Abstract
The proton pumping activity of bovine heart cytochrome c oxidase (CcO) is completely inhibited when all of the cardiolipin (CL) is removed from the enzyme to produce monomeric CcO containing only 11 subunits. Only dimeric enzyme containing all 13 subunits and 2–4 cardiolipin per CcO monomer exhibits a “normal” proton translocating stoichiometry of ~1.0 H+ per/e− when reconstituted into phospholipid vesicles. These fully active proteoliposomes have high respiratory control ratios (RCR=7–15) with 75–85% of the CcO oriented with the cytochrome c binding sites exposed to the external medium. In contrast, reconstitution of CL-free CcO results in low respiratory control ratios (RCR < 5) with the enzyme randomly oriented in the vesicles, i.e., ~50 percent oriented with the cytochrome c binding site exposed on the outside of the vesicle. Addition of exogenous CL to the CL-free enzyme completely restores electron transport activity, but restoration of proton pumping activity does not occur. This is true whether CL is added to CL-free CcO prior to reconstitution into phospholipid vesicles, or whether CL is included in the phospholipid mixture that is used to form the vesicles. Another consequence of CL removal is the inability of the 11-subunit, CL-free enzyme to dimerize upon exposure to either cholate or the cholate/PC/PE/CL mixture used during proteoliposome formation (monomeric, 13-subunit, CL-containing CcO completely dimerizes under these conditions). Therefore, a major difference between reconstitution of CL-free and CL-containing CcO is the incorporation of monomeric, rather than dimeric CcO into the vesicles. We conclude that bound CL is necessary for proper insertion of CcO into phospholipid vesicles and normal proton translocation.
Keywords: cytochrome c oxidase, proton pumping, cardiolipin, liposomes
1. Introduction
Cardiolipin (CL) is a major mitochondrial glycerophospholipid that is localized exclusively within the mitochondrial inner membrane [1,2,3]. CL is essential for the functional and/or structural integrity of a number of mitochondrial inner membrane protein complexes including cytochrome c oxidase (CcO) [4,5,6,7], cytochrome bc1 [8,9,10,11], succinate dehydrogenase (complex II) [12], NADH:CoQ oxidoreductase (complex I) [13,14], ADP/ATP carrier [15], and ATP synthase [16,17]. Among these proteins, CcO is the best characterized mitochondrial complex that depends upon CL.
Bovine heart cytochrome c oxidase is a member of the heme-copper oxidase super-family. It catalyzes the reduction of oxygen to water by ferrocytochrome c with coincident translocation of protons across the mitochondrial inner membrane [18,19,20]. The structural organization of bovine heart CcO is quite complicated. Within three dimensional crystals, bovine CcO is a dimer of 13-subunit monomers [21,22]. This organization is believed to reflect its structure within the inner mitochondrial membrane. Three of the thirteen subunits are mitochondrially encoded, ten are nuclearly encoded [23]. Mitochondrially encoded subunits I and II contain all four of the redox centers, (CuA, heme a, heme a3 and CuB), that participate in individual electron transfer steps involved in the reduction of oxygen by ferrocytochrome c [22]. The functional importance of the third mitochondrially encoded subunit, subunit III, is currently not completely understood, although thought to be involved in the proton pumping activity of CcO [24,25,26]. It also may function to stabilize the enzyme. For example, within the Rhodobacter sphaeroides enzyme, subunit III stabilizes the enzyme’s catalytic lifespan and thus prevents its suicide inactivation [27,28].
Purified, detergent-solubilized bovine heart CcO has a structural and functional requirement for 3–4 CL that are tightly bound to the proteins within each monomer [5,6,29,30]. Two of these CL are clearly visible in the crystal structure and are located between transmembrane helices of adjacent protein subunits [21]. If CL is completely removed and replaced by detergent or other phospholipids, electron transport activity decreases by 50–60 percent of normal [5,6]. Full electron-transport activity is restored by exogenous CL, but not by other phospholipids. Coincident with CL removal is the irreversible dissociation of subunits VIa and VIb [5,6]. Removal of CL also destabilizes the association of subunits III and VIIa making them more susceptible to dissociation by structural perturbants [6]. These effects are consistent with the fact that CL is bound adjacent to subunits III, VIb, and VIIa, while subunit VIIa rests on top of subunit VIIb [21]. Cardiolipin stabilizes all of these interactions by forming strong ionic interactions between the negatively charged polar head group of CL and the positively charged side chains of the protein while the fatty acid tails contact apolar amino acids. A direct consequence of CL removal and dissociation of subunits VIa and VIb is the inability of the CcO monomers to dimerize since subunits VIa and VIb participate in major protein contacts at the dimer interface. CL, therefore, acts as a type of “glue” that stabilizes the quaternary structure of CcO. CL has been suggested to serve a similar role in the formation of super-complexes within the mitochondrial inner membrane [31,32].
The importance of dimeric CcO to its function has been the subject of debate for several decades and is still not completely understood. Dimerization clearly is not essential for normal electron transfer activity since monomeric CcO has the same activity as the dimer. However, the possibility that dimerization plays a critical role in proton-translocation remains a topic of discussion. To address this problem, we have analyzed the effect of CL removal on proton translocation activity of CcO. Because CL removal prevents CcO self-association, these studies should help address the question whether such structural changes restrain alter, or inhibit the proton pumping activity of the enzyme.
2. Materials and Methods
2.1. Materials
Bovine cardiolipin (1,3-diphosphatidyl-sn-glycerol); dioleoylphosphatidylcholine (1,2-dioleoyl-sn-glycero-3-phosphocholine, DOPC); and dioleoylphosphatidylethanolamine (l,2-dioleoyl-sn-glycero-3-phosphoethanolamine, DOPE), each in chloroform, were obtained from Avanti Polar Lipids, Inc. Dodecyl maltoside (DM) was obtained from Anatrace. Horse heart cytochrome c (type III); Crotalus atrox venom; N,N,N',N'-tetramethyl-1,4-benzenediamine dihydrochloride (TMPD); carbonyl cyanide 3-chlorophenylhydrazone (CCCP); valinomycin; phenol red; sodium ascorbate; and sodium cholate were obtained from Sigma Chemical Co. Bio-Beads SM-2 were purchased from Bio-Rad Laboratories. All other chemicals were reagent grade.
2.2. Methods
Bovine cytochrome c oxidase was isolated from Keilin-Hartree particles [33] by the method of Fowler et al. [34] with modifications described previously [35]. HiTrapQ anion-exchange column chromatography was subsequently used to remove small amounts of contaminating cytochrome bc1 and all phospholipids except for 2–4 CL that remain tightly bound to the enzyme [5]. The purified complex had a molecular activity of 340 – 370 s−1 when assayed spectrophotometrically by following the pseudo-first order rate of ferrocytochrome c (30 µM) oxidation by 1.8 nM CcO in 50 mM phosphate buffer pH 7.2 containing 2 mM DM as described by Dale and Robinson [36]. Cardiolipin free detergent-solubilized CcO was obtained by treatment of CL–containing CcO with phospholipase A2 (PLA2) [6].
Incorporation of cytochrome c oxidase into liposomes
CcO-containing vesicles were prepared by a minor modification of the method of Casey [37]. The procedure was as follows: a suspension of 50 mg of phospholipids (defined below) in 1 mL of 0.1 M HEPES buffer, pH 7.4, containing 2% sodium cholate was sonicated on ice under nitrogen using a Branson model 250 Sonifier, until the solution became clear (~5 min). Unless otherwise specified, the suspension of phospholipids contained 25 mg DOPC and 25 mg DOPE (1:1 w/w). The purified CcO was exposed to Bio-Beads SM-2 to remove excess dodecyl maltoside before it was mixed with the phospholipid mixture. The procedure was to add 150 mg of Bio-Beads SM-2 per mL of CcO (2 mg/mL), stir the mixture for 15 min at 25 °C, and then remove the Bio-Beads by brief centrifugation [38]. The resulting detergent-depleted CcO was subsequently added to the phospholipid-cholate mixture described above at a final protein concentration of 1 mg/mL and a final protein to phospholipid ratio 1:40 (mg/mg). At the high molar ratio of phospholipid to protein that were used to prepare either CL-rich, or CL-free proteoliposomes, all of the enzyme was incorporated into the vesicles as indicated by the elution of a single peak when the products were analyzed by gel filtration chromatography (data not shown). The CcO-containing proteoliposomes were then formed by extensive dialysis of the CcO-phospholipid-cholate solution against 0.1 M HEPES, pH 7.4 for 8 hours; 10 mM HEPES, 40.0 mM KCl, 50.0 mM sucrose, pH 7.4 for 12 hours; and finally against 1 mM HEPES, 44.0 mM KCl, 55.0 mM sucrose, pH 7.4 for 8 hours.
Characterization of proteoliposomes
The orientation of CcO that had been incorporated into vesicles was determined using visible difference spectroscopy (ΔA606–630) after reduction of cytochrome a within a CcO-CN complex [39]. Two impermeant electron donors (5 mM sodium ascorbate and 3.5 µM cytochrome c), were added followed by the addition of a permeant electron donor (2.5 mM TMPD). The ratio of ΔA606–630 before and after addition of TMPD yields the fraction of CcO with its cytochrome c binding site exposed on the external surface of the proteoliposome. The RCR of reconstituted CcO was calculated from the ratio of the rate of oxidation of ferrocytochrome c before and after the addition of 3 µM CCCP plus 30 µM valinomycin recorded at 550 minus 540 nm [18].
Measurement of proton translocation
Proton pumping activity of CcO incorporated into vesicles was measured spectrophotometrically using phenol red as the absorption pH indicator. Extra-vesicular pH changes were detected at 600 nm minus 564 nm [40]. Absorbance changes were calibrated using a standard solution of HCl. The H+/e- ratio was calculated by dividing the moles of protons extruded by the moles of cytochrome c added.
Cytochrome c oxidase subunit composition analysis
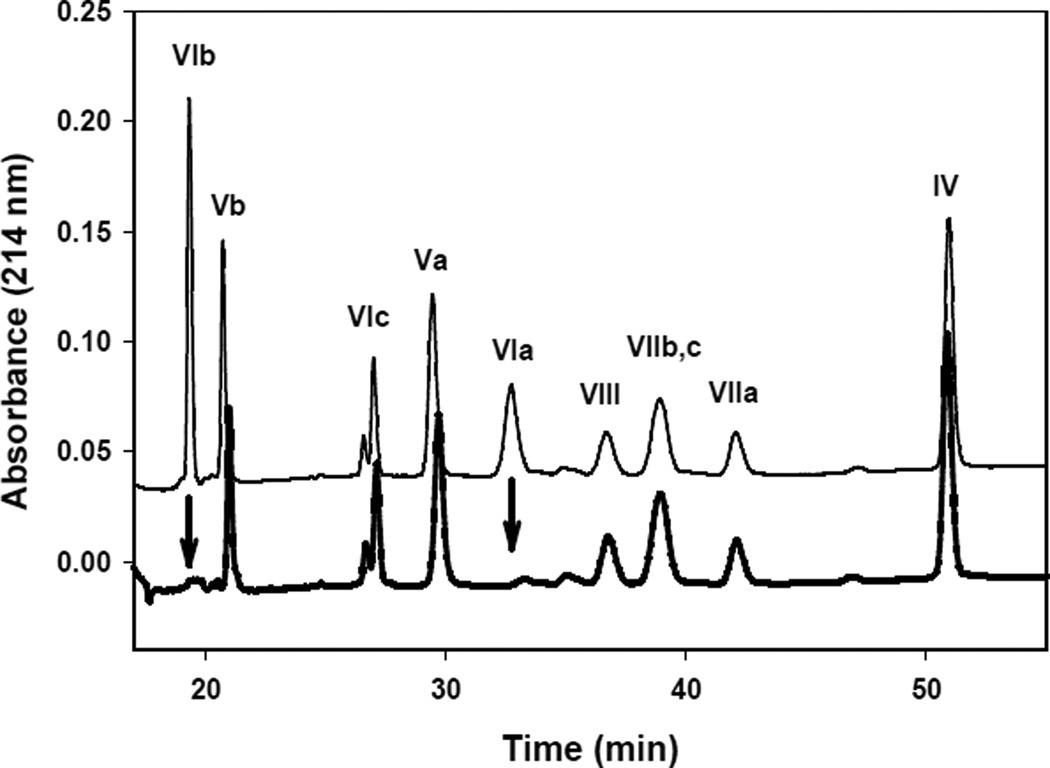
The content of the three mitochondrially encoded subunits was determined by quantitative scanning of Coomassie brilliant blue stained SDS-PAGE gels. The content of the 10 nuclearly encoded CcO subunits was determined using C18 reversed phase HPLC as described by Sedlák and Robinson [6] (Figure 1).
Figure 1. Separation of cytochrome c oxidase nuclear-encoded subunits by C18 reversed-phase-HPLC before (upper thin chromatogram) and after (lower thick chromatogram) cardiolipin removal.
In both cases 1.5 µM of cytochrome c oxidase was analyzed. The arrows indicate dissociated subunits VIa and VIb.
Sedimentation velocity
All analytical ultracentrifugation experiments were performed in a Beckman Optima XL-A analytical ultracentrifuge at the Center for Analytical Ultracentrifugation of Macromolecular Assemblies of the University of Texas Health Science Center at San Antonio. The sample preparation and experimental conditions were essentially the same as described previously [41,42]. CcO samples in dodecyl maltoside/cholate, or PL/cholate mixtures were prepared by dilution of CL-containing or CL-free enzyme (1 mg/mL) with the 20 mM Tris-SO4 buffer, pH 7.4, containing 2 mM sodium cholate and either: 1) 2 mM dodecyl maltoside; or 2) 20 mM PC/PE/CL (45% : 45% : 10%) mixture. The resulting solutions were then dialyzed for 12–16 h against buffer containing 0.2 mM dodecyl maltoside and 2 mM sodium cholate prior to centrifugation at 40 000 RPM in a Beckman XL/A centrifuge as described previously [42]. The resulting data were analyzed by the method of van Holde and Weischet using the UltraScan software developed by B. Demeler at Department of Biochemistry UTHSC at San Antonio and described in detailed at http://www.cauma.uthscsa.edu/
3. Results
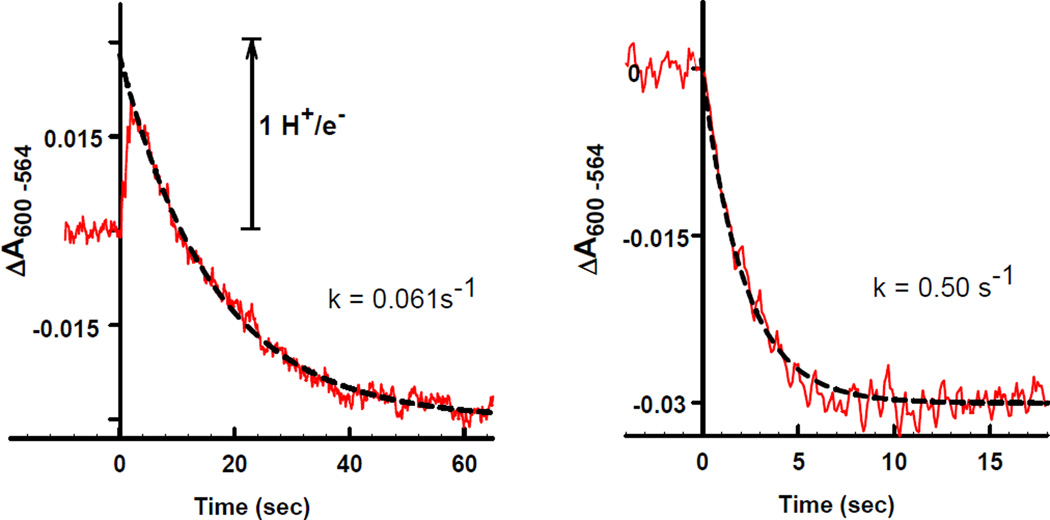
The addition of ferrocytochrome c to reconstituted CL-containing CcO results in very rapid acidification due to CcO proton translocation activity followed by much slower alkalinization (Figure 2, left panel). The rapid acidification is too rapid to be detected using this experimental approach, but can be determined by extrapolation of the slower alkalinization to the time of cytochrome c addition. The proton pumping stoichiometry is calculated from the magnitude of the rapid acidification. The slower alkalinization phase is due to proton leakage back into proteoliposomes [18]. The alkalinization phase exists whether proton translocation occurs or not due to chemical consumption of 1 H+/e− from the interior of the vesicle during the reduction of oxygen to water with subsequent equilibration of the alkaline vesicle interior with the bulk phase. The rate of alkalinization depends only upon how well vesicles are sealed, not upon the proton pumping activity of the enzyme.
Figure 2. Proton pumping activity of reconstituted 13-subunit cardiolipin-containing cytochrome c oxidase (left panel) and 11-subunit cardiolipin-free cytochrome c oxidase (right panel).
Concentration of CcO reconstituted into DOPC-DOPE vesicles was 0.1 µM. Rapid acidification occurred upon addition of 1 µM ferrocytochrome c to CL-free CcO (left panel). Unexpected alkalinization after addition of the same amount of ferrocytochrome c was observed with CL-free CcO (right panel). Rapid alkalinization was also observed in both cases in the presence of 5 µM CCCP (not shown). pH changes were calibrated by addition of standard HCl. Experimental results are in red; and fitted curves are dashed black lines.
Proteoliposomes prepared using CL-containing CcO exhibited “normal” proton translocating activity of ~1.0 H+/e−. The proteoliposomes are well sealed with a slow first-order rate constant for alkalinization of k = 0.061 s−1, RCR value of 10–12, with CcO asymmetrically inserted, i.e., 75–85% of the CcO is oriented with the cytochrome c binding sites exposed to the external medium (Table 1).
Table I.
Characteristics of reconstituted phospholipids vesicles
| Cytochrome c Oxidase | Orientation of CcO (% facing out) |
RCR | H+/e− | |||
|---|---|---|---|---|---|---|
| −CL | +CL | −CL | +CL | −CL | +CL | |
| CL-containing | 65–75 | 70–75 | 10–12 | 10–12 | 0.93 | 0.93 |
| CL-free | 45–50 | 60–65 | 2–4 | 5–7 | 0 | 0 |
CcO containing phospholipid vesicles were prepared using a mixture of DOPC and DOPE (50:50) or a mixture of DOPC:DOPE:CL (45:45:10). Orientation of CcO was calculated from the degree of reduction of cytochrome a within a CcO-cyanide complex by externally added ascorbic acid plus cytochrome c followed by the addition of TMPD. Respiratory control ratio (RCR) was determined from the ratio of the respiration rate in the presence of valinomycin plus CCCP to that in the absence of ionophores. H+/e− ratio was calculated as described in Figure 2.
In contrast to the results obtained with CL-containing CcO, CL-free enzyme did not exhibit any measurable proton translocation activity, i.e., the rapid acidification phase due to proton translocation was not detected (Figure 2, right panel). Only the slower alkalinization phase occurs, but at a rate ten times more rapidly than with the CL-containing CcO, i.e., k = 0.50 s−1. Occasionally a rate of alkalinization for reconstituted CL-free CcO was comparable to that observed for reconstituted CL-containing CcO, but proton pumping activity still was not detected. We, therefore, conclude that the rather large proton “leakage” rate observed for the CL-containing proteoliposomes is not responsible for the lack of an observable acidification phase. However, proteoliposomes prepared from CL-free CcO have a number of defects. They have very low RCR values (2–4) and are inserted in a random, rather than ordered orientation (i.e., only 45–50% of the cytochrome c binding sites of CcO are exposed to the exterior medium). If CL-free CcO does not pump protons, its poor RCR value is the result of translocation of only one electric charge across the membrane instead of the two charges by CL-containing CcO. The decreased number of translocated charges would make the CL-free enzyme much less sensitive to control by membrane potential. Occasionally slightly better RCR values of 5–7 were observed, but these preparations still did not exhibit any measureable proton pumping activity.
Addition of exogenous CL to detergent solubilized CL-free CcO restores normal electron transport activity, i.e., kcat is restored to ~90 percent of normal (320–340 s−1) from an initial value that is 45–50 percent of normal [6]. For this reason, the ability of exogenous CL to restore full proton pumping activity to CL-free CcO was tested. Inclusion of CL in the preparation of the proteoliposomes, i.e., vesicles made from 45% DOPC and 45% DOPE and 10% CL, had a positive effect since the orientation of CcO in the vesicles was improved by about 10 percent with either type of enzyme (Table I). Inclusion of CL also increased the RCR for CL-free CcO by about 2 fold (RCR = 5–7); however, it had no observable effect upon RCR values for CL-containing CcO. Nevertheless, inclusion of CL did not improve the proton pumping stoichiometry for either CL-containing, or CL-free CcO (Table I); the former remained at 0.93 H+/e−, while the latter could not be detected.
The self-association state of CL-free CcO was determined in dodecyl maltoside solution and under conditions known to induce dimerization of CL-containing CcO, i.e., a mixture of dodecyl maltoside and cholate. Dodecyl maltoside solubilized CL-free CcO sediments predominately as a monomer with approximately 70% of the enzyme having an s20,W of 10.5 S (Figure 3, filled circles). The rest of the enzyme population generates a slightly larger species with sedimentation coefficients ranging from 10.5 to 12 S, most likely due to a small amount of nonspecific aggregation. Inclusion of sodium cholate induces dimerization of 13-subunit, monomeric CcO [42] with s20,W of 15.5 – 16 S (Figure 3, open triangles). However, inclusion of cholate does not induce dimerization of the CL-free CcO (Figure 3, open circles). Of course, CL-free CcO is also missing subunits VIa and VIb, which almost certainly are required for dimerization; therefore, it is unclear whether removal of CL alone is sufficient to prevent dimerization. Essentially the same result was obtained when the aggregation state of CL free enzyme was analyzed in cholate/PC/PE/CL mixture (data not shown), a condition known to induce dimerization of the 13-subunit, CL-containing enzyme [42].
Figure 3. Cardiolipin-free cytochrome c oxidase does not dimerize.
CcO self-association was quantified by sedimentation velocity at 40 000 rpm and 20 °C. Both CL-containing (triangles) and CL-free CcO (circles) were analyzed in 20 mM Tris-SO4 buffer, pH 7.4, containing only 2 mM dodecyl maltoside with (filled symbols) or 2 mM dodecyl maltoside and 2 mM sodium cholate (open symbols).
Discussion
Cardiolipin has been resolved within the crystal structure of several of the electron transfer complexes: two CL’s within CcO [21,43]; one CL within cytochrome bc1 [9,44,45,46]; and one CL, within succinate dehydrogenase (Complex II) [47]. Protein bound CL has also been resolved in the three-dimensional structure of mitochondrial creatine kinase [48], as well as the Rhodobacter sphaeroides photosynthetic reaction center [49]. In each case, the CL is buried in crevices between transmembrane helices. It has been shown, at least for CcO and cytochrome bc1, that bound cardiolipin is important for the structural and functional integrity of the CL-containing enzymes. However, a conclusive mechanism by which this phospholipid might be directly or indirectly involved in either electron transfer and/or proton translocation is not evident.
In the present, work we have demonstrated that the removal of CL from bovine CcO prior to its incorporation into phospholipid vesicles, decreases the respiratory control ratio of the resulting proteoliposomes, prevents asymmetric insertion of CcO into the vesicles, and completely destroys proton translocation activity. CL, therefore, must either directly or indirectly alter or perturb the proton pumping apparatus. To analyze the possible explanation(s) of these phenomena, the localization and possible role of individual CL’s bound to CcO needs to be clarified.
Cytochrome c oxidase bound cardiolipin
Two molecules of CL are visible in the CcO crystal structure [21,43]. The first CL (CL1) is located between subunit VIa on one monomer and subunit III on the other monomer, and probably stabilizes the dimeric structure. The second CL (CL2) is localized between subunits VIIa and I. In our preparation of purified bovine heart CcO 3–4 CL are tightly bound to the enzyme. Analysis of the subunit labeling pattern with arylazido-CL photo-labels suggests that a third molecule of CL (CL3) is bound between subunits VIIa and VIIc near the entrance to the D-channel [30]. In addition, not one but two CL’s are possibly bound with lower affinity at the dimer interface of CcO near subunit VIa. Removal of these two CL’s near the dimer interface has very little or no effect upon electron-transport activity, but occurs coincident with the dissociation of both subunit VIa and VIb (Figure 1) [6]. Because subunits VIa and VIb form the major monomer to monomer contacts at the CcO dimer interface, their dissociation would be expected to disrupt the CcO dimer and produce an 11-subunit monomer. The 11-subunit monomer remains fully capable of catalyzing normal electron transfer [6]. Currently it is not known whether subunits VIa and VIb dissociation occurs coincident with CL removal, or whether CL removal destabilizes the enzyme so that these subunits are removed during purification of the enzyme by HiTrap Q, anion-exchange chromatography. What is known is that other subunits are not affected. The remaining two CL’s, those near subunit VIIa, can be removed only by phospholipase A2 digestion, which produces a CL-free preparation of CcO [6]. These latter two CL’s bind with high affinity near a group of subunits including VIIa and VIIc. One of these CL’s is almost certainly nestled between subunits VIIa and III and is visible in the crystal structure. The other, which is not visible in the crystal structure, is the one we proposed to be bound near the entrance to the D-channel between subunits VIIa and VIIc [30]. Together, these two CL’s near subunit VIIa are required for maximum electron-transport activity of CcO, possibly by regulating the import of protons into the D-channel. The removal of these 2 CL’s causes a 50–60% loss of electron-transport activity that is completely restored by re-association of one or two CL [5,6]; their removal, however, completely and irreversibly destroys proton pumping (present work).
How removal of bound cardiolipins affects cytochrome c oxidase proton pumping
Incorporation of CL-free CcO into liposomes results in a low fraction of enzyme incorporated with the cytochrome c binding site exposed to the bulk solution, a low RCR, and a complete absence of any proton translocation activity. The addition of CL to the DOPC-DOPE vesicles has little or no effect on either the orientation of incorporated enzyme, RCR value, or proton pumping. This is in contrast to the effect that CL has upon the electron transfer activity which is fully restored by addition of exogenous CL to detergent-solubilized CL-free CcO. This result can only be explained by the unique positions and properties of the bound CL’s. Indeed, coarse-grain molecular dynamic simulations [7] have confirmed the proposal of Sedlák et al. [30] that two CL bind with high affinity near the entrance to the D - and H-channels. It is well-known that even a small perturbation near CcO proton pathways can significantly affect the functionality of the enzyme. For example, site-directed, single-point, mutagenesis of amino-acids within the D-channel decouples proton translocation within CcO having either “normal” or high turnover [50,51]. Therefore, removal of CL from CcO appears to generate an enzyme with similar properties. If CL is bound near the entrance to the proton channels, and if its removal perturbs proton uptake, this would support the idea that phospholipid actively participates in proton trapping [52]. Very recently, using molecular dynamic simulation, essentially the same conclusion about CL’s unique position and possible role in proton uptake of cytochrome bc1 was made by Armarez et al. [10] and Pöyry et al. [11].
The alternative explanation, that only a CcO dimer can function as a proton pump, is less likely. Indeed, bacterial CcO is clearly capable of proton translocation although there is no evidence that it forms dimers. Additional support that monomeric CcO is sufficient for normal proton translocation is evident from studies on the structural organization of mammalian and yeast supercomplexes [53,54]. Only a single copy of CcO, i.e. a CcO monomer, is present within a functional supercomplex consisting of monomeric Complex I, dimeric Complex III, and monomeric CcO. Therefore, if these respiratory supercomplexes truly represent the functional units within the mitochondrion, CcO can be fully functional in a monomeric state. The proton pumping machinery does not depend upon a dimeric enzyme.
In summary, the present work examines the requirement of CcO for tightly bound CL for normal proton pumping activity. The experimental results provide evidence for either a direct or indirect participation of CcO bound CL in proton pumping. Removal of CL from CcO results in a number of irreversible changes that completely destroy the proton translocation ability of CcO; the electron transfer activity of CL free enzyme, however, is about half of normal CcO activity, and is completely restored by exogenous CL [5,6, present work]. The most plausible explanation is that the removal of CL’s (CL2 and CL3) perturbs the entrance to the D-channel preventing normal proton pumping. Such structural perturbation would have to be irreversible since the inhibition of proton translocation is irreversible and cannot be restored by the reassociation of CL. While some questions remain about the exact mechanism by which CL is involved in proton movement, our results indicate that bound CL is absolutely necessary for proper insertion of CcO into phospholipid vesicles and is essential for normal proton pumping translocation.
Research highlights.
Role of bound cardiolipin in cytochrome c oxidase proton pumping was investigated
Cardiolipin removal eliminates cytochrome c oxidase proton pumping activity
Cardiolipin removal prevents cytochrome c oxidase dimerization
The possible direct or indirect role of cardiolipin is discussed
Acknowledgements
We would like to thank Dr. Lawrence J. Prochaska from Wright State University for helpful suggestions. This work was supported by the National Institutes of Health (NIH 24795) and grant 2/0062/14 from the VEGA Agency of the Slovak Ministry of Education and Slovak Academy of Sciences.
Abbreviation
- CcO
bovine heart cytochrome c oxidase
- CL
cardiolipin
- RP-HPLC
reversed phase high-performance liquid chromatography
- RCR
respiratory control ratio
- PLA2
phospholipase A2
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- TMPD
N,N,N’,N’-tetramethyl-ρ-phenylenediamine
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ioannou PV, Golding BT. Cardiolipins: their chemistry and biochemistry. Prog Lipid Res. 1979;17(3):279–318. doi: 10.1016/0079-6832(79)90010-7. [DOI] [PubMed] [Google Scholar]
- 2.Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- 3.Krebs JJ, Hauser H, Carafoli E. Asymmetric distribution of phospholipids in the inner membrane of beef heart mitochondria. J Biol Chem. 1979;254:5308–5316. [PubMed] [Google Scholar]
- 4.Robinson NC, Strey F, Talbert L. Investigation of the essential boundary layer phospholipids of cytochrome c oxidase using Triton X- 100 delipidation. Biochemistry. 1980;19:3656–3661. doi: 10.1021/bi00557a003. [DOI] [PubMed] [Google Scholar]
- 5.Robinson NC. Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr. 1993;25:153–163. doi: 10.1007/BF00762857. [DOI] [PubMed] [Google Scholar]
- 6.Sedlák E, Robinson NC. Phospholipase A2 digestion of cardiolipin bound to bovine cytochrome c oxidase alters both activity and quaternary structure. Biochemistry. 1999;38:14966–14972. doi: 10.1021/bi9914053. [DOI] [PubMed] [Google Scholar]
- 7.Arnarez C, Marrink SJ, Periole X. Identification of cardiolipin binding sites on cytochrome c oxidase at the entrance of proton channels. Sci Rep. 2013 doi: 10.1038/srep01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez B, Jr, Robinson NC. Phospholipase digestion of bound cardiolipin reversibly inactivates bovine cytochrome bc1. Biochemistry. 1999;38:9031–9038. doi: 10.1021/bi990603r. [DOI] [PubMed] [Google Scholar]
- 9.Lange C, Nett JH, Trumpower BL, Hunte C. Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 2001;20:6591–6600. doi: 10.1093/emboj/20.23.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnarez C, Mazat JP, Elezgaray J, Marrink SJ, Periole X. Evidence for cardiolipin binding sites on the membrane-exposed surface of the cytochrome bc1. J Am Chem Soc. 2013;135:3112–3120. doi: 10.1021/ja310577u. [DOI] [PubMed] [Google Scholar]
- 11.Pöyry S, Oana Cramariuc O, Postila PA, Kaszuba K, Sarewicz M, Osyczka A, Vattulainen I, Róg T. Atomistic simulations indicate cardiolipin to have an integral role in the structure of the cytochrome bc1 complex. Biochim Biophys Acta. 2013;1827:769–778. doi: 10.1016/j.bbabio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Schwall CT, Greenwood VL, Alder NN. The stability and activity of respiratory Complex II is cardiolipin-dependent. Biochim Biophys Acta. 2012;1817:1588–1596. doi: 10.1016/j.bbabio.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Fry M, Green M. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- 14.Dröse S, Zwicker K, Brandt U. Full recovery of the NADH:ubiquinone activity of complex I (NADH:ubiquinone oxidoreductase) from Yarrowia lipolytica by the addition of phospholipids. Biochim Biophys Acta. 2002;1556:65–72. doi: 10.1016/s0005-2728(02)00307-9. [DOI] [PubMed] [Google Scholar]
- 15.Beyer K, Klingenberg M. ADP/ATP carrier protein from beef heart mitochondria has high amounts of tightly bound cardiolipin, as revealed by 31P nuclear magnetic resonance. Biochemistry. 1985;24:3821–3826. doi: 10.1021/bi00336a001. [DOI] [PubMed] [Google Scholar]
- 16.Eble KS, Coleman WB, Hantgan RR, Cunningham CC. Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J Biol Chem. 1990;265:19434–19440. [PubMed] [Google Scholar]
- 17.Acehan D, Malhotra A, Xu Y, Ren M, Stokes DL, Schlame M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J. 2011;100:2184–2192. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wikström MKF, Saari HT. The mechanism of energy conservation and transduction by mitochondrial cytochrome c oxidase. Biochim Biophys Acta. 1977;462:347–361. doi: 10.1016/0005-2728(77)90133-5. [DOI] [PubMed] [Google Scholar]
- 19.Wikström MKF. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977;266:271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- 20.Capitanio G, Martino PL, Capitanio N, Papa S. Redox Bohr effects and the role of heme a in the proton pump of bovine heart cytochrome c oxidase. Biochim Biophys Acta. 2011;1807:1287–1294. doi: 10.1016/j.bbabio.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Shinzawa-Itoh K, Aoyama H, Muramoto K, Terada H, Kurauchi T, Tadehara Y, Yamasaki A, Sugimura T, Kurono S, Tsujimoto K, Mizushima T, Yamashita E, Tsukihara T, Yoshikawa S. Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 2007;26:1713–1725. doi: 10.1038/sj.emboj.7601618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshikawa S, Muramoto K, Shinzawa-Itoh K, Mochizuki M. Structural studies on bovine heart cytochrome c oxidase. Biochim Biophys Acta. 2012;1817:579–589. doi: 10.1016/j.bbabio.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Kadenbach B, Jarausch J, Hartmann R, Merle P. Separation of mammalian cytochrome c oxidase into 13 polypeptides by a sodium dodecyl sulfate-gel electrophoretic procedure. Anal Biochem. 1983;129:517–521. doi: 10.1016/0003-2697(83)90586-9. [DOI] [PubMed] [Google Scholar]
- 24.Finel M, Wikström M. Studies on the role of the oligomeric state and subunit III of cytochrome oxidase in proton translocation. Biochim Biophys Acta. 1986;851:99–108. doi: 10.1016/0005-2728(86)90253-7. [DOI] [PubMed] [Google Scholar]
- 25.Wilson KS, Prochaska LJ. Phospholipid vesicles containing bovine heart mitochondrial cytochrome c oxidase and subunit III- deficient enzyme: analysis of respiratory control and proton translocating activities. Arch Biochem Biophys. 1990;292:413–420. doi: 10.1016/0003-9861(90)90137-n. [DOI] [PubMed] [Google Scholar]
- 26.Lincoln AJ, Donat N, Palmer G, Prochaska LJ. Site-specific antibodies against hydrophilic domains of subunit III of bovine heart cytochrome c oxidase affect enzyme function. Arch Biochem Biophys. 2003;416:81–91. doi: 10.1016/s0003-9861(03)00202-9. [DOI] [PubMed] [Google Scholar]
- 27.Bratton MR, Pressler MA, Hosler JP. suicide inactivation of cytochrome c oxidase: catalytic turnover in the absence of subunit III alters the active site. Biochemistry. 1999;38:16236–16245. doi: 10.1021/bi9914107. [DOI] [PubMed] [Google Scholar]
- 28.Varanasi L, Hosler JP. Subunit III-depleted cytochrome c oxidase provides insight into the process of proton uptake by proteins. Biochim Biophys Acta. 2012;1817:545–551. doi: 10.1016/j.bbabio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson NC, Zborowski J, Talbert LH. Cardiolipin-depleted bovine heart cytochrome c oxidase: binding stoichiometry and affinity for cardiolipin derivatives. Biochemistry. 1990;29:8962–8969. doi: 10.1021/bi00490a012. [DOI] [PubMed] [Google Scholar]
- 30.Sedlák E, Panda M, Dale MP, Weintraub ST, Robinson NC. Photolabeling of cardiolipin binding subunits within bovine heart cytochrome c oxidase. Biochemistry. 2006;45:746–754. doi: 10.1021/bi050870z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together: cardiolipin facilitates supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 32.Böttinger L, Horvath SE, Kleinschroth T, Hunte C, Daum G, Pfanner N, Becker T. Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. J Mol Biol. 2012;423:677–686. doi: 10.1016/j.jmb.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yonetani T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960;235:845–852. [PubMed] [Google Scholar]
- 34.Fowler LR, Richardson SH, Hatefi Y. A rapid method for the preparation of highly purified cytochrome oxidase. Biochim Biophys Acta. 1962;64:170–173. doi: 10.1016/0006-3002(62)90770-9. [DOI] [PubMed] [Google Scholar]
- 35.Capaldi RA, Hayashi H. The polypeptide composition of cytochrome oxidase from beef heart mitochondria. FEBS Lett. 1972;26:261–263. doi: 10.1016/0014-5793(72)80587-8. [DOI] [PubMed] [Google Scholar]
- 36.Dale MP, Robinson NC. Synthesis of cardiolipin derivatives with protection of the free hydroxyl: its application to the study of cardiolipin stimulation of cytochrome c oxidase. Biochemistry. 1988;27:8270–8275. doi: 10.1021/bi00421a042. [DOI] [PubMed] [Google Scholar]
- 37.Casey RP. Measurement of the H+ pumping activity of reconstituted cytochrome oxidase. Meth Enzymol. 1986;126:13–21. doi: 10.1016/s0076-6879(86)26004-8. [DOI] [PubMed] [Google Scholar]
- 38.Varhač R, Robinson NC, Musatov A. Removal of bound Triton X-100 from purified bovine heart cytochrome bc 1 . Anal Biochem. 2009;395:268–270. doi: 10.1016/j.ab.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thelen M, O'Shea PS, Petrone G, Azzi A. Proton translocation by a native and subunit III-depleted cytochrome c oxidase reconstituted into phospholipid vesicles. Use of fluorescein-phosphatidylethanolamine as an intravesicular pH indicator. J Biol Chem. 1985;260:3626–3631. [PubMed] [Google Scholar]
- 40.Vygodina TV, Pecoraro C, Mitchell D, Gennis R, Konstantinov AA. Mechanism of inhibition of electron transfer by amino acid replacement K362M in a proton channel of Rhodobacter sphaeroides cytochrome c oxidase. Biochemistry. 1998;37:3053–3061. doi: 10.1021/bi971876u. [DOI] [PubMed] [Google Scholar]
- 41.Musatov A, Ortega-Lopez J, Robinson NC. Detergent-solubilized bovine cytochrome c oxidase: dimerization depends on the amphiphilic environment. Biochemistry. 2000;39:12996–13004. doi: 10.1021/bi000884z. [DOI] [PubMed] [Google Scholar]
- 42.Musatov A, Robinson NC. Cholate induces dimerization of detergent or phospholipid solubilized cytochrome c oxidase. Biochemistry. 2002;41:4371–4376. doi: 10.1021/bi016080g. [DOI] [PubMed] [Google Scholar]
- 43.Yoshikawa S. Reaction mechanism and phospholipid structures of bovine heart cytochrome c oxidase. Biochem Soc Trans. 2005;33:934–937. doi: 10.1042/BST20050934. [DOI] [PubMed] [Google Scholar]
- 44.Hunte C, Koepke J, Lange C, Rossmanith T, Michel H. Structure at 2.3 Å resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Science. 2000;8:669–684. doi: 10.1016/s0969-2126(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 45.Hunte C, Palsdottir H, Trumpower BL. Protonmotive pathways and mechanisms in the cytochrome bc1 complex. FEBS Lett. 2003;545:39–46. doi: 10.1016/s0014-5793(03)00391-0. [DOI] [PubMed] [Google Scholar]
- 46.Palsdottir H, Hunte C. Lipids in membrane protein structures. Biochim Biopys Acta. 2004;1666:2–18. doi: 10.1016/j.bbamem.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- 48.Chevenal D, Carafoli E. Identification and primary structure of the cardiolipin-binding domain of mitochondrial creatine kinase. Eur J Biochem. 1988;171:1–9. doi: 10.1111/j.1432-1033.1988.tb13750.x. [DOI] [PubMed] [Google Scholar]
- 49.McAuley KE, Fyfe PK, Ridge JP, Isaacs NW, Cogdell RJ, Jones MR. Structural details of an interaction between cardiolipin and an integral membrane protein. Proc Natl Acad Sci. 1999;96:14706–14711. doi: 10.1073/pnas.96.26.14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfitzner U, Hoffmeier K, Harrenga A, Kannt A, Michel H, Bamberg E, Richter OMH, Ludwig B. Tracing the D-pathway in reconstituted site-directed mutants of cytochrome c oxidase from Paracoccus denitrificans. Biochemistry. 2000;39:6756–6762. doi: 10.1021/bi992235x. [DOI] [PubMed] [Google Scholar]
- 51.Pawate AS, Morgan J, Namslauer A, Mills D, Brzezinski P, Ferguson-Miller S, Gennis RB. A mutation in subunit I of cytochrome oxidase from Rhodobacter sphaeroides results in an increase in steady-state activity but completely eliminates proton pumping. Biochemistry. 2002;41:13417–13423. doi: 10.1021/bi026582+. [DOI] [PubMed] [Google Scholar]
- 52.Kates M, Syz JY, Gosser D, Haines TH. pH-dissociation characteristics of cardiolipin and its 2’-deoxy analogue. Lipids. 1993;28:877–882. doi: 10.1007/BF02537494. [DOI] [PubMed] [Google Scholar]
- 53.Althoff T, Mills DJ, Popot JL, Kühlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J. 2011;30(22):4652–64. doi: 10.1038/emboj.2011.324. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Böttinger L, Horvath SE, Kleinschroth T, Hunte C, Daum G, Pfanner N, Becker T. Phosphatidylethanolamine and cardiolipin differently affect the stability of mitochondrial respiratory chain supercomplexes. J Mol Biol. 2012;423:677–686. doi: 10.1016/j.jmb.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]