Abstract
Both otoacoustic emissions (OAEs) and auditory evoked potentials (AEPs) are sexually dimorphic, and both are believed to be influenced by prenatal androgen exposure. OAEs and AEPs were collected from people affected by 1 of 3 categories of disorders of sex development (DSD) – (1) women with complete androgen insensitivity syndrome (CAIS); (2) women with congenital adrenal hyperplasia (CAH); and (3) individuals with 46, XY DSD including prenatal androgen exposure who developed a male gender despite initial rearing as females (men with DSD). Gender identity (GI) and role (GR) were measured both retrospectively and at the time of study participation, using standardized questionnaires. The main objective of this study was to determine if patterns of OAEs and AEPs correlate with gender in people affected by DSD and in controls. A second objective was to assess if OAE and AEP patterns differed according to degrees of prenatal androgen exposure across groups. Control males, men with DSD, and women with CAH produced fewer spontaneous OAEs (SOAEs) – the male-typical pattern – than control females and women with CAIS. Additionally, the number of SOAEs produced correlated with gender development across all groups tested. Although some sex differences in AEPs were observed between control males and females, AEP measures did not correlate with gender development, nor did they vary according to degrees of prenatal androgen exposure, among people with DSD. Thus, OAEs, but not AEPs, may prove useful as bioassays for assessing early brain exposure to androgens and predicting gender development in people with DSD.
Keywords: auditory evoked potential (AEP), otoacoustic emission (OAE), disorders of sex development (DSD), congenital adrenal hyperplasia (CAH), complete androgen insensitivity syndrome (CAIS), gender identity (GI), gender role (GR), sex difference
Disorders of sex development (DSD) are congenital conditions in which genetic, hormonal and phenotypic sex are discordant (Hughes et al., 2006). For some types of DSD, a significant minority of affected individuals reject their assigned sex as they develop through childhood and adolescence, and this is the case especially in individuals possessing a 46, XY chromosomal complement and ambiguous external genitalia at birth due to atypical prenatal exposure to androgens who are reared female (Migeon et al., 2002). Currently, newborn genital phenotype is used as a bioassay to infer early brain exposure to androgens for people with DSD. However, evidence has been accumulating that genital phenotype alone does not predict who will develop a female or male gender (Migeon et al., 2002). Accordingly, there is clinical value in identifying measures of nervous system masculinization associated with gender development to aid in clinical decision-making regarding sex of rearing for newborns with certain types of DSD. Past attempts at identifying biomarkers of early hormone exposure other than genital phenotype, such as finger-length ratio, have resulted in mixed findings not amenable to clinical use (Berenbaum et al, 2009; Brown et al., 2002; Buck et al., 2003; Ökten et al., 2002).
The main goal of the present study was to assess if physiologic measures of the auditory system, otoacoustic emissions (OAEs) and auditory evoked potentials (AEPs), can be used to compare gender in people with DSD (Cohen-Bendahan et al., 2005). Previous attempts at exploring associations between sexually dimorphic auditory measures and sextyped behaviors other than sexual orientation, such as spatial ability, have not revealed relationships between OAEs, AEPs and behaviors; however, these studies did not include participants with DSD (Loehlin and McFadden, 2003). The current study includes participants with DSD, who historically report more atypical gender behavior than controls (Hughes et al., 2006), potentially increasing our ability to observe correlations between auditory measures and behaviors of interest.
A secondary goal of this study was to determine if OAEs and AEPs are influenced by prenatal androgen exposure in humans. Various measures of OAEs and AEPs differ between boys and girls at birth (Burns, Arehart & Campbell, 1992; Strickland, Burns & Tubis, 1985; Hall, 2007), and these sex differences have been shown to be influenced by prenatal androgen manipulations in nonhuman species (McFadden et al., 2006a; McFadden et al., 2006b; see McFadden 2008, 2009 for reviews). With the inclusion of participants affected by complete androgen insensitivity syndrome (CAIS) and congenital adrenal hyperplasia (CAH) we are able to investigate OAEs and AEPs in women exposed to less than, and greater than, female-typical levels of androgens early in development.
OAEs are sounds produced in the cochlea that propagate back through the middle ear into the external ear canal (Kemp, 2002; Lonsbury-Martin and Martin, 2008). One form of OAE, spontaneous OAEs (SOAEs), are tonal sounds that occur in the absence of acoustic stimulation; these are more common in females than in males, are more common in right ears than left ears, are present at birth with the same sex and ear differences as are observed in adults (Burns et al., 1992; Strickland et al., 1985), and (except for ears with acquired hearing loss) are relatively constant through life (Burns 2009). Click-evoked OAEs (CEOAEs) are echo-like sounds generated in the cochlea in response to clicks presented to the ear. The typical measure of CEOAEs is their rms amplitude expressed in decibels soundpressure level (dB SPL). For both infants and adults, CEOAEs are stronger in females than in males and stronger in right ears than in left (McFadden and Pasanen, 1998; Newmark et al., 2011; Snihur and Hampson, 2011).
AEPs are a series of brain waves that can be recorded using electrodes on the earlobes and the scalp when click stimuli are presented to the ears (Hall, 2007). Several of these early-occurring waves constitute the auditory brainstem response (ABR) and the latter constitute the middle-latency response (MLR), both of which were measured in the present study. Like OAEs, ABRs exhibit sex differences that are evident in newborns and adults (Hall, 2007). Specifically, ABRs of females generally have shorter latencies than those of males, and often exhibit larger amplitudes (Hall, 2007; McFadden and Champlin, 2000). Sex differences in the MLR have not been systematically explored (Goldstein and Aldrich, 1999).
Materials and Methods
Subjects
People with DSD were recruited from the SUCCEED Clinic at the University of Oklahoma Health Sciences Center (OUHSC) and from local and national DSD support groups in contact with the clinic. DSD categories studied included (1) women with CAIS including a 46, XY chromosomal complement, (2) women with CAH due to 21-hydroxylase deficiency including a 46, XX chromosomal complement, and (3) men with DSD including a 46, XY chromosomal complement and ambiguous genitalia at birth due to partial gonadal dysgenesis, partial testosterone biosynthetic defects, partial androgen insensitivity syndrome or unknown etiology (men with DSD). Although 46, XY DSD can also result in instances where prenatal androgen exposure is male-typical, such as in cases of cloacal exstrophy and aphallia, none of the men with DSD in this study were affected by these conditions. All participants with DSD, regardless of type, were initially reared female. People with CAIS were reared female, despite their possessing a male sex chromosome complement, because they were born with female external genitalia and typically develop a female GI. Historically, men with DSD were reared female at birth, despite possessing a male sex chromosome complement, because it was believed that their atypical external genitalia were incapable of performing penetrative sex, and also that they would develop a female GI through environmental influences and learning (Hughes et al., 2006). Control participants were men and women not affected by DSD and whose medical history included at least one urinary tract infection (UTI); they were recruited from the Department of Urology at OUHSC. All participants were 16 years of age or older, spoke English at an 8th grade level or higher and provided informed consent.
A total of 51 participants provided informed consent and completed the study. Among participants, 14 were control females with a mean age of 29 yr (range 16–54), 4 (29%) of whom reported the use of oral contraceptives (OCPs) at the time of testing. Twelve participants were women with CAIS (mean age = 41, range 16–59 yrs). All women with CAIS were gonadectomized prior to study participation; 4 (33%) reported estrogen replacement and 3 (25%) reported estrogen plus testosterone replacement. Nine participants were women with CAH (mean age = 28, range 16–56 yrs) and all reported both cortisol and mineralocorticoid replacement and no OCPs. Among male participants, 11 were controls with a mean age of 25 yr (range 16–38), none of whom reported testosterone replacement currently. Five participants were men with DSD (mean age = 17, range 16–18 yrs) including early androgen exposure, who were initially gonadectomized and assigned to a female sex of rearing in infancy. Three (60%) of these 5 men reported using testosterone replacement at the time of testing.
Gender questionnaires
All participants completed the Recalled Childhood Gender Identity/Gender Role Scale (RCGIS; Zucker et al., 2006), a validated 23-item self-report of GI and GR recalled from childhood and the Masculine Gender Identity Scale for Females, Part A (MGISF; Blanchard and Freund, 1983), a 20-item self-report of GR and cross gender wishes during the previous 12-month period. Control males and DSD men completed a modified version of the Masculine Gender Identity Scale where “man” was substituted for “woman” and “male” was substituted for “female.” Finally, all participants completed a medical history form that focused on ear/auditory health.
Physiologic measures
All participants received standard audiometric tests for hearing sensitivity and middle-ear function. Every individual ear having hearing loss greater than 20 dB at more than 1 audiometric test frequency (6 kHz or lower) was flagged, and data from that ear were not included in any analysis. An otoscope was used to verify that ear canals were clear prior to testing. During OAE and AEP recordings, participants sat in a recliner chair inside an electrically shielded, single-wall, sound-treated booth. A desktop computer (Dell Optiplex 380 Windows 7 OS) running LabVIEW software controlled a digital board (NI-PCI-4461) that generated the acoustic stimuli and digitized and stored the OAE and AEP signals. All signals were collected during a single test session lasting approximately 3 hrs. OAEs were measured first for half of the subjects and AEPs first for the other half. SOAEs were measured prior to CEOAEs, and for both OAEs and AEPs, the ear tested first was counter-balanced across subjects.
OAEs
For OAE measures, an Etymotic ER-10C insert microphone/earphone system was inserted into the external ear canal. This self-contained system consisted of 2 earphones for presenting acoustic stimuli plus 1 microphone for recording cochlear emissions. The output of the microphone was passed to a custom-built buffer/amplifier and high-pass filter (cut-off frequency = 400 Hz), and then to the National Instruments sound board to be digitized.
When measuring SOAEs, at least four 30-sec recordings were obtained from each ear canal, and this 2-min recording window was divided into segments of approximately 328 ms whose onsets were incremented by 82 ms (75% overlap). RMS level for each time segment was computed and a histogram of those levels was assembled for each ear of each participant. The 100 time segments having the lowest RMS levels were identified, a fast- Fourier transform (FFT) was computed for each of them, and the resulting 100 spectra were averaged. This averaged spectrum then was processed to eliminate all peaks exceeding a criterion magnitude and to estimate the local noise floor at each frequency in each ear. The smoothed spectrum then was compared with the initial averaged spectrum and peaks exceeding 5 standard deviations were defined as SOAEs (for additional detail see Pasanen and McFadden, 2000).
For measuring CEOAEs, a series of electrical pulses (100-µs duration and negative polarity) was delivered to 1 earphone channel of the ER-10C microphone/earphone system. Pulses (clicks) were delivered in groups of 10 at a rate of 10 clicks per second. Beginning at the onset of each click, 50 ms of the stimulus and echo-like response was collected. Those individual waveforms were summed with responses from the previous clicks until acceptable responses to 250 clicks were obtained. A 50-kHz sampling rate with 24-bit precision was used for stimulus generation and response recording. To characterize CEOAE magnitude, the average CEOAE response was band-pass filtered from 1.0–5.0 kHz, the rms amplitude of that response was calculated for a 20.48-ms segment beginning 6 ms after click onset, and that amplitude was converted to decibels sound-pressure level (dB SPL). CEOAE responses were collected using 3 click levels – 69, 75, and 81 dB SPL.
AEPs
Peaks in the AEP waveform are grouped according to their post-stimulus latency (Hall, 2007), with those between 0–10 ms corresponding to the auditory brainstem responses (ABRs), and those between 10–70 ms to the middle-latency responses (MLRs). The AEP recording procedure employed gold-plated surface electrodes placed on the vertex (Cz, the “positive” electrode), the forehead (Fpz, the “ground” electrode) and on each earlobe (A1 and A2, the “negative” electrodes) in accord with standard procedures (Hall, 2007). The four surface electrodes permitted the collection of two channels of data simultaneously, one from the electrode on the earlobe ipsilateral to the ear containing the earphone and one from the electrode on the contralateral earlobe. A train of electrical pulses (100-µs duration) was applied to a shielded insert earphone (Etymotic, model ER-3A) to produce rarefaction clicks with a level of 35- or 70-dB above mean behavioral threshold.
Time intervals between successive clicks were randomized over a range of +/− 10 ms, yielding average rates of 16.7 and 7.1 clicks per second for ABR and MLR, respectively. Scalp potentials were differentially amplified (Grass model P5; gain = 100,000) and bandpass filtered (−24 dB/octave rejection rate), with pass bands of 0.1 – 3.0 kHz and 10 – 300 Hz for the ABR and MLR potentials, respectively. During and following each click, the voltage waveform on each channel was digitized by a 24-bit analog-to-digital converter having a 50-kHz sampling rate. At each level, data collection was preceded by a 20-second silent period during which electrical activity was monitored to obtain a distribution of RMS values in the absence of stimuli. During data collection, if the RMS level ever exceeded the median value + 0.75 SDs, click presentation was suspended until the RMS level fell below the criterion or 5 seconds elapsed. Also during data collection, a maximum peak voltage (irrespective of polarity) in each sweep was recorded and compared to the distribution of peak voltages collected up to that point. If the peak value in the sweep exceeded 0.75 SDs above the median of the distribution, the sweep was discarded. These precautions reduced artifacts in the measures of interest. A total of 2000 and 800 click responses were averaged to obtain the ABRs and MLRs, respectively.
At the end of testing, 4 average waveforms were available for analyses: the ipsilateral and contralateral responses when the earphone was in the left and right ears. Latencies and amplitudes were measured for the same peaks in all 4 average waveforms. Of interest were peaks I and V of the ABR and peaks Po, Na, Pa, and Nb of the MLR. Amplitudes were measured from the peak to the following minimum for peaks I and V, and from Po to Na and Pa to Nb. Accordingly, there were 10 AEP measurements (6 latencies and 4 amplitudes) for each of the 4 average waveforms. In an effort to reduce variability, the data for earphone left/electrode left were averaged with the data for earphone right/electrode left (left hemisphere data), and the data for earphone right/electrode right were averaged with the data for earphone left/electrode right (right hemisphere data). This analysis was conducted by an individual with experience reading AEP waveforms who was blind to participants’ group membership. Author CAC was consulted when a waveform was ambiguous, occurring for < 5% of the waveforms.
Predictions
The prenatal-androgen-exposure argument posits that some sexually dimorphic measures of the auditory system are influenced by androgens during early development (McFadden, 2008; McFadden, 2009). This account presumes that androgen exposure exceeding female-typical levels during prenatal development permanently masculinizes structures or mechanisms in the cochlea and auditory pathway. This account also predicts that (1) the OAEs and AEPs in women with CAIS should be female-typical due to the absence of functioning androgen receptors associated with this DSD despite the presence of a 46, XY sex chromosome complement. (2) OAEs and AEPs in women with CAH should be masculinized as a result of their greater-than-typical prenatal androgen exposure secondary to 21-hydroxylase deficiency despite the presence of a 46, XX sex chromosome complement. (3) OAEs and AEPs in men with DSD should be at least partially masculinized due to their prenatal androgen exposure, inferred by their ambiguous genitalia at birth, that exceeds female-typical levels. Furthermore, comparisons between women with CAIS including a 46, XY chromosomal complement, women with CAH including a 46, XX chromosomal complement, and men with DSD including a 46, XY chromosomal complement allow for examining the influence of the sex chromosomes, independent of early androgen exposure, on the production of OAEs and AEPs (Cohen-Bendahan et al., 2005). If development of structures and neural circuits underlying auditory processing overlaps temporally with development of neural circuits that underlie gender development, then it is reasonable to expect that our auditory measures would correlate with gender development.
Data Analyses
Questionnaire items were coded 1 – 5 as indicated by Blanchard and Freund (1983) and Zucker et al. (2006) then recoded depending on the context of the question so that a “1” indicated more female-like/less male-like progressing to a “5” indicating more male-like/less female-like. This was done because under the standard scoring a male who has strongly accepted his male gender will score a 5, and similarly a female who has strongly accepted her female gender will score a 5. Even though one is very male and the other is very female there is no difference in their scores. To separate male from female genders reversing the standard scoring was necessary for one of these situations. For each item, differences between pairs of groups were tested using a Mann-Whitney U test (p-values are indicated in Tables 1 & 2). Note that Cohen’s d was 0.9 or greater for any comparison with a p-value of 0.05 or less.
Table 1.
Recalled Childhood Gender Identity/Gender Role Scale. Values shown are Mann-Whitney U test p-values for the pairwise group comparisons denoted at the top of each column. Questions were coded 1 – 5 depending on the context of the question so that a “1” indicated more female-like/less male-like progressing to a “5” indicating more male-like/less female-like. Only questions that showed a significant (p < 0.05) difference between female and male controls are shown in the table. All items with a p-value < 0.05 had an effect size > 0.9.
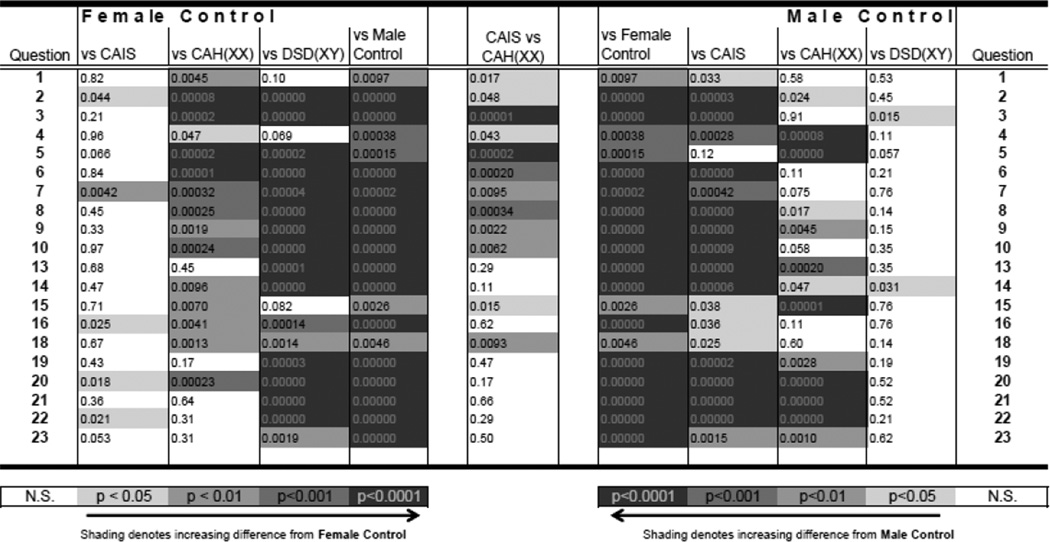 |
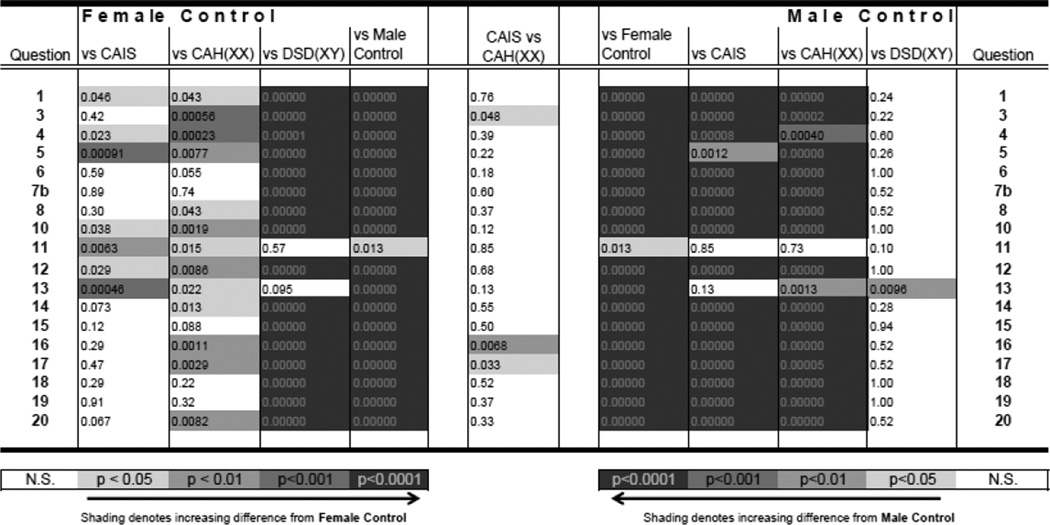
Table 2.
Masculine Gender Identity Scale for Females, Part A. Values shown are Mann-Whitney U test p-values for the pairwise group comparisons denoted at the top of each column. Values shown as "0.00000" are simply <0.000005. Questions were coded 1 – 5 depending on the context of the question so that a “1” indicated more female-like/less male-like progressing to a “5” indicating more male-like/less female-like. Only questions that showed a significant (p < 0.05) difference between female and male controls are shown in the table. All items with a p-value < 0.05 had an effect size > 0.9.
 |
A summary score, the average squared difference (ASD), was created for each participant to summarize the item differences of each participant from those of the average female control response (ASDf) and of the average male control response (ASDm). Only items that showed a significant (Mann-Whitney p < 0.05) difference between female and male controls were included in these summary scores: thus for the RCGIS, items 11, 12 and 17 were omitted, and for the MGIS, items 2, 9 and 11 were omitted. Note that a participant with an ASDf = 0 has a set of item responses that each equal the average item response of the female controls. Similarly, a participant with an ASDm = 0 has a set item responses that each equal the average item response of the male controls. For each questionnaire, discriminantfunction analysis was then used to assess differences between pairs of groups based on participants’ ASDf and ASDm.
For comparisons with OAEs and AEPs a one-dimensional measure of gender (masculinity) was created for each questionnaire by regressing ASDf on ASDm (r2 = 93%) and determining the distance of the projection of each individual on to this regression line from the point on this line determined by ASDf = 0 (the point of least masculinity). Thus, high values of this masculinity score (MS) are found for male controls and low values for female controls. Regression analyses of participants’ OAEs and AEPs on their MS were performed to determine if relationships were observed between self-reported gender scores and OAEs. Data were analyzed using IBM SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). A value of p < 0.05 was nominally accepted as significant.
Differences between groups for OAEs and AEPs were summarized using effect sizes. Cohen’s d was calculated as the difference between the means in the 2 conditions of interest divided by the square root of the weighted mean of the variances for the 2 conditions. Large effect sizes can arise from large differences between the means or small variability within the groups. By convention, effect sizes of 0.2, 0.5, and 0.8 are taken as small, medium, and large differences, respectively (Cohen, 1992).
To estimate how likely our outcomes were due to chance, a resampling procedure was used (McFadden et al., 2010). For each two-group comparison for the OAE and AEP measures (e.g., control females with women with CAIS, control males with men with DSD, etc.) measured values for all participants were pooled into one set. Then, individual values were reselected from the set at random without replacement until a subgroup of the same size as 1 of the original groups had been drawn. The remaining values formed a second subgroup, and the effect size for this random partitioning was calculated. This random partitioning was repeated 10,000 times for each measure, and the number of resamples where the absolute value of the effect size exceeded the original (actual) effect size was recorded. That number was divided by 10,000, and the resulting proportion was used to assess the (implied) significance for that 2-group comparison. In parallel with traditional tests of significance, a comparison was judged to be significantly different when that proportion was 0.05 or smaller. The use of the absolute value of the resampled effect sizes yields conservative (“two-tailed”) estimates of implied significance. Effect sizes for all group comparisons for OAEs and AEPs are provided in the online supplement to this paper.
Results
Gender questionnaires
Results from the Recalled Childhood Gender Identity/Gender Role Scale (see Table 1) reveal that control female participants were significantly more feminine/less masculine in their GR than control males during their childhood, as indicated by 20 of the 23 questionnaire items (Discriminant Function (DF) p < 0.0001). Concerning just those 20 questionnaire items that elicited sex differences for controls, women with CAIS were more feminine/less masculine in their GR than control men on 19 of 20 items (DF p < 0.0001). In contrast, women with CAIS responded differently from control women on measures of GR on only 5 of the 20 questionnaire items (Table 1, DF p = 0.002). Women with CAH were more feminine/less masculine in their GR than control men on 13 of 20 questionnaire items (DF p < 0.0001), and more masculine/less feminine than control women on 15 of the 20 questionnaire items (DF p < 0.001). Furthermore, women with CAH responded differently from women with CAIS on 11 of the 20 questionnaire items assessing GR (DF p = 0.022). Thus, women with CAIS reported a GR more similar to control women, while women with CAH reported a GR less similar to control women, in spite of the fact that all reported a female GI at study participation. Finally, men with DSD differed from control males on only 2 questionnaire items assessing GR (DF p = 0.14) and differed from control women on 17 of the 20 items (DF p < 0.0001) even though the men with DSD had been reared initially as females. All men with DSD and control males reported a male GI at study participation.
Results from the Masculine Gender Identity Scale for Females, Part A (see Table 2) reveal that control females were significantly more feminine/less masculine in their GI and GR than control males within the past 12 months of study participation on all 20 questions (DF p < 0.0001). Women with CAIS also were more feminine/less masculine in their GI and GR than control males on all but 2 of the 20 questions (DF p < 0.0001). Similarly, women with CAH were more feminine/less masculine in their GI and GR than control males on all but 2 of the items that differed by sex among controls (DF p < 0.0001). Women with CAH only differed on 3 questions from women with CAIS in their responses to this questionnaire (DF p = 0.086). In contrast, men with DSD differed from control women on all but 1 questionnaire items assessing GI and GR (DF p < 0.0001), but answered differently from control men on only 1 item (DF p = 0.34).
SOAEs and CEOAEs
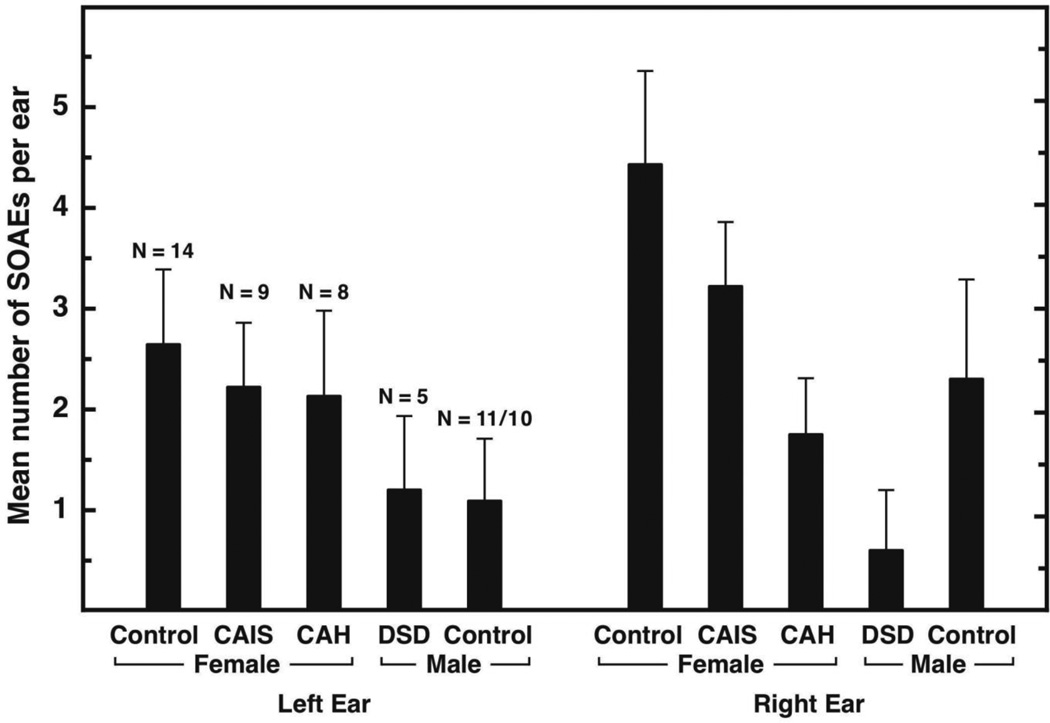
The number of SOAEs measured in controls was typical (e.g., Bilger et al., 1990; McFadden and Pasanen, 1999; Pasanen and McFadden, 2000), greater in control females than control males (effect sizes = 0.62 and 0.64 for left and right ears, respectively), and greater in right ears than in left ears (effect sizes = 0.57 and 0.46 for females and males, respectively; Figure 1). None of these differences achieved implied significance under resampling. For women with CAH, the effect size (women with CAH minus control women) for SOAEs was -0.91 (implied significance: p = 0.04) for the right ear. For men with DSD, the effect size (men with DSD men minus control women) for SOAEs was -1.25 for the right ear (implied significance: p = 0.03). No other group differences achieved implied significance for SOAEs.
Figure 1.
Mean (+SEM) number of SOAEs detected in left and right ears, shown separately for the 5 participant groups. The frequency range 0.55 – 9.0 kHz was examined for SOAEs in each ear. The number of left and right ears contributing data is shown for each group; right-ear data for 1 control male were omitted because of hearing loss. The “Female” and “Male” designations on the abscissa indicate the gender with which participants identified.
Mean strength of CEOAEs (control females minus control males) did not reveal a sex difference for either ear, contrary to previous reports (for reviews see McFadden, 2008, 2009). Additionally, none of the DSD groups differed significantly from control females for this measure. We have no explanation for the absence of the previously reported sex difference in CEOAEs.
Because group differences were observed for SOAEs according to degrees of prenatal androgen dose, regression analyses were performed comparing participants’ SOAEs to their responses to items in questionnaires 1 and 2 via their masculinity score (MS). Regression between SOAEs (both ears averaged) and MS on both questionnaires was significant (ps < 0.05), meaning that participants who reported more masculine/less feminine responses to the gender questionnaires also produced the smallest number of SOAEs. The Ns were too small to provide meaningful correlations between SOAEs and gender within participant groups.
AEPs
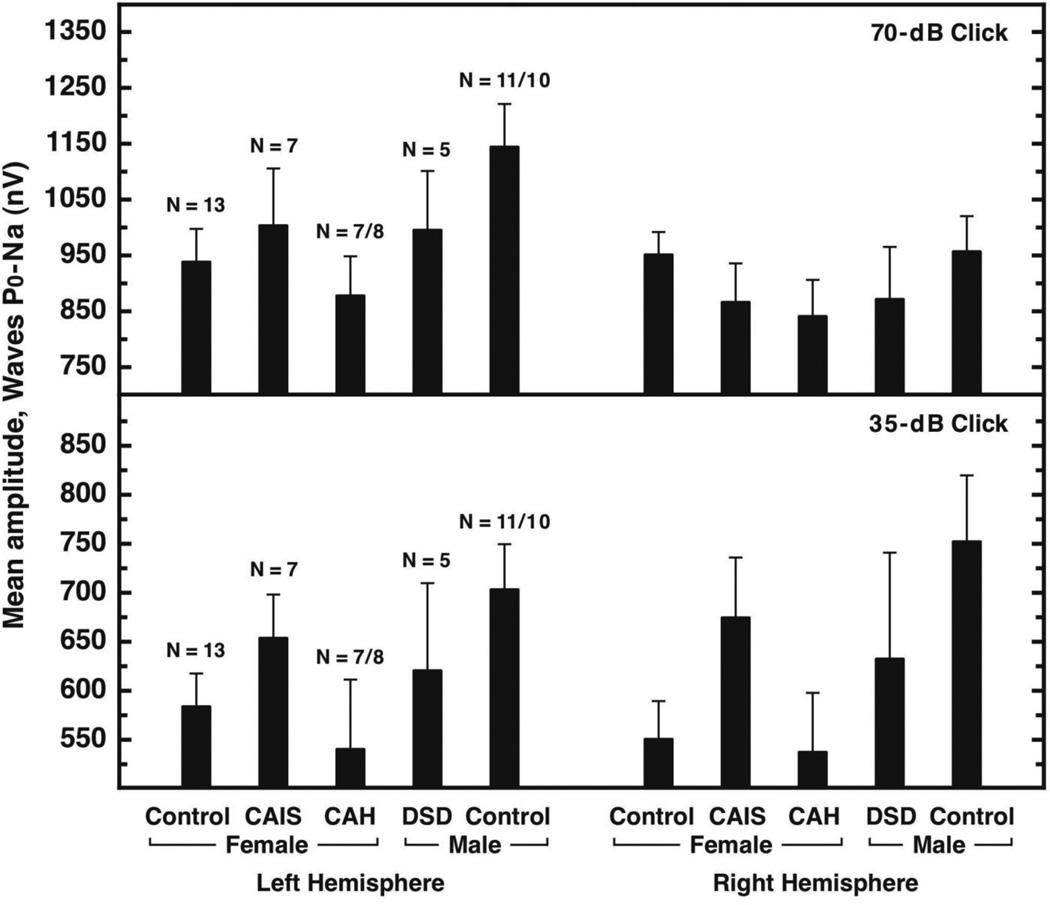
Also contrary to previous reports (see Hall, 2007), the sex differences commonly reported for ABR measures were absent in our control groups. The AEP measure that revealed the most consistent difference between control women and control men was the Po-Na component of the MLR (Figure 2). The Po-Na amplitude was greater in control men than control women for the 35-dB click level when recorded from both sides of the head (effect sizes = 0.87 and 1.1 for left and right hemispheres, respectively) and for the 70-dB click level when recorded from the left side of the head (effect size = 0.88 for left hemisphere). These differences reached statistical significance (p < 0.05) under re-sampling. However, none of the differences between the control female group and participants with DSD (women with CAIS, women with CAH and men with DSD) yielded large effect sizes, and none achieved implied significance during resampling.
Figure 2.
Mean (+SEM) amplitudes for the Po-Na component of the middle latency response acquired with the 70-dB click (top) and the 35-dB click (bottom) shown separately for the 5 participant groups recorded over the left and right hemispheres. The values shown for Left Hemisphere were averages of the data collected with earphone left/electrode left and earphone right/electrode left. The values shown for Right Hemisphere were averages of the data collected with earphone right/electrode right and earphone left/electrode right. The “Female” and “Male” designations on the abscissa indicate the gender with which participants identified.
Figures showing all of the OAE and AEP data can be found in the online supplement to this paper, along with effect sizes for all group comparisons.
Summary and Conclusions
Self-reported GR and GI by all groups of participants with DSD were consistent with previous studies (Dittmann et al., 1990a; Dittmann et al., 1990b; Hines et al., 2003; Hines et al., 2004; Hines, 2011; Mattila et al., 2012; Meyer-Bahlburg et al., 2006; Migeon et al, 2002; Pasterski et al., 2011; Wisniewski et al, 2000; Wisniewski et al, 2004; Zucker et al., 1996).
Concerning the auditory measures, women with CAH and men with DSD showed masculinized patterns of SOAEs in their right-ear measures. No other consistent patterns of differences were observed for the DSD groups for OAEs or AEPs. This pattern of results supports our prediction that sexually dimorphic patterns of SOAEs are influenced by prenatal androgen exposure, not a 46, XY chromosomal complement, and this effect is most evident for right ear measures. Our study was not designed to determine if a minimal or maximal number of SOAEs produced correlate with male or female GI. All interpretations of these outcomes must take into account the fact that the control groups did not exhibit sex differences previously reported for CEOAEs and AEPs. Replication of the current results is necessary to determine if SOAEs can be used as a bioassay, in addition to genital phenotype at birth, for assessing prenatal androgen exposure. Further studies to determine if SOAEs prospectively predict GI also are needed prior to utilizing these auditory measures to understand gender development.
Finally, the combined gender and auditory data from women with CAIS in the current study can be used to evaluate: (1) whether or not the aromatization hypothesis of brain/behavior masculinization observed in nonhuman species generalizes to gender and sexually dimorphic auditory measures in humans (Zuloaga et al., 2008), and (2) whether or not sex differences in cochlear function and gender development are influenced by sex chromosomes. Estrogen receptors exist in adult cochleas in rodents (Meltser et al., 2008), but have not been observed in fetal cochleas (Simonoska et al., 2009). Our data challenge the ideas that sex differences in human nervous system function (OAEs and AEPs) and behavior (GI and GR) result from aromatization of androgens to estrogens.
Supplementary Material
Highlights.
Spontaneous otoacoustic emissions vary according to prenatal androgen exposure.
SOAEs are correlated with gender behavior in humans.
People with Disorders of Sex Development exhibit different patterns of SOAEs.
Acknowledgments
This work was supported by a research grant awarded to ABW by the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD, R21HD067718). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We thank the study participants for their time and effort in completing this project.
List of Abbreviations
* Authors recommend deleting because they are common knowledge to readers
- ABR
auditory brainstem response
- AEP
auditory evoked potential
- CAH
congenital adrenal hyperplasia
- CAIS
complete androgen insensitivity syndrome
- CEOAE
click-evoked otoacoustic emission
- * dB
decibel
- DSD
disorder of sexual development
- FFT
fast Fourier transform
- * GI
gender identity
- * GR
gender role
- * kHz
kilohertz
- * ms
millisecond
- * n
number of subjects
- OAE
otoacoustic emission
- OCPs
oral contraceptive pills
- PAIS
partial androgen insensitivity syndrome
- * rms
root mean square
- SOAE
spontaneous otoacoustic emission
- SPL
sound-pressure level
- UTI
urinary tract infection
- * µs
microsecond
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous versions of this work were presented at the meetings of the American Academy of Pediatrics in 2012 and the Endocrine Society in 2014.
Contributor Information
Blas Espinoza-Varas, Email: Blas-Espinoza-varas@ouhsc.edu.
Christopher E. Aston, Email: chris-aston@ouhsc.edu.
Shelagh Edmundson, Email: sedmund608@aol.com.
Craig A. Champlin, Email: champlin@austin.utexas.edu.
Edward G. Pasanen, Email: pasanen@psy.utexas.edu.
Dennis McFadden, Email: mcfadden@psy.utexas.edu.
References
- Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. Fingers as a marker of prenatal androgen exposure. Endocrinology. 2009;150:5119–5124. doi: 10.1210/en.2009-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger R, Matthies ML, Hammel DR, Demorest ME. Genetic implications of gender differences in the prevalence of spontaneous otoacoustic emissions. J Speech Lang Hear Res. 1990;33:418–432. doi: 10.1044/jshr.3303.418. [DOI] [PubMed] [Google Scholar]
- Blanchard R, Freund K. Measuring masculine gender identity in females. J Counseling and Clinical Psychology. 1983;51:205–214. doi: 10.1037//0022-006x.51.2.205. [DOI] [PubMed] [Google Scholar]
- Brown WM, Hines M, Fane B, Breedlove SM. Masculinized finger length patterns in human males and females with congenital adrenal hyperplasia. Horm Behav. 2002;42:380–386. doi: 10.1006/hbeh.2002.1830. [DOI] [PubMed] [Google Scholar]
- Buck JJ, Williams RM, Hughes IA, Acerini CL. In-utero androgen exposure and 2nd and 4th digit length ratio-comparisons between healthy controls and females with classical congenital adrenal hyperplasia. Hum Reprod. 2003;18:976–979. doi: 10.1093/humrep/deg198. [DOI] [PubMed] [Google Scholar]
- Burns EM, Arehart KH, Campbell SL. Prevalence of spontaneous otoacoustic emissions in neonates. J Acoust Soc Am. 1992;91:1571–1575. doi: 10.1121/1.402438. [DOI] [PubMed] [Google Scholar]
- Burns EM. Long-term stability of spontaneous otoacoustic emissions. J Acoust Soc Am. 2009;125:3166–3176. doi: 10.1121/1.3097768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CCC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neuroscience and Biobehavioral Reviews. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Kappes MH, Kappes ME, Börger D, Stegner H, Willig RH, et al. Congenital adrenal hyperplasia I: Gender-related behavior and attitudes in female patients and sisters. Psychoneuroendocrinology. 1990a;15:401–420. doi: 10.1016/0306-4530(90)90065-h. [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Kappes MH, Kappes ME, Börger D, Meyer-Bahlburg HF, Stegner H, et al. Congenital adrenal hyperplasia II: Gender-related behavior and attitudes in female salt-wasting and simple-virilizing patients. Psychoneuroendocrinology. 1990b;15:421–434. doi: 10.1016/0306-4530(90)90066-i. [DOI] [PubMed] [Google Scholar]
- Goldstein R, Aldrich WM. Evoked Potential Audiometry: Fundamentals and Applications. Boston, MA: Allyn and Bacon; 1999. [Google Scholar]
- Hall JW., III . New Handbook of Auditory Evoked Responses. Boston, MA: Pearson Education; 2007. [Google Scholar]
- Hines M, Ahmed SF, Hughes IA. Psychological outcomes and gender-related development in complete androgen insensitivity syndrome. Arch Sex Behav. 2003;32:93–101. doi: 10.1023/a:1022492106974. [DOI] [PubMed] [Google Scholar]
- Hines M, Brook C, Conway GS. Androgen and psychosexual development: Core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH) J Sex Res. 2004;41:75–81. doi: 10.1080/00224490409552215. [DOI] [PubMed] [Google Scholar]
- Hines M. Prenatal endocrine influences on sexual orientation and on sexually differentiated childhood behavior. Front Neuroendo. 2011;32:170–182. doi: 10.1016/j.yfrne.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes IA, Houk C, Ahmed SF, Lee PA LWPES/ESPE Consensus Group. Consensus Statement on Management of Intersex Disorders. Archives of Diseases of Children. 2006;91(7):554–563. doi: 10.1136/adc.2006.098319. and Pediatrics 118, 488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DT. Otoacoustic emissions, their origin in cochlear function, and use. Brit Med Bull. 2002;63:223–241. doi: 10.1093/bmb/63.1.223. [DOI] [PubMed] [Google Scholar]
- Loehlin JC, McFadden D. Otoacoustic emissions, auditory evoked potentials, and traits related to sex and sexual orientation. Arch Sex Behav. 2003;32:115–127. doi: 10.1023/a:1022496207882. [DOI] [PubMed] [Google Scholar]
- Long D, Wisniewski AB, Migeon CJ. Gender role across development in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Ped Endocrinol Metab. 2004;17:167–172. doi: 10.1515/jpem.2004.17.10.1367. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Martin GK. Otoacoustic emissions: Basic studies in mammalian models. In: Manley GA, Popper AH, Fay RR, editors. Active Processes and Otoacoustic Emissions. Vol. 14. New York, NY: Springer Series in Auditory Research, Springer-Verlag; 2008. pp. 261–303. [Google Scholar]
- Mattila AK, Fagerholm R, Santtila P, Miettinen PJ, Taskinen S. Gender identity and gender role orientation in female assigned patients with disorders of sex development. J Urol. 2012;188:1930–1934. doi: 10.1016/j.juro.2012.07.018. [DOI] [PubMed] [Google Scholar]
- McFadden D. Masculinizing effects on otoacoustic emissions and auditory evoked potentials in women using oral contraceptives. Hear Res. 2000;142:23–33. doi: 10.1016/s0378-5955(00)00002-2. [DOI] [PubMed] [Google Scholar]
- McFadden D. What do sex, twins, spotted hyenas, ADHD, and sexual orientation have in common? Persp Psychol Sci. 2008;3:309–323. doi: 10.1111/j.1745-6924.2008.00082.x. [DOI] [PubMed] [Google Scholar]
- McFadden D. Masculinization of the mammalian cochlea. Hear Res. 2009;252:37–48. doi: 10.1016/j.heares.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Comparison of the auditory systems of heterosexuals and homosexuals: Click-evoked otoacoustic emissions. Proc Natl Acad Sci USA. 1998;95:2709–2713. doi: 10.1073/pnas.95.5.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Spontaneous otoacoustic emissions in heterosexuals, homosexuals, and bisexuals. J Acoust Soc Am. 1999;105:2403–2413. doi: 10.1121/1.426845. [DOI] [PubMed] [Google Scholar]
- McFadden D, Champlin CA. Comparison of auditory evoked potentials in heterosexual, homosexual, and bisexual males and females. J Assoc Res Otolaryngol. 2000;1:89–99. doi: 10.1007/s101620010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG, Raper J, Lange HS, Wallen K. Sex differences in otoacoustic emissions measured in rhesus monkeys (Macaca mulatta) Horm Behav. 2006a;50:274–284. doi: 10.1016/j.yhbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG, Weldele ML, Glickman SE, Place NJ. Masculinized otoacoustic emissions in female spotted hyenas (Crocuta crocuta) Horm Behav. 2006b;50:285–292. doi: 10.1016/j.yhbeh.2006.03.013. [DOI] [PubMed] [Google Scholar]
- McFadden D, Hsieh MD, Garcia-Sierra A, Champlin CA. Differences by sex, ear, and sexual orientation in the time intervals between successive peaks in auditory evoked potentials. Hear Res. 2010;270:56–64. doi: 10.1016/j.heares.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson J-A, Canlon B. Estrogen receptor protects against acoustic trauma in mice. J Clin Invest. 2008;118:1563–1570. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg HF, Dolezal C, Baker SW, Ehrhardt AA, New MI. Gender development in women with congenital adrenal hyperplasia as a function of disorder severity. Arch Sex Behav. 2006;35:667–684. doi: 10.1007/s10508-006-9068-9. [DOI] [PubMed] [Google Scholar]
- Migeon CJ, Wisniewski AB, Gearhart JP, Meyer-Bahlburg HFL, Rock JA, Brown TR, Money J. Ambiguous genitalia with perineoscrotal hypospadias in 46, XY individuals: Long-term medical, surgical and psychosexual outcome. Pediatrics. 2002;110:e31. doi: 10.1542/peds.110.3.e31. [DOI] [PubMed] [Google Scholar]
- Newmark M, Merlob P, Bresloff I, Olsha M, Attias J. Click evoked otoacoustic emissions: Inter-aural and gender differences in newborns. J Basic Clin Physiol Pharmacol. 2011;8:133–140. doi: 10.1515/jbcpp.1997.8.3.133. [DOI] [PubMed] [Google Scholar]
- Ökten A, Kalyoncu M, Yaris N. The ratio of second- and fourth-digit lengths and congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Early Hum Dev. 2002;70:47–54. doi: 10.1016/s0378-3782(02)00073-7. [DOI] [PubMed] [Google Scholar]
- Pappas KB, Wisniewski AB, Migeon CJ. Gender role across development in adults with 46, XY disorders of sex development including perineoscrotal hypospadias and small phallus raised male or female. J Pediatr Endocrinol Metab. 2008;21:625–630. doi: 10.1515/jpem.2008.21.7.625. [DOI] [PubMed] [Google Scholar]
- Pasanen EG, McFadden D. An automated procedure for identifying spontaneous otoacoustic emissions. J Acoust Soc Am. 2000;108:1105–1116. doi: 10.1121/1.1287026. [DOI] [PubMed] [Google Scholar]
- Pasterski V, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. Prenatal hormones and childhood sex segregation: Playmate and play style preferences in girls with congenital adrenal hyperplasia. Horm Behav. 2011;59:549–555. doi: 10.1016/j.yhbeh.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoska R, Stenberg A, Masironi B, Sahlin L, Hultcrantz Estrogen receptors in the inner ear during different stages of pregnancy and development in the rat. Acta Oto-Laryngol. 2009;129:1175–1181. doi: 10.3109/00016480802691150. [DOI] [PubMed] [Google Scholar]
- Snihur AWK, Hampson E. Sex and ear differences in spontaneous and click-evoked otoacoustic emissions in young adults. Brain Cogn. 2011;77:40–47. doi: 10.1016/j.bandc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Snihur AWK, Hampson E. Click-evoked otoacoustic emissions: Response amplitude is associated with circulating testosterone levels in men. Behav Neurosci. 2012;126:325–331. doi: 10.1037/a0027193. [DOI] [PubMed] [Google Scholar]
- Strickland EA, Burns EM, Tubis A. Incidence of spontaneous otoacoustic emissions in children and infants. J Acoust Soc Am. 1985;78:931–935. doi: 10.1121/1.392924. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Migeon CJ, Meyer-Bahlburg HFL, Gearhart JP, Berkovitz GD, Brown TR, Money J. Complete androgen insensitivity syndrome: Long-term medical, surgical and psychosexual outcome. JCEM. 2000;85:2664–2669. doi: 10.1210/jcem.85.8.6742. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Migeon CJ, Malouf MA, Gearhart JP. Psychosexual outcome in women affected by congenital adrenal hyperplasia due to 21-hydroxylase deficiency: an emphasis on long-term surgical results. J Urol. 2004;171:2497–2501. doi: 10.1097/01.ju.0000125269.91938.f7. [DOI] [PubMed] [Google Scholar]
- Zucker KJ, Bradley SJ, Oliver G, Blake J, Fleming S, Hood J. Psychosexual development of women with congenital adrenal hyperplasia. Horm Behav. 1996;30:300–318. doi: 10.1006/hbeh.1996.0038. [DOI] [PubMed] [Google Scholar]
- Zucker KJ, Mitchell JN, Bradley SJ, Tkachuk J, Cantor JM, Allin SM. The recalled childhood gender identity/gender role questionnaire: Psychometric properties. Sex Roles. 2006;54:469–483. [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: What we've learned from the testicular feminization mutation. Horm Behav. 2008;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.