Abstract
Despite the high prevalence and consequences associated with externalizing psychopathologies, little is known about their underlying neurobiological mechanisms. Studying multiple externalizing disorders, each characterized by compromised inhibition, could reveal both common and distinct mechanisms of impairment. The present study therefore compared individuals with intermittent explosive disorder (IED) (N=11), individuals with cocaine use disorder (CUD) (N=21), and healthy controls (N=17) on task performance and functional magnetic resonance imaging (fMRI) activity during an event-related color-word Stroop task; self-reported trait anger expression was also collected in all participants. Results revealed higher error-related activity in the two externalizing psychopathologies as compared with controls in two subregions of the dorsolateral prefrontal cortex (DLPFC) (a region known to be involved in exerting cognitive control during this task), suggesting a neural signature of inhibitory-related error processing common to these psychopathologies. Interestingly, in one DLPFC subregion, error-related activity was especially high in IED, possibly indicating a specific neural correlate of clinically high anger expression. Supporting this interpretation, error-related DLPFC activity in this same subregion positively correlated with trait anger expression across all participants. These collective results help to illuminate common and distinct neural signatures of impaired self-control, and could suggest novel therapeutic targets for increasing self-control in clinical aggression specifically and/or in various externalizing psychopathologies more generally.
Keywords: intermittent explosive disorder, aggression, cocaine addiction, inhibitory control, functional magnetic resonance imaging, Stroop, State-Trait Anger Expression Inventory
INTRODUCTION
Failures of self-control characterize many neuropsychiatric disorders, manifesting as chronic and relapsing behavioral tendencies that contribute to poor physical and mental health and evoke serious public health concerns. For example, drug addiction is a well-studied neuropsychiatric disorder marked by persistent neurocognitive dysfunction, including excessive salience attributed to drugs and drug-related stimuli (Luijten et al., 2013), disadvantageous and impulsive decision-making (Paulus, 2007), poor behavioral adaptation (Salo et al., 2009), and dysregulated inhibitory control (Kalivas and Volkow, 2005). Another externalizing disorder, far less studied but similarly marked by behavioral dysregulation, is intermittent explosive disorder (IED). As a clinical disorder, IED is defined by recurrent episodes of aggression and violence in response to disproportionately low provocation (Coccaro, 2004). Such reactive aggression has been associated with high emotional arousal in tandem with poor cortically-mediated response inhibition to halt a prepotent aggressive tendency (Raine et al., 1998, Siever, 2008). Thus, here we focus on both addiction and IED as clinical entities marked by Impaired Response Inhibition and Salience Attribution (iRISA) (Goldstein and Volkow, 2002, 2011), an empirically supported and theoretically-bound model of addiction that has mapped core self-regulatory dysfunction onto aberrant corticostriatal circuitry. Studying both of these externalizing disorders can provide novel neurobehavioral signatures of compromised inhibitory control, which have the potential to enrich our understanding of a broad spectrum of neuropsychiatric disorders characterized by iRISA.
To focus our efforts in this initial study, we concentrated on response inhibition. Participants performed an event-related color-word Stroop task (Stroop, 1935), a classical cognitive paradigm of conflict- and error-related processing, while undergoing functional magnetic resonance imaging (fMRI). This task consistently engages select prefrontal cortical subregions that participate in response inhibition and cognitive control, including the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) (Carter and van Veen, 2007, Kerns et al., 2004, MacDonald et al., 2000, Roberts and Hall, 2008) – regions that are also perturbed in addiction and aggression. Indeed, numerous studies have documented abnormalities in the ACC and DLPFC as underlying core neurocognitive dysfunctions in drug addiction (Goldstein and Volkow, 2011). These regions also show reactivity to laboratory aggression challenges in healthy individuals (Denson et al., 2009, New et al., 2009). Importantly, a meta-analyses revealed functional and structural deficits of the right ACC and left DLPFC [and the orbitofrontal cortex (OFC)] in aggressive/antisocial individuals compared with healthy individuals or psychiatric controls (Yang and Raine, 2009). Specifically, the color-word Stroop task (and related Stroop task variants) has been previously used to probe the functioning of the ACC, DLPFC, and other regions comprising the frontoparietal network, tapping into the core phenomenology of drug addiction as further associated with clinical outcome (Barros-Loscertales et al., 2011, Brewer et al., 2008, Devito et al., 2012, Mayer et al., 2013, Moeller et al., 2014a, Worhunsky et al., 2013). However, we are not aware of any prior studies that used the color-word Stroop task in IED, an understudied impulse control disorder.
Here, we hypothesized that group differences [i.e., between healthy controls versus individuals with IED and/or individuals with cocaine use disorder (CUD)] would emerge in PFC regions that are typically engaged by the color-word Stroop task, and that are impaired in these psychopathologies (e.g., ACC, DLPFC). Based on our prior experience (Moeller, Honorio, 2014a), we expected especially robust PFC group effects during error-related processing (i.e., instead of Stroop conflict processing), whereby a more optimized (i.e., reduced) response would characterize the healthy controls versus IED and CUD. We further expected that IED and CUD would show comparable hyperactivity of these regions, possibly indicating a common neural signature of compromised inhibitory/PFC functioning. Finally, beyond comparing IED and CUD, we also probed for regions that would uniquely track pathological aggression (i.e., abnormal activations specific to IED) – regions that we further hypothesized would correlate with trait anger expression.
METHODS
Participants
Participants were 17 healthy controls, 11 individuals with IED, and 21 individuals with CUD, recruited from local newspaper- and website advertisements, and by word-of mouth. All were male, right-handed, native English speakers, not currently taking medications, and able to understand all study procedures and provide written consent in accordance with Stony Brook University’s Institutional Review Board. Exclusion criteria were: (A) history of head trauma or loss of consciousness (> 30 min) or other neurological disease of central origin (including seizures); (B) abnormal vital signs; (C) history of major medical conditions, encompassing cardiovascular (including high blood pressure), endocrinological (including metabolic), oncological, or autoimmune diseases; (E) contraindications to MRI; and (F) except for cocaine in CUD, positive urine screens for psychoactive drugs or their metabolites (10/21 CUD participants tested positive for cocaine on the day of scanning). Eleven participants (7 controls, 4 IED, 0 CUD) were drawn from a study (unpublished) during which they received a single-dose challenge of methylphenidate or counterbalanced placebo during fMRI (for details of methylphenidate administration, and analyses showing this procedural difference did not drive our effects, see Supplementary Data). None of the study participants demonstrated or endorsed signs or symptoms of intoxication from alcohol (determined by breathalyzer) or cocaine (determined by trained study staff).
Participants underwent a comprehensive clinical interview, consisting of: (A) Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1996, Ventura et al., 1998); (B) Addiction Severity Index (ASI) (McLellan et al., 1992); (C) Cocaine Selective Severity Assessment Scale (Kampman et al. , 1998); (D) Severity of Dependence Scale (Gossop et al. , 1992); (E) Cocaine Craving Questionnaire (Tiffany et al., 1993); (F) Structured Clinical Interview for Axis II personality disorders, specifically of Cluster B; (G) assessment for Intermittent Explosive Disorder, which enables IED diagnosis according to DSM-IV criteria (Coccaro, 2004); and (H) Life History of Aggression, which tallies the amount of aggressive behavior across the lifespan (Coccaro et al., 1997). Because components F-H were not administered to this sample of CUD (i.e., these procedures were not yet in place for these individuals), alternative measures/interviews were examined in their stead (see Supplementary Data for these measures and analyses, which provide evidence that these CUD were dispositionally non-aggressive).
Based on this interview, all IED participants met criteria for intermittent explosive disorder (current: N=7; remitted: N=4), all CUD participants met criteria for cocaine use disorder (current: N=17; remitted: N=4) (Table 1, which also shows that the groups did not differ on remission status), and no IED participants met criteria for cocaine use disorder. We allowed IED into the study who had comorbid substance use disorders (SUDs), which occurred for 4/11 IED, because of the high overlap of these two disorders in the general population (approximately 35% in adults) (Kessler et al., 2006), enhancing generalizability of our results; nevertheless, we accounted for the presence of comorbid SUDs in the Supplementary Data. Also of importance, IED and CUD did not differ on current or lifetime comorbidities (Table 1) (see Supplementary Data for specific comorbidities in each group). No controls met criteria for either disorder, which is an important consideration for comparison purposes (i.e., we did not expect IED and CUD to differ from one another, but both psychopathologies to differ from controls). Across all groups, 27 participants had a history of smoking, and 23 participants were current smokers (Table 1).
Table 1.
Demographics, trait aggression, drug use, and task behavior of the study sample.
| Healthy Controls (N=17) |
Intermittent Explosive Disorder (N=11) |
Cocaine Use Disorder (N=21) |
Group Effect (without covariates) |
P (without covariates) |
Group Effect (with covariates)a |
P (with covariates)a |
Source of Omnibus Group Effect |
|
|---|---|---|---|---|---|---|---|---|
| Age (years) | 32.6 ± 6.4 | 33.5 ± 7.1 | 43.2 ± 6.5 | F=14.6 | <0.001 | --- | --- | CUD > HC + IED |
| Race (African-American/Other) | 7/10 | 6/5 | 15/6 | Χ2=3.6 | 0.17 | --- | --- | --- |
| Education (years) | 13.5 ± 1.4 | 13.5 ± 1.7 | 13.0 ± 2.0 | F=0.5 | 0.64 | --- | --- | --- |
| Verbal IQ: Wide Range Achievement Test III – Reading Scale |
105.6 ± 10.1 | 96.9 ± 16.6 | 96.9 ± 8.9 | F=2.9 | 0.063 | --- | --- | --- |
| Nonverbal IQ: Wechsler Abbreviated Scale of Intelligence – Matrix Reasoning Scale |
9.9 ± 3.4 | 10.4 ± 3.0 | 10.4 ± 2.4 | F=0.1 | 0.88 | --- | --- | --- |
| Depressive Symptoms: Beck Depression Inventory II |
2.3 ± 3.1 | 6.4 ± 5.1 | 5.4 ± 5.0 | F=3.1 | 0.054 | --- | --- | --- |
| Cigarette Smoking History (No/Yes) | 13/4 | 6/5 | 3/18 | Χ2=15.2 | <0.001 | --- | --- | CUD ≠ HC, IED |
| Current Cigarette Smoker (No/Yes) | 13/4 | 7/4 | 6/15 | Χ2=9.3 | 0.010 | --- | --- | CUD ≠ HC |
| Cigarettes Per Day (Current Smokers: N=23) | 5.5 ± 4.4 | 5.5 ± 1.3 | 7.9 ± 5.4 | F=0.6 | 0.55 | --- | --- | --- |
| Years of Alcohol Use | 8.2 ± 6.4 | 10.6 ± 9.9 | 18.8 ± 10.2 | F=0.0 | 0.98 | --- | --- | --- |
| Number Alcohol Use Days (Last 30 Days) | 5.2 ± 8.0 | 3.6 ± 5.4 | 6.5 ± 8.3 | F=0.3 | 0.74 | --- | --- | --- |
| Years of Cocaine/Crack Useb | 0.0 ± 0.0 | 0.0 ± 0.0 | 17.8 ± 7.3 | --- | --- | --- | --- | --- |
| Number of Cocaine/Crack Use Days (Last 30 Days)b |
0.0 ± 0.0 | 0.0 ± 0.0 | 9.0 ± 8.7 | --- | --- | --- | --- | --- |
| Presence of Comorbidities, Current (No/Yes) |
--- | 7/4 | 19/2 | Χ2=3.4 | 0.07 | --- | --- | --- |
| Presence of Comorbidities, Lifetime (No/Yes) |
--- | 3/8 | 7/14 | Χ2=0.1 | 0.73 | --- | --- | --- |
| Disease Remission Status (Current/Remitted) |
--- | 7/4 | 17/4 | Χ2=1.2 | 0.28 | |||
| STAXI Anger Expression Out | 12.7 ± 3.4 | 24.2 ± 4.6 | 14.0 ± 3.1 | F=36.5 | <0.001 | F=35.6 | <0.001 | IED > HC, CUD |
| STAXI Anger Expression In | 15.6 ± 5.1 | 21.4 ± 5.3 | 16.3 ± 5.0 | F=4.5 | 0.017 | F=4.9 | 0.012 | IED > HC, CUD |
| Stroop Congruent Percent Correct (Per Run) | 0.88 ± 0.12 | 0.86 ± 0.13 | 0.92 ± 0.06 | Main Effect: F=0.5 |
Main Effect: p=0.63 |
Main Effect: F=1.5 |
Main Effect: 0.23 |
|
| Stroop Incongruent Percent Correct (Per Run) | 0.59 ± 0.18 | 0.57 ± 0.29 | 0.60 ± 0.21 | --- | ||||
| Stroop Incongruent>Congruent Percent Correct (Per Run) |
−0.29 ± 0.19 | −0.29 ± 0.25 | −0.31 ± 0.18 | Interaction: F=0.1 |
Interaction: 0.94 |
Interaction: F=0.3 |
Interaction: 0.77 |
--- |
| Stroop Correct Congruent RT (ms) | 651.4 ± 79.1 | 632.3 ± 67.5 | 691.5 ± 62.7 | Main Effect: F=3.3 |
Main Effect: p=0.047c |
Main Effect: F=0.4 |
Main Effect: 0.68 |
--- |
| Stroop Correct Incongruent RT (ms) | 843.6 ± 122.7 | 846.9 ± 118.8 | 905.8 ± 85.0 | --- | ||||
| Stroop Correct Incongruent>Congruent RT (ms) |
191.9 ± 66.2 | 212.9 ± 83.1 | 221.9 ± 75.9 | Interaction: F=0.8 |
Interaction: 0.47 |
Interaction: F=0.4 |
Interaction: 0.69 |
--- |
| Stroop Incorrect Congruent RT (ms) | 667.1 ± 120.2 | 708.3 ± 106.5 | 663.6 ± 120.5 | Main Effect: F=1.4 |
Main Effect: 0.26 |
Main Effect: F=0.1 |
Main Effect: 0.91 |
------ |
| Stroop Incorrect Incongruent RT (ms) | 710.9 ± 233.8 | 763.8 ± 269.6 | 857.7 ± 186.6 | ------ | ||||
| Stroop Incorrect Incongruent>Congruent RT (ms) |
43.8 ± 202.6 | 72.0 ± 240.3 | 182.5 ± 197.3 | Interaction: F=2.2 |
Interaction: 0.13 |
Interaction: F=1.1 |
Interaction: 0.35 |
--- |
Note. Numbers are frequencies or means ± standard deviation;
covariates were age and cigarette smoking history, which differed among the groups (included in the analyses of trait aggression and task behavior);
ANOVA was not conducted because neither controls nor individuals with intermittent explosive disorder reported cocaine use;
No follow-up comparisons were significant; RT=reaction time, HC = healthy controls, IED = individuals with intermittent explosive disorder, CUD = individuals with cocaine use disorder.
fMRI
Task
Participants performed three runs of an event-related fMRI color word Stroop task, which has been described in detail elsewhere (Moeller, Honorio, 2014a, Moeller et al., 2014b, Moeller et al., 2012). Briefly, participants pressed for ink color of color words printed in their congruent (94% of trials) or incongruent colors (6% of trials, spaced by ≥ 5 congruent stimuli). Each word was presented for 1300 ms, with an intertrial interval of 350 ms. Remuneration for task completion was $25. Accuracy and reaction time (RT) were continuously collected.
MRI Data Acquisition
Magnetic resonance imaging scanning was performed on a 4T whole-body Varian/Siemens MRI scanner. The blood oxygenation level dependent (BOLD)-fMRI responses were measured as a function of time using a T2*-weighted single-shot gradient-echo planar sequence (TE/TR=20/1600 ms, 4 mm slice thickness, 1 mm gap, typically 33 coronal slices, 20 cm FOV, 64×64 matrix size, 90°-flip angle, 200kHz bandwidth with ramp sampling, 207 time points, and 4 dummy scans to avoid non-equilibrium effects in the fMRI signal). Earplugs (28 dB sound attenuation; Aearo Ear TaperFit 2; Aearo Company) and headphones (30 dB sound attenuation; Commander XG MRI Audio System, Resonance Technology Inc.) were used to minimize scanner noise (Tomasi et al., 2005).
MRI Data Processing
Analyses were performed with Statistical Parametric Mapping (SPM8) (Wellcome Trust Centre for Neuroimaging, London, UK). Images were reconstructed by using an iterative phase correction method that produces minimal signal-loss artifacts in echo-planar images (Caparelli and Tomasi, 2008). A six-parameter rigid body transformation (3 rotations, 3 translations) was used for image realignment and correction of head motion. Criteria for acceptable motion were 2 mm displacement and 2° rotation. The realigned datasets were spatially normalized to the standard stereotactic space of the Montreal Neurological Institute (MNI) using a 12-parameter affine transformation (Ashburner et al., 1997) and a voxel size of 3×3×3 mm. An 8-mm full-width-half-maximum Gaussian kernel spatially smoothed the data.
BOLD-fMRI Analyses
Calculation of the fMRI contrasts followed our prior work (Moeller, Honorio, 2014a, Moeller, Konova, 2014b, Moeller, Tomasi, 2012). Briefly, two general linear models (Friston et al. , 1995), each with six motion regressors (3 translation and 3 rotation) and up to three task conditions (incongruent correct events, congruent error events, and/or incongruent error events) convolved with a canonical hemodynamic response function and high-pass filtered (cut-off frequency: 1/90 s), were used to calculate the following 1st Level main effect contrasts: (Incongruent Error + Incongruent Correct) – (Congruent Error + Congruent Correct) (incongruent>congruent); and (B) (Incongruent Error + Congruent Error) – (Incongruent Correct + Congruent Correct) (error>correct).
At the 2nd Level, we tested how each of these 1st Level contrasts differed as a function of group (for exploratory cluster analyses, where groups were formed on the basis of brain activations, see Supplementary Data). We estimated two separate one-way analyses of covariance (ANCOVAs) at the whole-brain level in SPM8: one model for error>correct, one model for incongruent>congruent; for each model, controls and IED were each compared with the other two groups [note that since results of this CUD sample have been previously reported (Moeller, Tomasi, 2012), analyses were not conducted in this group alone]; age and cigarette smoking history, which were higher in CUD than the other groups (Table 1), were included in the models as covariates of no interest to account for their potential contributions to results. Whole-brain fMRI analyses were performed using a height threshold of p=0.005 voxel-level uncorrected (T=2.68). We then used a Monte Carlo procedure (Slotnick et al., 2003) to identify the number of contiguous voxels necessary for a p<0.05 cluster-corrected threshold (i.e., given our imaging parameters and a height threshold of T=2.68), which was calculated to be 26 contiguous voxels. To provide additional confirmation of effects, we also examined the respective conjunction contrasts. For example, if controls showed less activation than both CUD and IED in a particular region, then we also conducted a follow-up conjunction analysis to ensure that both CUD and IED differed from controls in that region – thus speaking against the idea that the effect is driven by one study group. All significant clusters identified in both the whole-brain and conjunction analyses were further extracted and evaluated to identify outliers. These extracted BOLD signals were used in SPSS correlation analyses, testing for their association with select behavioral measures as described in more detail below.
In addition to these main analyses, supplementary analyses were also performed, which together were meant to rule out potential confounds and alternative interpretations (see Supplementary Data for these analyses). In particular, we tested the impact on results of the following considerations: procedural differences including methylphenidate administration, substance use histories in IED, possible aggression in CUD. We also tested whether we could identify study groups based not on their diagnosis, but rather by their BOLD activity.
Brain-Behavior Correlations
We correlated the extracted fMRI-BOLD signals with five dependent measures of interest: on the color-word Stroop task, (A) average task errors, (B) average RT for correct trials, and (C) average RT for error trials; and on the reliable, well-validated State-Trait Anger Expression Inventory (STAXI-2) (Spielberger, 1988) (Table 1), the (D) Anger Expression-Out (AX-O) and (E) Anger Expression-In (AX-I) subscales. The AX-O subscale measures outward aggression against others and destruction of property; the AX-I subscale measures internalization or suppression of anger. In the current sample, these two subscales were moderately correlated across all participants (r=0.52, p<0.001), showing that these variables are related yet unique (i.e., thus supporting their separate inspection). Correlation analyses were considered significant at p<0.01 to minimize Type I error, and with these five variables corrected for age and cigarette smoking history.
RESULTS
Behavior (Table 1)
Task
Percent accuracy and RT (the latter inspected separately for error and correct trials) were each analyzed using 2 (Trial: congruent, incongruent) × 3 (Diagnosis: control, IED, CUD) mixed ANCOVAs, controlling for age (continuous) and cigarette smoking history (smoker, nonsmoker). There were no main effects or interactions for percent accuracy (p>0.081). For RT on correct or incorrect trials, there was only a main effect of Trial on correct trials, revealing the reliable Stroop interference effect [incongruent>congruent; F(1,44)=11.57, p=0.001].
Trait Anger
Two separate one-way ANCOVAs, which similarly controlled for age and cigarette smoking history, revealed differences among the groups on the STAXI AX-O and AX-I subscales [F(2,41)>4.77, p<0.014]: IED reported more anger expression (STAXI AX-O) and internalization (STAXI AX-I) than either CUD or controls (Table 1); CUD and controls did not differ.
fMRI (Table 2)
Table 2.
Color-word Stroop between-group differences during error and conflict.
| Region | BA | Side | Voxels | Peak T | Uncorrected P | x | y | z |
|---|---|---|---|---|---|---|---|---|
| TASK ERROR (ERROR>CORRECT) | ||||||||
| Control > IED, CUD | ||||||||
| ---- | ||||||||
| Control < IED, CUD | ||||||||
| Middle Frontal Gyrus: DLPFC | 9,46 | L | 70 | 4.2 | <0.001 | −39 | 47 | 28 |
| −30 | 59 | 22 | ||||||
| Anterior Cingulate | 32 | L | 29 | 3.6 | <0.001+ | −6 | 23 | 37 |
| Cerebellum | -- | 62 | 4.5 | <0.001+ | 21 | −58 | −44 | |
| IED > Control, CUD | ||||||||
| Superior Frontal Gyrus: DLPFC | 9 | L | 26 | 3.9 | <0.001 | −15 | 59 | 34 |
| IED < Control, CUD | ||||||||
| ---- | ||||||||
| TASK CONFLICT (INCONGRUENT>CONGRUENT) | ||||||||
| Control > IED, CUD | ||||||||
| Supramarginal Gyrus | 40 | R | 146 | 4.8 | <0.001 | 51 | −34 | 31 |
| Inferior Parietal Lobule | 40 | L | 60 | 4.0 | <0.001+ | −54 | −46 | 46 |
| Control < IED, CUD | ||||||||
| Superior Temporal Gyrus | 13 | R | 33 | 3.5 | <0.001+ | 45 | −10 | 1 |
| Hippocampus | -- | L | 27 | 3.4 | 0.001 | −27 | −25 | −5 |
| IED < Control, CUD | ||||||||
| ---- | ||||||||
| IED < Control, CUD | ||||||||
| ---- | ||||||||
Note. Analyses are one-way ANCOVAs (controlling for age and cigarette smoking history), thresholded at p<0.05 corrected (p<0.005 voxel-uncorrected and 26 voxels); all effects were also significant using follow-up conjunction analysis (p<0.01, two-tailed); +does not survive correction for history of substance use disorder (see Supplementary Data for analyses); BA=Brodmann Area; R=right, L=left, B=bilateral. As this sample of cocaine subjects has been previously reported, we did not analyze these individuals as a stand-alone group.
Error>Correct
Whole-brain SPM8 analyses revealed that the two psychopathologies of impaired self-control (IED and CUD), compared with healthy controls, had increased error>correct activations in the DLPFC (Figures 1A–C) (Table 2). IED and CUD also had more error>correct activity than controls in the left ACC and right cerebellum (Table 2). A conjunction analysis of the contrasts ‘control versus IED’ and ‘control versus CUD’ revealed overlap in the DLPFC, ACC, and cerebellum, showing that both IED and CUD had more error>correct activity in these regions than controls. However, the ACC and cerebellum results did not survive correction for SUD (Table 2) (Supplementary Data). A conjunction analysis of the contrasts ‘IED versus control’ and ‘IED versus CUD’ revealed significant overlap in the DLPFC, showing that IED had more error>correct activity in this region than the other groups.
Figure 1.
Regions showing error-related group differences between healthy controls, individuals with intermittent explosive disorder (IED), and individuals with cocaine use disorder (CUD). Higher percent blood-oxygenation-level dependent (BOLD) signal to the contrast error>correct was observed in CUD versus controls and IED versus controls in the (A-C) dorsolateral prefrontal cortex (DLPFC). (D) In the slightly more anterior and medial DLPFC region, error>correct activity also positively correlated with outward anger expression across participants. Images are in neurological view (left=left).
Incongruent>Congruent
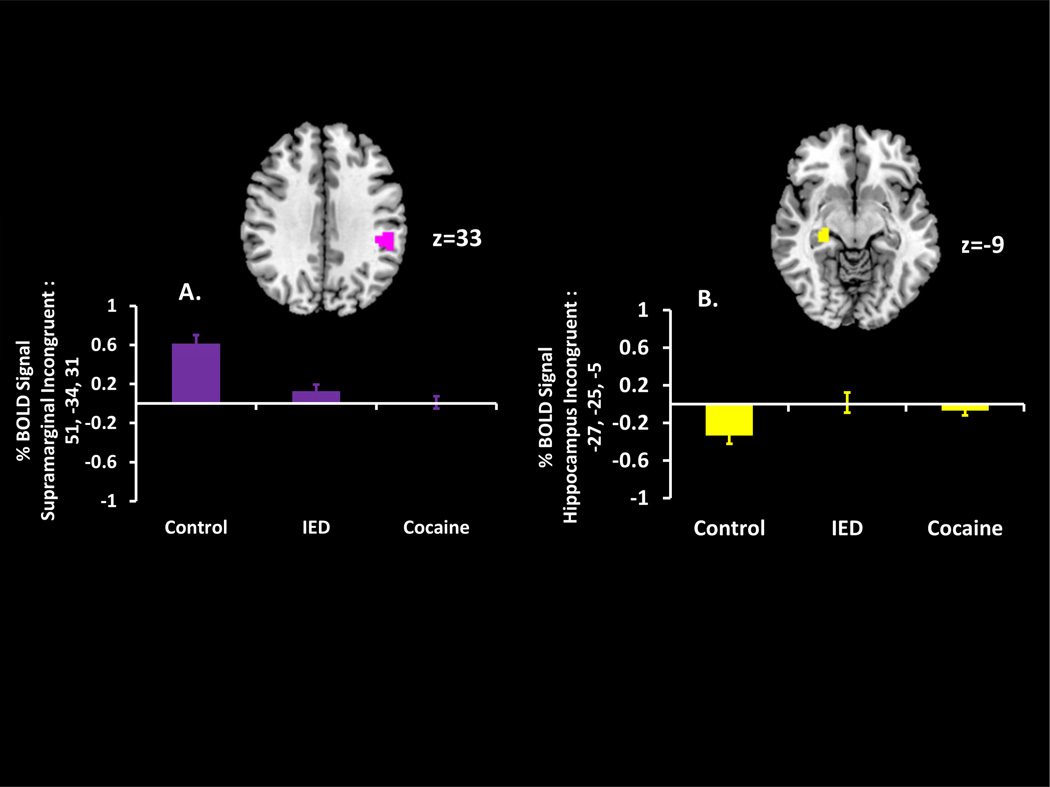
Controls differed in incongruent>congruent activity from both IED and CUD in attention-related regions including the inferior parietal lobule and supramarginal gyrus (controls>IED+CUD) (Figure 2A) (Table 2), and in the superior temporal gyrus and hippocampus (controls< IED+CUD) (Figure 2B) (Table 2). Conjunction analyses of the contrasts ‘control versus IED’ and ‘control versus CUD’ revealed significant overlap in all four regions. However, the superior temporal gyrus and inferior parietal lobule results did not survive correction for SUD (Table 2) (Supplementary Data).
Figure 2.
Regions showing conflict-related group differences between healthy controls, individuals with intermittent explosive disorder (IED), and individuals with cocaine use disorder (CUD). Higher percent blood-oxygenation-level dependent (BOLD) signal to the contrast incongruent>congruent was observed in controls versus CUD and controls versus IED in the (A) supramarginal gyrus and (B) hippocampus. Images are in neurological view (left=left).
Brain-Behavior Correlations
Across all participants, the higher the expressed outward anger (STAXI AX-O), the higher was the (more anterior) DLPFC activity to error (r=0.41, p=0.004) (Figure 1D), suggesting that these DLPFC hyperactivations to error could be possible neural correlates of trait anger expression as continuously measured. No additional brain-behavior correlations, including with task variables, reached significance.
DISCUSSION
Our goal was to interrogate a core component of the iRISA model (impaired response inhibition), related to aberrant PFC circuitry, in two externalizing psychopathologies. Using a classical color-word Stroop task in combination with fMRI, we investigated the neural correlates of inhibition and error processing in individuals with IED and CUD, and compared such responding with that of healthy controls. Consistent with our hypotheses about shared neural signatures of compromised self-regulation, results revealed that the two psychopathologies were generally more similar than different.
During error processing (versus correct trials), both IED and CUD had more activity than healthy controls in the left DLPFC; the ACC, while expected (and indeed initially observed), did not survive correction for covariates (Table 2). The DLPFC has been implicated in non-emotional conflict resolution (Egner et al., 2008) and the implementation of cognitive control (Kerns, Cohen, 2004), and in some studies has been shown to be hyperactive in select psychopathologies including cocaine addiction (Mayer, Wilcox, 2013). Similarly, our prior work has indicated that lower (i.e., more efficient) error>correct fMRI Stroop response is more beneficial. Specifically, we showed that the indirect dopamine agonist methylphenidate decreased error>correct DLPFC activity in CUD, which further correlated with better task performance (fewer errors) (Moeller, Honorio, 2014a) . Moreover, reduced fMRI DLPFC response during the color-word Stroop predicted better clinical outcome (treatment retention) in a prospective study of CUD (Brewer, Worhunsky, 2008). Taken together, our results could suggest that, to successfully implement this region’s cognitive functions, controls did not need to activate this region as intensely as the individuals with the select externalizing psychopathologies. Because the error-related DLPFC hyperactivity was driven by IED (especially in the superior frontal gyrus, where IED showed activation in this region that eclipsed both healthy controls and CUD), and given this region’s correlation with expressed outward aggression (STAXI AX-O), it is possible that this unique pattern of error-related DLPFC activation during error commission in IED (as distinguished from CUD) could serve as a neural signature of clinically-relevant reactive aggression.
During conflict processing (versus congruent trials), controls again differed from both IED and CUD. Here, controls showed higher incongruent>congruent activation than the other groups in the supramarginal gyrus. This region forms part of the frontoparietal attention network, which participates in attentional/top-down control (Corbetta and Shulman, 2002, Weidner et al., 2009) and response selection (Hackley, 2009, Yalachkov et al., 2010), both important cognitive functions for resolving Stroop interference. While some recent investigations have reported higher activation within or adjacent to this region in addicted samples, these studies either used different contrasts (commission errors) (Castelluccio et al., 2014) or studied different addictions (nicotine) (Froeliger et al., 2013). Here, higher supramarginal activity in controls may have reflected healthy cognitive/attentional control. In contrast, because no behavioral differences between the groups emerged on incongruent errors, CUD and IED may have compensated to achieve comparable interference resolution with activations in regions not typically associated with Stroop performance, such as the hippocampus (Price et al., 2011). Nevertheless, given that we did not have a priori hypotheses about group differences in these regions, and given that these activations were uncorrelated with task behavior or trait anger, the mechanisms and functional consequences of these effects should be followed up in future studies.
There are several limitations pertaining to the current IED sample. First, the sample size was relatively low (both when considered in absolute terms and when compared with the other groups), which may have diminished our statistical power. However, despite this small and unequal sample of IED, and although other regions may have emerged with a larger sample size, we are confident in the current fMRI effects, which indeed achieved significance when corrected for multiple comparisons and square with the known cognitive functions engaged by this task. Second, there are multiple factors that increase heterogeneity of the sample including: (A) lifetime SUD comorbidities, (B) other comorbidities, and (C) remission status of either CUD or IED. We addressed (A) by controlling for SUD history in our analyses (which, as described in Supplementary Data, indeed helped clarify and refine the results) (Table 2). Moreover, we highlight the unique pattern of DLPFC activation to error in IED, meaning that IED and CUD did not completely overlap in results (which would have been expected had SUD driven the results). We view (B) as unlikely to drive our results, since IED and CUD did not differ on either current or lifetime comorbidities (Table 1). Similarly, IED and CUD did not differ on (C) (remission status) (Table 1). Furthermore, we believe that (C) would work against our hypotheses, with remission bringing IED and/or CUD closer in function to healthy controls; instead, most results were in the opposite direction (Table 2). In addition, many of these concerns about heterogeneity are addressed by the DLPFC-STAXI correlation, which showed that across all individuals (regardless of study group, comorbidity, etc.), DLPFC activity during error tracked individual differences in aggression. Nevertheless, future studies should aim to recruit samples of IED and CUD that are more homogeneous and matched. Third, several IED and controls (but not CUD) were drawn from an ongoing study during which participants received methylphenidate or counterbalanced placebo (which raises potential issues of expectation and, if their used scan was their second, habituation). Importantly, however, controlling for placebo status did not attenuate our effects (Supplementary Data). Another limitation is that our CUD sample, studied earlier, did not complete the IED interview, meaning it is possible that some CUD could have also met criteria for IED. However, we consider this possibility unlikely in light of the low STAXI aggression (Table 1) and the low incidence of conflicts/violence reported by this CUD sample (Supplementary Data). For these collective reasons/limitations, our results, while novel, are preliminary and await replication.
In conclusion, we used a classical cognitive control task in combination with fMRI to interrogate the brain regions that differentiate healthy controls from individuals with externalizing disorders (individuals with IED and CUD). Including individuals with IED, a largely understudied disorder of self-regulation, is an important step toward elucidating the neuroscientific basis of clinical aggression. In particular, unlike the group differences in the ACC, cerebellum, superior temporal gyrus, and inferior parietal lobule [which may be attributable to SUD, given that these regions did not survive correction for this variable) (Table 2) (Supplementary Data)], the DLPFC, hippocampus, and supramarginal gyrus appear to be tapping something unique to inhibitory control even after controlling for SUD (i.e., potentially tracking aggression). Future studies can extend these efforts by investigating in IED versus CUD the other major component of iRISA (salience attribution), using tasks that tap into this function [e.g., exposure to emotionally prohibitive/frustrating stimuli (Alia-Klein et al., 2009, Alia-Klein et al., 2007); facial emotion recognition (Best et al., 2002, Coccaro et al., 2007)]. More generally, by comparing these two externalizing psychopathologies, we employed a potentially powerful approach to investigate shared and unique signatures of impulse dysregulation. Although the groups did not differ on comorbidities, therefore indicating that different disorders not defined by impulse control dysfunction (e.g., depression, PTSD) are unlikely to drive our findings, we acknowledge that abnormalities in the regions observed here (e.g., DLPFC) are also prominent in other psychopathologies. To establish the error-induced DLPFC activity as a true neural signature of dysfunctional impulse control, future studies can test for double dissociation with another neurocognitive dysfunction not marked by impaired self-control (i.e., whether abnormalities emerge in different brain regions or during a different task). Future studies also need to resolve the competing hypotheses of whether the current abnormalities reflect a state or trait marker of dysfunctional impulse control, using a priori recruitment and stratification based on current disease state. Even with these caveats, the current results provide an important first step for the relatively new and exciting approach of studying these psychopathologies by their underlying neurocognitive dysfunctions. Instead of studying the multifarious symptoms/behavioral of traditional clinical diagnoses, this approach, if verified and extended, might yield new, more effective treatment options. Thus, beyond addiction and IED, our results could inform other neuropsychiatric disorders of impaired self-control (e.g., binge eating disorder, borderline personality).
Supplementary Material
Highlights.
We studied two self-control disorders: pathological aggression and cocaine abuse.
A color-word fMRI Stroop task examined their similarities and differences.
The most robust group effects emerged in the dorsolateral prefrontal cortex.
Higher activity in this region also positively correlated with trait aggression.
Abnormal fMRI activity in this region may therefore track pathological aggression.
Acknowledgements
The authors gratefully acknowledge the contributions of Dardo Tomasi, Thomas Maloney, Patricia A. Woicik, Pias Malaker, Daniel Carrero, Jason Frank, Tanya Turkewitz, and all members and volunteers of the Neuropsychoimaging of Addiction and Related Conditions Research Group.
Role of the Funding Source
This study was supported by grants from the National Institute on Mental Health (NIMH): R01MH090134 (NAK); and the National Institute on Drug Abuse (NIDA): R21DA034954 (RZG), 1F32DA030017-01 (SJM), and 1F32DA033088-01 (MAP). NIMH and NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alia-Klein N, Goldstein RZ, Tomasi D, Woicik PA, Moeller SJ, Williams B, et al. Neural mechanisms of anger regulation as a function of genetic risk for violence. Emotion. 2009;9:385–396. doi: 10.1037/a0015904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Goldstein RZ, Tomasi D, Zhang L, Fagin-Jones S, Telang F, et al. What is in a word? No versus Yes differentially engage the lateral orbitofrontal cortex. Emotion. 2007;7:649–659. doi: 10.1037/1528-3542.7.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Neelin P, Collins DL, Evans A, Friston K. Incorporating prior knowledge into image registration. Neuroimage. 1997;6:344–352. doi: 10.1006/nimg.1997.0299. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, Avila C. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Res. 2011;194:111–118. doi: 10.1016/j.pscychresns.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Best M, Williams JM, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci U S A. 2002;99:8448–8453. doi: 10.1073/pnas.112604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparelli E, Tomasi D. K-space spatial low-pass filters can increase signal loss artifacts in Echo-Planar Imaging. Biomedical Signal Processing and Control. 2008;3:107–114. doi: 10.1016/j.bspc.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Castelluccio BC, Meda SA, Muska CE, Stevens MC, Pearlson GD. Error processing in current and former cocaine users. Brain imaging and behavior. 2014;8:87–96. doi: 10.1007/s11682-013-9247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF. Intermittent explosive disorder and impulsive aggression: the time for serious study is now. Current psychiatry reports. 2004;6:1–2. doi: 10.1007/s11920-004-0027-7. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Res. 1997;73:147–157. doi: 10.1016/s0165-1781(97)00119-4. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Denson TF, Pedersen WC, Ronquillo J, Nandy AS. The angry brain: neural correlates of anger, angry rumination, and aggressive personality. J Cogn Neurosci. 2009;21:734–744. doi: 10.1162/jocn.2009.21051. [DOI] [PubMed] [Google Scholar]
- Devito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug Alcohol Depend. 2012;122:228–235. doi: 10.1016/j.drugalcdep.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J, Williams J. Structured Clinical Interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Froeliger B, Modlin LA, Kozink RV, Wang L, Garland EL, Addicott MA, et al. Frontoparietal attentional network activation differs between smokers and nonsmokers during affective cognition. Psychiatry Res. 2013;211:57–63. doi: 10.1016/j.pscychresns.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Hackley SA. The speeding of voluntary reaction by a warning signal. Psychophysiology. 2009;46:225–233. doi: 10.1111/j.1469-8986.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, et al. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Coccaro EF, Fava M, Jaeger S, Jin R, Walters E. The prevalence and correlates of DSM-IV intermittent explosive disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2006;63:669–678. doi: 10.1001/archpsyc.63.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Field M, Franken IH. Pharmacological interventions to modulate attentional bias in addiction. CNS spectrums. 2013:1–8. doi: 10.1017/S1092852913000485. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Wilcox CE, Teshiba TM, Ling JM, Yang Z. Hyperactivation of the cognitive control network in cocaine use disorders during a multisensory Stroop task. Drug Alcohol Depend. 2013;133:235–241. doi: 10.1016/j.drugalcdep.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Honorio J, Tomasi D, Parvaz MA, Woicik PA, Volkow ND, et al. Methylphenidate enhances executive function and optimizes prefrontal function in both health and cocaine addiction. Cereb Cortex. 2014a;24:643–653. doi: 10.1093/cercor/bhs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Konova AB, Parvaz MA, Tomasi D, Lane RD, Fort C, et al. Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiatry. 2014b;71:61–70. doi: 10.1001/jamapsychiatry.2013.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Honorio J, Volkow ND, Goldstein RZ. Dopaminergic involvement during mental fatigue in health and cocaine addiction. Transl Psychiatry. 2012;2:e176. doi: 10.1038/tp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Newmark RE, Zhang J, Triebwasser J, Meyerson D, et al. Laboratory induced aggression: a positron emission tomography study of aggressive individuals with borderline personality disorder. Biol Psychiatry. 2009;66:1107–1114. doi: 10.1016/j.biopsych.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Price RB, Eldreth DA, Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Transl Psychiatry. 2011;1:e46. doi: 10.1038/tp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Meloy RJ, Bihrle S, Stoddard J, LaCasse L, Buchsbaum MS. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behavioral Sciences and the Law. 1998;16:319–332. doi: 10.1002/(sici)1099-0798(199822)16:3<319::aid-bsl311>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Roberts KL, Hall DA. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. J Cogn Neurosci. 2008;20:1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:706–709. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165:429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source for visual shapes. Brain Res Cogn Brain Res. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. STAXI. State-Trait Anger Expression Inventory. Tampa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage. 2005;27:377–386. doi: 10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Weidner R, Krummenacher J, Reimann B, Muller HJ, Fink GR. Sources of top-down control in visual search. J Cogn Neurosci. 2009;21:2100–2113. doi: 10.1162/jocn.2008.21173. [DOI] [PubMed] [Google Scholar]
- Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, et al. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2013;27:477–488. doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Sensory and motor aspects of addiction. Behav Brain Res. 2010;207:215–222. doi: 10.1016/j.bbr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. 2009;174:81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.