Abstract
Hedgehog (Hh) is a paracrine signaling protein with major roles in development and disease. In vertebrates and invertebrates, Hh signal transduction is carried out almost entirely by evolutionarily conserved components, and in both, intercellular movement of Hh is mediated by cytonemes – specialized filopodia that serve as bridges that bring distant cells into contact. A significant difference is the role of primary cilia, a slender, tubulin-based protuberance of many vertebrate cells. Although the primary cilium is essential for Hh signaling in cells that have one, most Drosophila cells lack a primary cilium. This perspective addresses the roles of primary cilia and cytonemes, and proposes that for Hh signaling, the role of primary cilia is to provide a specialized hydrophobic environment that hosts lipid-modified Hh and other components of Hh signal transduction after Hh has traveled from elsewhere in the cell. Implicit in this model is the idea that initial binding and uptake of Hh is independent of and segregated from the processes of signal transduction and activation.
Keywords: Hedgehog, primary cilium, cytoneme, lipid raft
Text
Cytonemes are specialized types of signaling filopodia that are actin-based (Ramírez-Weber and Kornberg, 1999) (Fig. 1). They extend from both the apical and basal surfaces of polarized cells (Hsiung et al., 2005) and they ferry signaling proteins such as Hedgehog (Hh) and Decapentaplegic (Dpp) between source and target cells (Bilioni et al., 2012; Bischoff et al., 2013; Callejo et al., 2011; Kornberg, 2011b; Roy et al., 2010). The primary cilium is a microtubule-based structure that emanates from a cell’s basal body. Almost every vertebrate cell has one, and primary cilia have roles in many signaling processes, including Hh signaling (reviewed in Goetz and Anderson, 2010). Yet, although both cytonemes and primary cilia are specialized cytoplasmic extensions and both function in Hh signaling, their roles in Hh signaling are probably distinct. The primary cilium is not a cytoneme in the sense that a primary cilium is not a conduit for transporting Hh between cells. And unlike a primary cilium, a cytoneme does not house components of Hh signal transduction and it is not a structure in which signals are transduced.
Figure 1. Primary cilia and cytonemes in polarized cells.
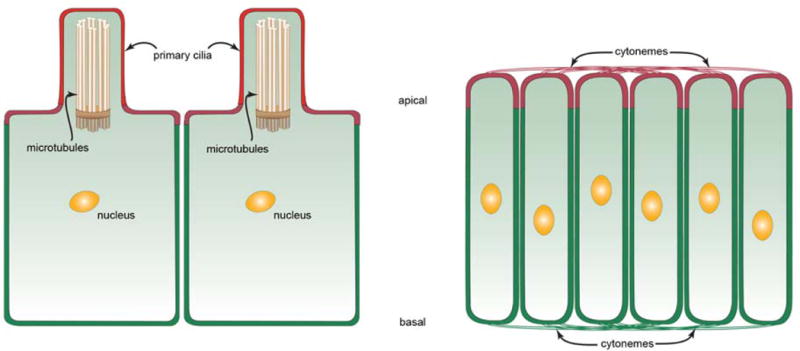
Drawings show ciliated cells (left) and non-ciliated cells (right) in a polarized epithelium, apical up and basal down. Ciliated cells extend a single primary cilium apically that contains 9 doublet pairs of microtubules linked to a basal body. These cells presumably have cytonemes that are not depicted in this drawing. Apical and basal cytonemes extend across both the apical and basal surfaces of the non-ciliated cells such as those of the wing imaginal disc.
The importance of primary cilia to Sonic Hedgehog signal transduction was discovered when mouse mutants lacking functional primary cilia were found to be defective in Hh signaling (Huangfu et al., 2003) (for simplicity, the Hh abbreviation will be used here for both Hedgehog and Sonic Hedgehog). Subsequent work revealed that primary cilia contain some components of the Hh signal transduction pathway, including the Gli transcription factors, the Patched 1 (Ptc1) Hh receptor and the seven-transmembrane protein Smoothened (Smo) (Corbit et al., 2005; Haycraft et al., 2005; Rohatgi et al., 2007), and that Hh co-localizes with Ptc in the primary cilium (Rohatgi et al., 2007). Although these findings do not show whether Hh-dependent Ptc function controls pathway activation in cilia or elsewhere, they have supported the idea that the primary cilium has two roles in Hh signaling – to receive Hh after it has been released by Hh-producing cells and to initiate signal transduction in responding cells. There are a number of reasons to propose that the primary cilium does not have a direct role in Hh reception.
Although primary cilia have been implicated as signal sensing organelles, for instance in vertebrate left-right axis specification (Field et al., 2011; Kamura et al., 2011; McGrath et al., 2003; Pennekamp et al., 2002), they do not appear to be the site of Hh binding and uptake, for instance, in the neural tube of the mouse embryo where Hh patterns cell types in a concentration and time-dependent manner (reviewed in Jacob and Briscoe, 2003). At embryo stage E8.5, Hh is produced by the notochord, which is next to the basal surface of the cells of the ventral neural tube (Fig. 2A). Hh protein, detected with α-Hh antibody (Gritli-Linde et al., 2001) or by fluorescence of Hh:GFP (Chamberlain et al., 2008), distributes most prominently along the basal surface of the neural tube, with highest concentrations ventrally. Hh is also detected apically in the lumen of the neural tube, again with highest concentrations ventrally. These distributions are consistent with the idea that Hh emanates basally from the ventral notochord and then disperses basally along the basal surface of the neural tube. The route that leads Hh to the apical distribution in the neural tube is less obvious and must involve additional steps.
Figure 2. Hh distributions in the neural tube of the mouse embryo and model for signaling in a ciliated mouse cell.
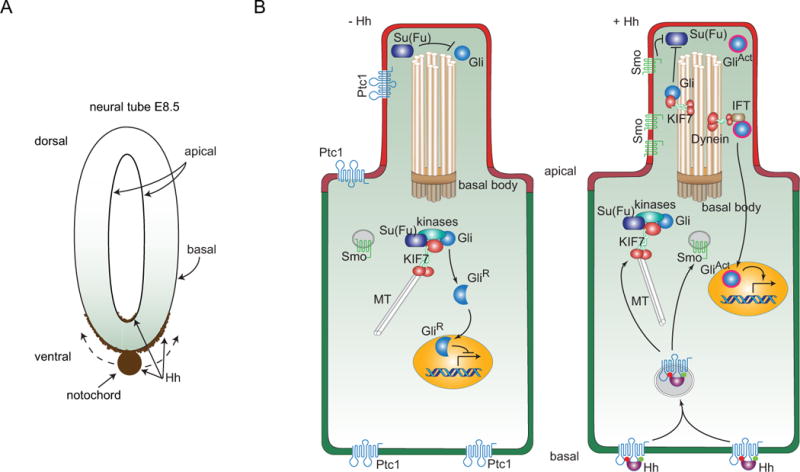
(A) Drawing depitcs Hh expression (brown) in the notochord of an E8.5 mouse embryo. Hh expressed in the notochord moves dorsally and distributes along the basal surface of the neural tube; some accumulates at the apical surface. (B) A subset of the components involved in Hh signal transduction is depicted in a cell prior to receipt of Hh (−Hh; left), and in a cell active for Hh signal transduction (+ Hh; right). The primary cilium contains a 9+0 bundle of doublet microtubules that extend from a basal body, and ferry components of Hh signal transduction with microtubule-binding KIF7 and Dynein motor proteins. The Ptc1 receptor (blue) is present in the apical plasma membrane, and in in non-signaling cells, in the plasma membrane of the primary cilium. Smo is only present in the plasma membrane of the primary cilium in Hh-signaling cells. In non-signaling cells, Smo is in intracellular vesicles and Gli is present both in the primary cilium where its inactive state is Su(Fu)-dependent and in the cytoplasm. In the cytoplasm it is associated with KIF7, Su(Fu) and several kinases and is processed to a proteolyzed repressor form that translocates to the nucleus. In signaling cells, shown as receiving Hh at the basal membrane, intracellular vesicles containing Ptc1 and Hh form, the process that generates GliR is inhibited, and in the primary cilium, Su(Fu)-dependent inactivation of Gli is inhibited and the transcriptional activator form of Gli is generated.
Steady state distributions may suggest a path from producing to recipient cells, but they can be misleading if a route is indirect and if intermediate steps are rate-limiting. The steady state distributions of Hh in the neural tube do not distinguish whether the apical accumulation forms from Hh taken up from a basal pool, or if it arrives by a different route. Chamberlain et al (Chamberlain et al., 2008) propose a version of the latter – an independent route that involves endocytic uptake by ventral midline cells, transcytosis to their apical surface, and release. Dispersion within the apical lumen might then generate the observed apical concentration gradient and might provide access to the primary cilia, which are exclusively at the apical surface. Although there are no kinetic data that either support or invalidate this model, a process that distributes Hh apically is attractive given the presence of Ptc1, Smo and Gli in the primary cilia of a variety of cell types. (Corbit et al., 2005; Haycraft et al., 2005; Rohatgi et al., 2007). Nevertheless, there are several considerations that are consistent with a different model in which all Hh uptake is basal.
Although histological studies have shown that Ptc1 is enriched at the primary cilium in mammalian cultured cells (Rohatgi et al., 2007), suitable antibody reagents have not been available to directly monitor Ptc1 distributions in the neural tube or in other tissues. Nevertheless, even if the studies of cultured cells can be assumed to be relevant, enrichment of Ptc1 in the cilia of the neural tube and other tissues is not evidence that Hh is received at cilia because intracellular trafficking may move Ptc1 between various locations and these trafficking pathways may not be obvious from steady state distributions. Certainly if Hh first encounters Ptc1 at the basal membrane, an intracellular transport pathway that moves Hh from the basal compartment to the apical surface is implied (Fig. 2B), and if Hh transport within the cell, either as a complex with Ptc1 or together with co-receptors (e.g. Cdon (cell-adhesion-molecule-related), Boc (bioregional Cdon-binding protein) and Gas1 (Growth arrest specific 1) proteins (Izzi et al., 2011)), is rapid relative to the basal and apical residence times, the relationship between these pools will not be apparent in the steady state distributions. Polarized cells have efficient pathways to selectively move proteins between basal and apical compartments. In the Drosophila wing disc, steady state distributions have led to proposals that Hh spreads apically (Ayers et al., 2010; Eugster et al., 2007), kinetic studies show that Hh-expressing cells first insert Hh into the apical membrane where it is exposed to the external environment, but it is not released (Fig. 3A). Rather, it is internalized, packaged into endocytic vesicles and moved to the basolateral compartment for export (Callejo et al., 2011). The key point is that the Drosophila system offers a precedent for intracellular transcytosis of Hh, and for an indirect pathway of Hh movement and processing. If the vertebrate systems also move Hh between intracellular compartments, then the site where signal transduction is activated may not be the site of uptake, and if Hh is taken up basally, the primary cilium is not the site of Hh reception. It is worth noting that the primary cilia of cells in the paraxial mesenchyme of mouse embryos also do not orient toward the cells in the notochord that produce the Hh they receive (Rohatgi et al., 2007).
Figure 3. Model for Hh signaling and distribution in polarized Drosophila disc cells.
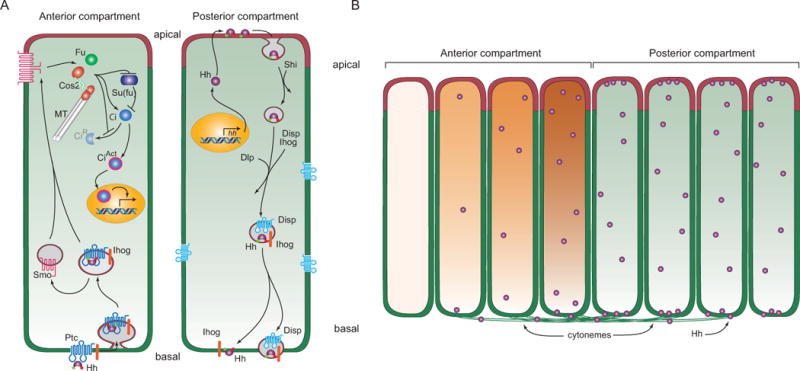
(A) Drawing depicts a model of production and signal transduction in Hh-producing Posterior compartment and Hh-responding Anterior compartment cells. In Hh-producing cells, Hh (purple) that has lipid modifications (red and green circles) is packaged in vesicles that invaginate in a Shibire-dependent (Shi) process from the apical plasma membrane. These vesicles incorporate Ihog (orange) and Dispatched (Disp, blue) and deliver vesicular Hh to the basal plasma membrane. Hh is taken up basally by Hh-responding cells, generating vesicles that contain Ptc and Ihog. Smo redistributes from intracellular vesicles to the plasma membrane and interacts with components of Hh signal transduction (Fused (Fu), microtube (MT)-associated Costal-2 (Cos2) and Su(fu)) to both inhibit production of the repressor form of Ci (CiR) and induce production of the activator form of Ci (CiAct). (B) Drawing depicts an epithelium with Hh-producing (right, green) and Hh-responding (left, orange) cells, and Hh transport (left to right) from Posterior compartment Hh-producing cells by basal cytonemes to Anterior compartment Hh-responding cells. Both the concentration gradient of Hh (purple spheres) and the concentration-dependent response (orange) in the Anterior cells are represented.
Most Drosophila cells do not have primary cilia, but because most components of Drosophila Hh signaling are homologous to vertebrate counterparts, it seems likely that Drosophila has a functionally analogous way to organize them (Figs. 2B, 3A). The principal output of the Drosophila Hh signal transduction pathway is Ci activity, which is homologous to the Gli transcription factors (von Mering and Basler, 1999). Prior to activation, Ci is part of a complex of cytoplasmic proteins (Aza-Blanc et al., 1997; Robbins et al., 1997), one of which is Costal-2, a kinesin-related microtubule binding protein (reviewed in Hooper and Scott, 2005). Costal-2 mobility is essential for Ci activation and signaling (Farzan et al., 2008). As part of this complex, and presumably tethered in some manner to the cytoskeleton (Robbins et al., 1997), Ci activation is regulated by Hh (Aza-Blanc et al., 1997; Methot and Basler, 1999). Histological studies have not identified an intracellular location for the Ci cytoplasmic complex, but this result may be attributable to the fact that at least some of the components of the complex are present in 10-300X molar excess relative to Smo (Farzan et al., 2009) – if some fraction is a functionally distinct sub-population that is localized within the cells, currently available reagents may not distinguish them. In Drosophila cells that are activated by Hh, Ptc redistributes from the plasma membrane to internal vesicles and Smo redistributes from internal cytoplasmic stores to the cell membrane. Smo also associates with Costal-2 (Lum et al., 2003; Ogden et al., 2003; Ruel et al., 2003), together with Ptc, Ci and other components of Hh signal transduction (Hooper and Scott, 2005; Ogden et al., 2004). These behaviors of Ptc and Smo are similar to those of the homologous vertebrate Ptc1 and Smo proteins.
In vertebrate cells, Ptc1 cycles between the primary cilium and the plasma membrane, and in cells that are not exposed to Hh, some Ptc1 is membrane-associated at the primary cilium and most Smo has a cytoplasmic distribution. Upon stimulation by Hh, however, the relative distributions of Ptc1 and Smo reverse, and Smo concentrates at the tubulin-based primary cilium (Corbit et al., 2005; Rohatgi et al., 2007). Presumably, Smo has joined a tethered Hh signaling complex that includes the Gli transcription factors and other components. The homologies between the two systems are clearly extensive despite the absence of a primary cilium in most fly cells.
If vertebrates have adopted the primary cilium as a site to house the Hh signaling machinery, what might the attributes of the primary cilium be that contribute to Hh signal transduction? One possibility is the composition of the ciliary membrane, which is specialized and may change in response to Hh (Chailley and Boisvieux-Ulrich, 1985; Senin et al., 2004; Tyler et al., 2009). The extreme curvature of the ciliary membrane will require distinct lipids in the inner and outer leaflets, and both leaflets should have compositions that are distinct from the lipids of the plasma membrane elsewhere. The cholesterol and lipid modifications of Hh presumably dictate its associations and movements, and are responsible for its presence in lipid rafts (Rietveld et al., 1999). The “Hh barcoding model”, which proposes that the role of Hh’s cholesterol modification is to target Hh to an intracellular, vesicular trafficking pathway, is based on this idea (Kornberg, 2011a). If Smo is also sensitive to lipid composition, the lipids of the primary cilium must be favorable for both Hh and activated Smo, and recent evidence indicates that the association of Smo with lipid rafts is essential for Smo activation and Hh signal transduction (Shi et al., 2013). It is possible that Smo’s affinity for particular lipids changes and/or that the lipids in the primary cilium change upon activation.
Lipid-dependent localization of Hh signal transduction components has several implications that are relevant here. For one, it would limit the interface between primary cilium lipids and plasma membrane lipids to the base of the cilium such that the circumference of the interface would be independent of the length of the primary cilium and the size of its specialized lipid domain. It is interesting to note that while the diameter of primary cilia appear to be constant, the lengths of primary cilia vary, and primary cilia of the floor plate, whose cells are closest to the source of Hh, are longer than primary cilia of cells that are farther away (Cruz et al., 2010).
Second, for cells that lack primary cilia, the model for lipid-dependent localization suggests that Hh signal transduction may adopt a region of the plasma membrane that has similar composition and confers similar functionality as the membrane of the primary cilium. Studies that have the resolution and sensitivity to follow Hh signaling in real time may identify such regions in these cells.
And third, the idea that cells have a specialized region to process a response to Hh also implies that the arrival of Hh from an exogenous source may occur elsewhere. Current evidence suggests that Hh movement and uptake is mediated by cytonemes independently of primary cilia or association with microtubules (Fig. 3B). Cytonemes make synaptic contacts and ferry signaling proteins between signaling cells (Kornberg and Roy, 2014a, b; Roy et al., 2014). They are actin-based filopodia and do not contain microtubules (Ramírez-Weber and Kornberg, 1999). In Drosophila, cytonemes that extend from Hh-producing cells as well as cytonemes that extend from Hh-receiving cells have been implicated in Hh transport and signaling (Bilioni et al., 2012; Bischoff et al., 2013; Callejo et al., 2011; Chen and Kornberg, 2014), and there is evidence that cytonemes that extend from Hh-producing cells in chick embryos transport Hh to target cells (Sanders et al., 2013). In the Drosophila wing disc, cytonemes that emanate from Hh-receiving cells extend along the basal surface of the polarized epithelium, mediating the exchange and movement of Hh that is released basally from producing cells (Bischoff et al., 2013; Chen and Kornberg, 2014). Thus, whereas Hh processing and modification are associated with the apical compartment of producing cells, export and transport are basal (Callejo et al., 2011; Kornberg, 2011a). Given the microtubule association of Costal-2 and other components of Hh signal transduction (Fig. 3A) (Hooper and Scott, 2005; Robbins et al., 1997), it seems reasonable to suggest that these proteins are not present in cytonemes and that cytonemes do not function directly in signal transduction. Hh processing, export and transport have not been as well characterized in vertebrate systems such as the neural tube, and have not been carried out in conditions that might detect cytonemes. Nevertheless, it seems likely that transport and signal transduction are similarly segregated in this system.
These arguments are predicated on the idea that despite the importance of the primary cilium to Hh signaling, ciliated and non-ciliated cells use similar mechanisms for Hh transport, uptake and signal transduction. This proposition is consistent with a recent study of Hh signaling in ciliated Drosophila cells (Kuzhandaivel et al., 2014). As noted above, most Drosophila cells do not have primary cilia, but olfactory sensory neurons (OSNs) in the Drosophila antenna have non-motile cilia that not only structurally resemble mammalian primary cilia (Jana et al., 2011; Keil, 2012), but also function similarly in Hh signaling (Kuzhandaivel et al., 2014). The OSN cilia localize Ptc and Smo, requiring the intraflagellar transport system and the kinesin-like Cos2. These findings show that the key distinction is not the functionality of the signal transduction components – the same proteins are essential for Hh signaling in both OSNs and non-ciliated Drosophila cells – but the role of the primary cilium. How, then, to account for the critical importance of primary cilia only in ciliated cells? The answer may be found in the process that directs the signal transduction components to either the primary cilium (in ciliated cells) or to a different site in non-ciliated cells. If mutant cells that lack functional primary cilia nevertheless target signal transduction components to an address that is not present, Hh signaling system may be compromised. This model is consistent with the finding that mouse embryo fibroblasts have both primary cilium-independent and primary cilium-dependent responses to Hh, and that primary cilium-dependent responses are impaired in cells with a mutant Smo that can activate the primary cilium-independent but does not localize to the primary cilium (Bijlsma et al., 2012).
Closing
In cells that have a primary cilium, Hh signaling is dependent on normal ciliary function. The issue that has been addressed here is the nature of this requirement – whether the primary cilium has two distinct roles – as an organelle that receives Hh from an extracellular source and as a site for signal transduction components. The argument is that it does not, that similar processes take up Hh in ciliated and non-ciliated cells but that the sites for signal processing differ in these types of cells. The primary cilium may be one way to organize the signal transduction components in a favorable environment.
The roles of primary cilia and cytonemes in Hh signaling are compared and contrasted.
Cytonemes are organelles that move Hh between Hh-producing and Hh-receiving cells.
I propose that primary cilia do not receive Hh directly from producing cells.
I propose that primary cilia are specialized lipid domains for Hh signal transduction components.
Acknowledgments
I thank S. Roy, R. Rohatgi, D. Robbins, K. Simon, H. Roelink, B. Shilo, P-T Chuang, and J. Reiter for helpful comments and discussions, and acknowledge funding from NIH GM030637 and GM105987.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayers KL, Gallet A, Staccini-Lavenant L, Therond PP. The long-range activity of Hedgehog is regulated in the apical extracellular space by the glypican Dally and the hydrolase Notum. Developmental cell. 2010;18:605–620. doi: 10.1016/j.devcel.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Bijlsma MF, Damhofer H, Roelink H. Hedgehog-stimulated chemotaxis is mediated by smoothened located outside the primary cilium. Science signaling. 2012;5:ra60. doi: 10.1126/scisignal.2002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilioni A, Sanchez-Hernandez D, Callejo A, Gradilla AC, Ibanez C, Mollica E, Carmen Rodriguez-Navas M, Simon E, Guerrero I. Balancing hedgehog, a retention and release equilibrium given by Dally, Ihog, Boi and shifted/dWif. Dev Biol. 2012 doi: 10.1016/j.ydbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Bischoff M, Gradilla AC, Seijo I, Andres G, Rodriguez-Navas C, Gonzalez-Mendez L, Guerrero I. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat Cell Biol. 2013;15:1269–1281. doi: 10.1038/ncb2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejo A, Bilioni A, Mollica E, Gorfinkiel N, Andres G, Ibanez C, Torroja C, Doglio L, Sierra J, Guerrero I. Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1106881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chailley B, Boisvieux-Ulrich E. Detection of plasma membrane cholesterol by filipin during microvillogenesis and ciliogenesis in quail oviduct. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1985;33:1–10. doi: 10.1177/33.1.3965567. [DOI] [PubMed] [Google Scholar]
- Chamberlain CE, Jeong J, Guo C, Allen BL, McMahon AP. Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development. 2008;135:1097–1106. doi: 10.1242/dev.013086. [DOI] [PubMed] [Google Scholar]
- Chen W, Kornberg TB. Dispersion via cytonemes: how the Hedgehog gradient forms in the Drosopohila wing imaginal disc. 55th Annual Drosophila Research Conference; San Diego, CA. 2014. [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Cruz C, Ribes V, Kutejova E, Cayuso J, Lawson V, Norris D, Stevens J, Davey M, Blight K, Bangs F, Mynett A, Hirst E, Chung R, Balaskas N, Brody SL, Marti E, Briscoe J. Foxj1 regulates floor plate cilia architecture and modifies the response of cells to sonic hedgehog signalling. Development. 2010;137:4271–4282. doi: 10.1242/dev.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster C, Panakova D, Mahmoud A, Eaton S. Lipoprotein-heparan sulfate interactions in the Hh pathway. Developmental cell. 2007;13:57–71. doi: 10.1016/j.devcel.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Ascano M, Jr, Ogden SK, Sanial M, Brigui A, Plessis A, Robbins DJ. Costal2 functions as a kinesin-like protein in the hedgehog signal transduction pathway. Curr Biol. 2008;18:1215–1220. doi: 10.1016/j.cub.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Stegman MA, Ogden SK, Ascano M, Jr, Black KE, Tacchelly O, Robbins DJ. A quantification of pathway components supports a novel model of Hedgehog signal transduction. J Biol Chem. 2009;284:28874–28884. doi: 10.1074/jbc.M109.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field S, Riley KL, Grimes DT, Hilton H, Simon M, Powles-Glover N, Siggers P, Bogani D, Greenfield A, Norris DP. Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development. 2011;138:1131–1142. doi: 10.1242/dev.058149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nature reviews Genetics. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Developmental cell. 2011;20:788–801. doi: 10.1016/j.devcel.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Briscoe J. Gli proteins and the control of spinal-cord patterning. EMBO Rep. 2003;4:761–765. doi: 10.1038/sj.embor.embor896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana SC, Girotra M, Ray K. Heterotrimeric kinesin-II is necessary and sufficient to promote different stepwise assembly of morphologically distinct bipartite cilia in Drosophila antenna. Molecular biology of the cell. 2011;22:769–781. doi: 10.1091/mbc.E10-08-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura K, Kobayashi D, Uehara Y, Koshida S, Iijima N, Kudo A, Yokoyama T, Takeda H. Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left-right axis. Development. 2011;138:1121–1129. doi: 10.1242/dev.058271. [DOI] [PubMed] [Google Scholar]
- Keil TA. Sensory cilia in arthropods. Arthropod structure & development. 2012;41:515–534. doi: 10.1016/j.asd.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Kornberg TB. Barcoding Hedgehog for intracellular transport. Science signaling. 2011a;4:pe44. doi: 10.1126/scisignal.2002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg TB. Direct delivery mechanisms of morphogen dispersion. Sci Signal. 2011b;4:pt8. doi: 10.1126/scisignal.2002434. [DOI] [PubMed] [Google Scholar]
- Kornberg TB, Roy S. Communicating by touch – neurons are not alone. Trends in cell biology. 2014a doi: 10.1016/j.tcb.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg TB, Roy S. Cytonemes as specialized signaling filopodia. Development. 2014b;141:729–736. doi: 10.1242/dev.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhandaivel A, Schultz SW, Alkhori L, Alenius M. Cilia-Mediated Hedgehog Signaling in Drosophila. Cell reports. 2014 doi: 10.1016/j.celrep.2014.03.052. [DOI] [PubMed] [Google Scholar]
- Lum L, Zhang C, Oh S, Mann RK, von Kessler DP, Taipale J, Weis-Garcia F, Gong R, Wang B, Beachy PA. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell. 2003;12:1261–1274. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Methot N, Basler K. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell. 1999;96:819–831. doi: 10.1016/s0092-8674(00)80592-9. [DOI] [PubMed] [Google Scholar]
- Ogden SK, Ascano M, Jr, Stegman MA, Robbins DJ. Regulation of Hedgehog signaling: a complex story. Biochemical pharmacology. 2004;67:805–814. doi: 10.1016/j.bcp.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden SK, Ascano M, Jr, Stegman MA, Suber LM, Hooper JE, Robbins DJ. Identification of a functional interaction between the transmembrane protein Smoothened and the kinesin-related protein Costal2. Curr Biol. 2003;13:1998–2003. doi: 10.1016/j.cub.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol. 2002;12:938–943. doi: 10.1016/s0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- Ramírez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- Rietveld A, Neutz S, Simons K, Eaton S. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. Journal of Biological Chemistry. 1999;274:12049–12054. doi: 10.1074/jbc.274.17.12049. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–234. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Roy S, Hsiung F, Kornberg TB. Specificity of Drosophila cytonemes for distinct signaling pathways. Science. 2011 Apr 15;332(6027):354–358. doi: 10.1126/science.1198949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Huang H, Liu S, Kornberg TB. Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science. 2014;343:1244624. doi: 10.1126/science.1244624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel L, Rodriguez R, Gallet A, Lavenant-Staccini L, Therond PP. Stability and association of Smoothened, Costal2 and Fused with Cubitus interruptus are regulated by Hedgehog. Nat Cell Biol. 2003;5:907–913. doi: 10.1038/ncb1052. [DOI] [PubMed] [Google Scholar]
- Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–632. doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senin II, Hoppner-Heitmann D, Polkovnikova OO, Churumova VA, Tikhomirova NK, Philippov PP, Koch KW. Recoverin and rhodopsin kinase activity in detergent-resistant membrane rafts from rod outer segments. J Biol Chem. 2004;279:48647–48653. doi: 10.1074/jbc.M402516200. [DOI] [PubMed] [Google Scholar]
- Shi D, Lv X, Zhang Z, Yang X, Zhou Z, Zhang L, Zhao Y. Smoothened oligomerization/higher order clustering in lipid rafts is essential for high Hedgehog activity transduction. J Biol Chem. 2013;288:12605–12614. doi: 10.1074/jbc.M112.399477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, Hazlett TL, Engman DM. Flagellar membrane localization via association with lipid rafts. Journal of cell science. 2009;122:859–866. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mering C, Basler K. Distinct and regulated activities of human Gli proteins in Drosophila. Current Biology. 1999;9:1319–1322. doi: 10.1016/s0960-9822(00)80054-8. [DOI] [PubMed] [Google Scholar]


