Abstract
A subset of acute promyelocytic leukemia (APL) cases have been characterized by the t(5;17)(q35;q21) translocation variant which fuses nucleophosmin (NPM) to retinoic acid receptor alpha (RARA). The resultant NPM-RAR fusion protein blocks myeloid differentiation, and leads to a leukemic phenotype similar to that caused by the t(15;17)(q22;q21) PML-RAR fusion. The contribution of the N-terminal 117 amino acids of NPM contained within NPM-RAR has not been well studied. As a molecular chaperone, NPM interacts with a variety of proteins implicated in leukemogenesis. Therefore, a proteomic analysis was conducted to identify novel NPM-RAR associated proteins. Tumor necrosis factor receptor type 1-associated DEATH domain protein (TRADD) was identified as a relevant binding partner for NPM-RAR. This interaction was validated by co-precipitation and co-localization analysis. Biological assessment found that NPM-RAR expression impaired TNF-induced signaling through TRADD, blunting TNF-mediated activation of caspase 3 (CASP3) and caspase 8 (CASP8), to ultimately block apoptosis.
Implications
This study identifies a novel mechanism through which NPM-RAR impacts leukemogenesis.
Keywords: acute promyelocytic leukemia, nucleophosmin, NPM-RAR, TRADD, apoptosis
INTRODUCTION
Acute Promyelocytic Leukemia (APL) is a malignant proliferation of differentiation-competent myeloblasts and promyelocytes(1). In the vast majority of cases, APL is characterized by t(15;17)(q22;q21), which introduces the gene for the retinoic acid receptor alpha (RARA) into the locus encoding the PML protein. The resultant PMLRAR fusion encodes the N-terminal protein-interaction and leucine-zipper domains of PML fused to the DNA-binding Zinc finger, leucine-rich dimerization, and C-terminal ligand-binding and co-activator/co-repressor domains of RARA(1). Forced expression of PML-RAR in mice results in an APL-like phenotype(2-4).
The molecular basis by which PML-RAR disrupts normal myeloid development is complex(1). PML-RAR, having greater affinity for co-repressors than RARA, is capable of binding to retinoic acid responsive promoters, and suppressing transcription of retinoic-acid target genes. PML-RAR also has unique DNA binding properties, and may act as a rogue transcriptional activator or repressor. PML-RAR may impact transcription pathways indirectly, through its ability to bind with and sequester RXR, a key binding partner for many members of the nuclear hormone receptor family. PML itself localizes to nuclear structures known as PML Oncogenic Domains (PODS), and within these nuclear bodies PML interacts with a diverse series of proteins, including DAXX, p53, Rb, CREB-binding protein, ski, MYB, mdm2, and SUMO: by virtue of its ability to delocalize PML and disrupt the structure of PML-containing nuclear bodies, PML-RAR may impact a wide variety of cellular functions contributing to apoptosis, cellular senescence, and cell cycle regulation. In addition, PML-RAR, through recruitment of co-repressor containing histone deacetylase activity to PML-containing complexes, may also affect the acetylation and function of proteins that bind to PML, as has been shown for p53(5).
We have been investigating the rare cases of APL that do not express the PML-RAR fusion. These leukemias manifest similar phenotype, but different genotype, and as such represent “experiments of nature” with which to test mechanistic hypotheses(6). Seven variant translocations have been characterized on a molecular basis: all express fusion proteins containing the same C-terminal sequences of RARA as are expressed in PMLRAR: t(11;17)q(23;q21) which fuses the PLZF transcriptional repressor to RARA(7); t(5;17)(q35;q21) that joins nucleophosmin (NPM) to RARA(8); t(11;17)(q13;q21) that fuses the nuclear matrix protein NUMA to RARA(9); der17 that fuses the Signal Transducer and Activator of Transcription STAT5b with RARA(10); fusion of RARA with the regulatory subunit of the cyclic adenosine monophosphate dependent protein kinase PRKAR1A on 17q24 (11); t(4;17) which fuses FIP1L1 to RARA(12); and t(X;17)(p11;q21) which fuses the BCL-6 co-repressor protein BCOR to RARA(13). We have focused our studies on t(5;17), which, after PLZF-RAR is the second most common of the variants, and manifests a similar phenotype to t(15;17) APL, including the ability of t(5;17) blasts to differentiate in the presence of all-trans retinoic acid(14).
The t(5;17) translocation fuses the same C-terminal sequences of RARA expressed in PML-RAR to the N-terminal 117 amino acids of nucleophosmin (NPM) (8). We have shown in both in vitro and in vivo models that, like PML-RAR, ectopic expression of NPM-RAR induces an APL-like phenotype(15, 16). We have previously shown that NPM-RAR localizes throughout the nucleoplasm(17) and interacts with co-activator and co-repressor molecules(18). NPM-RAR binds to DNA both as homodimers and heterodimers with RXR, and similar to PML-RAR, its activity as a transcriptional activator varies by cell-type and target sequence(18).
NPM (also known as B23, numatrin, and NO38) is a molecular chaperone originally identified through its ability to bind and transport nascent ribosomal units in the nucleolus(19). NPM shuttles between nucleolus, nucleus, and cytoplasm, and participates in a myriad of cellular functions. NPM binds to centrosomes: phosphorylation by CDK2/cyclin E dissociates NPM from centrosomes and initiates centrosomal duplication. NPM is redistributed from the nucleolus in response to cytotoxic drugs and genotoxic stress. NPM binds several cellular and viral proteins, including human immunodeficiency virus (HIV) Rev and Tat proteins, hepatitis delta virus delta antigens, retinoblastoma protein, p53, mDM2, ARF, YY1 and IRF-1 (for review, see Colombo et al. (19)).
All of the APL variant fusion proteins, including NPM-RAR, contain dimerization domains within the sequences of the N-terminal fusion partner(6), and it has been proposed that dimerization of RARA alone might be sufficient for development of APL(20-22). However, Sternsdorf et al. (23) have shown that transgenic mice expressing a construct containing artificial dimerization domains fused to RARA fail to develop the full APL phenotype, suggesting that the N-terminal fusion partners provide an important gain of function, in addition to their ability to induce dimerization.
Limited information is available concerning the functionality of the N-terminal 117 amino acids of NPM expressed in NPM-RAR. This region contains two nuclear export signals, as well as elements important for dimerization(19). In this manuscript we test the hypothesis that the N-terminal sequences of NPM-RAR express a protein-interaction domain that contributes to NPM-RAR-leukemogenesis. Through a proteomic analysis of NPM-RAR-interacting proteins, we identified the Tumor necrosis factor receptor type-I – associated DEATH domain protein TRADD as specifically binding to NPM-RAR.
MATERIALS and METHODS
Cell Culture and Reagents
HEK293, HeLa, and U937 cells were obtained from the American Type Culture Collection (ATCC; Bethesda, MD). These cells were not further authenticated in our laboratory. HEK293 and HeLa cells were maintained in a humidified 5% CO2 atmosphere in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Herndon, VA) supplemented with 10% Fetal Bovine Serum (GIBCO, Grand Island, NY), 2mMglutamine, 100 U/ml penicillin, and 100 microg/ml streptomycin. U937 cells were grown in RPMI 1640 (Mediatech) containing the same supplements. HEK293 or HeLa cells were transfected by CaPO4 co-precipitation, as described (18). Pools of stably transfected cells were selected in medium containing 1 mg/ml G418 (Gibco). The generation and characterization of the stably-transfected U937 cells has been previously described(24).
Antibodies and reagents
Rabbit polyclonal anti-RARA and anti-TRADD antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-NPM antibody was obtained from Invitrogen (Carlsbad, CA). Anti-PARP was obtained from Cell Signaling Technology (Danvers, MA), and anti-caspase-3 was from Assay Designs (Ann Arbor, MI). Anti-β-actin and anti-FLAG mouse monoclonal antibodies were purchased from Sigma (St. Louis, MO).
Proteomic analysis
pCDNA vector containing coding regions for the calmodulin binding peptide (CBP) and the IgG binding domains of protein A was a gift of R. Steinman. NPM-RAR or RARA was subloned in the appropriate reading frame to generate C-terminal TAP-tag fusions. Pools of clones of HEK293 cells stably expressing the TAP-tagged NPMs-RAR, RAR proteins and control were washed two times with ice cold PBS and then lysed in 10 mL of ice cold NP-40 lysis buffer containing 20 mM Tris, pH 8.0, 137 mM NaCl, 10% Glycerol, 1% (v/v) NP-40, 10 mM NaF, 5 mM NaVO4 and protease inhibitor cocktail (Sigma). After incubation on ice for 30 min, the lysates were centrifuged at 14,000 rpm for 15 min at 4C. The resulting supernatants were incubated with 100 μL of packed IgGSepharose (GE Healthcare) for 4 h at 4°C. After extensively washing with ice cold lysis buffer containing 500 mM KCl, proteins were eluted with PBS containing 1% SDS. The eluate was concentrated and desalted using Amicon Microcon (Millipore) centrifugal filter devices (3,000 MWCO) and the proteins were resolved by 1D SDS-PAGE. Bands were visualized by Coomassie staining and the entire lane was excised into ten gel bands and subjected to in-gel trypsin digestion as described previously (25). Tryptic digests were analyzed in duplicate by nanoflow reversed-phase liquid chromatography (LC)-MS/MS using a nanoflow LC (Dionex Ultimate 3000, Dionex Corporation, Sunnyvale, CA) coupled online to an linear ion trap MS (LTQ-XL, ThermoFisher Scientific, San Jose, CA) and peptide/protein identifications were derived as described previously (25).
Eptiope-tagged Constructs
The coding region of TRADD or NPM-RAR was subcloned into pcDNA3/V5-His (Invitrogen) in the appropriate reading frame to generate fusion proteins bearing a V5-His tag at the C terminus. Fidelity was confirmed by DNA sequencing and Western blotting.
Co-immunoprecipitation assays
To test for binding between the V5-His-tagged TRADD and NPM-RAR, transiently transfected HEK293 cells were lysed with NP-40 lysis buffer containing 10 mM Imidazole. Complexes were captured on Ni-NTA beads (Qiagen). The beads were washed with NP-40 buffer containing 20 mM Imidazole before elution with 2× SDS sample buffer. Samples were analyzed by immunoblotting with anti-RARA or anti-TRADD antibodies. To determine association between FLAG-tagged NPM-RAR and TRADD, transiently-transiently transfected cells were lysed in NP40 lysis bufer and incubated with the M2 anti-FLAG antibody. Complexes were captured on protein A sepharose beads, and separated on SDS-PAGE. Immunoblots were prepared and probed as described(18).
Western blotting
After treatment with cyclohexamide (Sigma) and human recombinant TNF-alpha (R & D System Minneapolis, MN) cells were washed with ice-cold PBS and resuspended in NP-40 lysis buffer. After 30 min incubation on ice, lysates were cleared by centrifugation. 50 μg of lysate were resolved by 8% or 12% SDS-PAGE and transferred onto Nitrocellulose membranes (Bio-Rad). After blocking with 5% non-fat milk, membranes were incubated with primary antibodies as recommended by the supplier. The immune complexes were detected by the ECL detection system (Invitrogen).
Annexin staining
Cells were exposed to 10 ng/mL TNF alpha (R & D System Minneapolis, MN) and 1 ug/mL of cycloheximide (Sigma) for one hour. Annexin V staining was determined using an FITC-conjugated Annexin V antibody and propidium iodide (PI) according to instructions provided by the manufacturer (BD Biosceince), using an Accuri C6 flow cytometer (Accuri Cytometers, Ann Arbor, MI), gating on the Annexin Vpostive/PI negative population. The population of apoptotic cells with sub-G0/G1 DNA content was determined by PI staining of cells fixed in ice-cold 70% ethanol before staining with 1 mg/mL PI and analysis by flow cytometry (Accuri C6 flow cytometer, Accuri Cytometers, Ann Arbor, MI).
Caspase-8 activity
U937 and U937 transfected vector only or NPM-RAR expressing cells were seeded into 96-well plates at a density of 1.5 × 104 cells/well. After 24 h, cells were treated with 10 ng/mL TNF-alpha and 1 μg/ml cycloheximide. Activity of caspase-8 was measured using caspase-Glo 8 detection assay kit (Promega, Madison, WI). Luminescence was measured using the 1420 VICTOR3 micro-plate reader (Perkin Elmer, Waltham, MA).
Immunofluorescence
Hela cells were grown on glass cover-slips and transiently transfected with FLAG-NPMRAR and V5/His-TRADD. Cells were fixed with 2% para-formaldehyde in PBS for 15 min at room temperature, permeabilized with 0.1% Triton X-100 in PBS for 15 min, and non-specific binding sites were blocked with PBS containing 2% BSA for 45 min. After blocking, cells were incubated with a 1:200 dilution of mouse monoclonal anti-V5 antibody (Invitrogen) and/or a 1:500 dilution of rabbit polyclonal anti-FLAG antibody (Sigma). After washing with PBS containing 0.5% BSA, cells were incubated for 1 h with a 1:1000 dilution of Alexa 488-conjugated anti-rabbit antibody and 1:500 dilution of Alexa-546-conjugated anti-mouse antibody (Invitrogen). Nuclei were stained with 1:5000 dilution of DRAQ5 (Cell Signaling Technologies, Danvers, MA). Confocal images were captured using a Leica TCS SL microscope (Leica, Heidelberg, Germany).
RESULTS
NPM-RAR binds TRADD
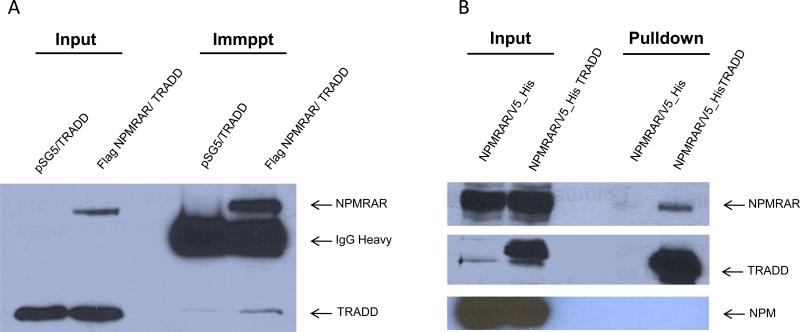
To identify binding partners of NPM-RAR, we engineered constructs expressing NPM-RAR or RARA fused at the C-terminus to sequences encoding the calmodulin-binding domain and protein A. These were introduced into HEK293 cells, and transfected cells were lysed. Interacting proteins were captured on Ig-coated Sepharose beads. The beads were washed extensively with high stringency buffers (500 mM Kl) to minimize nonspecific protein interactions. The eluted proteins were size-separated on one-dimensional polyacrylamide gels. Bands were excised from the gel, eluted, and subjected to tryptic digestion and LC-MS/MS. Amongst proteins uniquely interacting with NPM-RAR, but not RARA, we identified TRADD. The specificity of the interaction was validated by co-precipitation of transiently-transfected HEK cell lysates, using either TRADD as the target and NPM-RAR as the bait (Figure 1A), or alternatively NPM-RAR as the target and TRADD as the bait (figure 1B).
Figure 1. NPMRAR interacts with TRADD.
A. HEK293 cells were co-transfected with plasmids expressing TRADD and Flag-tagged NPMRAR or empty vector. Lysates were incubated with anti-FLAG antibody, and complexes captured on Protein A sepharose beads. Complexes were separated on SDS-PAGE, and immunoblots probed with anti-TRADD and anti-RARA antibodies.
B. HEK293 cells were co-transfected with expression plasmids for NPMRAR and V5_His-TRADD or V5_His empty vector. His-TRADD or His-empty vector complexes were captured on Ni-agarose beads and separated on SDS-PAGE. Immunoblots were probed with antibodies against RARA, TRADD, and the C-terminus of NPM.
TRADD and NPM-RAR co-localize in cells
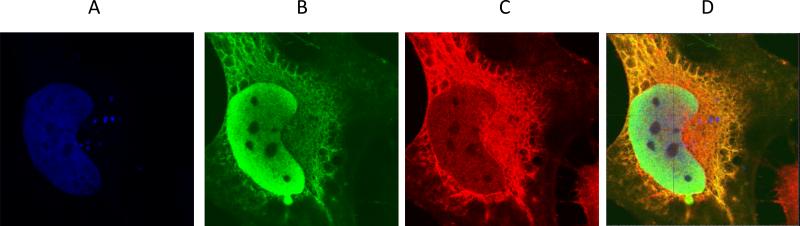
To confirm that TRADD and NPM-RAR interact in cells, we transiently co-transfected HeLa cells with epitope-tagged expression plasmids for NPM-RAR and TRADD. We found that NPM-RAR localizes primarily to the nucleoplasm with a small component expressed in the cytoplasm. TRADD colocalized with NPM-RAR in the cytoplasm (Figure 2).
Figure 2. Colocalization of NPM-RAR and TRADD.
Confocal microscopic images of FLAG-NPM-RAR and V5-TRADD co-transfected Hela Cells. A. DRAQ5 stain of nucleus B. NPM-RAR C. TRADD D. Merged image.
NPM-RAR/TRADD interaction is independent of wild-type NPM
NPM-RAR contains N-terminal sequences implicated in NPM dimerization. We have previously determined that NPM-RAR can form NPM-RAR/NPM-RAR homodimers as well as NPMRAR/NPM heterodimers(18). TRADD has not been previously reported as interacting with NPM. To test whether NPM was present in the NPM-RAR/TRADD complex, we probed immunoblots of TRADD-precipitated proteins with an antibody that recognizes the C-terminal sequences of NPM. We did not find signal for wild-type NPM in the complex (Figure 1) indicating that the ability to bind NPM is a novel attribute of the NPM-RAR fusion protein.
NPM-RAR inhibits TNF-induced apoptosis
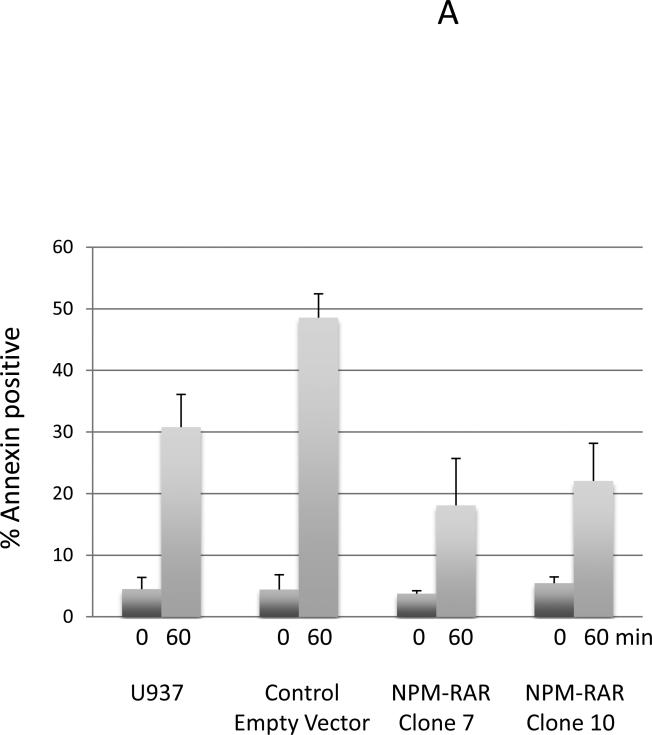
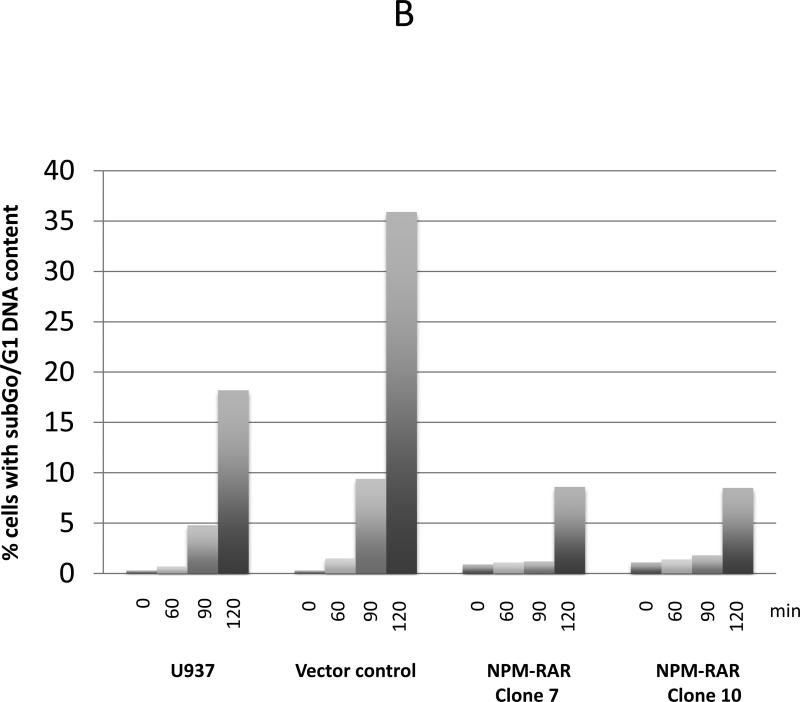
TRADD is a death-domain containing adapter molecule that interacts with the Tumor Necrosis Factor (TNF) type 1Recptor (TNFR1). TRADD plays an integral part in activation of the extrinsic apoptotic pathway. To determine whether NPM-RAR-expression impacted TNF-induced apoptosis, we tested the TNF-sensitivity of U937 cells stably transfected with NPM-RAR. The engineering and characterization of these clones are described elsewhere (24). Independent U937-NPM-RAR clones, or as controls parental U937 cells or clones stably transfected with vector alone, were treated with TNF and assayed by PI-exclusion and Annexin V staining. This assay was performed three times with similar results: averaging all three experiments, 30.8% (+/− 5.3%) of parental U937 cells and 48.6% (+/− 3.9%) of a vector-control clone, but only 18.1% (+/− 7.6%) and 22% (+/− 6.1%) of two independent NPM-RAR expressing clones became Anenxin V positive at 60 minutes, indicating a muted response of NPM-RAR expressing cells to TNF-induced apoptosis (Figure 3A). To confirm these results, we also analyzed cells for fragmentation of nuclei, as assessed by the percentage of cells staining with PI with sub-G0/G1 DNA content (Figure 3B). Flow cytometry of PI stained cells also indicated a muted apoptotic response of the NPM-RAR expressing clones, with apoptosis occurring in 18.7% of parental U937 cells 120 minutes after exposure to TNFalpha; 35.9% in the vector-transfected clone, but only 8.6% and 8.5% in the two NPM-RAR expressing U937 clones. Repetition of the assay generated similar results (see Supplementary Figure 1).
Figure 3. TNF-alpha induced apoptosis.
A: Annexin V staining: U937 clones stably expressing NPM-RAR or vector control were treated with 10 ng/mL TNF-alpha and 1 ug/mL CHX for 60 minutes, stained with annexin V-PE and 7-AAD, and analyzed by flow cytometry. The average of three separate experiments is depicted.
B: PI staining: Cells were treated with 2.5 ng/ml TNF-alpha and 250 ng/ml CHX, ethanol fixed, and stained with PI. The percentage of cells containing sub-G/G1 DNA content was assessed by flow cytometry at time 0, 60, 90, and 120 minutes after exposure to TNF-alpha.
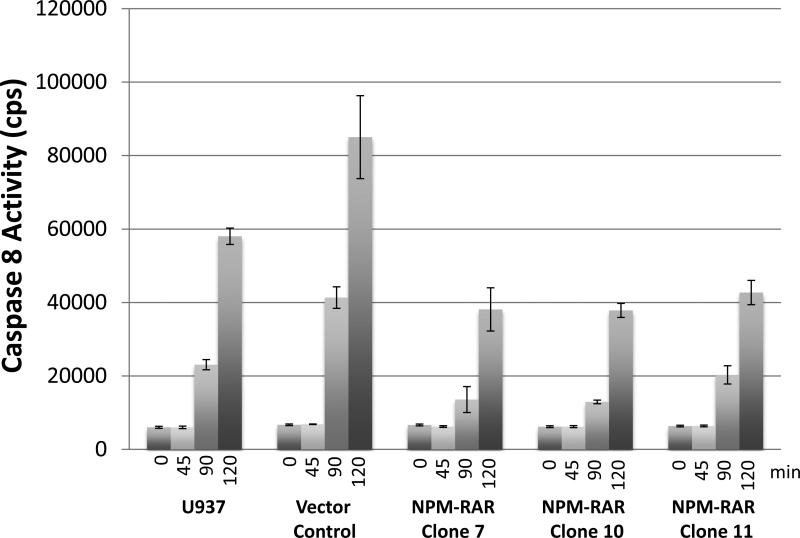
NPM-RAR inhibits TNF-induced activation of the initiator Caspase 8
TNF activation of the TNF-receptor leads to TRADD-dependent cleavage and activation of the initiator procapsase 8. To assess the effect of NPM-RAR on this pathway, we treated NPM-RAR or control U937 cells with TNF, and harvested cell lysates for immunoblots. Figure 4 indicates retention of non-cleaved procaspase 8 in NPM-RAR expressing cells, compared with parental U937 cells or a control U937 clone.
Figure 4. Caspase-8 mediated apoptosis induced by TNF alpha is suppressed by NPMRAR.
U937 clones stably expressing NPM-RAR or vector only control were treated with 10 ng/mL TNF-alpha and 1 ug/mL CHX for 0, 45, 90, or 120 minutes. Conversion of substrate into luminescent product was determined using the caspase-Glo 8 detection kit. The assay was performed in triplicate.
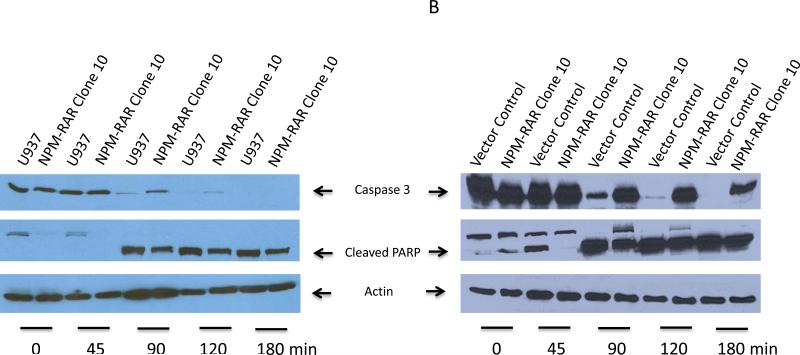
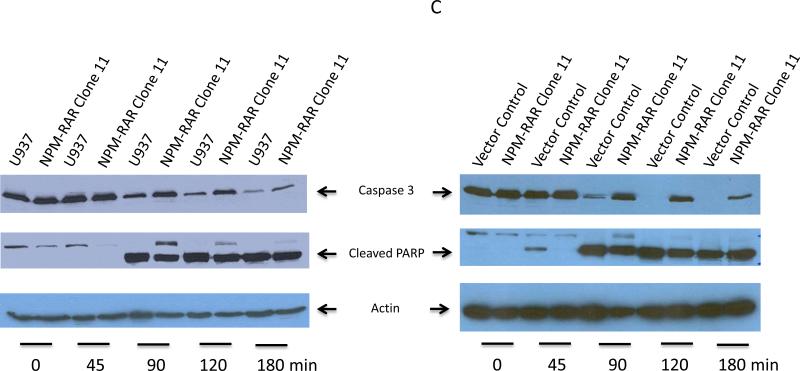
NPM-RAR inhibits TNF-induced activation of the effector caspase 3
Activation of the initiator caspase 8 leads to cleavage and activation of the effector caspase 3. If NPMRAR inhibits activation of caspase 8, we would also predict decreased activation of caspase 3 upon exposure to TNF in NPM-RAR expressing cells. Figure 5 indicates lack of activation of procaspase 3 upon stimulation with TNF in the NPM-RAR expressing cells.
Figure 5. NPMRAR suppresses TNF-alpha induced activation of Caspase 3.
Clones were treated with TNF-alpha (10 ng/mL) and cycloheximide (1 ug/mL). Immunoblots of lysates were probed with anti-caspase 3, anti-PARP, and anti-actin antibodies. Arrows indicate bands for Caspase 3, Cleaved PARP, and Actin.
Panel A: Uncleaved Caspase 3 and Activated PARP in NPM-RAR clone 7, compared with parental U937 cells and vector control clone.
Panel B: Uncleaved Caspase 3 and Activated PARP in NPM-RAR clone 10, compared with parental U937 cells and vector control clone.
Panel C: Uncleaved Caspase 3 and Activated PARP in the NPM-RAR clone 11, compared with parental U937 cells and vector control clone.
NPM-RAR inhibits TNF-induced activation of PARP
Activation of the effector caspase 3 initiates a series of late apoptotic events, including cleavage and inactivation of poly ADP ribose polymerase (PARP). If indeed NPM-RAR inhibited TNF-mediated apoptosis, we would expect retention of the full-length form of PARP in NPM-RAR-expressing cells upon stimulation with TNF. Figure 5 indicates the refractoriness of NPM-RAR cells to TNF-induced cleavage of PARP.
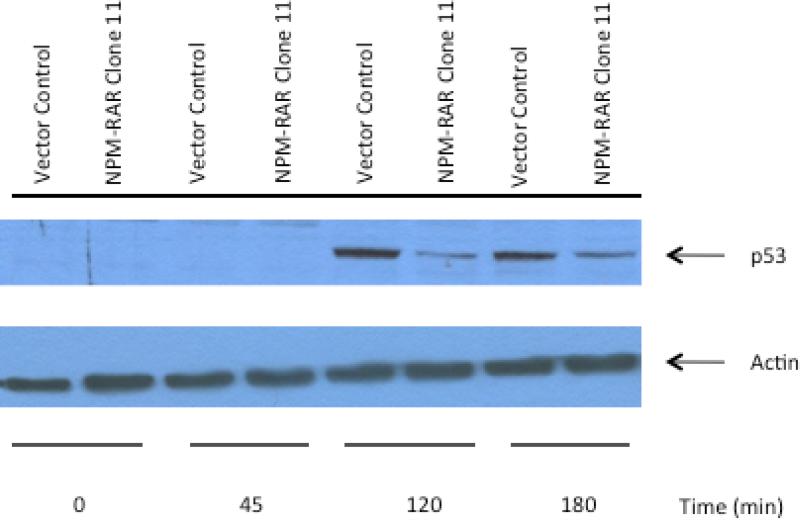
NPM-RAR suppresses TNF-induced of p53
Though the molecular pathway has not been fully characterized, it has been observed that one of the events associated with TNF-mediated apoptosis is accumulation of p53 protein(26). To assess the effects of NPMRAR on TNF-induced accumulation of p53, we prepared immunoblots of lysates of the U937 clones stimulated with TNF, and probed them with an anti-p53 antibody. Figure 6 shows increased accumulation of p53 in control cones treated with TNF, consistent with prior reports. NPM-RAR expressing clones failed to demonstrate TNF-induced upregulation of p53.
Figure 6. NPM-RAR blocks TNF-mediated upregulation of p53.
NPM-RAR expressing U937 clone or vector-transfected control were treated with TNF-alpha (10 ng/mL) and cycloheximide (1 ug/mL) for the indicated times. Immunoblots were probed with antibodies against p53 and actin (as loading control).
DISCUSSION
We performed proteomic analysis to identify proteins that bind to NPM-RAR. We identified TRADD as a binding partner of NPM-RAR. This interaction was validated by reciprocal co-precipitation and immunofluorescent colocalization. TRADD is an adapter protein that interacts with the TNFR1 receptor, and mediates the activation of programmed cell death and NFkB activation. We found that cells that expressed NPMRAR were less sensitive to TNF-induction of apoptosis.
The N-terminal 117 amino acids of NPM that are represented in NPM-RAR contain a putative protein interaction domain. Within the N-terminal 117 amino acids of NPM are two nuclear export signals that interact with the nuclear exportin CRM(19). Indeed, the N-terminal region of NPM has been implicated in oligomerization(27), and we have shown that NPM-RAR binds unrearranged NPM(28). Our data suggest direct association between NPM-RAR and TRADD. We did not find evidence for an interaction between TRADD and RARA in our proteomic screen of RARA-interacting proteins, nor did we find evidence for direct interaction between wild-type NPM and TRADD. This leads us to propose that TRADD interaction is unique to the fusion protein, possibly due to a modified configuration of the N-terminal NPM domain that occurs as a result of fusion with RARA.
NPM-ALK in t(2;5) anaplastic lymphoma is derived from the same breakpoint in the NPM locus, and expresses the same domains of NPM as in NPM-RAR(29). The N-terminus of NPM is also contained in the NPM-MLF1 fusion of t(3;5)(q25;q34) that is expressed in rare cases of myelodysplastic syndrome and acute myeloid leukemia(30);in t(3;5) the breakpoint involves a different intron, to generate expression of the 175 N-terminal amino acids of NPM. The same N-terminal sequences are also expressed in NPMc mutations, found in a majority of cases of AML with normal karyotype, in which a variety of frameshift mutations lead to an altered C-terminus(31). It is intriguing to speculate whether these other oncoproteins also bind to TRADD.
Several mechanisms may explain the observation that NPM-RAR binding to TRADD inhibits downstream pro-apoptotic actions of TRADD. TRADD is localized both in the cytoplasm, where it can interact with the TNF receptor and other cytoplasmic proteins through which it activates the extrinsic panoptic pathway, as well as in the nucleus, where it apparently mediates apoptosis through a poorly defined pathway that does not depend on activation of caspase 8(32, 33). We have previously reported that NPM-RAR localizes primarily throughout the nucleoplasm, with a small component in the cytoplasm (17). PML-RAR has also been observed to localize to both the nucleus and cytoplasm (34). Our immunofluorescence data indicates that NPM-RAR and TRADD co-localize primarily within the cytoplasm. Thus, we propose that NPM-RAR interaction in the cytoplasm blocks TRADD from interacting with FADD and caspase 8 to participate in TNF-mediated apoptosis. Our model is reminiscent of that proposed for the tumorigenic properties of the EBV latent membrane protein 1 (LMP1), which similarly binds to TRADD to inhibit apoptotic signaling (35, 36).
The intertwining of the extrinsic and intrinsic apoptotic pathways is complex. Several reports have indicated that TNF activation, through the TNFR1 receptor and the adapter protein TRADD, leads to increased expression of p53, possibly through modulation of p19ARF (26). We found that NPM-RAR blocked TNF-mediated P53 expression as well. We have previously shown that NPM-RAR modulates the activity of p53 in a direct fashion (manuscript in preparation); the mechanism by which NPM-RAR affects apoptotic pathways could thus be diverse.
The significance of the TNF pathway in AML has not been studied in depth. Nevertheless, Sawanobori has reported that TNFR1 is expressed on the majority of hematopoietic precursors, and Rushworth has suggested that altered heme-oxidase mediated pathways in leukemic cells could quell the pro-apoptotic effects of TNFR1 (37, 38). The recent finding that TRADD has tumor suppressor functions (39) leads us to speculate that it may have an important role in leukemogenesis. NPM-RAR and LMP1 (35, 36) are the only oncogenic proteins yet identified as directly interacting with and inhibiting the function of TRADD. Our work suggests a novel pathway through which NPM-RAR modulates TNF signaling in the t(5;17) variant.
The majority of APL cases express PML-RAR, not NPM-RAR. Testa et al. have shown that PML-RAR inhibits TNF-induced apoptosis, though through a different mechanism of downregulation of expression of the TNF-receptor(40). Witcher et al. has shown that TNF enhances ATRA-induced differentiation in APL cells(41, 42). Nuclear TRADD co-localizes to PML-nuclear bodies (43), and PML is necessary for nuclear TRADD-mediated apoptosis. PML-nuclear bodies have been shown to regulate the availability of the apoptotic adapter protein FLASH (FLICE-associated Huge Protein) (44), as well as the proapoptotic protein DAXX (45, 46). Inhibition of the function of wild-type PML by PML-RAR, and the disruption of PML-nuclear bodies, most likely affect these mediators of the extrinsic pathway. Thus, interference with the extrinsic apoptotic pathway may be a common feature by which APL fusion proteins contribute to leukemogenesis.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Richard Steinman MD PhD and Daniel Johnson PhD for valuable discussions and sharing of reagents. This work was supported by NIH R01 CA67346 and P30 CA047904.
Footnotes
The authors declare no conflicts of interest.
DISCLOSURE OF CONFLICTS OF INTEREST:
The authors declare no conflicts of interest.
REFERENCES
- 1.Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- 2.Brown D, Kogan S, Lagasse E, Weissman I, Alcalay M, Pelicci PG, et al. A PMLRARalpha transgene initiates murine acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogan SC. Acute promyelocytic leukemia: a view from a mouse. Blood Cells Mol Dis. 2000;26:620–625. doi: 10.1006/bcmd.2000.0345. [DOI] [PubMed] [Google Scholar]
- 4.Kogan SC. Mouse models of acute promyelocytic leukemia. Curr Top Microbiol Immunol. 2007;313:3–29. doi: 10.1007/978-3-540-34594-7_2. [DOI] [PubMed] [Google Scholar]
- 5.Insinga A, Monestiroli S, Ronzoni S, Carbone R, Pearson M, Pruneri G, et al. Impairment of p53 acetylation, stability and function by an oncogenic transcription factor. Embo J. 2004;23:1144–1154. doi: 10.1038/sj.emboj.7600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redner RL. Variations on a theme: the alternate translocations in APL. Leukemia. 2002;16:1927–1932. doi: 10.1038/sj.leu.2402720. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Brand NJ, Chen A, Chen SJ, Tong JH, Wang ZY, et al. Fusion between a novel Kruppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. Embo J. 1993;12:1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redner RL, Rush EA, Faas S, Rudert WA, Corey SJ. The t(5;17) variant of acute promyelocytic leukemia expresses a nucleophosmin-retinoic acid receptor fusion. Blood. 1996;87:882–886. [PubMed] [Google Scholar]
- 9.Wells RA, Catzavelos C, Kamel-Reid S. Fusion of retinoic acid receptor alpha to NuMA, the nuclear mitotic apparatus protein, by a variant translocation in acute promyelocytic leukaemia. Nat Genet. 1997;17:109–113. doi: 10.1038/ng0997-109. [DOI] [PubMed] [Google Scholar]
- 10.Arnould C, Philippe C, Bourdon V, Gr goire MJ, Berger R, Jonveaux P. The signal transducer and activator of transcription STAT5b gene is a new partner of retinoic acid receptor alpha in acute promyelocytic-like leukaemia. Hum Mol Genet. 1999;8:1741–1749. doi: 10.1093/hmg/8.9.1741. [DOI] [PubMed] [Google Scholar]
- 11.Catalano A, Dawson MA, Somana K, Opat S, Schwarer A, Campbell LJ, et al. The PRKAR1A gene is fused to RARA in a new variant acute promyelocytic leukemia. Blood. 2007;110:4073–4076. doi: 10.1182/blood-2007-06-095554. [DOI] [PubMed] [Google Scholar]
- 12.Kondo T, Mori A, Darmanin S, Hashino S, Tanaka J, Asaka M. The seventh pathogenic fusion gene FIP1L1-RARA was isolated from a t(4;17)-positive acute promyelocytic leukemia. Haematologica. 2008;93:1414–1416. doi: 10.3324/haematol.12854. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Tsuzuki S, Tsuzuki M, Handa K, Inaguma Y, Emi N. BCOR as a novel fusion partner of retinoic acid receptor alpha in a t(X;17)(p11;q12) variant of acute promyelocytic leukemia. Blood. 2010;116:4274–4283. doi: 10.1182/blood-2010-01-264432. [DOI] [PubMed] [Google Scholar]
- 14.Redner RL, Corey SJ, Rush EA. Differentiation of t(5;17) variant acute promyelocytic leukemic blasts by all-trans retinoic acid. Leukemia. 1997;11:1014–1016. doi: 10.1038/sj.leu.2400661. [DOI] [PubMed] [Google Scholar]
- 15.Du C, Redner RL, Cooke MP, Lavau C. Overexpression of wild-type retinoic acid receptor alpha (RARalpha) recapitulates retinoic acid-sensitive transformation of primary myeloid progenitors by acute promyelocytic leukemia RARalpha-fusion genes. Blood. 1999;94:793–802. [PubMed] [Google Scholar]
- 16.Rego EM, Ruggero D, Tribioli C, Cattoretti G, Kogan S, Redner RL, et al. Leukemia with distinct phenotypes in transgenic mice expressing PML/RAR alpha, PLZF/RAR alpha or NPM/RAR alpha. Oncogene. 2006;25:1974–1979. doi: 10.1038/sj.onc.1209216. [DOI] [PubMed] [Google Scholar]
- 17.Rush EA, Schlesinger KW, Watkins SC, Redner RL. The NPM-RAR fusion protein associated with the t(5;17) variant of APL does not interact with PML. Leuk Res. 2006;30:979–986. doi: 10.1016/j.leukres.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 18.Redner RL, Chen JD, Rush EA, Li H, Pollock SL. The t(5;17) acute promyelocytic leukemia fusion protein NPM-RAR interacts with co-repressor and co-activator proteins and exhibits both positive and negative transcriptional properties. Blood. 2000;95:2683–2690. [PubMed] [Google Scholar]
- 19.Colombo E, Alcalay M, Pelicci PG. Nucleophosmin and its complex network: a possible therapeutic target in hematological diseases. Oncogene. 2011;30:2595–2609. doi: 10.1038/onc.2010.646. [DOI] [PubMed] [Google Scholar]
- 20.Kwok C, Zeisig BB, Dong S, So CW. Forced homo-oligomerization of RARalpha leads to transformation of primary hematopoietic cells. Cancer Cell. 2006;9:95–108. doi: 10.1016/j.ccr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Lin RJ, Evans RM. Acquisition of oncogenic potential by RAR chimeras in acute promyelocytic leukemia through formation of homodimers. Mol Cell. 2000;5:821–830. doi: 10.1016/s1097-2765(00)80322-6. [DOI] [PubMed] [Google Scholar]
- 22.Minucci S, Maccarana M, Cioce M, De Luca P, Gelmetti V, Segalla S, et al. Oligomerization of RAR and AML1 transcription factors as a novel mechanism of oncogenic activation. Mol Cell. 2000;5:811–820. doi: 10.1016/s1097-2765(00)80321-4. [DOI] [PubMed] [Google Scholar]
- 23.Sternsdorf T, Phan VT, Maunakea ML, Ocampo CB, Sohal J, Silletto A, et al. Forced retinoic acid receptor alpha homodimers prime mice for APL-like leukemia. Cancer Cell. 2006;9:81–94. doi: 10.1016/j.ccr.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Rush EA, Pollock SL, Abecassis I, Redner RL. Interaction with RXR is necessary for NPM-RAR-induced myeloid differentiation blockade. Leuk Res. 2013;37:1704–1710. doi: 10.1016/j.leukres.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hood BL, Grahovac J, Flint MS, Sun M, Charro N, Becker D, et al. Proteomic analysis of laser microdissected melanoma cells from skin organ cultures. J Proteome Res. 2010;9:3656–3663. doi: 10.1021/pr100164x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chio, Sasaki M, Ghazarian D, Moreno J, Done S, Ueda T, et al. TRADD contributes to tumour suppression by regulating ULF-dependent p19(Arf) ubiquitylation. Nat Cell Biol. 2012;14:625–633. doi: 10.1038/ncb2496. [DOI] [PubMed] [Google Scholar]
- 27.Lee HH, Kim HS, Kang JY, Lee BI, Ha JY, Yoon HJ, et al. Crystal structure of human nucleophosmin-core reveals plasticity of the pentamer-pentamer interface. Proteins. 2007;69:672–678. doi: 10.1002/prot.21504. [DOI] [PubMed] [Google Scholar]
- 28.Swaney EM, Chattopadhyay A, Rush EA, Redner RL. Dominant-negative effect of the NPM-RAR fusion protein on NPM-mediated regulation of p53. preparation [Google Scholar]
- 29.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 30.Yoneda-Kato N, Look AT, Kirstein MN, Valentine MB, Raimondi SC, Cohen KJ, et al. The t(3;5)(q25.1;q34) of myelodysplastic syndrome and acute myeloid leukemia produces a novel fusion gene, NPM-MLF1. Oncogene. 1996;12:265–275. [PubMed] [Google Scholar]
- 31.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 32.Bender LM, Morgan MJ, Thomas LR, Liu ZG, Thorburn A. The adaptor protein TRADD activates distinct mechanisms of apoptosis from the nucleus and the cytoplasm. Cell Death Differ. 2005;12:473–481. doi: 10.1038/sj.cdd.4401578. [DOI] [PubMed] [Google Scholar]
- 33.Morgan M, Thorburn J, Pandolfi PP, Thorburn A. Nuclear and cytoplasmic shuttling of TRADD induces apoptosis via different mechanisms. J Cell Biol. 2002;157:975–984. doi: 10.1083/jcb.200204039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao RH, Berkova Z, Wise JF, Rezaeian AH, Daniluk U, Ao X, et al. PMLRARalpha binds to Fas and suppresses Fas-mediated apoptosis through recruiting c-FLIP in vivo. Blood. 2011;118:3107–3118. doi: 10.1182/blood-2011-04-349670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Z, Xin B, Yang X, Chan C, Cao L. Nuclear factor-kappaB activation is involved in LMP1-mediated transformation and tumorigenesis of rat-1 fibroblasts. Cancer Res. 2000;60:1845–1848. [PubMed] [Google Scholar]
- 36.Ndour PA, Brocqueville G, Ouk TS, Goormachtigh G, Morales O, Mougel A, et al. Inhibition of latent membrane protein 1 impairs the growth and tumorigenesis of latency II Epstein-Barr virus-transformed T cells. J Virol. 2012;86:3934–3943. doi: 10.1128/JVI.05747-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heasman SA, Zaitseva L, Bowles KM, Rushworth SA, Macewan DJ. Protection of acute myeloid leukaemia cells from apoptosis induced by front-line chemotherapeutics is mediated by haem oxygenase-1. Oncotarget. 2011;2:658–668. doi: 10.18632/oncotarget.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rushworth SA, MacEwan DJ. HO-1 underlies resistance of AML cells to TNF-induced apoptosis. Blood. 2008;111:3793–3801. doi: 10.1182/blood-2007-07-104042. [DOI] [PubMed] [Google Scholar]
- 39.Chio, Sasaki M, Ghazarian D, Moreno J, Done S, Ueda T, et al. TRADD contributes to tumour suppression by regulating ULF-dependent p19(Arf) ubiquitylation. Nat Cell Biol. 2012 doi: 10.1038/ncb2496. [DOI] [PubMed] [Google Scholar]
- 40.Testa U, Grignani F, Samoggia P, Zanetti C, Riccioni R, Lo Coco F, et al. The PML/RARalpha fusion protein inhibits tumor necrosis factor-alpha-induced apoptosis in U937 cells and acute promyelocytic leukemia blasts. J Clin Invest. 1998;101:2278–2289. doi: 10.1172/JCI1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witcher M, Ross DT, Rousseau C, Deluca L, Miller WH., Jr. Synergy between alltrans retinoic acid and tumor necrosis factor pathways in acute leukemia cells. Blood. 2003;102:237–245. doi: 10.1182/blood-2002-09-2725. [DOI] [PubMed] [Google Scholar]
- 42.Witcher M, Shiu HY, Guo Q, Miller WH., Jr Combination of retinoic acid and tumor necrosis factor overcomes the maturation block in a variety of retinoic acid-resistant acute promyelocytic leukemia cells. Blood. 2004;104:3335–3342. doi: 10.1182/blood-2004-01-0023. [DOI] [PubMed] [Google Scholar]
- 43.Wu WS, Xu ZX, Hittelman WN, Salomoni P, Pandolfi PP, Chang KS. Promyelocytic leukemia protein sensitizes tumor necrosis factor alpha-induced apoptosis by inhibiting the NF-kappaB survival pathway. J Biol Chem. 2003;278:12294–12304. doi: 10.1074/jbc.M211849200. [DOI] [PubMed] [Google Scholar]
- 44.Milovic-Holm K, Krieghoff E, Jensen K, Will H, Hofmann TG. FLASH links the CD95 signaling pathway to the cell nucleus and nuclear bodies. Embo J. 2007;26:391–401. doi: 10.1038/sj.emboj.7601504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salomoni P, Pandolfi PP. The role of PML in tumor suppression. Cell. 2002;108:165–170. doi: 10.1016/s0092-8674(02)00626-8. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi Y, Lallemand-Breitenbach V, Zhu J, de The H. PML nuclear bodies and apoptosis. Oncogene. 2004;23:2819–2824. doi: 10.1038/sj.onc.1207533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.