Abstract
The frizzled/starry night pathway regulates planar cell polarity in a wide variety of tissues in many types of animals. It was discovered and has been most intensively studied in the Drosophila wing where it controls the formation of the array of distally pointing hairs that cover the wing. The pathway does this by restricting the activation of the cytoskeleton to the distal edge of wing cells. This results in hairs initiating at the distal edge and growing in the distal direction. All of the proteins encoded by genes in the pathway accumulate asymmetrically in wing cells. The pathway is a hierarchy with the Planar Cell Polarity (PCP) genes (aka the core genes) functioning as a group upstream of the Planar Polarity Effector (PPE) genes which in turn function as a group upstream of multiple wing hairs. Upstream proteins, such as Frizzled accumulate on either the distal and/or proximal edges of wing cells. Downstream PPE proteins accumulate on the proximal edge under the instruction of the upstream proteins. A variety of types of data support this hierarchy, however, we have found that when over expressed the PPE proteins can alter both the subcellular location and level of accumulation of the upstream proteins. Thus, the epistatic relationship is context dependent. We further show that the PPE proteins interact physically and can modulate the accumulation of each other in wing cells. We also find that over expression of Frtz results in a marked delay in hair initiation suggesting that it has a separate role/activity in regulating the cytoskeleton that is not shared by other members of the group.
Keywords: Planar Cell Polarity, Planar cell polarity effectors, Drosophila, Wing, Protein interactions
Introduction
The epidermis of many animals is polarized within the plane of the tissue. This tissue planar cell polarity (PCP) is dramatic in the cuticle of insects such as Drosophila, which is decorated with arrays of hairs and sensory bristles. Tissue polarity in Drosophila is under the control of the frizzled (fz)/starry night (stan) signaling pathway and in recent years it has become clear that this conserved pathway also controls planar polarity in many aspects of vertebrate development (Goodrich and Strutt, 2011; Wang and Nathans, 2007) including cell and tissue movements during gastrulation (Goto and Keller, 2002; Jessen et al., 2002; Takeuchi et al., 2003; Wallingford et al., 2001), the differentiation of stereocilia in the inner ear (Montcouquiol et al., 2003; Wang et al., 2005; Wang et al., 2006), the morphogenesis of the fur of mice(Chang and Nathans, 2013), kidney development (Babayeva et al., 2013; Goggolidou, 2013) and the polarity of the cilia in the respiratory epithelium (Vladar et al., 2012).
Genetic studies focused on the fly wing led to the identification and characterization of a number of genes essential for PCP that comprise the fz/stan signaling/signal transduction pathway (Adler et al., 1987; Chae et al., 1999; Das et al., 2004; Gubb and Garcia-Bellido, 1982; Held et al., 1986; Taylor et al., 1998; Usui et al., 1999; Wolff and Rubin, 1998; Wong and Adler, 1993). These genes consist of three phenotypic groups that are also epistasis groups (Wong and Adler, 1993). They are the PCP genes (aka, core group), the PPE (Planar Polarity Effecter) genes and the multiple wing hairs (mwh) gene. frizzled (fz), disheveled (dsh), prickle/spiny leg (pk/sple), Van Gogh (Vang) (aka strabismus), starry night (stan) (aka flamingo) and diego (dgo) are all members of the PCP group. A distinctive feature of these genes is that their protein products accumulate asymmetrically on the distal (Fz, Dsh and Dgo)(Axelrod, 2001; Das et al., 2004; Feiguin et al., 2001; Strutt, 2001), proximal (Vang, Pk) (Bastock et al., 2003; Jenny et al., 2003; Tree et al., 2002) or both distal and proximal (Stan) edges of wing cells (Usui et al., 1999). These proteins are a functional group and are co-requirements for the asymmetric accumulation of the others.
The Planar Polarity Effecter (PPE) group includes inturned (in), fuzzy (fy) and fritz (frtz). The abnormal polarity patterns seen in PPE mutants are similar to those seen in PCP mutants but PPE mutants differ in several ways from PCP mutants (Collier and Gubb, 1997; Collier et al., 2005; Park et al., 1996; Wong and Adler, 1993). The vast majority of PCP mutant wing cells form a single hair at a relatively central location on the apical surface, while in contrast many PPE mutant wing cells form 2 or 3 hairs at abnormal locations along the cell periphery. A variety of evidence argues that the PPE genes function downstream of the PCP genes. Loss of function mutations in PPE genes are epistatic to mutations in PCP genes and the function of the PPE genes is not required for the asymmetric accumulation of PCP proteins (Strutt, 2001; Usui et al., 1999; Wong and Adler, 1993). In contrast, the proximal accumulation of the PPE proteins requires the function of and is instructed by PCP genes (Adler et al., 2004; Strutt and Warrington, 2008). Similar experimental results indicate that mwh functions downstream of the PPE genes (Strutt and Warrington, 2008; Wong and Adler, 1993; Yan et al., 2008).
The PPE genes share almost identical mutant phenotypes, double null mutants do not show a stronger phenotype and the genes show strong genetic interactions (Adler et al., 2004; Collier et al., 2005; Wong and Adler, 1993). For example, any double mutant combination of weak PPE alleles results in a strong PPE mutant phenotype (Collier et al., 2005). These observations suggest that the PPE genes function as a unit. Consistent with this hypothesis all three are co-requirements for the proximal accumulation of the others and the level of In and Frtz are dramatically reduced when animals are mutant for another PPE gene (e.g. fy) (Adler et al., 2004; Strutt and Warrington, 2008). We report here that these proteins can be coimmunoprecipated in a variety of experimental conditions and that In interacted with both Fy and Frtz in the yeast two hybrid system (Lee, 2002; Yan, 2008). We also observed that the over expression (oe) of one PPE protein could affect the accumulation of a second. Our data argue these proteins form a protein complex that functions in wing tissue polarity.
One of the hallmarks of the PCP genes is that over expression (oe) leads to a gain of function (gof) hair polarity phenotype that is superficially similar to the loss of function phenotype (Axelrod et al., 1998; Boutros et al., 1998; Feiguin et al., 2001; Gubb et al., 1999; Krasnow and Adler, 1994; Usui et al., 1999). This appears to be due to a loss of their asymmetric distribution, perhaps due to saturating binding sites. In contrast the oe of In or Fy did not lead to a gof phenotype even though that led to the loss or degradation of their asymmetric accumulation. A possible explanation for these results is that only properly localized protein is active. Alternatively, activity could require that the proteins are in the proper protein complex and the amount of the other component(s) is limiting when only one is over expressed. Interestingly, we found that oe In and Fy together resulted in a relatively weak but consistent gof phenotype. This is consistent with the latter hypothesis. We also found that the oe of frtz by itself led to a gof planar polarity phenotype. What could be the mechanism by which the oe of PPE proteins can alter hair polarity? One possibility is that the oe of PPE proteins uncouples the site of hair initiation from the side of the cell where Fz, Dsh and Dgo accumulate, much as loss of function mutations in PPE genes do. Another possibility is that the oe of the PPE proteins could re-pattern the accumulation of the upstream PCP proteins. This seemed less likely as it requires putative downstream proteins to affect the localization of upstream proteins. Surprisingly, we found the latter hypothesis was correct. This establishes that the PPE proteins functioning downstream of the PCP proteins is context dependent and blurs the line between the two groups of genes.
Materials and Methods
Fly Genetics
All flies were raised at 25°C unless otherwise stated. Mutant stocks were either obtained from the Bloomington Drosophila stock center at Indiana University, from the VDRC, generated in our lab or were generous gifts from J. Axelrod, D. Strutt, or T. Uemura. The FLP/FRT technology was used to generate genetics mosaics. To direct transgene expression, we used the Gal4/UAS system (Brand and Perrimon, 1993). For temperature shift experiments the relevant animals were collected as white prepupae, placed into fresh food vials and moved to different temperatures at the desired time. Several of the genes used in this paper have multiple names. In line with FlyBase usage we use starry night instead of flamingo and Van Gogh instead of strabismus.
Transgenes and antibodies
We attempted to determine if Frtz and Fy, like In localized to the proximal side of wing cells by raising antibodies against these two proteins. The antibodies generated were able to specifically detect over-expressed proteins but were not able to detect the endogenous proteins. As an alternative we constructed transgenes that encoded tagged versions of these genes. For fy we generated GFP, Flag and Flag-Ollas tagged transgenes driven by the following promoter/regulatory sequences: UAS, hsp70, armadillo, ubiquitin and the endogenous fy promoter. All of these constructs were able to provide complete rescue of a fy null allele. Immunostaining of the tagged Fy proteins did not yield consistent data that Fy accumulated in the stereotypic zig zag pattern, although we saw this on occasion. Strutt and colleagues using a different construct found Fy to be asymmetrically localized (Strutt and Warrington, 2008).
We also generated transgenic flies that expressed myc-Frtz-GFP from the ubiquitin promoter (ubi-Myc-Frtz-GFP) (Sekelsky et al., 1995). These transgenes provided complete rescue of frtz phenotypic null alleles and in experiments using them we determined that Frtz was localized to the proximal side of wing cells. In z sections we found that Frtz was localized near the adherens junctions as is the case for other proteins involved in tissue polarity). These results confirm those previously published by Strutt and colleagues (Strutt and Warrington, 2008). We also generated transgenic lines that expressed frtz, myc-Frtz or Frtz-GFP under the direction of UAS. For reasons that are unclear the myc-frtz transgene drove expression at a lower level than the other two and did not result in a gain of function phenotype. Perhaps this is due to the amino terminal tag lowering protein accumulation. For in we used previously described transgenes that express HA-In driven by UAS (Park et al., 1996).
A surprising feature of the PPE UAS transgenes is that they often provided substantial genetic rescue in the absence of a GAL4 driver or in regions outside of the normal domain of Gal4 expression (Park et al., 1996). The degree of rescue was more complete for in and fy than frtz and varied as a function of body region and insert. For example, rescue was usually not seen for the abdominal bristle polarity phenotype. The rescue is likely due to enhancer trapping driving expression of the transgenes. In some cases (for example for a frtz-GFP insert) we were able to detect this by immunostaining. Since we have not seen equivalent rescue of mutant wing phenotypes with other UAS-transgenes (e.g. UAS-mwh, UAS-trc, UAS-fz) we suggest that PPE function can be provided by relatively low levels of protein.
In our initial experiments standard P element vectors were used that resulted in relatively random insertions (Klemenz et al., 1987; Pirrotta, 1988 ). Later experiments utilized phi-C31insertion vectors and insertion sites(Groth et al., 2004). For UAS-constructs we used the attp2 and attp40 landing sites (Bischof et al., 2007).
Immunostaining
A standard paraformaldehyde fixation and staining procedure was used (Adler et al., 2004).
Plasmid Constructs
The full length frtz cDNA with a Myc tag at its N-terminus and the full length fy cDNA with both OLLAS and FLAG tags at its C-terminus were generated by PCR and subcloned into pPUAST-attB vector. The full length frtz cDNA with a GFP tag on its C-terminus was subcloned into pPUM6 (Jeff Sekelsky's Lab) using EcoRI and SacII sites.
The following primers were used in the constructions:
PUAST-attB-(myc)6-frtz (from KpnI to XbaI) 5’ primer: GAGGGTACCCCACCATGGGGCAGGGAT 3’ primer: CTAGTCTAGACTAGACCACGCCGAAGTGGACCACC PUAST-fy-FLAG-OLLAS (from XhoI to XbaI) 5’ primer: CCGCTCGAGATGTCCATCTATTTGTTATGTTTGACAACAAACGGCGGATTG 3’ primer: TGCTCTAGACTACTTGCCCATCAACCTAGGTCCCAATTCATTCGCAAAGCCACT PUM6-frtz-GFP (from EcoRI to SacII) 5’ primer: CCGGAATTCAATGCTGCTCAGCGAGAC 3’ primer: TCCCCGCGGCTACTTGTACAGCTCGTCCATG The UAS-HA-in construct was described previously (Lu et al., 2010).
Construct Subcloning for Two-hybrid Assays.
Full-length of inturned cDNA was subcloned into pGADT7 from NdeI-BamHI. The following primers were used:
Inturn-th5: TCTAGGGAATTTCCATATGCGCAAATCGCCGGCCAG; Inturn-th3: GATCGCGGATCCATGTCATCCCATTGAGAAGAAGGA. Full-length of fuzzy or fritz cDNA was subcloned into pGBKT7 from NcoI-BamHI or NdeIEcoRI respectively. The following primers were used:
pGBKT7-5’: CATGCCATGGAGATGTCCATCTATTTGTTATG; pGBKT7-3’: CGCGGATCCTTATCACCAACATACTGACTTC; 5’ frtz-hybrid: GGGAATTCCATATGCTGCTCAGCGAGACC; 3’fritz-hybrid: CCGGAATTCTTATTAGACCACGCCGAAGTGGA.
To get truncated forms of fritz cDNA, we used the following primers and the PCR products were subcloned into pGBKT7 from NdeI-EcoRI sites. We used the same 5’fritz-hybrid primer and DBD-frtzN-3’: CCGGAATTCTTAGTGCGACAGATCCAGCAAG to amplify the N-terminus of fritz cDNA (encoding 1-400 amino acid); we used DBD-frtzC-5’:
GGGAATTCCATATGTACTTCGTGGCCCAGCCA and the same 3’frtz-hybrid primer to amplify the C-terminus of fritz (encoding 401- 951 amino acid). 5’frtz-hybrid primer and Dfrtz200-3’: CCGGAATTCTTAAACTGTGAGGTGGCGG were used to amplify the fritz cDNA encoding 1-200 amino acids; Dfrzt400-5’:
GGGAATTCCATATGAACGCCAGCTTCGATC and 3’fritz-hybrid primer were used to PCR frtz cDNA encoding 201-400 amino acid. To Amplify the frtz cDNA encoding WD40 repeats, the following primers were used: DBDFrtz-WD5: GGAATTCCATATGCAGATCTGCTCCTTTGCCTTC; DBD-Frtz-WD3: CGGAATTCTTACAGCTGATGGCCAATGGTG.
To examine interactions between truncated forms of Inturned and Fuzzy or Fritz, full-length of fuzzy or fritz cDNA was subcloned into pGADT7 from NcoI-BamHI or NdeI-EcoRI respectively. We used the same pGBKT7-5’, pGBKT7-3’, 5’frtz-hybrid and 3’frtz-hybrid primers as described before. Truncated forms of inturned cDNA were generated by PCR and subcloned into pGBKT7 from NdeI-BamHI. We used the same Inturn-th5’ primer and in-Nterm1-3’: CGCGGATCCTCACAAGATCACGGCCAGACTC to amplify the N-terminal part of Inturned (1- 550 amino acid); we used in-Cterm1-5’: GGGAATTCCATATGAAGATCTTCGATGCTCCAG and the same Inturn-th3’ primer to amplify the C-terminal part of Inturned (551-869 amino acid). The fragment encoding Inturned 551-731 amino acid was cut from pGBKT-inC (551-869 aa) construct by restriction enzymes NdeI and EcoRI and inserted into pGBKT7 or pGADT7 from the same sites. We used AD-in-731-5’: GGAATTCCATATGGTTCTGCACTACGTCTACA and the same Inturnth3’ primer to amplify inturned cDNA encoding 731-869 amino acid, the PCR product was subcloned into pGBKT7 or pGADT7 from NdeI-BamHI.
For the yeast two hybrid assays, we used yeast strain AH109 and Matchmaker Two-Hybrid System 3 from Clontech. Briefly, full length or truncated cDNA was subcloned into pGADT7 or pGBKT7 by specific restriction enzyme sites. To test the interaction of defined protein partners, pGADT7 and pGBKT7 plasmids, which each encoding one of the two protein partners were co-transformed into yeasts. Transformed yeasts were selected by growing on two-marker dropout medium (SD/-Leu/-Trp). Colonies from previous dropout medium were transferred to a four-marker dropout medium (SD/-Ade/-His/-Leu/-Trp) with X-alpha-Gal. Only colonies growing on four-marker plates were considered as positive colonies.
Antibodies
Monoclonal anti-armadillo and anti-Flamingo antibodies were obtained from Developmental Studies Hybridoma Bank at the University of Iowa. Anti-HA, anti-Flag and anti-Myc antibodies were obtained from Cell Signaling Technology. Anti-Ollas monoclonal antibody was purchased from Novus Biologicals. Anti-GFP antibody was obtained from Molecular Probes. Alexa 488- and Alexa 568-conjugated secondary antibodies were purchased from Molecular Probes. Alexa 568 and 647 phalloidin were purchased from Molecular Probes. Anti-Pk antibodies were kindly provided by Drs. J. Axelrod and D. Strutt. Anti-Frtz antibody was generated in rats using a Frtz protein made in E. coli and his tagged to facilitate purification. A similar strategy was used to obtain an anti-Fy antibody. In both cases the expressed proteins were found in inclusion bodies and solubilized and we do not know if they were in a native conformation. Neither antibody was able to detect the endogenous protein.
Coimmunoprecipitation and western blotting
The following genotypes were used in these experiments:
w; ptc-Gal4/UAS-HA-in UAS-fy-GFP;UAS-frtz-GFP
w; ptc-Gal4/UAS-HA-in; UAS-frtz-GFP
w; ptc-Gal4/ UAS-fy-GFP;UAS-frtz-GFP
w; ptc-Gal4/UAS-HA-in; UAS-(myc)6-frtz
w; ptc-Gal4/UAS-HA-in UAS-fy-GFP
w; ptc-Gal4/UAS-fy-GFP; UAS-(myc)6-frtz
w; ptc-Gal4/UAS-HA-in; UAS-fy-FLAG-OLLAS
w; ptc-Gal4/UAS-fy-FLAG-OLLAS;UAS-(myc)6-frtz
w hs-flp; UAS-HA-in UAS-fy-GFP
Co-immunoprecipitation experiments were done in a variety of different ways. The most common procedure was as follows. 150-200 wing discs of transgenic flies were dissected from third instar larvae and homogenized in pre-chilled lysis buffer containing protease inhibitors. The extract was then spun to remove cell debris. Protein A agarose beads (Roche) were added to the extract and incubated for at least 3 hours to reduce the background of non-specific binding. The extract was spun again and 7-10μl desired antibody was added to the supernatant and incubated for 3-4 hr. New protein A agarose beads were then added and the sample was incubated overnight. The beads were pelleted and washed. Protein was released from the beads and analyzed by standard western blot procedures. In other experiments we used as starting material S2 cells, whole larvae or larval “heads” (these were dissected anterior sections of larvae that contained the brain, imaginal discs, salivary glands, anterior larval epidermis and other tissues). Similar results were obtained from all three sources.
Imaging
Adult wings were mounted in Euparal and bright field images obtained using a Spot RT camera and a Zeiss Axiskop II microscope. In most cases a 40X Apochromatic objective was used (oil, 1.4 NA). When wings were not flat enough to allow a good final image from a single optical plane a Z stack was obtained using Metamorph and a final image was obtained either using a minimal projection (most cases) or by using an Image J extended focus plug in. In a few very difficult preparations a Z stack was obtained and prior to carrying out the minimal projection for the dorsal wing hairs the ventral hairs were erased manually from some optical planes. Quantitation of images was accomplished using ImageJ.
Confocal images of immunostained pupal wings were obtained using either a Zeiss 510 or a Leica SP5 WLL confocal microscope. Final panels were assembled using Adobe Photoshop or Microsoft PowerPoint.
Results
Many of the experiments reported in this paper used transgenes to express PPE proteins. Most of these have not been described previously and details are provided in the Methods and Materials. All of the tagged (and untagged) transgenes used were able to provide complete rescue of null alleles when expressed at appropriate levels (e.g. using the ubiquitin promoter/enhancer to drive expression). Tagged In and Frtz proteins encoded by the transgenes localized to the proximal side of wing cells as the endogenous proteins do (Fig S1)(Adler et al., 2004; Strutt and Warrington, 2008). This was also reported to be the case for Fy (Strutt and Warrington, 2008). Using tagged transgenes we have on occasion seen evidence of asymmetric Fy accumulation but we have been unable to get this result consistently. Hence, we have not studied factors that mediate Fy localization.
Gain of function phenotypes of PPE genes
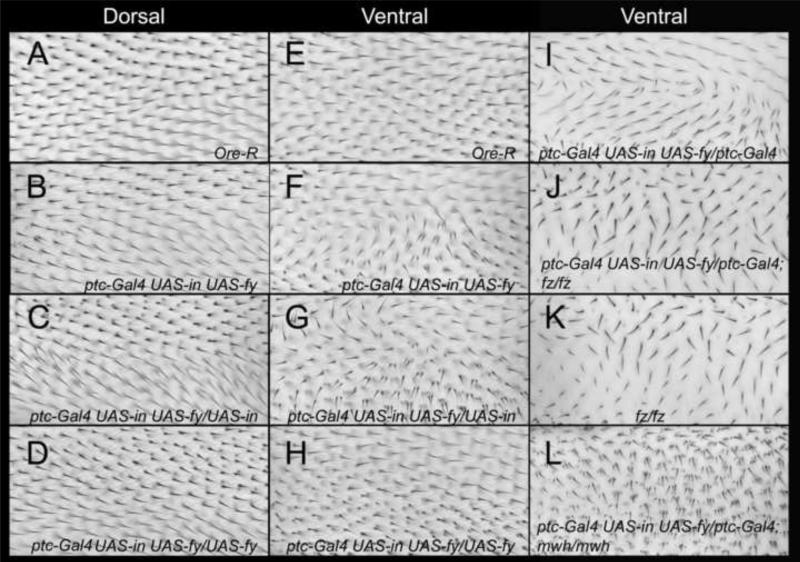
The expression of either in or fy driven by a variety of Gal4 drivers (e.g. ptc-Gal4, act-Gal4, ap-Gal4) did not result in a gain of function (gof) phenotype. This was surprising as the endogenous In (and Fy) protein accumulated at the proximal side of wing cells and when over expressed (oe) it was found throughout the cell (Fig S1B-D) (Adler et al., 2004). Presumably, In (or Fy) is not active in that context. It is worth noting however that neither of these proteins accumulated to as high levels as we have seen for many other proteins in similar experiments. We found that co-over expressing in and fy together consistently resulted in a gain of function phenotype that consisted of cells that formed hairs that did not point distally and some of these cells formed multiple hairs (Fig 1B-H). When ptc-GAL4 was used to drive expression this was typically manifested as a characteristic swirl in a relatively proximal region on the ventral surface of the wing (Fig 1F). A multiple hair cell (mhc) phenotype and a milder polarity disruption were seen on the dorsal surface of such wings (Fig 1B). Both the hair polarity and mhc phenotypes were enhanced by the addition of a second Gal4 expressing gene or a second UAS-in transgene (Fig 1C,G,I, Table S1, Table S2). It was also enhanced when a second copy of both UAS-HA-in and UAS-fy-GFP were present. As is described later, conditions that lead to a stronger hair phenotype also lead to a higher level of In protein accumulation. In contrast the addition of a second UAS-fy-GFP or UAS-fy-Flag-Ollas transgene partly suppressed the in/fy gof (Fig 1D,H, Table S1, Table S2). Thus, while the oe of both genes is needed to generate a gain of function the In/Fy ratio is also important. We did not see clear evidence of a suppression of either phenotype when the flies were heterozygous for a loss of function mutation in the endogenous in gene or for the mhc phenotype when mutant for fy (Table S1, Table S2). We suggest that this is due to the level of oe being great enough that a 50% reduction in the endogenous protein was relatively insignificant.
Figure 1.
The in/fy gain of function wing phenotype. All images are from the C region of the wing (between the 3rd and 4th veins just anterior to the posterior cross vein. This region is in the ptc domain. A-D show the dorsal surface of the wing and E- L the ventral surface. The genotypes are noted in each panel. Ore-R shows the wild type hair pattern.
Heterozygosity for a frtz mutation weakly but significantly suppressed the In/Fy gof and a low level of additional frtz supplied by a Ubi-myc-frtz-gfp transgene increased the strength of the phenotype but in this case the change was not significant (Table S1, S2). We also observed that heterozygosity for several of the PCP genes enhanced the in/fy gof mhc phenotype (Table S1,S2). pk, Vang also enhanced the polarity gof phenotype. Further, supplying a modest amount of extra Dsh partly suppressed both in/fy gof phenotypes (Table S1).
To determine if the in/fy gof polarity phenotype required the function of the fz/stan pathway we examined flies that oe in and fy while also being mutant for a third genes and determined if the characteristic in/fy swirl was still obvious. The characteristic swirl seen in ptc-Gal4 UAS-HA-in UAS-fy-GFP/ptc-Gal4 wings was not detected in ptc-Gal4 UAS-HA-in UAS-fy-GFP/ptc-Gal4; fzP21/fzK21 wings (Fig 1I,J) and these latter wings were similar to the ptc-Gal4/+; fzP21/fzK21wings (Fig 1K). We concluded from this experiment that the function of the upstream fz/stan genes was essential for generating the in/fy gof. We carried out similar experiments substituting mwh for fz and obtained equivalent results (Fig 1L). That is, mwh function was essential for the in/fy gof phenotype implying that Mwh is essential for the transduction of the gof signal to the cytoskeleton.
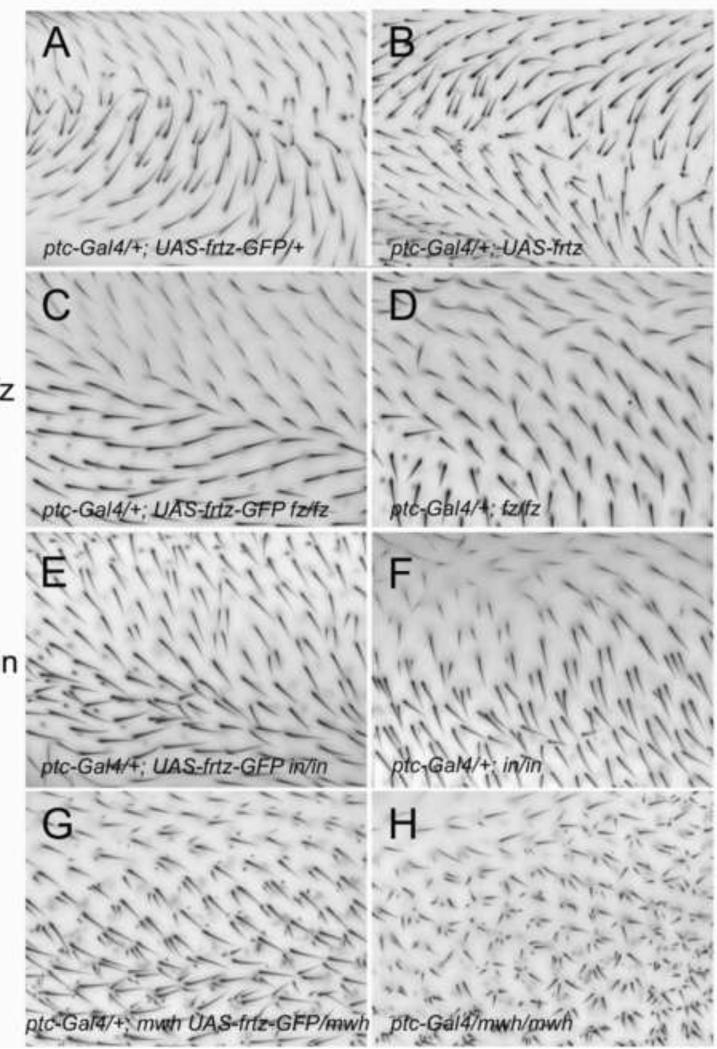
In contrast to the results seen with in and fy, frtz over expression by itself resulted in a consistent gof (Fig 2B). When untagged frtz was oe using ptc-gal4 hairs pointed away from the midline of the wing; the location where ptc-G4 drives the highest level of expression (Fig 2B). Hence, wing hairs appeared to be pointing from cells of higher toward cells of lower Frtz levels, much as they do when frizzled is over expressed in this way (Adler et al., 1997). The wing hair phenotype was qualitatively similar with two different UAS-frtz insertions (at attp2 and attp40) and dose sensitive. The ability of frtz oe to induce a PCP phenotype was also seen when alternate drivers were used. Ay-GAL4 flip out clones that drove expression of UAS-frtz resulted in a PCP gain of function and it was clear from stained pupal wings that frtz oe resulted in non-cell autonomous PCP abnormalities (Fig 3EF). The hairs produced by neighboring cells pointed away from the clone, once again away from the higher frtz levels. Flip out clones examined in pupal wings during early stages of hair morphogenesis consistently showed delayed/slowed hair growth. In contrast to that seen for in and fy the oe of frtz using some Gal4 drivers such as ap-Gal4 or act-Gal4 resulted in extensive lethality. In addition to the tissue polarity phenotype we also observed a distortion of the wing blade that led to a fold when frtz was oe with ptc-Gal4 (Fig S2). The frequency and severity of this fold was generally more dramatic in genotypes that gave stronger polarity phenotypes. In wings with a severe fold it was difficult to accurately assess the polarity phenotype. The frtz oe phenotype was sensitive to the dose of both other PPE genes and upstream PCP genes. Heterozygosity for in, fy, fz and pk resulted in an enhanced polarity phenotype (Fig S3A,B) and heterozygosity for in, fy and pk enhanced the number of mhc.
Figure 2.
The over expression of frtz and frtz-GFP result in opposite gain of function PCP phenotypes. All images are from the C region on the dorsal surface of the wing (between the 3 and 4th veins just anterior to the posterior cross vein. This region is in the ptc domain. The genotypes are noted in each panel.
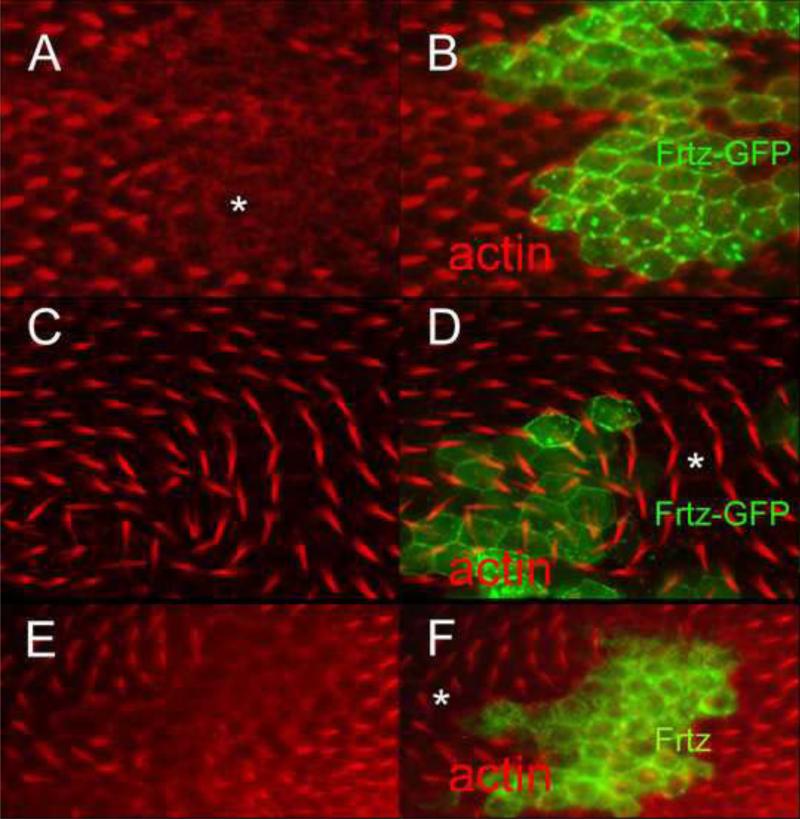
Figure 3.
The gain of function PCP phenotypes associated with over expression of frtz and frtz-GFP can affect the polarity of neighboring wing cells. A and B show a flip out clone that expresses Frtz-GFP. This results in a cell autonomous delay in hair formation. The asterisk marks the region showing hair delay. C and D show a clone in an older wing. The asterisk marks a region of wild type cells distal to the clone that form hairs that point toward the clone. E and F show a flip out clone that over expresses Frtz. Note the delay in hair formation of clone cells. The asterisk marks a region of wild type cells proximal to the clone that form hairs that point away from the clone.
When expressed at a low level frtz-GFP provided complete rescue of a null frtz allele (Yan, 2008). Thus, our expectation was that oe Frtz-GFP would act like the oe Frtz, but that proved not to be the case. When frtz-GFP was expressed using ptc-gal4 hairs pointed toward the midline of the wing (Fig 2A); the location where ptc-G4 drives the highest level of expression. Thus hairs pointed from cells of lower toward cells of higher Frtz-GFP levels. This is also seen when PCP genes such as pk or stan are expressed in this pattern (Tree et al., 2002; Usui et al., 1999) and is the opposite of what we observed with the over expression of frtz. A qualitatively similar phenotype was seen with different UAS-frtz-GFP inserts. Flip out clones that expressed frtz-GFP displayed a non-cell autonomous PCP phenotype (Fig 3CD) that was the opposite of that seen with frtz flip out clones. The hairs formed by neighboring cells pointed toward the clone, once again toward cells expressing higher levels of Frtz-GFP. We also observed a delay in hair formation in the Frtz-GFP over expression clones (Fig 3AB) as well as increased cytoplasmic general F-actin staining. The delay and the increased cytoplasmic staining appeared cell autonomous. Thus, the oe of frtz and frtz-GFP produced a similar effect on hair growth despite their opposite effects on polarity. As was the case for frtz, the oe of frtz-GFP using some Gal4 drivers such as actin-Gal4 resulted in extensive lethality.
When Frtz-GFP was oe using ptc-Gal4 in flies that were simultaneously mutant for null (or strong) alleles of fz, dsh, in or mwh the distinctive frtz-GFP polarity phenotype was lost (Fig 2). Thus, the frtz-GFP gof phenotype is dependent on the activity of the fz/stan pathway. It was also obvious that the resulting pattern was not a copy of the mutant fz/stan pathway phenotype. Hence, when oe frtz-GFP can also influence hair polarity by a mechanism that is independent of other components of the fz/stan pathway.
The over expression of PPE genes can re-pattern the accumulation of the PCP proteins.
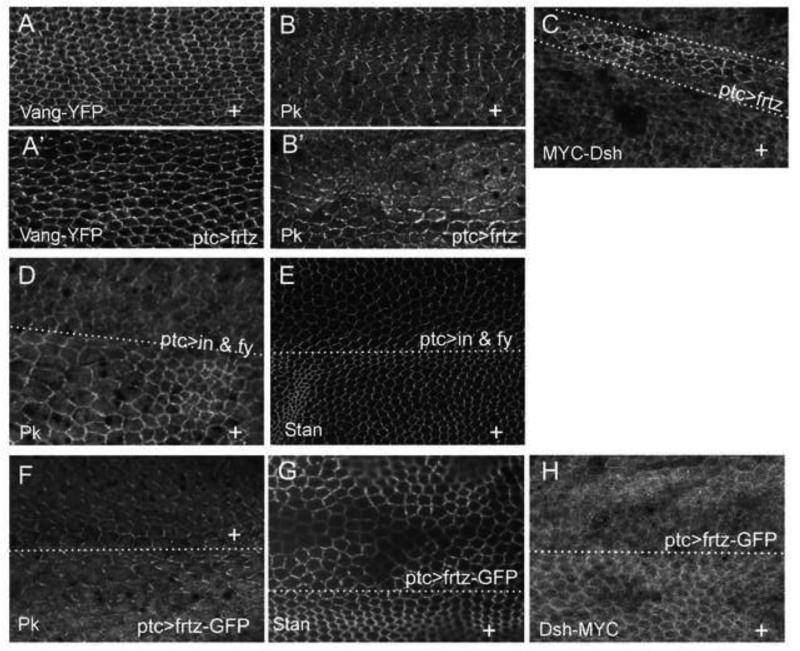
The ability of oe of in + fy, frtz and frtz-GFP to produce abnormal wing hair polarity could be explained by either hair polarity becoming uncoupled from the accumulation of PCP proteins in these cells (as is the case in a PPE loss of function mutant) or by the over expression of the PPE genes resulting in repatterning the accumulation of PCP proteins. The latter but not the former hypothesis can explain the non-autonomy seen in flip out clones that oe frtz or frtz-GFP. To distinguish between these two hypotheses we examined the distribution of several PCP proteins (Fz, Stan, Vang, Dsh and Pk) in pupal wings that oe either frtz, frtz-GFP or in + fy. In all cases where the oe of a PPE protein produced gof hair polarity phenotype the zig zag accumulation pattern of PCP proteins was altered. In some cases there was a re-patterning so the high level edges were now rotated 90° (e.g Fig 4 A’,B’) and in some cases there also appeared to be a change in the level of the PCP protein (e.g. Fig 4C-H). For example the oe of Frtz lead to alterations in the zigzag accumulation of Vang and Pk (Fig 4A’,B’) and an increase in the level of Dsh (Fig 4C). However, as was seen for hair polarity the oe of Frtz-GFP lead to the opposite effect. That is the decreased accumulation of Stan (Fig. 4G) and Dsh (Fig 4H).
Figure 4.
The over expression of PPE genes can alter the accumulation of PCP proteins. A and A’ are from the same ptc-Gal4/+; UAS-frtz/act-Vang-YFP wing. A is from outside the ptc domain and A’ from inside the domain. Vang-YFP immunostaining is shown. Outside the ptc domain Vang accumulates in the typical proximal/distal zig-zag pattern. Inside the ptc domain Vang accumulation is more punctate and is preferentially found on the anterior/posterior sides of cells. B and B’ are from the same ptc-Gal4/+; UAS-frtz wing. B is from outside the ptc domain and B’ from inside the domain. Endogenous Pk accumulation is shown by immunostaining. Outside the ptc domain Pk accumulates in the typical proximal/distal zig-zag pattern. Inside the ptc domain Pk accumulation is more punctate and is preferentially found on the anterior/posterior sides of cells. C shows a ptc-Gal4/dsh-myc; UAS-frtz/+ wing that was immunostained with anti-Myc antibody to show the accumulation of Dsh-myc. E shows a ptc-Gal4 UAS-HA-in UAS-fy-GFP/+ wing immunostained to show the accumulation of the endogenous Pk protein. Note the increased Pk accumulation and altered zigzag accumulation. E shows a ptc-Gal4 UAS-HA-in UAS-fy-GFP/+ wing immunostained to show the accumulation of the endogenous Stan protein. Note the loss of the distinct proximal/distal zigzag in the ptc domain. F shows a ptc-Gal4/+; UAS-frtz-GFP/+ wing immunostained to show the accumulation of the endogenous Pk protein. Note the slightly increased accumulation of Pk and the loss of the proximal/distal zigzag in the ptc domain.
The PPE genes form a physically interacting group
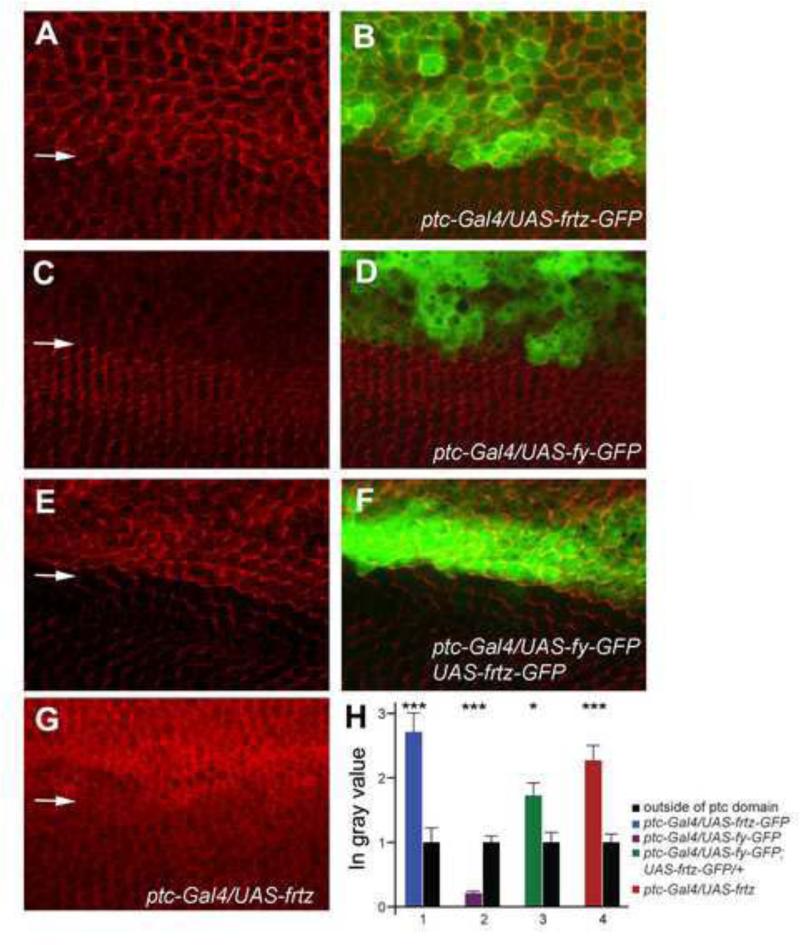
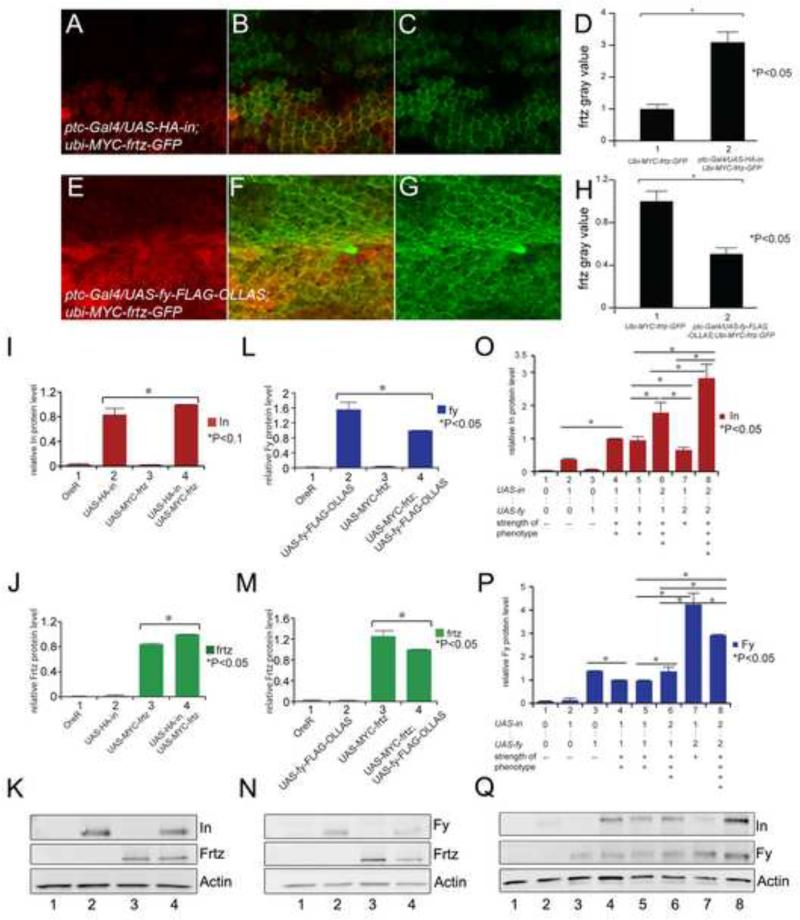
The proximal accumulation of In (and Frtz) required the function of the other PPE genes (Adler et al., 2004; Strutt and Warrington, 2008). Based on such observations it seemed possible that at least In and Frtz were stabilized by binding to other PPE proteins. If that was the case we predicted that increased levels of Frtz would result in increased In levels and visa versa. We examined the accumulation of the endogenous In protein by immunostaining wings where either frtz-GFP, frtz or fy-GFP were expressed using ptc-GAL4. We saw an increase in the level of endogenous In in cells that over expressed Frtz-GFP (Fig 5A,H) or Frtz (Fig 5G,H) consistent with the stabilization model. Consistent with the hair polarity phenotype seen with these genotypes the increased In was no longer found in a zigzag oriented along the anterior posterior axis. Rather, the accumulation of In was rotated 90° (Fig 5A). Thus, Frtz-GFP (and Frtz) not only stabilized In but also re-patterned its accumulation. Surprisingly, the expression of Fy-GFP caused a decrease in the accumulation of endogenous In (Fig 5C,H). This was a decrease and not a complete loss, consistent with the lack of effect of over expressing Fy on hair polarity. When both frtz and fy were over expressed In levels increased, thus higher Frtz levels were epistatic to higher Fy levels with respect to In accumulation (Fig 5E,H). We carried out similar experiments where we examined the effects of in and fy over expression on the accumulation of Frtz expressed at a low level from a Ubi-my-frtz-GFP transgene. We used this transgene since we have not succeeded in generating an anti-Frtz antibody that can reliably detect the endogenous protein. We found that the over expression of in lead to increased accumulation of Frtz (Fig 6A-D) while the over expression of fy lead to decreased accumulation (Fig 6E-H), although the results were not as dramatic as for In (Fig 5).
Figure 5.
In accumulation is altered in wing cells by the over expression of frtz and fy. A and B show a ptc-Gal4/+; UAS-frtz-GFP/+ wing immunostained to show the endogenous In protein. The arrow marks the approximate location of the edge of the ptc-domain. Note the increased accumulation of In and its altered accumulation pattern associated with the over expression of frtz-GFP. C and D show a ptc-Gal4/+; UAS-fy-GFP/+ wing immunostained to show the endogenous In protein. The arrow marks the approximate location of the edge of the ptc-domain. Note the decreased accumulation of In associated with the over expression of fy-GFP. E and F show a ptc-Gal4/UAS-fy-GFP; UAS-frtz-GFP/+ wing immunostained to show the endogenous In protein. The arrow marks the approximate location of the edge of the ptc-domain. Note the increased accumulation of In and its altered accumulation pattern associated with the over expression of frtz-GFP. G shows a ptc-Gal4/ UAS-frtz wing immunostained to show the endogenous In protein. The arrow marks the approximate location of the edge of the ptc-domain. Note the increased accumulation of In where frtz was overexpressed. H shows the quantitation of the changes in In immunostaining inside and outside of the ptc domain. Average grey scale values in the ptc domain are normalized to the level outside of the ptc domain. A t-test was used to compare the grey values inside and outside of the ptc domain. * p = 0.05-0.01, ** p = 0.01-0.001, *** p<0.001.
Figure 6.
The over expression of one PPE protein affects the accumulation of the others. A, B and C show a ptc-Gal4/UAS-HA-in; ubi-myc-frtz-GFP/+ wing immunostained for both In (red) and GFP (green-Frtz). This ubi-myc-frtz-GFP transgene gives variegated expression. D shows the quantitation of GFP (Frtz) staining inside (2) and outside (1) of the ptc domain. (Note the quantitation was done on the peripheral accumulation in cells that expressed high levels of Frtz). The increase in Frtz accumulation was significant (p<0.05). E, F and G shows a ptc-Gal4/UAS-fy-Flag-ollas; ubi-myc-frtz-GFP/+ wing immunostained for both Ollas (red-Fy) and GFP (green-Frtz). H shows the quantitation of GFP (Frtz) staining inside (2) and outside (1) of the ptc domain. The decrease in Frtz accumulation was significant (p<0.05). I, J and K show the results of Western blots of wing disc samples where the expression of UAS-HA-in and UAS-myc-frtz were driven either singly or together using ptc-Gal4. I and J show the quantitation and K the blot. M, N and L show the results of Western blots of wing disc samples where the expression of UAS-fy-Flag-Ollas and UAS-myc-frtz were driven either singly or together using ptc-Gal4. L and M show the quantitation and N the blot. O, P and Q show the results of Western blots of wing disc samples where the expression of UAS-HA-in and UAS-fy-GFP were driven either singly or together in varying doses using ptc-Gal4. O and P show the quantitation and Q the blot.
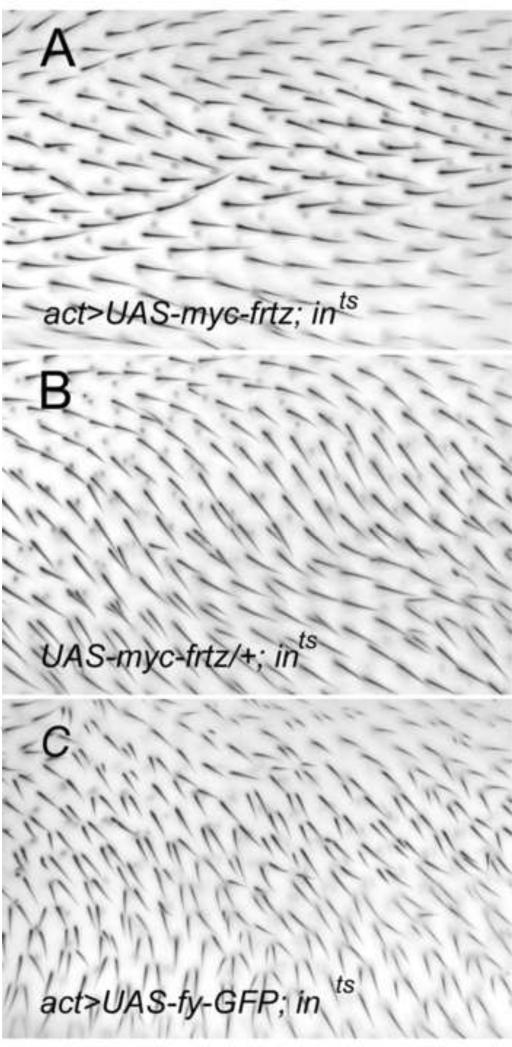
To test if the ability of over expression of fy or frtz to modulate the level of endogenous In could be functionally important we over expressed fy or frtz in flies that carried a temperature sensitive hypomorphic allele of in. Our hypothesis was that increased or decreased levels of the Ints protein would suppress or enhance the mutant phenotype. Consistent with this hypothesis we found that the over expression of fy-GFP enhanced the phenotype of ints (Fig 7BC, while the over expression of myc-frtz almost completely suppressed the phenotype (Fig 7 AB). Similar results were seen using both act-Gal4 and ptc-Gal4 to drive transgene expression.
Figure 7.
The over expression of fy and frtz modifies a hypomorphic in phenotype. Panel B is a control showing the in-ts phenotype at 25°C. The wings in A and C used actin-Gal4 to drive the expression of myc-frtz and fy-GFP respectively.
We also carried out a similar set of experiments where we simultaneously over expressed two PPE proteins and determined by quantitative western blot analysis if one protein had an effect on the accumulation of the second. The simultaneous over expression of both in and frtz resulted in increased accumulation of both compared to single over expression controls (Fig 6I,K,O). In contrast when we simultaneously over expressed frtz and fy the accumulation of both was decreased compared to single over expression controls (Fig 6J,L,P). The situation was more complicated for the simultaneous over expression of in and fy (Fig 6M,N,Q). The presence of a single UAS-fy gene resulted in increased accumulation of In, however the presence of two UAS-fy genes resulted in decreased accumulation of In. Thus the effects of Fy on In accumulation was dose sensitive. This dose sensitivity is similar to that seen for the in/fy gain of function hair polarity phenotype described earlier and provides an explanation for it.
As noted previously when over expressed the proximal localization of In and Frtz was lost and the proteins were found throughout wing cells, although often enriched at the cell periphery. In co-imunolocalization experiments we found substantial co-localization of all three protein pairs (see fig S4). In other experiments we examined the localization of co-expressed proteins in the large salivary gland cells. We observed a clear co-localization of In and Fy in salivary gland cells. These were primarily at the cell periphery but in an irregular fashion. Similar results were seen for In and Frtz and Fy and Frtz (Fig S4)
In several experiments we over expressed all 3 PPE genes. This resulted in the accumulation of the In, Fy and Frtz proteins in spherical puncta in pupal wing cells. The significance of these puncta is unclear. They were also seen in other cell types such as the salivary gland and third instar wing discs (Fig S4).
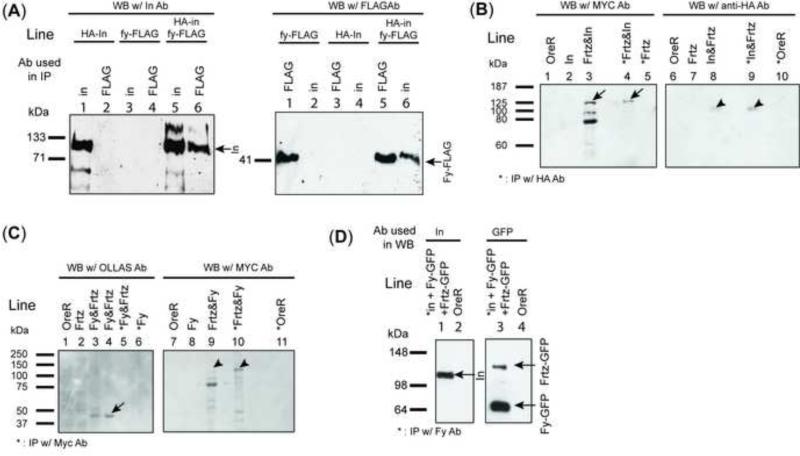
The requirements for other PPE proteins for the proper accumulation of In and Frtz suggested the possibility that these proteins would interact physically in vivo. To test this hypothesis we determined if these proteins could be co-immunoprecipitated. In our initial experiments we used extracts of larvae that carried hs-HA-In and hs-fy-FLAG transgenes 4 hrs after heat shock. We were able to detect In (using anti-HA) in immunoprecipitates of Fy (using anti-Flag) and Fy (using anti-Flag) in immunopreciptates of In (using anti-In)(Fig 8A). In a second set of experiments we used Flag tagged Fy and wild type In and expressed both behind hsp70 promoters in S2 cells. We were able to detect In (using anti-In) in immunoprecipitates of Fy (using anti-Flag) (Fig S5A) and in complementary experiments we could detect Fy (using anti-Flag) in immunopreciptates of In (using anti-In) (Fig S6A). In another set of experiments we used UAS-HA-In and UAS-Fy-Flag-Ollas transgenes and wing discs and once again detected In in Ollas immunoprecipitates of Fy and Fy in immunoprecipitates of In (HA antibody) (Fig S5B).
Figure 8.
Co-immunoprecipitation of PPE proteins. A shows an experiment where HA-In and Fy-Flag were co-immunoprecipitated by either anti-In or anti-Flag antibodies. In this experiment a heat shock was used to induce the expression of transgenes subcloned behind the hsp70 promoter. B shows the co-immunoprecipitation of In and Frtz. This experiment used UAS-HA-in and UAS-myc-frtz transgenes driven by ptc-Gal4. Wing disc samples were immunoprecipitated using Rabbit anti-HA antibody. Arrows point to myc-Frtz and HA-In on the Western blots. C shows an experiment where we tested the co-immunoprecipitation of Fy and Frtz. Samples from ptc-Gal4/UAS-fy-Flag-Ollas; UAS-myc-frtz/+ wing discs were IP with anti-Myc antibody and then assayed by western blotting. Lane 3 and 4 (on the left side) are duplicates. The arrows point to Fy-Flag-Ollas (left) and myc-Frtz (right). Note that Fy was not preciptiated with Frtz. D shows an experiment where all three of the PPE proteins were co-expressed (ptc-Gal4/UAS-HA-in UAS-fy-GFP; UAS-frtz-GFP/+). Samples were made from wing discs and immunoprecipitated using anti-Fy antibody. The Western blots were probed with anti-GFP (to detect both Fy-GFP and Frtz-GFP) and anti-In. Control experiments established that anti-Fy antibody could not pull down either HA-in or Frtz-GFP (data not shown).
Similar but less extensive experiments were done with Frtz and In. We found that anti-HA antibodies could immunoprecipitate Frtz from ptc-Gal4 UAS-HA-in/+; UAS-myc-frtz/+ wing disc extracts (Fig 8B). Similarly, we found that anti-Frtz antibodies could co-immunoprecipitate In from ptc-Gal4 UAS-HA-in/+; UAS-frtz-GFP/+ wing discs (Fig S5C).
In contrast to the results noted above we did not see the co-immunoprecipitation of Fy and Frtz when we tested extracts from ptc-Gal4/UAS-fy-flag-ollas; UAS-myc-ftrz wing discs (anti-Frtz did not precipitate Fy-flag-ollas (Fig 8C)).
Finally we found that anti-Fy antibodies could co-immunoprecipitate both In and Frtz from wing disc extracts from ptc-Gal4/ UAS-HA-in UAS-fy-GFP; UAS-frtz-GFP/+ larvae (Fig 8D) suggesting all 3 proteins are present in a common complex.
In can interact directly with Fy and Frtz
We tested the three PPE proteins for direct interactions using the yeast two hybrid system. We found that In could interact with both Fy and Frtz but we did not see any evidence for a direct interaction between Fy and Frtz (Fig S6).
We also carried out a limited set of experiments to determine if the known protein-protein interaction domains found in In and Frtz were essential for the interactions between PPE proteins. The Frtz protein contains a pair of WD40 repeats (aa304-384) (Collier et al., 2005) in the amino terminal half of the protein as well as a predicted coiled coil region. There is also an atrophin 1 homology in the carboxy terminal half of the protein (aa644-879). In the yeast two-hybrid system the amino terminal (aa1-550) but not the carboxy terminal region (aa 550-904) of Frtz could interact with In. We further split the amino terminal region into several parts (Fig S7). The WD40 containing fragment interacted the most strongly, but we also obtained evidence for a weaker interaction between In and the coiled coil containing fragment of Frtz (Fig S6, Fig S7). Our data suggest there are two binding interactions between In and Frtz.
The In protein contains a PDZ domain (aa158-213) (Cho et al., 1992; Te Velthuis et al., 2007) that is found in the amino terminal region (aa 1-550), while the C terminal half (aa 551-869) contains no recognizable domains. We found that the C terminal domain of In could interact with both Frtz and Fy, while no interaction was seen with the amino terminal domain (Fig S6). Thus, the PDZ domain does not appear to be important for the ability of In to interact with Frtz or Fy.
Discussion
PPE genes appear to be a functional and biochemical group
A variety of genetic data suggested that the PPE genes in, fy and frtz comprised a functional group in Drosophila (Adler et al., 2004; Collier et al., 2005; Strutt and Warrington, 2008; Wong and Adler, 1993). Our data establishes that In binds directly to both Fy and Frtz. Our analysis of gain of function phenotypes suggested some functional differences between the genes, thus their physical interactions may not be obligate in some contexts, although caution is needed when interpreting gain of function phenotypes.
The vertebrate homologs of in, fy and frtz are known as intu, fuz and WDPCP (aka frtz). Mutations in these genes show both PCP and ciliogenesis phenotypes (Cui et al., 2013; Gray et al., 2009; Heydeck et al., 2009; Kim et al., 2010; Park et al., 2006; Zilber et al., 2013). In the mouse both intu and fuz mutations result in neural tube defects (Heydeck and Liu, 2011; Heydeck et al., 2009) and double mutants appear similar to single mutants (Heydeck and Liu, 2011) mirroring the lack of phenotypic additivity seen in flies (Collier et al., 2005). The similarity of these phenotypes is consistent with the interactions detected in the fly proteins being conserved in mammals. The ciliogenesis phenotype is not seen in flies where very few cells contain primary cilia. In humans frtz mutations have been implicated in Bardet-Biedl (BBS) syndrome (Kim et al., 2010) and fuz mutations have been implicated in neural tube defects (Seo et al., 2011). The defect in ciliogenesis results in defects in hedgehog signaling (Gray et al., 2009; Heydeck et al., 2009) and defects in Wnt and FGF signaling have also been reported in mutants (Tabler et al., 2013; Zhang et al., 2011; Zilber et al., 2013) although these could be secondary to the hedgehog effect. Defects in key signaling pathways that function in many locations and times in development complicate the interpretation of mutant phenotypes. A variety of data has suggested that intu and fuz might not function downstream of the PCP genes in some vertebrates and that the vertebrate homologs of the PPE genes might not share all of the same functions. A typical feature of PCP mutants in vertebrates is a convergent extension phenotype in early embryos (Goodrich and Strutt, 2011; Goto and Keller, 2002; Jessen et al., 2002; Wallingford et al., 2001) and this was also seen in intu and fuz morphants in Xenopus (Park et al., 2006). However, no evidence for such a convergent-extension phenotype was seen in intu and fuz muose mutants (Heydeck and Liu, 2011). Further, a cochlea stereocilia phenotype seen in PCP mutants is present in frtz mutants (Cui et al., 2013) but has not been reported in intu and fuz mutants. The fuz gene has been found to be important for membrane trafficking (Gray et al., 2009) and retrograde intraflagellar transport (Brooks and Wallingford, 2012) and similar finding have not been reported for the other PPE genes.
Mutations in the Drosophila PPE genes produce notable planar polarity phenotypes in a subset of the tissues where such phenotypes are associated with mutations in the upstream PCP genes (Adler, 1992; Gubb and Garcia-Bellido, 1982). This could be due to the PPE genes only functioning in some cellular contexts or to the PPE proteins functioning downstream of the PCP genes in all tissues but not being important in some. For example, this could be due to redundancy with an alternative gene module in some cell types. In support of this latter hypothesis, when we examined the eye of in mutants we detected a very weak PCP phenotype (only a few ommatidia per eye were affected) that had been missed due to the lack of a rough eye phenotype (Lee and Adler, 2002). Some of the differences between the phenotypes of vertebrate PCP and PPE genes could have a similar basis.
An interesting observation is that frtz mutant mice show a loss of Vangl2 in the cochlea (Cui et al., 2013). Thus, in the murine cochlea frtz is not “downstream” of Vangl2. Similarly, both intu and fuz regulate Dsh localization to the cilium (Park et al., 2006; Zilber et al., 2013). Thus, in these vertebrate tissues the PPE genes do not appear to be downstream of the PCP genes. These observations are reminiscent of our observations that the fly PPE genes are not obligatorily downstream of the PCP genes. Further, both intu and fuz being required for normal Dsh localization to the cilium suggests these two proteins functioning together in vertebrates.
In addition to the shared phenotypes frtz has also been found to interact with septin7 and septin2 and to affect focal adhesions and cell migration (Cui et al., 2013; Kim et al., 2010). It was suggested that through its interaction with septins frtz could regulate the actin cytoskeleton (Cui et al., 2013; Kim et al., 2010). Our observation that the over expression of Frtz leads to a substantial delay in hair initiation and to an altered F-actin distribution in wing cells prior to hair initiation also suggests an effect on the actin cytoskeleton independent of its role in PCP. Neither of these phenotypes were seen with in and fy. Thus, in both Drosophila and vertebrates Frtz appears to have activity that is not shared with In and Fy.
From co-immunoprecipitation experiments we obtained evidence that all three proteins are found in the same protein complex. However, in the conditions these experiments were carried out under all of the proteins accumulated in large puncta and this may reflect an abnormal complex. However, it is worth noting that when only two of the proteins are oe they colocalize in both wing and salivary gland cells in the absence of any large puncta and they can be coimmunoprecipitated. We and others have also found that when In and Frtz are expressed at normal or close to normal levels, they co-localize at the proximal edge of pupal wing cells (Adler et al., 2004; Strutt and Warrington, 2008). Strutt and colleagues also were able to show that Fy also localized at the proximal edge of wing cells (Strutt and Warrington, 2008). These observations all support the hypothesis that the 3 proteins are found in a common complex.
The role of Fy seems in some ways different from that of In and Frtz. At least its interaction with the other two PPE proteins stands out. In the absence of fy function In accumulation is sharply decreased however when oe Fy also decreases the accumulation of endogenous In. Further when In and Fy are both oe the level of In accumulation can be increased or decreased depending on the relative dose of Fy. Thus, the stoichiometry of the two proteins appears to be the key. The importance of the relative In/Fy dose may also explain the requirement for the oe of both to produce a gof wing hair polarity phenotype. The relative ratio of Frtz and Fy also appears to be important as oe of Fy leads to decreased accumulation of Frtz encoded by either the ubiquitin promoter driven gene or by the UAS/Gal4 system. Nothing is currently known about the stoichiometry of a PPE protein complex and the number of types of such complexes. Such information might provide an explanation for the complex relationship between Fy and In (and Frtz). In vertebrates Fuz functions in intraflagellar transport, membrane vesicle fusion and secretion (Brooks and Wallingford, 2012; Gray et al., 2009). Perhaps in flies it functions in the intracellular trafficking of In and Frtz.
PPE and PCP proteins
Our experiments established that the oe of either In + Fy or Frtz by itself could alter the level and/or subcellular location where PCP proteins accumulated. These results were surprising as the PPE genes are generally thought to function downstream of the PCP proteins. As noted earlier in the mouse a frtz mutation altered Vangl2 accumulation in hair cells (Cui et al., 2013) and Intu and Fuz mutations altered Dsh recruitment to the cilium (Park et al., 2006; Zilber et al., 2013). Those and our results have blurred the hierarchy between these two groups of genes.
The formation of the proximal and distal protein domains in wing cells is thought to involve both inter and intra cellular signaling. It is generally thought that negative interactions between PCP proteins that accumulate on the proximal and distal edges of wing cells are part of an intracellular negative feedback loop that helps establish and maintain the localized protein domains (Amonlirdviman et al., 2005; Bastock et al., 2003; Das et al., 2004; Tree et al., 2002). One way in which the oe of the cytoplasmic PPE proteins might repattern the upstream proteins would be to interfere with this negative feedback loop. For example, the binding of a PPE protein to a PCP protein such as Pk might block Pk's antagonistic interaction with Dsh. Alternatively, PPE proteins when oe could bind to and stabilize one or more of the PCP proteins (e.g. Pk or Dsh). For example, such an interaction might prevent the degradation of the PCP protein. Ubiquitin dependent Pk degradation has been implicated in regulating PCP (Narimatsu et al., 2009) and increased protein levels could result in the hair polarity phenotypes as the oe of any of the PCP proteins leads to alterations in wing hair polarity (Adler et al., 1997; Tree et al., 2002; Usui et al., 1999). There is also good precedent for the over expression of one PCP protein altering the levels of others. For example, it is known that the oe of Pk leads to increased accumulation of Fz, Dsh and Stan (Tree et al., 2002). Further studies will be needed to elucidate the mechanism by which the PPE proteins can influence the upstream proteins.
Frtz and the cytoskeleton
Our experiments on frtz also provided several novel and surprising results. The oe of frtz and frtz-GFP led to complementary effects on hair polarity. This was unexpected since when expressed at close to normal levels both proteins provide complete rescue of null frtz alleles. The basis for the complementary gof phenotypes remains unclear. Perhaps the folding of the GFP tagged protein is slowed and when over expressed much of it ends up in an abnormal conformation with abnormal activity with respect to PCP. Despite the complementary polarity phenotypes we detected a similar dramatic delay in hair outgrowth in flipout clones of both. This suggested the possibility that at least when over expressed Frtz could inhibit the activation of the actin cytoskeleton. A second set of observations consistent with an effect on the actin cytoskeleton are bristle morphology abnormalities associated with over expression of frtz (or frtz-GFP) (pna, unpublished). Some effects on hair and bristle morphogenesis can be seen with other PCP and PPE genes but none as dramatic as those we have seen for frtz. Driving expression of frtz by ptc-Gal4 also led to a fold in the wing that runs down the ptc domain. The basis for this is unclear but could be due to changes in cell size, cell number or shape. We have not detected similar phenotypes in either gain or loss of function experiments with other fz/stan pathway mutants. These observations suggest that Frtz may modulate the cytoskeleton independently of the fz/stan pathway as a whole.
Several other observations suggest that Frtz plays multiple roles during development and that at least one in planar cell polarity is likely to be independent of the fz/stan pathway. In ptc>frtz-GFP wings a very distinctive hair polarity phenotype was seen. When wings were ptc>frtz-GFP and also mutant for another fz/stan pathway gene this distinctive polarity pattern was lost. However, the hair polarity pattern also did not closely resemble that of the fz/stan pathway mutant. This indicates that frtz-GFP can influence hair polarity in a fz/stan pathway independent manner. The mechanism and the identity of a presumptive second pathway remain to be elucidated. One possibility is that frtz might function as an effector of the dachsous/fat pathway in a fz/stan independent manner. A second is that Frtz might regulate the actin cytoskeleton (Cui et al., 2013; Kim et al., 2010) independently of its role in the fz/stan pathway.
Supplementary Material
Figure S1. Accumulation of endogenous and overexpressed PPE proteins in pupal wing cells. All are confocal images that are single optical sections or maximal projections of a small number of optical sections from the sub-apical region. Panel A shows the asymmetric accumulation of Frtz-GFP when expressed from a ubiquitin promoter vector. Panel B shows the endogenous In protein detected by anti-In antibodies. C to E’ show the accumulation of Fy, In and Frtz when over expressed by ptc-Gal4. Note the wide spread accumulation of the over expressed proteins.
Figure S2. The over expression of Frtz by ptc-Gal4 results in a fold in the wing. A ptc-Gal4/+; UAS-frtz wing is shown. The arrow points to the fold.
Figure S3. Genetic interactions of fz/stan pathway genes with frtz over expression. In the experiments described here the UAS-frtz transgene is not as highly expressed as the one used in Fig 2. In A we scored each wing for the furthest region that showed a 45° polarity disruption. In B we scored wings for the greatest extent of polarity disruption. 0-30° = 1, 30° - 60° = 2 and >60° = 3. The non-parametric Mann Whitney test was used to compare each condition to puc-Gal4 UAS-frtz/+ controls.
Figure S4. The co-localization of In, Fy and Frtz. Shown is a ptc-Gal4/UAS-HA-in; UAS-myc-frtz/+ salivary gland (A-C) and pupal wing (D-F) immunostained for HA (red)(In) and myc (green) (Frtz). In the next row is a ptc-Gal4 UAS-HA-in UAS-fy-GFP/+ saliavary gland (GHI) and pupal wing (JKL) immunostained for GFP (green)(Fy) and HA (red)(In). In the third row is a ptc-Gal4/UAS-fy-GFP; UAS-myc-frtz/+ salivary gland (MNO) and pupal wing (PQR) immunostained for GFP (green) and Myc (red). The next row of images is a ptc-Gal4/UAS-HA-in UAS-fy-GFP; UAS-frtz-GFP pupal wing (STU) immunostained for HA (red) and GFP (green). Below (VWX) are higher magnification images of a small region of STU. Note the particulate staining in S-X). The magnification in STU is the same as in DEF.
Figure S5. Co-immunoprecipitation of PPE proteins. A. S2 cell extracts where both HA-in and fy-Flag were expressed by the hsp70 promoter were tested by immunoprecipitation. The approximately 60KD band found in both the upper and lower western blots are presumably immunoglobin heavy chain contamination from the IP. Note that anti-In can pull down Fy and that anti-Flag can precipitate In. B. Extracts of wing discs where ptc-Gal4 driven expression of HA-In and Fy-Flag-Ollas were tested for co-immunoprecipitation. Anti-HA was able to pull down Fy-Flag-Ollas and anti-Ollas was able to pull down In. C. Wing disc extracts from ptc-Gal4 UAS-HA-in/+; UAS-frtz-GFP/+ animals were tested for co-immunoprecipitation. Anti-Frtz antibodies pulled down In.
Figure S6 Yeast two hybrid assays that provided evidence for In directly interacting with both Frtz and Fy.
Figure S7– The WD40 region of Frtz interacts with In. Shown is a summary of a set of yeast two hybrid experiments that indicate that the WD40 motif of Fritz interacts strongly with In.
Highlights.
The PCP effector genes are thought to function downstream of the PCP core genes.
We find PCP effector proteins can affect the function of “upstream” PCP proteins.
The PCP effector proteins In, Fy and Frtz interact physically.
These interactions can alter protein accumulation.
The Frtz protein has independent functions in PCP.
Acknowledgements
This work was supported by a grant from the NIGMS to pna and also by a National Research Foundation of Korea (NRF-2013R1A1A2011441) grant funded by the Korean Government (to hl). We thank our colleagues in the fly community for generously sharing reagents and the reviewers for their helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler PN. The genetic control of tissue polarity in Drosophila. BioEssays : news and reviews in molecular, cellular and developmental biology. 1992;14:735–741. doi: 10.1002/bies.950141103. [DOI] [PubMed] [Google Scholar]
- Adler PN, Charlton J, Vinson C. Allelic Variation at the Frizzled Locus of Drosophila. Dev Genet. 1987;8:99–119. [Google Scholar]
- Adler PN, Krasnow RE, Liu JC. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Current Biology. 1997;7:940–949. doi: 10.1016/s0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- Adler PN, Zhu CM, Stone D. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Current Biology. 2004;14:2046–2051. doi: 10.1016/j.cub.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of dishevelled mediates frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babayeva S, Rocque B, Aoudjit L, Zilber Y, Li J, Baldwin C, Kawachi H, Takano T, Torban E. Planar cell polarity pathway regulates nephrin endocytosis in developing podocytes. J Biol Chem. 2013;288:24035–24048. doi: 10.1074/jbc.M113.452904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targetted gene expression as a means of altering cell fate and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brooks ER, Wallingford JB. Control of vertebrate intraflagellar transport by the planar cell polarity effector Fuz. The Journal of cell biology. 2012;198:37–45. doi: 10.1083/jcb.201204072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae J, Kim MJ, Goo JH, Collier S, Gubb D, Charlton J, Adler PN, Park WJ. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development. 1999;126:5421–5429. doi: 10.1242/dev.126.23.5421. [DOI] [PubMed] [Google Scholar]
- Chang H, Nathans J. Responses of hair follicle-associated structures to loss of planar cell polarity signaling. Proc Natl Acad Sci U S A. 2013;110:E908–917. doi: 10.1073/pnas.1301430110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Collier S, Gubb D. Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development. 1997;124:4029–4037. doi: 10.1242/dev.124.20.4029. [DOI] [PubMed] [Google Scholar]
- Collier S, Lee H, Burgess R, Adler P. The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the drosophila epidermis. Genetics. 2005;169:2035–2045. doi: 10.1534/genetics.104.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Chatterjee B, Lozito TP, Zhang Z, Francis RJ, Yagi H, Swanhart LM, Sanker S, Francis D, Yu Q, San Agustin JT, Puligilla C, Chatterjee T, Tansey T, Liu X, Kelley MW, Spiliotis ET, Kwiatkowski AV, Tuan R, Pazour GJ, Hukriede NA, Lo CW. Wdpcp, a PCP protein required for ciliogenesis, regulates directional cell migration and cell polarity by direct modulation of the actin cytoskeleton. PLoS biology. 2013;11:e1001720. doi: 10.1371/journal.pbio.1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- Goggolidou P. Wnt and planar cell polarity signaling in cystic renal disease. Organogenesis. 2013:10. doi: 10.4161/org.26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, Lee I, Weiss GS, Liu KJ, Marcotte EM, Wallingford JB, Finnell RH. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol. 2009;11:1225–1232. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phi C31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- Gubb D, Green C, Huen D, Coulson D, Johnson G, Tree D, Collier S, Roote J. The balance between isoforms of the Prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 1999;13:2315–2327. doi: 10.1101/gad.13.17.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held LI, Duarte CM, Derakhshanian K. Extra Tarsal Joints and Abnormal Cuticular Polarities in Various Mutants of Drosophila-Melanogaster. Rouxs Archives of Developmental Biology. 1986;195:145–157. doi: 10.1007/BF02439432. [DOI] [PubMed] [Google Scholar]
- Heydeck W, Liu A. PCP effector proteins inturned and fuzzy play nonredundant roles in the patterning but not convergent extension of mammalian neural tube. Dev Dyn. 2011;240:1938–1948. doi: 10.1002/dvdy.22696. [DOI] [PubMed] [Google Scholar]
- Heydeck W, Zeng H, Liu A. Planar cell polarity effector gene Fuzzy regulates cilia formation and Hedgehog signal transduction in mouse. Dev Dyn. 2009;238:3035–3042. doi: 10.1002/dvdy.22130. [DOI] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. Embo J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, Wallingford JB. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R, Weber U, Gehring WJ. The White Gene as a Marker in a New P-Element Vector for Gene-Transfer in Drosophila. Nucleic Acids Res. 1987;15:3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow RE, Adler PN. A Single Frizzled Protein Has a Dual Function in Tissue Polarity. Development. 1994;120:1883–1893. doi: 10.1242/dev.120.7.1883. [DOI] [PubMed] [Google Scholar]
- Lee H. Dissertation. University of Virginia; 2002. Genetic and Biochemical Studies of Planar Polarity Genes in Drosophila. [Google Scholar]
- Lee H, Adler PN. The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics. 2002;160:1535–1547. doi: 10.1093/genetics/160.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Yan J, Adler PN. The Drosophila planar polarity proteins inturned and multiple wing hairs interact physically and function together. Genetics. 2010;185:549–558. doi: 10.1534/genetics.110.114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, Wrana JL. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Park WJ, Liu J, Sharp EJ, Adler PN. The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development. 1996;122:961–969. doi: 10.1242/dev.122.3.961. [DOI] [PubMed] [Google Scholar]
- Pirrotta V. Vectors for P-mediated transformation in Drosophila. Biotechnology. 1988;10:437–456. doi: 10.1016/b978-0-409-90042-2.50028-3. [DOI] [PubMed] [Google Scholar]
- Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM. Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics. 1995;139:1347–1358. doi: 10.1093/genetics/139.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Zilber Y, Babayeva S, Liu J, Kyriakopoulos P, De Marco P, Merello E, Capra V, Gros P, Torban E. Mutations in the planar cell polarity gene, Fuzzy, are associated with neural tube defects in humans. Human molecular genetics. 2011;20:4324–4333. doi: 10.1093/hmg/ddr359. [DOI] [PubMed] [Google Scholar]
- Strutt D. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Strutt D, Warrington SJ. Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development. 2008;135:3103–3111. doi: 10.1242/dev.025205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler JM, Barrell WB, Szabo-Rogers HL, Healy C, Yeung Y, Perdiguero EG, Schulz C, Yannakoudakis BZ, Mesbahi A, Wlodarczyk B, Geissmann F, Finnell RH, Wallingford JB, Liu KJ. Fuz mutant mice reveal shared mechanisms between ciliopathies and FGF-related syndromes. Dev Cell. 2013;25:623–635. doi: 10.1016/j.devcel.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, Ueno N. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr Biol. 2003;13:674–679. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: A new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Velthuis AJ, Isogai T, Gerrits L, Bagowski CP. Insights into the molecular evolution of the PDZ/LIM family and identification of a novel conserved protein motif. PLoS One. 2007;2:e189. doi: 10.1371/journal.pone.0000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree DRP, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. Microtubules enable the planar cell polarity of airway cilia. Curr Biol. 2012;22:2203–2212. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Vogeli KM, Harland RM. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int J Dev Biol. 2001;45:225–227. [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Wolff T, Rubin G. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- Wong LL, Adler PN. Tissue Polarity Genes of Drosophila Regulate the Subcellular Location for Prehair Initiation in Pupal Wing Cells. Journal of Cell Biology. 1993;123:209–221. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J. Dissertation. University of Virginia; 2008. The study of downstream components in the frizzled signaling pathway in planar cell polarity. [Google Scholar]
- Yan J, Huen D, Morely T, Johnson G, Gubb D, Roote J, Adler PN. The multiple-wing-hairs Gene Encodes a Novel GBD-FH3 Domain-Containing Protein That Functions Both Prior to and After Wing Hair Initiation. Genetics. 2008;180:219–228. doi: 10.1534/genetics.108.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wlodarczyk BJ, Niederreither K, Venugopalan S, Florez S, Finnell RH, Amendt BA. Fuz regulates craniofacial development through tissue specific responses to signaling factors. PLoS One. 2011;6:e24608. doi: 10.1371/journal.pone.0024608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilber Y, Babayeva S, Seo JH, Liu JJ, Mootin S, Torban E. The PCP effector Fuzzy controls cilial assembly and signaling by recruiting Rab8 and Dishevelled to the primary cilium. Molecular biology of the cell. 2013;24:555–565. doi: 10.1091/mbc.E12-06-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Accumulation of endogenous and overexpressed PPE proteins in pupal wing cells. All are confocal images that are single optical sections or maximal projections of a small number of optical sections from the sub-apical region. Panel A shows the asymmetric accumulation of Frtz-GFP when expressed from a ubiquitin promoter vector. Panel B shows the endogenous In protein detected by anti-In antibodies. C to E’ show the accumulation of Fy, In and Frtz when over expressed by ptc-Gal4. Note the wide spread accumulation of the over expressed proteins.
Figure S2. The over expression of Frtz by ptc-Gal4 results in a fold in the wing. A ptc-Gal4/+; UAS-frtz wing is shown. The arrow points to the fold.
Figure S3. Genetic interactions of fz/stan pathway genes with frtz over expression. In the experiments described here the UAS-frtz transgene is not as highly expressed as the one used in Fig 2. In A we scored each wing for the furthest region that showed a 45° polarity disruption. In B we scored wings for the greatest extent of polarity disruption. 0-30° = 1, 30° - 60° = 2 and >60° = 3. The non-parametric Mann Whitney test was used to compare each condition to puc-Gal4 UAS-frtz/+ controls.
Figure S4. The co-localization of In, Fy and Frtz. Shown is a ptc-Gal4/UAS-HA-in; UAS-myc-frtz/+ salivary gland (A-C) and pupal wing (D-F) immunostained for HA (red)(In) and myc (green) (Frtz). In the next row is a ptc-Gal4 UAS-HA-in UAS-fy-GFP/+ saliavary gland (GHI) and pupal wing (JKL) immunostained for GFP (green)(Fy) and HA (red)(In). In the third row is a ptc-Gal4/UAS-fy-GFP; UAS-myc-frtz/+ salivary gland (MNO) and pupal wing (PQR) immunostained for GFP (green) and Myc (red). The next row of images is a ptc-Gal4/UAS-HA-in UAS-fy-GFP; UAS-frtz-GFP pupal wing (STU) immunostained for HA (red) and GFP (green). Below (VWX) are higher magnification images of a small region of STU. Note the particulate staining in S-X). The magnification in STU is the same as in DEF.
Figure S5. Co-immunoprecipitation of PPE proteins. A. S2 cell extracts where both HA-in and fy-Flag were expressed by the hsp70 promoter were tested by immunoprecipitation. The approximately 60KD band found in both the upper and lower western blots are presumably immunoglobin heavy chain contamination from the IP. Note that anti-In can pull down Fy and that anti-Flag can precipitate In. B. Extracts of wing discs where ptc-Gal4 driven expression of HA-In and Fy-Flag-Ollas were tested for co-immunoprecipitation. Anti-HA was able to pull down Fy-Flag-Ollas and anti-Ollas was able to pull down In. C. Wing disc extracts from ptc-Gal4 UAS-HA-in/+; UAS-frtz-GFP/+ animals were tested for co-immunoprecipitation. Anti-Frtz antibodies pulled down In.
Figure S6 Yeast two hybrid assays that provided evidence for In directly interacting with both Frtz and Fy.
Figure S7– The WD40 region of Frtz interacts with In. Shown is a summary of a set of yeast two hybrid experiments that indicate that the WD40 motif of Fritz interacts strongly with In.