial excluded to examine the effects of individual BT for TTM. Results identified a large treatment effect (SMD= 1.56, 95% CI: 1.00, 2.13, z= 5.43, p< 0.001), with less heterogeneity [Q(5)= 11.47, p= 0.04, I2= 56.47%].
Figure 2.
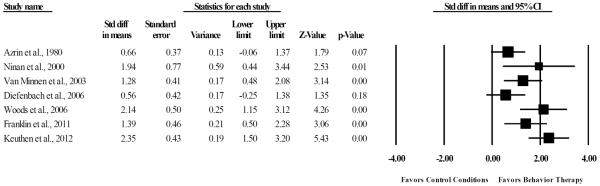
Efficacy of behavior therapy relative to control conditions for the treatment of hair pulling severity.
3.4 Moderators of BT
Table 2 presents results for moderator analyses. First, no significant association was observed between mean participant age and ES (p= 0.98). Furthermore, there were no significant difference among BT trials that included only adults (SMD= 1.71), only children (SMD= 1.39), or included both youth and adults (SMD= 0.94). Second, there was no significant association between ES and the percentage of study participants with either co-occurring anxiety disorders (p= 0.33) or depressive disorders (p= 0.61). Third, trials using treatment-blind clinician-raters (SMD= 1.90) had greater ES relative to unblind self-report raters (SMD= 1.11); however, this difference was not statistically significant (p= 0.09). Fourth, there was a significant association between number of therapeutic contact hours and ES (p< 0.01). Fifth, there was no significant association between study methodological quality and ES (p= 0.94). Finally, when examining BT treatment subtypes, a significant difference emerged between trials that used core BT (SMD= 1.02) compared to trials that used mood-enhanced BT (SMD= 2.26, p= 0.002).
Table 2.
Regression Analyses and Analog to ANOVA Examining Moderators of Treatment Effects in BT and SRI Trials
| BT Trials (n = 7) | SRI Trials (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study Characteristics | B | SE | z | p | B | SE | z | p |
| Average participant age | −0.001 | 0.04 | −0.02 | 0.98 | 0.02 | 0.06 | 0.26 | 0.79 |
| Percentage of comorbid anxiety disorders | −0.03 | 0.03 | −0.97 | 0.33 | −0.01 | 0.01 | −0.87 | 0.39 |
| Percentage of comorbid depressive disorders | −0.02 | 0.03 | −0.51 | 0.61 | −0.02 | 0.02 | −0.84 | 0.40 |
| Average number of therapeutic contact hours | 0.17 | 0.07 | 2.58 | 0.01 | NA | NA | NA | NA |
| Study methodological quality rating | −0.004 | 0.06 | −0.08 | 0.94 | 0.01 | 0.03 | 0.09 | 0.92 |
| Q | (df) | p | Q | (df) | p | |||
| Youth, Adult, or Mixed Participants | 2.03 | 2 | 0.36 | 1.13 | 1 | 0.29 | ||
| Clinician versus self-report ratings | 2.93 | 1 | 0.09 | 0.03 | 1 | 0.87 | ||
| Therapy subtype comparisons | 10.01 | 1 | 0.002 | 1.09 | 1 | 0.30 | ||
| Control Condition Comparison | 2.09 | 2 | 0.35 | 0.90 | 1 | 0.34 | ||
Note: BT = Behavior Therapy, SRI = Serotonin Reuptake Inhibitors, NA = not applicable
3.5 Publication Bias and Sensitivity Analyses for BT Trials
Although visual inspection of the funnel plot suggested publication bias may exist, Eggers' test for bias indicated that publication bias was not significant (t= 1.31, p= 0.25). Duval and Tweedie's trim-and-fill method trimmed no studies, and a large treatment effect remained (SMD= 1.41). Rosenthal's and Orwin's Fail-safe N calculations identified that at least 115 and 25 unretrieved studies with an effect size of zero were needed to reduce the summary ES of BT to a non-significant and/or trivial effect, respectively. Finally, no significant difference was identified across comparison conditions for WL trials (SMD= 2.14), PLBO trials (SMD= 1.43), and AC trials (SMD= 1.23, see Table 2).
3.6 Treatment Effects of SRIs
As seen in Figure 3, a random effects meta-analysis identified a moderate summary effect of SRI medications relative to control conditions (SMD= 0.41, 95% CI: 0.06, 0.75, z=2.29, p=0.02). Visual inspection of the forest plot, Q statistic, and I2 indicated minimal heterogeneity among ES across trials [Q(5)= 1.74, p= 0.88, I2= 0%]. Given that two of the six RCTs used unstandardized outcome measures (Christenson et al., 1991a; Streichenwein and Thornby, 1995), the summary effect was re-calculated with these two trials excluded to examine the effects of SRIs for TTM using psychometrically validated rating scales. Results identified a moderate treatment effect (SMD= 0.46, 95% CI: 0.06, 0.86, z= 2.24, p= 0.03), with little heterogeneity [Q(3)= 1.38, p= 0.71, I2= 0%].
Figure 3.
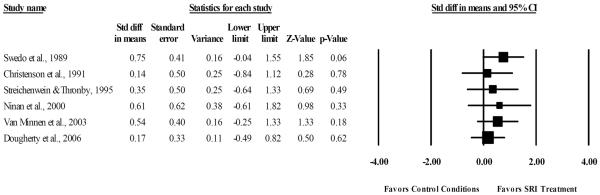
Efficacy of serotonin reuptake inhibitors compared to control conditions for the treatment of hair pulling severity.
3.7 Moderators of SRIs
Table 2 presents results for moderator analyses. First, no significant association was observed between mean participant age and ES (p= 0.79). Furthermore, there were no significant differences between SRI trials that included only adults (SMD= 0.26) relative to SRI trials that included both older adolescents and adults SMD= 0.65). Second, there was no significant association between ES and the percentage of study participants with co-occurring anxiety disorders (p= 0.39) or depressive disorders (p= 0.40). Third, no significant difference was identified between trials using treatment blind clinician-raters (SMD= 0.43) relative to unblind self-report informants (SMD= 0.37). Fourth, there was no significant association between study methodological quality and ES (p= 0.92). Finally, when specific SRI medication classes were examined, CMI trials (SMD= 0.71) exhibited greater ES relative to SSRI trials (SMD= 0.29); however, the difference between medication classes was not statistically significant (p= 0.30).
3.8 Publication Bias and Sensitivity Analyses for SRI Trials
Visual inspection of the funnel plot and Egger's test for bias both indicated that publication bias was not significant (t= 0.46, p= 0.67). Duval and Tweedie's trim-and-fill method trimmed no studies, and a moderate significant effect remained (SMD= 0.41). Rosenthal's and Orwin's Fail-safe N calculations similarly identified that only three unretrieved studies with an effect size of zero were needed to reduce the summary ES of SRI to a non-significant and/or trivial effect respectively. Finally, no significant difference was found across comparison conditions for PLBO trials (SMD= 0.32), and AC trials (SMD= 0.75, see Table 2).
4. DISCUSSION
This study examined the efficacy of BT and SRIs for the treatment of TTM, and explored treatment moderators. A large effect for BT was found across RCTs for TTM. This is consistent with the prior meta-analysis (Bloch et al., 2007), and supports expert consensus that BT is an efficacious treatment for individuals with TTM (Flessner et al., 2010). Moderator analyses revealed that trials with a greater number of therapeutic contact hours exhibited larger ES. The relationship between increased number of therapy session and ES is consistent with findings on the dose-response relationship observed in behavior therapy trials (McGuire et al., 2014) and medication trials of related disorders (Bloch et al., 2010). Additionally, moderator analyses revealed that trials using mood-enhanced BT outperformed studies that utilized only core BT components. This suggests that there is added benefit to including mood enhanced therapeutic components in treatment. Notably, the moderating effects of therapeutic contact and mood-enhanced BT are confounded due to the greater level of therapeutic contact provided by mood-enhanced BT trials. This confounding factor prohibits definitive interpretation of these individual moderator findings, and highlights the need for a head-to-head comparison controlling for therapeutic contact to clarify this relationship. Sensitivity analyses failed to identify publication bias, and calculations for Fail-safe N's suggested that between 25–115 unretrieved studies were needed with non-significant effects to make these findings non-significant and/or trivial. Thus, findings from BT trials yield large treatment effects for TTM, and appear to be robust to publication bias and file drawer phenomena.
This meta-analysis found a moderate treatment effect for SRIs (ES= 0.41). While CMI trials exhibited greater treatment effects (ES= 0.71) relative to SSRI trials (ES= 0.29), the difference between medication classes was not statistically significant. This is somewhat contrary to previous meta-analytic findings (Bloch et al., 2007), with differential findings being attributed to different outcome measures used to calculate treatment effects. While the prior meta-analysis placed preference on blinded treatment ratings, this meta-analysis placed a preference on clinician-rated measures due to standardized administration and objectivity (Lewin et al., 2012). Thus, outcome measures differed for four of the six SRI trials between meta-analyses (Christenson et al., 1991a; Dougherty et al., 2006; Streichenwein and Thornby, 1995; van Minnen et al., 2003). Although publication bias was not observed, sensitivity analyses revealed that only three unretrieved studies were needed with non-significant effects to make this summary effect non-significant and/or trivial. These findings demonstrate the moderate efficacy of SRIs for TTM and highlight the need for further exploration of SRI efficacy using psychometrically validated outcome measures.
Several limitations should be considered. First, only 11 RCTs were included and thus these two meta-analyses had modest power to detect moderators (Borenstein et al., 2009). Proposed moderators that did not reach statistical significance should not be interpreted as a conclusive lack of association. Conversely, moderators that were significant suggest that the identified association was robust enough to be detected amidst limited power. Second, medication status among BT trials was largely unknown. Given that there is some evidence supporting combined BT and SRI therapy for TTM (Dougherty et al., 2006), this trial characteristic warrants further examination. However, given medications were stabilized prior to trial participation and would likely have been equally distributed across treatment groups due to random assignment, it may not significantly impact treatment outcome across included trials. Third, there were limited characteristics available for extraction across RCTs. Although theoretically driven variables were selected, there may be unexamined factors (e.g., homework compliance, medication adherence) omitted from these reports that influence treatment effects. Finally, when drawing comparisons between trial types, it's important to consider that participants' characteristics may differ between BT and SRI trials. A head-to-head comparison trial would prove useful to determine comparative efficacy between these two treatment options.
Despite these limitations, this meta-analysis provides evidence that BT is an efficacious treatment that produces large treatment effects for individuals with TTM (Bloch et al., 2007; Flessner et al., 2010). Given that findings in related disorders indicate the limited number of BT treatment providers (Woods et al., 2010), future research should examine strategies to increase the availability of BT that have demonstrated preliminary success (Flancbaum et al., 2011; Himle et al., 2012). While individual moderators of BT were confounded due to the greater therapeutic contact provided by mood-enhanced BT trials, these findings suggest that either of these two treatment characteristics (or their combination) may play an important in enhancing BT therapeutic outcomes. Although providing some evidence for treatment moderators, a large scale RCT would prove useful to explore treatment moderators with greater statistical power. Comparatively, SRI medications present another treatment option that has a more modest effect relative to BT. Although not statistically significantly, CMI trials exhibited larger treatment effects compared to SSRI trials. Sensitivity analyses highlight the further need for evaluation of SRI medications.
While these findings demonstrate the benefit of existing treatment options, they also highlight several challenges confronting the treatment of TTM. For instance, relative to related conditions like obsessive-compulsive disorder that exhibit a comparable prevalence rate, there have been few RCTs examining the efficacy of treatments for individuals with TTM. Moreover, as many individuals with TTM report that symptom onset during youth (Odlaug et al., 2012), it presents a particular concern that only two RCTs have examined interventions targeted at youth with TTM (Bloch et al., 2013; Franklin et al., 2011). Aside from increasing the number of treatment studies focused on TTM, there has been minimal psychometric examination of TTM rating scales that has led to a limited consensus concerning a primary outcome measure to assess TTM symptom severity. Although this study placed an emphasis on clinician rating scales, there is a clear need for further examination of TTM rating scales across multiple informants to consistently identify reliable and valid measures. Finally, most of these trials focus on acute treatment outcomes, with minimal examination of the durability of acute treatment gains and/or long-term outcome. As most of these RCTs have brief acute treatment period, longer term studies would prove beneficial to examine when optimal treatment response is achieved for interventions and explore the long-term durability of initial treatment gains. Overall, these meta-analytic findings highlight the benefit of existing treatment options, but note that further research is still needed to overcome the challenges confronting the treatment of TTM.
HIGHLIGHTS.
Behavior therapy (BT) yields large treatment effects for trichotillomania (TTM).
Therapeutic contact and mood-enhanced components moderated BT treatment effects.
Serotonin reuptake inhibitors (SRIs) demonstrated modest treatment effects.
No significant difference was found between clomipramine (CMI) and selective SRIs.
Sensitivity analyses highlight the need for further evaluation of SRIs for TTM.
Acknowledgements
The authors would like to express their gratitude to Dr. Darin D. Dougherty M.D. for his assistance in obtaining data not published in his original article.
Mr. McGuire receives support from the National Institute Of Mental Health of the National Institutes of Health under award R01MH093381.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Mental Health or the National Institutes of Health. Dr. Lewin has served as a consultant for Otsuka America Pharmaceutical and ProPhase, Inc. He has received grant support from International Obsessive Compulsive Disorder Foundation; National Alliance for Research on Schizophrenia and Depression; University of South Florida Research Foundation, Inc. He has received travel support from University of South Florida Research Foundation, Inc. Dr. Murphy has received research funding from NIH/NIMH, CDC, Otsuka Pharmaceuticals, NARSAD, IOCDF, Ortho-McNeil Janssen Pharmaceuticals, Shire Pharmaceuticals, Pfizer, Inc. and Indevus Pharmaceuticals. She has received travel support from the Tourette Syndrome Association and honorarium from grand rounds lectures. Dr. Storch has received grant funding in the last 2 years from the National Institutes of Health, Centers for Disease Control, Agency for Healthcare Research and Quality, National Alliance for Research on Schizophrenia and Affective Disorders, International OCD Foundation, Tourette Syndrome Association, and Janssen Pharmaceuticals. He receives textbook honorarium from Springer publishers, American Psychological Association, and Lawrence Erlbaum. Dr. Storch has been an educational consultant for Rogers Memorial Hospital. He is a consultant for Prophase, Inc. and CroNos, Inc., and is on the Speaker's Bureau and Scientific Advisory Board for the International OCD Foundation. He receives research support from the All Children's Hospital Guild Endowed Chair.
Role of the funding Source None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors J. McGuire designed the meta-analysis, analyzed data, and wrote the first draft of the manuscript.
D. Ung co-designed the meta-analysis, assisted with data collection, and contributed to manuscript preparation.
R. Selles co-designed the meta-analysis, assisted with data collection, and contributed to manuscript preparation.
O. Rahman assisted with the design of the meta-analysis and manuscript preparation.
A. Lewin assisted with the design of the meta-analysis and manuscript preparation.
T. Murphy assisted with the design of the meta-analysis and manuscript preparation.
E. Storch assisted with the design of the meta-analysis and manuscript preparation.
Disclosures Ms. Ung, Mr. Selles, and Dr. Rahman report no relevant disclosures.
5.0 REFERENCES
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Azrin NH, Nunn RG, Frantz SE. Treatment of hairpulling (trichotillomania): A comparative study of habit reversal and negative practice training. J Behav Ther Exp Psychiatry. 1980;11:13–20. [Google Scholar]
- Bloch MH, Landeros-Weisenberger A, Dombrowski P, Kelmendi B, Wegner R, Nudel J, et al. Systematic review: Pharmacological and behavioral treatment for trichotillomania. Biol Psychiatry. 2007;62:839–46. doi: 10.1016/j.biopsych.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15:850–5. doi: 10.1038/mp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Panza KE, Grant JE, Pittenger C, Leckman JF. N-acetylcysteine in the treatment of pediatric trichotillomania: a randomized, double-blind, placebo-controlled add-on trial. J Am Acad Child Adolesc Psychiatry. 2013;52:231–40. doi: 10.1016/j.jaac.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis. 2 ed Biostat; Englewood, NJ: 2005. [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Wiley; 2009. [Google Scholar]
- Bouwer C, Stein DJ. Trichobezoars in trichotillomania: Case report and literature overview. Psychosom Med. 1998;60:658–60. doi: 10.1097/00006842-199809000-00025. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Sahakian BJ, Fineberg NA. Lifting the veil on trichotillomania. The American Journal of Psychiatry. 2007;164:568–74. doi: 10.1176/ajp.2007.164.4.568. [DOI] [PubMed] [Google Scholar]
- Christenson GA, Mackenzie TB, Mitchell JE, Callies AL. A placebo-controlled, double-blind crossover study of fluoxetine in trichotillomania. The American Journal of Psychiatry. 1991a;148:1566–71. doi: 10.1176/ajp.148.11.1566. [DOI] [PubMed] [Google Scholar]
- Christenson GA, Pyle RL, Mitchell JE. Estimated lifetime prevalence of trichotillomania in college students. J Clin Psychiatry. 1991b;52:415–7. [PubMed] [Google Scholar]
- Diefenbach GJ, Tolin DF, Crocetto J, Maltby N, Hannan S. Assessment of Trichotillomania: A Psychometric Evaluation of Hair-Pulling Scales. J Psychopathol Behav Assess. 2005a;27:169–78. [Google Scholar]
- Diefenbach GJ, Tolin DF, Hannan S, Crocetto J, Worhunsky P. Trichotillomania: Impact on psychosocial functioning and quality of life. Behav Res Ther. 2005b;43:869–84. doi: 10.1016/j.brat.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Diefenbach GJ, Tolin DR, Hannan S, Maltby N, Crocetto J. Group Treatment for Trichotillomania: Behavior Therapy Versus Supportive Therapy. Behav Ther. 2006;37:353–63. doi: 10.1016/j.beth.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Loh R, Jenike MA, Keuthen NJ. Single modality versus dual modality treatment for trichotillomania: Sertraline, behavioral therapy, or both? J Clin Psychiatry. 2006;67:1086–92. doi: 10.4088/jcp.v67n0711. [DOI] [PubMed] [Google Scholar]
- Duke DC, Bodzin DK, Tavares P, Geffken GR, Storch EA. The phenomenology of hairpulling in a community sample. J Anxiety Disord. 2009;23:1118–25. doi: 10.1016/j.janxdis.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Duke DC, Keeley ML, Geffken GR, Storch EA. Trichotillomania: A current review. Clin Psychol Rev. 2010;30:181–93. doi: 10.1016/j.cpr.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Ferrão YA, Miguel E, Stein DJ. Tourette's syndrome, trichotillomania, and obsessive–compulsive disorder: How closely are they related? Psychiatry Res. 2009;170:32–42. doi: 10.1016/j.psychres.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Flancbaum M, Rockmore L, Franklin ME. Intensive behavior therapy for tics: Implications for clinical practice and overcoming barriers to treatment. Journal of Developmental and Physical Disabilities. 2011;23:61–9. [Google Scholar]
- Flessner CA, Penzel F, Keuthen NJ. Current treatment practices for children and adults with trichotillomania: Consensus among experts. Cognitive and Behavioral Practice. 2010;17:290–300. [Google Scholar]
- Franklin ME, Edson AL, Ledley DA, Cahill SP. Behavior Therapy for Pediatric Trichotillomania: A Randomized Controlled Trial. J Am Acad Child Adolesc Psychiatry. 2011a;50:763–71. doi: 10.1016/j.jaac.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin ME, Flessner CA, Woods DW, Keuthen NJ, Piacentini JC, Moore P, et al. The Child and Adolescent Trichotillomania Impact Project: Descriptive psychopathology, comorbidity, functional impairment, and treatment utilization. J Dev Behav Pediatr. 2008;29:493–500. doi: 10.1097/DBP.0b013e31818d4328. [DOI] [PubMed] [Google Scholar]
- Franklin ME, Zagrabbe K, Benavides KL. Trichotillomania and its treatment: A review and recommendations. Expert Rev Neurother. 2011b;11:1165–74. doi: 10.1586/ern.11.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: A double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66:756–63. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- Himle MB, Freitag M, Walther M, Franklin SA, Ely L, Woods DW. A randomized pilot trial comparing videoconference versus face-to-face delivery of behavior therapy for childhood tic disorders. Behav Res Ther. 2012;50:565–70. doi: 10.1016/j.brat.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Iancu I, Weizman A, Kindler S, Sasson Y, Zohar J. Serotonergic drugs in trichotillomania: Treatment results in 12 patients. J Nerv Ment Dis. 1996;184:641–4. doi: 10.1097/00005053-199610000-00011. [DOI] [PubMed] [Google Scholar]
- Keuthen NJ, O'Sullivan RL, Ricciardi JN, Shera D, et al. The Massachusetts General Hospital (MGH) Hairpulling Scale: I. Development and factor analyses. Psychother Psychosom. 1995;64:141–5. doi: 10.1159/000289003. [DOI] [PubMed] [Google Scholar]
- Keuthen NJ, Rothbaum BO, Falkenstein MJ, Meunier S, Timpano KR, Jenike MA, et al. DBT-enhanced habit reversal treatment for trichotillomania: 3- and 6-month follow-up results. Depress Anxiety. 2011;28:310–3. doi: 10.1002/da.20778. [DOI] [PubMed] [Google Scholar]
- Keuthen NJ, Rothbaum BO, Fama J, Altenburger E, Falkenstein MJ, Sprich SE, et al. DBT-enhanced cognitive-behavioral treatment for trichotillomania: A randomized controlled trial. Journal of Behavioral Addictions. 2012;1:106–14. doi: 10.1556/JBA.1.2012.003. [DOI] [PubMed] [Google Scholar]
- Lewin AB, Peris TS, De Nadai AS, McCracken JT, Piacentini J. Agreement between therapists, parents, patients, and independent evaluators on clinical improvement in pediatric obsessive-compulsive disorder. J Consult Clin Psychol. 2012;80:1103–7. doi: 10.1037/a0029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini C, Van Ameringen M, Patterson B, Simpson W, Truong C. Trichotillomania in youth: A retrospective case series. Depress Anxiety. 2009;26:661–5. doi: 10.1002/da.20579. [DOI] [PubMed] [Google Scholar]
- McGuire JF, Kugler BB, Park JM, Horng B, Lewin AB, Murphy TK, et al. Evidence-based assessment of compulsive skin picking, chronic tic disorders and trichotillomania in children. Child Psychiatry Hum Dev. 2012;43:855–83. doi: 10.1007/s10578-012-0300-7. [DOI] [PubMed] [Google Scholar]
- McGuire JF, Piacentini J, Brennan EA, Lewin AB, Murphy TK, Small BJ, et al. A meta-analysis of behavior therapy for Tourette Syndrome. J Psychiatr Res. 2014;50:106–12. doi: 10.1016/j.jpsychires.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Moncrieff J, Churchill R, Drummond DC, McGuire H. Development of a quality assessment instrument for trials of treatments for depression and neurosis. International Journal of Methods in Psychiatric Research. 2001;10:126–33. [Google Scholar]
- Murad MH, Montori VM. Synthesizing evidence: shifting the focus from individual studies to the body of evidence. JAMA. 2013;309:2217–8. doi: 10.1001/jama.2013.5616. [DOI] [PubMed] [Google Scholar]
- Ninan PT, Rothbaum BO, Marsteller FA, Knight BT, Eccard MB. A placebo-controlled trial of cognitive-behavioral therapy and clomipramine in trichotillomania. J Clin Psychiatry. 2000;61:47–50. doi: 10.4088/jcp.v61n0111. [DOI] [PubMed] [Google Scholar]
- O'Sullivan RL, Keuthen NJ, Hayday CF, Ricciardi JN. The Massachusetts General Hospital (MGH) Hairpulling Scale: II. Reliability and validity. Psychother Psychosom. 1995;64:146–8. doi: 10.1159/000289004. [DOI] [PubMed] [Google Scholar]
- Odlaug BL, Chamberlain SR, Harvanko AM, Grant JE. Age at onset in trichotillomania:clinical variables and neurocognitive performance. Prim Care Companion CNS Disord. 2012;14 doi: 10.4088/PCC.12m01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odlaug BL, Kim SW, Grant JE. Quality of life and clinical severity in pathological skin picking and trichotillomania. J Anxiety Disord. 2010;24:823–9. doi: 10.1016/j.janxdis.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Orwin RG. A fail-safe N for effect size in meta-analysis. Journal of Educational Statistics. 1983;8:157–9. [Google Scholar]
- Panza KE, Pittenger C, Bloch MH. Age and gender correlates of pulling in pediatric trichotillomania. J Am Acad Child Adolesc Psychiatry. 2013;52:241–9. doi: 10.1016/j.jaac.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard CA, Ibe IO, Krojanker DN, Kitchen AD, Bronson SS, Flynn TM. Clomipramine treatment of trichotillomania: A follow-up report on four cases. J Clin Psychiatry. 1991;52:128–30. [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. Rev ed Sage Publications, Inc; Thousand Oaks, CA: 1991. [Google Scholar]
- Stanley MA, Breckenridge JK, Snyder AG, Novy DM. Clinician-rated measures of hair pulling: A preliminary psychometric evaluation. J Psychopathol Behav Assess. 1999;21:157–82. [Google Scholar]
- Stein DJ, Grant JE, Franklin ME, Keuthen N, Lochner C, Singer HS, et al. Trichotillomania (hair pulling disorder), skin picking disorder, and stereotypic movement disorder: Toward DSM-V. Depress Anxiety. 2010;27:611–26. doi: 10.1002/da.20700. [DOI] [PubMed] [Google Scholar]
- Stemberger RMT, Thomas AM, Mansueto CS, Carter JG. Personal toll of trichotillomania: Behavioral and interpersonal sequelae. J Anxiety Disord. 2000;14:97–104. doi: 10.1016/s0887-6185(99)00028-6. [DOI] [PubMed] [Google Scholar]
- Stewart RS, Nejtek VA. An open-label, flexible-dose study of olanzapine in the treatment of trichotillomania. J Clin Psychiatry. 2003;64:49–52. doi: 10.4088/jcp.v64n0110. [DOI] [PubMed] [Google Scholar]
- Streichenwein SM, Thornby JI. A long-term, double-blind, placebo-controlled crossover trial of the efficacy of fluoxetine for trichotillomania. The American Journal of Psychiatry. 1995;152:1192–6. doi: 10.1176/ajp.152.8.1192. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Lenane MC, Leonard HL. Long-term treatment of trichotillomania (hair pulling) The New England Journal of Medicine. 1993;329:141–2. doi: 10.1056/NEJM199307083290220. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Rapoport JL, Lenane MC. A double-blind comparison of clomipramine and desipramine in the treatment of trichotillomania (hair pulling) The New England Journal of Medicine. 1989;321:497–501. doi: 10.1056/NEJM198908243210803. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Diefenbach GJ, Flessner CA, Franklin ME, Keuthen NJ, Moore P, et al. The Trichotillomania Scale for Children: Development and validation. Child Psychiatry Hum Dev. 2008;39:331–49. doi: 10.1007/s10578-007-0092-3. [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Patterson B, Bennett M, Oakman J. A randomized, double-blind, placebo-controlled trial of olanzapine in the treatment of trichotillomania. J Clin Psychiatry. 2010;71:1336–43. doi: 10.4088/JCP.09m05114gre. [DOI] [PubMed] [Google Scholar]
- van Minnen A, Hoogduin KAL, Keijsers GPJ, Hellenbrand I, Hendriks G-J. Treatment of trichotillomania with behavioral therapy or fluoxetine: A randomized, waiting-list controlled study. Arch Gen Psychiatry. 2003;60:517–22. doi: 10.1001/archpsyc.60.5.517. [DOI] [PubMed] [Google Scholar]
- Wetterneck CT, Woods DW, Norberg MM, Begotka AM. The social and economic impact of trichotillomania: Results from two nonreferred samples. Behavioral Interventions. 2006;21:97–109. [Google Scholar]
- White MP, Koran LM. Open-label trial of aripiprazole in the treatment of trichotillomania. J Clin Psychopharmacol. 2011;31:503–6. doi: 10.1097/JCP.0b013e318221b1ba. [DOI] [PubMed] [Google Scholar]
- Winchel RM, Jones JS, Molcho A, Parsons B. The Psychiatric Institute Trichotillomania Scale (PITS) Psychopharmacol Bull. 1992;28:463–76. [PubMed] [Google Scholar]
- Woods DW. Treating trichotillomania across the lifespan. J Am Acad Child Adolesc Psychiatry. 2013;52:223–4. doi: 10.1016/j.jaac.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Woods DW, Conelea CA, Himle MB. Behavior therapy for Tourette's disorder: Utilization in a community sample and an emerging area of practice for psychologists. Professional Psychology: Research and Practice. 2010;41:518–25. [Google Scholar]
- Woods DW, Flessner CA, Franklin ME, Keuthen NJ, Goodwin RD, Stein DJ, et al. The trichotillomania impact project (TIP): Exploring phenomenology, functional impairment, and treatment utilization. J Clin Psychiatry. 2006a;67:1877–88. doi: 10.4088/jcp.v67n1207. [DOI] [PubMed] [Google Scholar]
- Woods DW, Wetterneck CT, Flessner CA. A controlled evaluation of acceptance and commitment therapy plus habit reversal for trichotillomania. Behav Res Ther. 2006b;44:639–56. doi: 10.1016/j.brat.2005.05.006. [DOI] [PubMed] [Google Scholar]