Abstract
Zinc (Zn2+) has been recently recognized as a crucial element for male gamete function in many species although its detailed mechanism of action is poorly understood. In sea urchin spermatozoa, Zn2+ was reported as an essential trace ion for efficient sperm motility initiation and the acrosome reaction by modulating intracellular pH (pHi). In this study we found that submicromolar concentrations of free Zn2+ change membrane potential (Em) and increase the concentration of intracellular Ca2+ ([Ca2+]i) and cAMP in Lytechinus pictus sperm. Our results indicate that the Zn2+ response in sperm of this species mainly involves an Em hyperpolarization caused by K+ channel activation. The pharmacological profile of the Zn2+-induced hyperpolarization indicates that the cGMP-gated K+ selective channel (tetraKCNG/CNGK), which is crucial for speract signaling, is likely a main target for Zn2+. Considering that Zn2+ also induces [Ca2+]i fluctuations, our observations suggest that Zn2+ activates the signaling cascade of speract, except for an increase in cGMP, and facilitates sperm motility initiation upon spawning. These findings provide new insights about the role of Zn2+ in male gamete function.
Keywords: Zinc, calcium, membrane potential, potassium channels, cAMP, pH
Introduction
Although Zn2+ is a trace element, it is known to be essential for a wide range of biological processes: transcription, cell division, proliferation, immune function, defense against free radicals and fertilization (Pluth et al., 2011). In mammals, it has been known for long time that Zn2+ concentration in the seminal fluid is notably high (Hidiroglou and Knipfel, 1984; Mawson and Fischer, 1953). In fact, the highest concentration in the human body is in the seminal plasma (3 mM; (Saaranen et al., 1987)). Zn2+ has also been proposed to stabilize chromatin (Bjorndahl and Kvist, 2010) and regulate sperm capacitation (Andrews et al., 1994; Aonuma et al., 1978; Lishko et al., 2010). Furthermore, it was recently reported that Zn2+ is also important for male gamete motility regulation in the nematode C. elegans (Liu et al., 2013).
Upon dilution of sea urchin sperm in seawater, metal chelators such as EDTA, EGTA, phenantroline and cysteine, at concentrations that do not alter the Ca2+ or Mg2+ levels (0.1-1 mM), delay motility initiation and inhibit the AR induced by egg jelly (Clapper et al., 1985; Johnson and Epel, 1983). Among the trace ions in seawater (such as Cu2+ and Ni2+), only Zn2+ can rescue these chelator inhibitory effects, indicating a crucial role of this divalent cation in sea urchin sperm physiology. In addition, Zn2+ is able to induce the AR of Lytechinus pictus spermatozoa, although its physiological relevance is unknown. The action of Zn2+ in sea urchin spermatozoa has been attributed to pHi regulation (Clapper et al., 1985).
It is well established that echinoderm spermatozoa respond to sperm-activating peptides (SAPs) derived from the egg jelly of the same species which regulate motility (Suzuki, 1995). Speract is the first structurally identified SAP from the egg jelly of Hemicentrotus pulcherrimus (Suzuki et al., 1981) and Strongylocentrotus purpuratus (Hansbrough and Garbers, 1981), and also stimulates L. pictus spermatozoa (Guerrero et al., 2010; Nishigaki and Darszon, 2000). Speract binding to its receptor in the flagella plasma membrane stimulates the synthesis of cGMP (Garbers, 1989) which directly activates the cGMP-gated K+ selective channel (tetraKCNG/CNGK) inducing a K+ efflux and a membrane hyperpolarization (Babcock et al., 1992; Bonigk et al., 2009; Galindo et al., 2007). This hyperpolarization leads to increases in pHi (Lee, 1984), [Ca2+]i, and cAMP levels (Beltran et al., 1996). All these changes induced by speract, except for the cGMP increase, are inhibited by elevating the K+ concentration in seawater (Harumi et al., 1992).
In this study we show that submicromolar free Zn2+ concentrations induce similar sperm responses as speract does including Em changes, cAMP elevation and [Ca2+]i fluctuations except for the cGMP increase in L. pictus spermatozoa. We discuss the molecular mechanisms and physiological significance of Zn2+ for sea urchin spermatozoa.
Material and Methods
Gametes and reagents
L. pictus sea urchins were obtained from Marinus (Long Beach, CA, USA). Dry sperm were collected after intracelomic injection of 0.5 M KCl and kept on ice until used. The fluorescent dyes 3,3'-dipropylthiadicarbocyanine iodide (DiSC3(5)), Fluo-4-AM, Quin-2 and 5-(and-6)-Carboxyfluorescein diacetate were obtained from Molecular Probes (Eugene, OR, USA). Anhydrous dimethylsulfoxide (DMSO), tolbutamide, glibenclamide, were from Sigma-Aldrich. ZnSO4 was from Merck. Charybdotoxin and Iberiotoxin were from Alomone Labs. The Kits to measure cAMP (TRK 432) and cGMP (TRK 500) were from Amersham. Speract was synthesized in Professor Possani's Laboratory (IBT-UNAM) and fucose sulfate polymer (FSP) was prepared according to the previous report (Garbers et al., 1983). The rest of the reagents used were of the highest quality available.
Composition of artificial seawater (ASW)
Normal ASW was prepared with the following composition (in mM): 465 NaCl, 26 MgCl2, 10 KCl, 30 MgSO4, 10 CaCl2, 2.5 NaHCO3 and 0.1 EDTA (or otherwise indicated) with pH 8.0 by NaOH. Ca2+-free pH 8.0 (0CaSW) or 1 mM Ca2+ pH 7.0 (1CaSW) ASWs were the same except for the indicated CaCl2 concentration and pH. High K+ ASW (50KSW) contained 50 mM KCl. Cl--free ASW (0ClSW) was prepared by substituting NaCl and KCl with each metanesulphonate salt and substitution of MgCl2 and CaCl2 with each sulfate salt. In all cases the osmolarity was 950-1000 mOsm.
Fluorescence measurements with sperm suspension
Fluorometric measurements were performed in an OLIS-upgraded SLM 8000 Aminco spectrofluorometer with a temperature-controlled cell holder (14 °C) and a magnetic stirrer. Spermatozoa [Ca2+]i and pHi measurements were done according to Rodríguez and Darszon (Rodriguez and Darszon, 2003). Briefly, diluted (1:5) dry sperm in 1CaSW containing 20 μM of Quin-2-AM or Fluo 4-AM without or with 0.5 % Pluronic F-127 or 10 μM Carboxyfluorescein diacetate were incubated for 3 h at 14 °C in the dark. After loading with these fluorescent dyes, 10 ml of 1CaSW were added to the sperm suspension. Coelomocytes and spines were precipitated by mild centrifugation (121 g for 7 min at 4 °C) and the sperm suspension was applied to further centrifugation (1000 g for 8 min at 4 °C) to eliminate the dyes remaining in the media. The sperm pellet was resuspended in the original volume of 1CaSW and kept on ice in the dark until used. A 10 μl aliquot of the loaded sperm was added to a flat-bottom glass tube containing 800 μl ASW. After 30 seconds, an agonist was added using a Hamilton syringe. Fluorescence intensities (excitation/emission wavelength) for Quin-2 (340/490 nm), Fluo-4 (505/525 nm) and Carboxyfluorescein diacetate (490/535 nm) were recorded every 0.5 s.
For Em experiments, 10 μl of diluted (1:10 in 1CaSW) sperm were added to 800 μl of ASW plus 0.7 mM EDTA and 0.5 μM DisC3(5). The EDTA concentration for Em measurements was increased because DisC3(5) induces the acrosomal reaction in ASW with 0.1 mM EDTA, but not in 0.8 mM EDTA. Fluorescence intensities of DisC3(5) (640/670 nm) were registered every 0.5 s. After 2-3 minutes, a protonophore (CCCP 1 μM) was added to avoid mitochondrial membrane potential interference. Once the fluorescence reached the equilibrium, an agonist was added. DisC3(5) fluorescence was calibrated using 1 μM valinomycin and sequential additions of KCl. Em was calculated according to the Nernst equation assuming that the intracellular K+ concentration is 180 mM (Babcock et al., 1992). For fluorometric experiments, stock solutions of Fluo-4-AM (1 mM) and Carboxyfluorescein diacetate (1 mM) and DiSC3(5) (0.8 mM) were made in anhydrous DMSO.
Single cell fluorescence imaging
Fluorescence imaging was carried out as previously reported (Nishigaki et al., 2004). Briefly, Fluo-4-labeled spermatozoa were adhered to glass coverslips coated with 50 Pg/ml poly-L-lysine solution (Sigma) and mounted into a micro incubator, PDMI-2 (Harvard Apparatus, Holliston, MA, USA) maintained at 14 °C. Fluorescence images were acquired using a Nikon DIAPHOT 300 inverted microscope with a Nikon Plan Apo 60X objective lens (1.4 NA) and a custom-built stroboscopic illumination system (Nishigaki et al., 2006) with a Chroma filter set (Ex, HQ470/40x; DC, 505DCXRU; Em, HQ510LP (Chroma Technology)). Images were acquired with a Quantix 57 camera (Photometrics Inc.) under the continuous (stream) acquisition mode.
Determination of cAMP and cGMP levels
Sperm diluted (1:200) in 0CaSW with 1 mM 3-isobutyl-1-methylxanthine (IBMX) were incubated with a ligand (Zn2+ or speract) for 1 min at 14 °C. Subsequently, the sperm suspension was transferred to a boiling bath to quench the enzymatic reaction and extract nucleotides. TRK 432 or TRK 500 Kits of Amersham were used to measure cAMP or cGMP concentration, respectively.
Estimation of free Zn2+ concentration
The free Zn2+ concentration in ASW after various Zn2+ additions was estimated using a WinMaxC 2.4 software package (Stanford University, Chris Patton http://www.stanford.edu/~cpatton/downloads.htm). DisC3(5) contains a certain amount of Zn2+ as a contaminant and though small amount of DisC3(5) (0.5 PM) should not significantly affect the total Zn2+, in most Em experiments ASW containing 0.8 mM EDTA was used. Addition of 10 μM ZnSO4 to ASW containing 0.8 mM EDTA yields ~ 0.2 nM free Zn2+.
Data normalization
Although the absolute changes in the sperm Em obtained within one season are very reproducible, there is variability between experiments with cells from different seasons. For this reason we normalized the data in order to compare them. Furthermore, in experiments where high K+ (50KSW) was used to block the speract responses it is difficult to calibrate Em using the Nernst equation. Therefore, Em changes in 50KSW were expressed with F/F0, where F is fluorescence intensity of DisC3(5) and F0 is a F value just before addition of Zn2+.
Statistics
Student's t test or one-way ANOVA were used for statistical evaluations. Differences among experimental conditions were considered significant when p < 0.05.
Results
Zn2+ changes membrane potential (Em) and increases pHi and [Ca2+]i
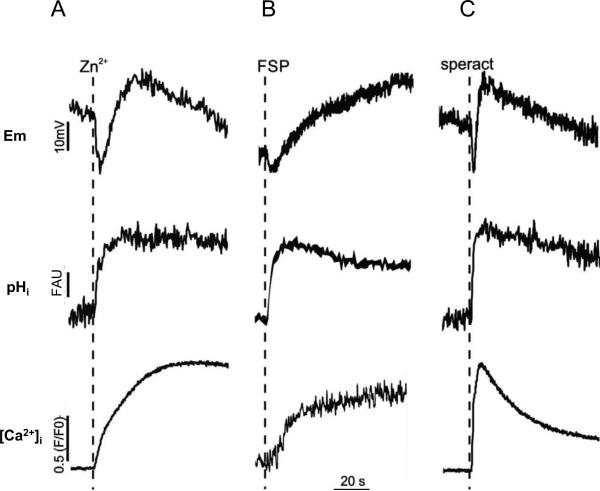
It is known in sea urchin sperm that Zn2+ increases pHi (Clapper et al., 1985). Since Zn2+ is also known to induce the AR in L. pictus spermatozoa, we determined changes of Em, pHi, and [Ca2+]i triggered by Zn2+ as well as those induced by FSP, the natural AR inducer, and speract. The upper records in Figure 1 show that addition of 10 μM Zn2+ (0.2 nM free Zn2+), FSP (~100 nmol fucose/ml) and 100 nM speract causes a transient Em hyperpolarization followed by a depolarization. The Zn2+-induced Em changes are different from those triggered by FSP (middle upper trace), but rather similar to those induced by speract. The three inducers altered pHi in a somehow similar fashion (center traces). While the [Ca2+]i increase induced by speract is a transient, those induced by Zn2+ and FSP are relatively permanent probably due to the induction of AR (lower records). It is worth noting that the FSP induced [Ca2+]i changes presented in Fig. 1B were performed using Quin-2 (see Methods) and thus appear smaller than those induced by Zn2+ and speract where Fluo-4 was used.
Fig. 1.
Zn2+ changes Em and increases pHi and [Ca2+]i as FSP and Speract.
Em (upper row records), pHi (middle row records) and [Ca2+]i (lower row records) of sea urchin sperm were measured in cell populations as described in methods. Dotted lines indicate a point of agonist addition: A, Zn2+ (10 M total); B, FSP (~100 nmol fucose/ml); C, 100 nM Speract. The free concentration of Zn2+ was estimated to be 0.2 nM in Em measurement (0.8 mM EDTA) and 1.8 nM in pHi and [Ca2+]i measurements (0.1 mM EDTA). Downward and upward deflections of each parameter indicate hyperpolarization and depolarization of Em and decreases and increases of [Ca2+]i and pHi, respectively. The scale of each parameter Em (mV), pHi (FAU, Fluorescence Arbitrary Units) and [Ca2+]i ((F/F0); where F is Fluo-4 (or Quin-2) fluorescence intensity and F0 is the fluorescence before adding speract or Zn2+) is indicated with vertical bars. The time scale was indicated by horizontal bars. Records shown in this figure are representative of at least three different animals. Zn2+ and FSP induced similar amounts of acrosome reaction that were ≥57%.
Zn2+hyperpolarizes L. pictus sperm through K+ channel activation
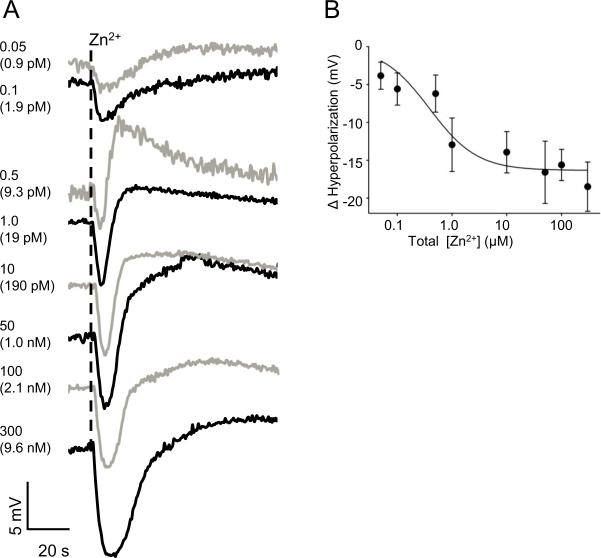
As a next step, we measured Em changes with different concentrations of Zn2+. Figure 2A shows representative records of Em changes induced by different concentrations of Zn2+ and Figure 2B, the average of Em hyperpolarization. Using the results from Figure 2B, we estimated a DL50 of Zn2+ for the L. pictus sea urchin sperm hyperpolarization of 0.38 μM.
Fig. 2.
Zn2+ hyperpolarizes the sperm Em in a dose dependent manner.
Representative traces of the sperm Em changes triggered by different Zn2+ concentrations (0.05-300 μM) are shown in panel A. Each number indicates the total concentration of Zn2+ in PM and the estimated free Zn2+ concentration is in parenthesis. To avoid trace confusion, black and gray lines were used alternatively. Panel B shows the best fit curve of the hyperpolarization change as a function of log[Zn2+] for three different animals with triplicate measurements. One way ANOVA was used to examine if the differences between different Zn2+ concentrations was significant at *p < 0.05.
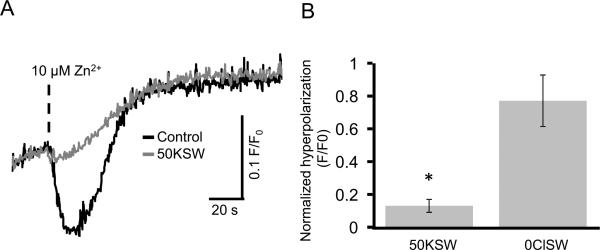
A simple mechanism for the Em hyperpolarization might be the activation of electrogenic K+ efflux or Cl- influx. Therefore, we explored these two possibilities by altering the ionic compositions of artificial seawater. As shown in Figure 3A, high K+ ASW (50KSW) effectively inhibited the Em hyperpolarization induced by Zn2+, although the subsequent Em depolarization was not affected significantly. In contrast, Cl--free ASW (0ClSW) had a small effect on Em hyperpolarization induced by Zn2+ (Fig. 3B). These results suggest that one of the principal targets for Zn2+ may be a K+ channel of sea urchin spermatozoa.
Fig. 3.
High K+ ASW (50KSW) inhibits the Zn2+-induced hyperpolarization.
Panel A shows representative sperm Em responses to 10 μM Zn2+ (0.2 nM free Zn2+) in normal ASW (black line) and in 50KSW (gray line). Panel B shows the magnitude of Em hyperpolarization (mean ± SE) induced by 10 μM Zn2+ in 50KSW and Cl- free ASW (0ClSW). The magnitude of hyperpolarization (delta F/F0) was normalized against control experiments performed in normal ASW. Statistically significant differences are indicated by asterisks (n = 5 for 50KSW and n = 4 for 0ClSW; *p 0.05).
Effects of K+ channel blockers on Zn2+-induced Em hyperpolarization
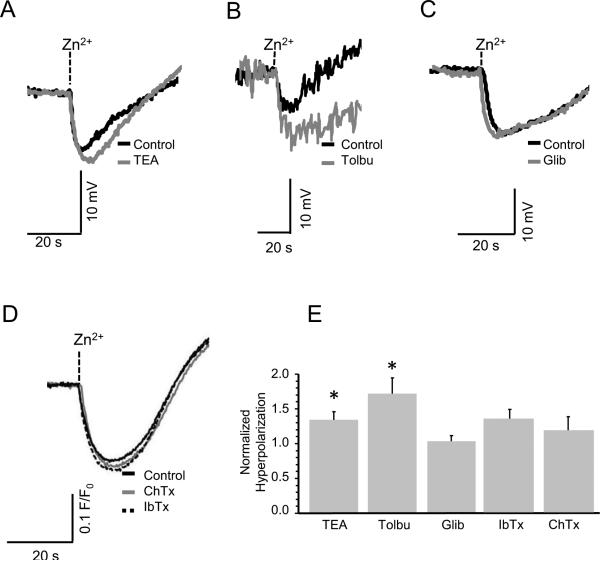
In order to determine the type of K+ channel involved in the Zn2+-induced Em changes, we treated the spermatozoa with several K+ channel blockers. As seen in Figure 4A, 20 mM of TEA (tetraethylammonium), which blocks many types of K+ channel including one activated by FSP (Guerrero and Darszon, 1989), did not inhibit the Zn2+-induced hyperpolarization. It was reported that Zn2+ activates KATP channels in pancreatic beta cells and brain nerve terminals (Bancila et al., 2005; Bloc et al., 2000; Matias et al., 2010; Prost et al., 2004), but two KATP channel blockers, either tolbutamide (Fig. 4B) or glibenclamide (Fig. 4C), did not inhibit the Zn2+-induced hyperpolarization. Moreover, neither Charybdotoxin, a blocker of BK/Slo1 (Gimenez-Gallego et al., 1988; Miller et al., 1985) and Kv1 K+ channels (Grissmer et al., 1994), nor Iberiotoxin another blocker for BK/Slo1 K+ channel (Galvez et al., 1990) inhibited the Zn2+-induced hyperpolarization (Fig. 4D). Notably, none of these K+ channel inhibitors were able to significantly decrease the magnitude of the Zn2+ induced Em hyperpolarization; instead some reagents rather enhanced it (Fig. 4E). These results better correspond to the pharmacological profile of tetraKCNG/CNGK, which is key to the speract signaling cascade, reported before (Bonigk et al., 2009; Galindo et al., 2007).
Fig. 4.
The Zn2+-triggered hyperpolarization is not inhibited by several K+ channel blockers. Traces A-D indicate representative Em changes induced by 10 μM Zn2+ (0.2 nM free Zn2+) in ASW containing 20 mM tetraethylammonium (TEA) in panel A, 300 μM Tolbutamide (Tolbu) in panel B, 20 μM Glibenclamide (Glib) in panel C, or 100 nM Charybdotoxin (ChTx) or 100 nM Iberiotoxin (IbTx) in panel D. Black traces are in the absence of blockers (control) and gray traces are in the presence of each K+ blocker. In panel D, broken trace indicates in the presence of IbTx. Panel E shows the mean ± SE of the normalized hyperpolarization change of sperm from three different animals with triplicate measurements. Asterisks (*) indicate statistically significant differences compared to controls using Student's t test (n= 3; p ≤ 0.05). We also analyzed the data with ANOVA comparing each condition, however no significant differences between groups were found.
Zn2+ induces an increase in cAMP but not in cGMP
Since cyclic nucleotides play fundamental roles in the speract signaling (Darszon et al., 2008; Darszon et al., 2011; Kaupp et al., 2008), we determined the effect of Zn2+ on cyclic nucleotides in the cells. Speract elevates the cGMP concentration and this occurs more prominently in high K+ (50 mM) condition (Fig. 5A), which appears to prevent guanylyl cyclase inactivation (Harumi et al., 1992; Ward et al., 1986). In contrast, Zn2+ did not alter the level of cGMP either in normal K+ (10 mM) or high K+ condition (Fig. 5A). However, Zn2+ was able to induce an increase in the cAMP concentration, as speract does (Fig. 5B and Fig. S1). Furthermore, the increase in cAMP induced by Zn2+ was inhibited in high K+ (Fig. 5B), as in the case of speract. These results suggest that Zn2+ activates a K+ channel, possibly tetraKCNG/CNGK, without altering the cGMP concentration.
Fig. 5.
Zn2+ and speract raise cAMP levels but only speract increases cGMP.
Concentration of cGMP (panel A) and cAMP (panel B) in spermatozoa diluted in 0CaSW with 1 mM IBMX were measured in the presence or absence of 100 nM Speract or 10 μM Zn2+ (0.07 nM free Zn2+). Also, the same measurements were performed in high K+ (50 mM) condition. The mean ± SE of three experiments with sperm from different animals with triplicate measurements were normalized against the control (the resting level of cyclic nucleotides). Asterisks (*) indicate statistically significant differences compared to controls (p < 0.05).
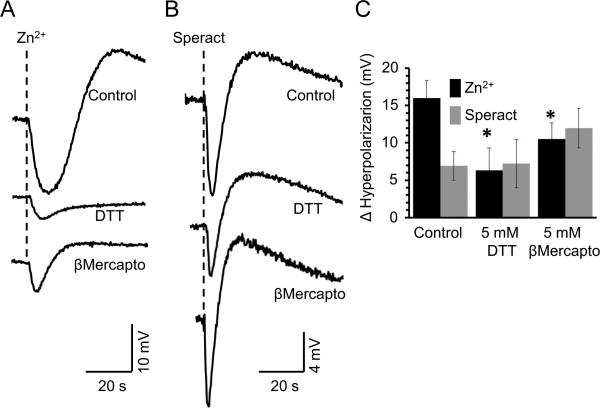
Reducing agents partially inhibit the Zn2+-induced hyperpolarization
Previous work implicates cysteine residues as part of the Zn2+ binding site that regulates some ionic channels (Choi et al., 2001; Gore et al., 2004; Hou et al., 2010). The role of cysteines in the Zn2+-triggered hyperpolarization was assessed under reducing and non-reducing conditions. DTT is a strong reducing agent that also acts as a metal chelator. As shown in figures 6A&C, 5 mM DTT diminishes (~60%) the Zn2+-triggered hyperpolarization, but does not affect the one induced by Speract (Figs. 6B&C). As DTT is a Zn2+ chelator, we tested another cysteine reducing agent such as E-mercaptoethanol that is not considered a Zn2+ chelator. This latter reducing agent also inhibited the Zn2+-triggered hyperpolarization but to a lesser extent (~34%) (Fig. 6A&C). These findings suggest that cysteines do participate in this event. It is worth pointing out that the Speract induced hyperpolarization was not significantly sensitive to -mercaptoethanol or DTT. Notably, the depolarization and repolarization that follow the initial hyperpolarization induced by Zn2+ was differentially inhibited by DTT (~90%) and β-mercaptoethanol (~70%), while the one triggered by Speract was insensitive to both reducing agents.
Fig. 6.
The Zn2+-triggered hyperpolarization is inhibited by DTT and β-mercaptoethanol , two reducing agents. Sperm diluted in ASW containing or not (Control) 5 mM of DTT or β-mercaptoethanol, were exposed to either 10 μM Zn2+ (A) or 100 nM Speract (B). The mean ± SE of the hyperpolarization change (mV) is shown (n ≥ 5; *p ≤ 0.05).
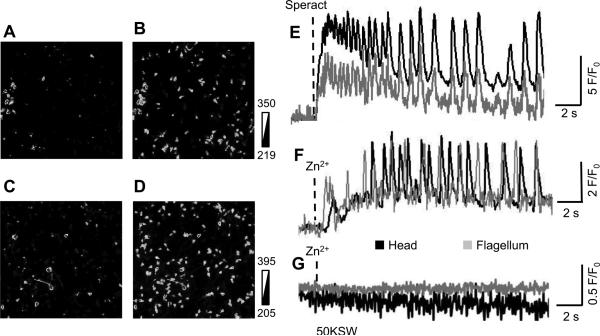
Zn2+ triggers [Ca2+]i fluctuations
One of the striking effects of speract on sea urchin sperm is the induction of [Ca2+]i fluctuations (Wood et al., 2003). Surprisingly, we observed that Zn2+ induces [Ca2+]i fluctuations in a similar way as speract does (Fig. 7). As expected, 50KSW completely blocked the [Ca2+]i fluctuations induced by Zn2+ (Fig. 7) as in the case of speract (Wood et al., 2003). These results support our hypothesis, namely, that Zn2+ activates the tetraKCNG/CNGK channel.
Fig. 7.
Speract and Zn2+ induce [Ca2+]i oscillations in sea urchin sperm.
Ca2+ images of individual spermatozoa were acquired using Fluo-4 as described in materials and methods. Panel A and B show representative fluorescence images before (A) and 3 s after addition of 100 nM speract (B). Panel C and D show representative fluorescence images before (C) and 2.5 s after addition of 10 μM Zn2+ (total) (D). Fluorescence images (A-D) are expressed as gray scales. Panel E, F and G indicate fluorescence changes (F/F0) of single spermatozoon (black lines are from the head and gray ones are from the tail). Spermatozoa were stimulated by 100 nM speract (E), 10 μM Zn2+ (F) and 10 μM Zn2+ in high K+ ASW, 50KSW (G). The results are representative of 5 independent experiments.
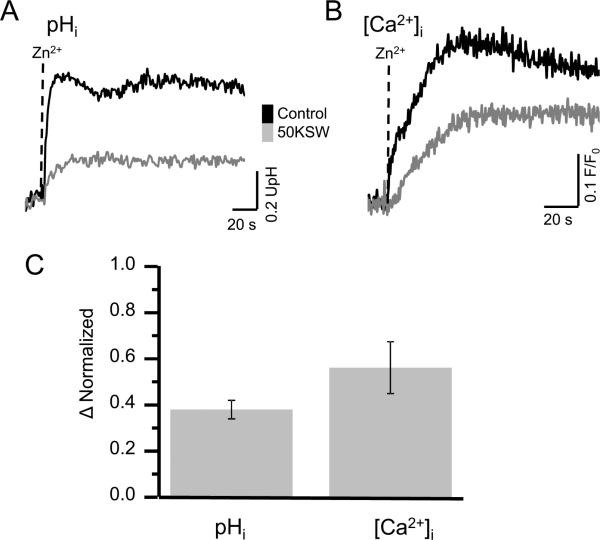
High K+ ASW only partially inhibits Zn2+-induced increases in pHi and [Ca2+]i
It is known that all speract-induced sperm responses, except for the cGMP increase, are suppressed in high K+ seawater (Harumi et al., 1992). However, we observed that Zn2+ only mildly inhibits an Em depolarization under these conditions (Fig. 3A). Therefore, we explored the effects of Zn2+ on [Ca2+] and pHi changes in the presence of high external K+. Unexpectedly, although the Zn2+ induced increases in pHi (Fig. 8A&C) and [Ca2+]i (Fig. 8B&C) were smaller in 50KSW, a significant fraction of them persisted. These results suggest that there may be another target (or targets) for Zn2+ besides a K+ channel in L. pictus sperm.
Fig. 8.
High K+ ASW (50KSW) partially inhibits the Zn2+ induced increase in pHi and [Ca2+]i. Sperm pHi and [Ca2+] changes induced by 10 μM Zn (1.8 nM free Zn2+) were measured in normal ASW (black line) and 50KSW (gray line) by fluorometry as in Figure 1. Panel A and B show representative traces of pHi and [Ca2+]I measurements, respectively. Panel C summarizes the inhibition of the pHi and [Ca2+]i increases induced by Zn2+ in 50KSW normalized against the values obtained in normal ASW (mean ± SE, n=3 for [Ca2+]i and n=4 for pHi).
Discussion
The tetraKCNG/CNGK channel as a principal target of Zn2+
It was known that Zn2+ is involved in pHi regulation in sea urchin sperm, but it had not been explored whether this divalent cation affects other physiological parameters such as Em or [Ca2+]i. Therefore, we first determined how Zn2+ affects sperm Em, pHi and [Ca2+]i, and compared these responses to those induced by FSP and speract. Remarkably, our results show that Zn2+ also triggers sperm Em and Ca2+ changes besides alkalinizing pHi (Fig. 1), as FSP and speract do. Zn2+ potently hyperpolarized sperm Em in a dose-dependent manner (Fig. 2). This Zn2+-induced hyperpolarization is likely due to the activation of K+ channels since 50KSW efficiently inhibited the hyperpolarization, but 0ClSW did not (Fig. 3B). However, none of the K+ channel blockers so far tested efficiently inhibited the Zn2+-induced hyperpolarization. TEA, a general K+ channel blocker, did not affect Zn2+ induced hyperpolarization. Two KATP channel blockers, Tolbutamide or Glibenclamide, were unable to inhibit the Zn2+-induced hyperpolarization although KATP channels are known to be activated by low Zn2+ concentrations (Bloc et al., 2000; Prost et al., 2004). Considering the proposed speract signaling cascade of sea urchin sperm, at least two K+ channels could potentially be responsible for the main component of the Zn2+- induced hyperpolarization; the TetraKCNG (Galindo et al., 2007) and a Ca2+ dependent K+ channel (CaKC) (reviewed in Darszon et al., 2011). The fact that the Zn2+-triggered hyperpolarization in L. pictus sperm was ~30 % reduced in the absence of external Ca2+ (Fig. S2) is, in principle, consistent with the participation of CaKCs. However, two peptide blockers of BK/Slo1, Charibdotoxin (also for Kv1 channels) and Iberiotoxin, did not significantly alter the hyperpolarization magnitude. These results may indicate the minor participation of another Ca2+ dependent K+ channel such as the small conductance SK channel (Adelman et al., 2012). Aside from this minor component, the pharmacological profile determined here coincides with the one displayed by the cGMP-gated K+ channel in sea urchin sperm reported by Galindo et al. previously (Galindo et al., 2000) and thereafter identified as tetraKCNG/CNGK channel (Bonigk et al., 2009; Galindo et al., 2007).
If Zn2+ directly activates tetraKCNG/CNGK channel, this divalent cation should stimulate the speract signaling cascade. This proposal is further supported by two different lines of experimental evidence. One is that Zn2+ increases cAMP levels depending on the extracellular K+ concentration (Fig. 5B). The other is that Zn2+ induces sperm [Ca2+]i fluctuations as speract does (Fig. 7), in spite of not increasing cGMP levels. These findings altogether are consistent with the proposal that Zn2+ activates tetraKCNG/CNGK channels in sea urchin sperm. However, since there is no specific channel blocker for this channel, its heterologous expression will be required to establish if Zn2+ directly activates tetraKCNG/CNGK or not.
Cysteine residues have been found as part of the Zn2+ binding site that regulates several membrane proteins including ionic channels (Choi et al., 2001; Gore et al., 2004). Our experiments showed that neither DTT nor -mercaptoethanol, two reducing agents, decreased the hyperpolarization triggered by Speract (Fig. 6B&C), however, both compounds inhibited the Zn2+- induced hyperpolarization (Fig. 6A&C; ~60 % and ~35 %, respectively). It is important to point out that the TetraKCNG contains three extracellular cysteines (C214 in IS5-S6; C1311 in IIIS5-S6 and C1946 in IVS5-S6) one of which could be a Zn2+ target. Our findings thus suggest that the K+ channel activated by Zn2+ has a cysteine binding site and if it is tetraKCNG, the Zn2+ binding site and the one for speract are independent. Certainly it is possible that a sea urchin sperm K+ channel still not described could be the main target of Zn2+.
Other targets of Zn2+ besides tetraKCNG/CNGK channel
Although the tetraKCNG/CNGK channel is likely to be the principal target of Zn2+ in the sea urchin sperm plasma membrane, it is probably not the only one. High [K+]e inhibited only ~50 % of the Zn2+-triggered Ca2+ increase (Fig. 4C&D) and ~40 % of the pHi alkalinization. This divalent cation could activate directly or indirectly Ca2+ channels besides K+ channels and the sNHE. It is important to stress that the Zn2+ induced increase in pHi does not by itself cause a hyperpolarization ((Garcia-Soto et al., 1987) and experiments not shown). Furthermore, Zn2+ could affect a carbonic anhydrase found in the S. purpuratus genome (similar to carbonic anhydrase 8, NCBI Reference Sequence: XP_795365.2). It is therefore clear that Zn2+ has multiple targets in sea urchin sperm as it does in many other cell types.
In high K+ seawater the speract signaling cascade is completely blocked, except for the cGMP elevation (Harumi et al., 1992). Namely, both the pHi and [Ca2+]i increases induced by speract are completely suppressed in this condition. In contrast, those induced by Zn2+ were only significantly reduced (60 and 40 %, respectively), but not eliminated. Actually, this difference may distinguish the action of Zn2+ and speract on L. pictus spermatozoa where Zn2+ is able to trigger a significantly higher % of AR than speract (Fig. S3A) as previously reported (Clapper et al., 1985). In this regard we examined if Zn2+ triggers the AR through the same mechanism as FSP does.
Interestingly, while Ca2+ in seawater is essential for the FSP-induced AR, it is not for the Zn2+-induced AR (Fig. S3B). Furthermore, though Ca2+ channel blockers, Ni2+ (300 μM) and nifedipine (20 μM) efficiently inhibit the AR induced by FSP, neither treatments inhibited the Zn2+- induced AR (Fig. S3B). Nifulmic acid, a Cl- channel blocker, only inhibited the AR induced by FSP, but not the AR induced by Zn2+ (Fig. S3B). Although 50KSW efficiently inhibited the AR in both cases, the K+ channels involved in these two events seems different since TEA inhibits FSP-induced AR in S. purpuratus sea urchin sperm (Guerrero and Darszon, 1989), in contrast this blocker did not inhibit the Zn2+-induced AR. This pharmacological profile coincides with that of the Zn2+-induced hyperpolarization (Fig. 4). These findings altogether indicate that Zn2+ and FSP trigger the AR through different signaling pathways. It is worth pointing out that in S. purpuratus spermatozoa, Zn2+ does not induce the AR although it causes Em hyperpolarization and increases in pHi and [Ca2+]i (data not shown). The differential ability of Zn2+ to induce AR in the two species probably stems from the fact that L. pictus sperm have a lower threshold to trigger this reaction.
Physiological role of Zn2+ for sea urchin sperm
It is known that Zn2+ plays crucial roles for the initiation of sperm motility and the AR in L. pictus sea urchins. In both cases, pHi regulation might be a key point. It is known that sperm motility is suppressed in the testis by low pHi principally produced by a high partial pressure of CO2 (Johnson et al., 1983). Upon spawning, spermatozoa are exposed to seawater that has low partial pressure of CO2 and high pH (around 8.0), which results in the elevation of pHi.
It was reported that a Na+/H+ exchanger (NHE) is also involved in the alkalinization of the pHi upon sperm motility initiation (Lee et al., 1983). It has been known that sea urchin sperm possess a unique NHE which is regulated by membrane potential (hyperpolarization) (Lee, 1984; Lee and Garbers, 1986). Now, this NHE is thought to be a sea urchin homolog of sperm specific NHE (sNHE), initially identified in mouse (Nomura and Vacquier, 2006; Wang et al., 2003). Compared to other NHEs in somatic cells, this sNHE contains an extra domain composed of 4 trans-membrane segments in the C-terminus (Wang et al., 2003), which is structurally similar to the voltage sensor domain of voltage-gated channels and voltage-regulated phosphatases (Okamura, 2007). Therefore, it is believed that sNHE is regulated by membrane potential using this putative voltage sensor domain. In the speract signaling cascade, Em hyperpolarization caused by K+ efflux through tetraKCNG/CNGK channel is supposed to activate the sHNE and lead to pHi alkalinization (Darszon et al., 2008). Results obtained in this study suggest that the same signaling cascade can be activated by Zn2+ upon sperm dilution when seawater contains Zn2+. It is known that low pH seawater can suppress sperm motility (Ohtake, 1976), but the sperm flagella still slightly vibrate in this condition. Related to this fact, low pH (6.0) and high K+ (50 mM) seawater has been employed as a condition to store immotile spermatozoa for long period (Bracho et al., 1997). Taking all information into account, the Em hyperpolarization caused by Zn2+-induced K+ efflux might be relevant for sperm motility initiation upon spawning in seawater. It is worth mentioning that, in the ascidian Ciona intestinalis, an egg-derived single ligand, a sulfated steroid named SAAF, induces both sperm motility initiation and sperm chemotaxis (Yoshida et al., 1993; Yoshida et al., 2002). SAAF is known to hyperpolarize sperm Em through K+ efflux (Izumi et al., 1999). Furthermore, it was reported, in gill cells of the mollusk Mytilus galloprovincialis, that Zn2+ activates a NHE and increases pHi (Kaloyianni et al., 2006; Koutsogiannaki et al., 2006). Since we observed that Zn2+ still increases sperm pHi in high K+ seawater (Fig. 8), it cannot be ruled out the possibility that this divalent cation directly activates the sNHE.
Conclusions
We demonstrated that submicromolar free Zn2+ in seawater can activate a K+ channel of sea urchin spermatozoa. Pharmacological profiles suggest that the tetraKCNG/CNGK channel, which plays the fundamental role in the early part of the speract signaling cascade, is a principal target of Zn2+. Therefore, Zn2+ can promote sperm motility initiation upon spawning by sharing actions of speract: increases in pHi , [Ca2+]i and cAMP. Further detailed studies, particularly heterologous expression of tetraKCNG/CNGK channel, are necessary to define the molecular action of Zn2+ in sea urchin spermatozoa.
Supplementary Material
Zn2+ hyperpolarizes sea urchin sperm membrane potential.
The cGMP-gated K+ channel seems to be a main target of Zn2+.
Zn2+ induces [Ca2+]i fluctuations.
Zn2+ increases cAMP, but not cGMP.
Acknowledgements
This work was supported by CONACyT (49113 to AD and CB, 177138 to TN), DGAPA-UNAM (IN211809 & IN109210 to AD, IN217409 & IN204112 to CB and IN203513 to TN), the National Institutes of Health (R01 HD038082-07A1) and PROMEP-SEP (UGTO-PTC-228 to ERM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelman JP, et al. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. 2012;74:245–69. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- Andrews JC, et al. Role of zinc during hamster sperm capacitation. Biol Reprod. 1994;51:1238–47. doi: 10.1095/biolreprod51.6.1238. [DOI] [PubMed] [Google Scholar]
- Aonuma S, et al. The effect of zinc ions on fertilization of mouse ova in vitro. J Reprod Fertil. 1978;53:179–83. doi: 10.1530/jrf.0.0530179. [DOI] [PubMed] [Google Scholar]
- Babcock DF, et al. Early persistent activation of sperm K+ channels by the egg peptide speract. Proc Natl Acad Sci U S A. 1992;89:6001–5. doi: 10.1073/pnas.89.13.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancila V, et al. Two SUR1-specific histidine residues mandatory for zinc-induced activation of the rat KATP channel. J Biol Chem. 2005;280:8793–9. doi: 10.1074/jbc.M413426200. [DOI] [PubMed] [Google Scholar]
- Beltran C, et al. Membrane potential regulates sea urchin sperm adenylylcyclase. Biochemistry. 1996;35:7591–8. doi: 10.1021/bi952806v. [DOI] [PubMed] [Google Scholar]
- Bjorndahl L, Kvist U. Human sperm chromatin stabilization: a proposed model including zinc bridges. Mol Hum Reprod. 2010;16:23–9. doi: 10.1093/molehr/gap099. [DOI] [PubMed] [Google Scholar]
- Bloc A, et al. Zinc-induced changes in ionic currents of clonal rat pancreatic -cells: activation of ATP-sensitive K+ channels. J Physiol. 529 Pt. 2000;3:723–34. doi: 10.1111/j.1469-7793.2000.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonigk W, et al. An atypical CNG channel activated by a single cGMP molecule controls sperm chemotaxis. Sci Signal. 2009;2:ra68. doi: 10.1126/scisignal.2000516. [DOI] [PubMed] [Google Scholar]
- Bracho GE, et al. A method for preparation, storage, and activation of large populations of immotile sea urchin sperm. Biochem Biophys Res Commun. 1997;237:59–62. doi: 10.1006/bbrc.1997.7074. [DOI] [PubMed] [Google Scholar]
- Clapper DL, et al. Involvement of zinc in the regulation of pHi, motility, and acrosome reactions in sea urchin sperm. J Cell Biol. 1985;100:1817–24. doi: 10.1083/jcb.100.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, et al. Three pairs of cysteine residues mediate both redox and zn2+ modulation of the nmda receptor. J Neurosci. 2001;21:392–400. doi: 10.1523/JNEUROSCI.21-02-00392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darszon A, et al. Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int J Dev Biol. 2008;52:595–606. doi: 10.1387/ijdb.072550ad. [DOI] [PubMed] [Google Scholar]
- Darszon A, et al. Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev. 2011;91:1305–55. doi: 10.1152/physrev.00028.2010. [DOI] [PubMed] [Google Scholar]
- Galindo BE, et al. Participation of a K(+) channel modulated directly by cGMP in the speract-induced signaling cascade of strongylocentrotus purpuratus sea urchin sperm. Dev Biol. 2000;221:285–94. doi: 10.1006/dbio.2000.9678. [DOI] [PubMed] [Google Scholar]
- Galindo BE, et al. Sp-tetraKCNG: A novel cyclic nucleotide gated K(+) channel. Biochem Biophys Res Commun. 2007;354:668–75. doi: 10.1016/j.bbrc.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Galvez A, et al. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083–90. [PubMed] [Google Scholar]
- Garbers DL. Molecular basis of fertilization. Annu Rev Biochem. 1989;58:719–42. doi: 10.1146/annurev.bi.58.070189.003443. [DOI] [PubMed] [Google Scholar]
- Garbers DL, et al. Elevation of sperm adenosine 3′:5′-monophosphate concentrations by a fucose-sulfate-rich complex associated with eggs: I. Structural characterization. Biol Reprod. 1983;29:1211–20. doi: 10.1095/biolreprod29.5.1211. [DOI] [PubMed] [Google Scholar]
- Garcia-Soto J, et al. Internal pH can regulate Ca2+ uptake and the acrosome reaction in sea urchin sperm. Dev Biol. 1987;120:112–20. doi: 10.1016/0012-1606(87)90109-6. [DOI] [PubMed] [Google Scholar]
- Gimenez-Gallego G, et al. Purification, sequence, and model structure of charybdotoxin, a potent selective inhibitor of calcium-activated potassium channels. Proc Natl Acad Sci U S A. 1988;85:3329–33. doi: 10.1073/pnas.85.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A, et al. Inhibitory mechanism of store-operated Ca2+ channels by zinc. J Biol Chem. 2004;279:11106–11. doi: 10.1074/jbc.M400005200. [DOI] [PubMed] [Google Scholar]
- Grissmer S, et al. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994;45:1227–34. [PubMed] [Google Scholar]
- Guerrero A, Darszon A. Evidence for the activation of two different Ca2+ channels during the egg jelly-induced acrosome reaction of sea urchin sperm. J Biol Chem. 1989;264:19593–9. [PubMed] [Google Scholar]
- Guerrero A, et al. Tuning sperm chemotaxis by calcium burst timing. Dev Biol. 2010;344:52–65. doi: 10.1016/j.ydbio.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Hansbrough JR, Garbers DL. Speract. Purification and characterization of a peptide associated with eggs that activates spermatozoa. J Biol Chem. 1981;256:1447–52. [PubMed] [Google Scholar]
- Harumi T, et al. Effects os sperm-activating peptide I on Hemicentrotus pulcherrimus spermatozoa in high potassium sea water. Dev. Growth Differ. 1992;34:163–172. doi: 10.1111/j.1440-169X.1992.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Hidiroglou M, Knipfel JE. Zinc in mammalian sperm: a review. J Dairy Sci. 1984;67:1147–56. doi: 10.3168/jds.S0022-0302(84)81416-2. [DOI] [PubMed] [Google Scholar]
- Hou S, et al. Zn2+ activates large conductance Ca2+-activated K+ channel via an intracellular domain. J Biol Chem. 2010;285:6434–42. doi: 10.1074/jbc.M109.069211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H, et al. Membrane hyperpolarization by sperm-activating and -attracting factor increases cAMP level and activates sperm motility in the ascidian Ciona intestinalis. Dev Biol. 1999;213:246–56. doi: 10.1006/dbio.1999.9367. [DOI] [PubMed] [Google Scholar]
- Johnson CH, et al. A volatile inhibitor immobilizes sea urchin sperm in semen by depressing the intracellular pH. Dev Biol. 1983;98:493–501. doi: 10.1016/0012-1606(83)90378-0. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Epel D. Heavy metal chelators prolong motility and viability of sea urchin sperm by inhibiting spontaneous acrosome reactions. J Exp Zool. 1983;226:431–40. doi: 10.1002/jez.1402260314. [DOI] [PubMed] [Google Scholar]
- Kaloyianni M, et al. The influence of Zn on signaling pathways and attachment of Mytilus galloprovincialis haemocytes to extracellular matrix proteins. Comp Biochem Physiol C Toxicol Pharmacol. 2006;144:93–100. doi: 10.1016/j.cbpc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, et al. Mechanisms of sperm chemotaxis. Annu Rev Physiol. 2008;70:93–117. doi: 10.1146/annurev.physiol.70.113006.100654. [DOI] [PubMed] [Google Scholar]
- Koutsogiannaki S, et al. Cytotoxic mechanisms of Zn2+ and Cd2+ involve Na+/H+ exchanger (NHE) activation by ROS. Aquat Toxicol. 2006;78:315–24. doi: 10.1016/j.aquatox.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Lee HC. A membrane potential-sensitive Na+-H+ exchange system in flagella isolated from sea urchin spermatozoa. J Biol Chem. 1984;259:15315–9. [PubMed] [Google Scholar]
- Lee HC, Garbers DL. Modulation of the voltage-sensitive Na+/H+ exchange in sea urchin spermatozoa through membrane potential changes induced by the egg peptide speract. J Biol Chem. 1986;261:16026–32. [PubMed] [Google Scholar]
- Lee HC, et al. Changes in internal pH associated with initiation of motility and acrosome reaction of sea urchin sperm. Dev Biol. 1983;95:31–45. doi: 10.1016/0012-1606(83)90004-0. [DOI] [PubMed] [Google Scholar]
- Lishko PV, et al. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–37. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- Liu Z, et al. The micronutrient element zinc modulates sperm activation through the SPE-8 pathway in Caenorhabditis elegans. Development. 2013;140:2103–7. doi: 10.1242/dev.091025. [DOI] [PubMed] [Google Scholar]
- Matias CM, et al. Blockade of presynaptic K ATP channels reduces the zinc-mediated posttetanic depression at hippocampal mossy fiber synapses. Brain Res. 2010;1320:22–7. doi: 10.1016/j.brainres.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Mawson CA, Fischer MI. Zinc and carbonic anhydrase in human semen. Biochem J. 1953;55:696–700. doi: 10.1042/bj0550696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, et al. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985;313:316–8. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Nishigaki T, Darszon A. Real-time measurements of the interactions between fluorescent speract and its sperm receptor. Dev Biol. 2000;223:17–26. doi: 10.1006/dbio.2000.9734. [DOI] [PubMed] [Google Scholar]
- Nishigaki T, et al. Stroboscopic illumination using light-emitting diodes reduces phototoxicity in fluorescence cell imaging. Biotechniques. 2006;41:191–7. doi: 10.2144/000112220. [DOI] [PubMed] [Google Scholar]
- Nishigaki T, et al. A sea urchin egg jelly peptide induces a cGMP-mediated decrease in sperm intracellular Ca(2+) before its increase. Dev Biol. 2004;272:376–88. doi: 10.1016/j.ydbio.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Nomura M, Vacquier VD. Proteins associated with soluble adenylyl cyclase in sea urchin sperm flagella. Cell Motil Cytoskeleton. 2006;63:582–90. doi: 10.1002/cm.20147. [DOI] [PubMed] [Google Scholar]
- Ohtake H. Respiratory behaviour of sea-urchin spermatozoa. I. Effect of pH and egg water on the respiratory rate. J Exp Zool. 1976;198:303–11. doi: 10.1002/jez.1401980303. [DOI] [PubMed] [Google Scholar]
- Okamura Y. Biodiversity of voltage sensor domain proteins. Pflugers Arch. 2007;454:361–71. doi: 10.1007/s00424-007-0222-6. [DOI] [PubMed] [Google Scholar]
- Pluth MD, et al. Biochemistry of mobile zinc and nitric oxide revealed by fluorescent sensors. Annu Rev Biochem. 2011;80:333–55. doi: 10.1146/annurev-biochem-061009-091643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost AL, et al. Zinc is both an intracellular and extracellular regulator of KATP channel function. J Physiol. 2004;559:157–67. doi: 10.1113/jphysiol.2004.065094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E, Darszon A. Intracellular sodium changes during the speract response and the acrosome reaction in sea urchin sperm. J Physiol. 2003;546:89–100. doi: 10.1113/jphysiol.2002.030510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaranen M, et al. Lead, magnesium, selenium and zinc in human seminal fluid: comparison with semen parameters and fertility. Hum Reprod. 1987;2:475–9. doi: 10.1093/oxfordjournals.humrep.a136573. [DOI] [PubMed] [Google Scholar]
- Suzuki N. Structure, function and biosynthesis of sperm-activating peptides and fucose sulfate glycoconjugate in the extracellular coat of sea urchin eggs. Zoolog Sci. 1995;12:13–27. doi: 10.2108/zsj.12.13. [DOI] [PubMed] [Google Scholar]
- Suzuki N, et al. Purification and the primary structure of sperm-activity peptides from the jelly coat of sea urchin eggs. Biochem Biophys Res Commun. 1981;99:1238–44. doi: 10.1016/0006-291x(81)90752-x. [DOI] [PubMed] [Google Scholar]
- Wang D, et al. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol. 2003;5:1117–22. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- Ward GE, et al. Phosphorylation of membrane-bound guanylate cyclase of sea urchin spermatozoa. J Cell Biol. 1986;103:95–101. doi: 10.1083/jcb.103.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CD, et al. Speract induces calcium oscillations in the sperm tail. J Cell Biol. 2003;161:89–101. doi: 10.1083/jcb.200212053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, et al. Sperm chemotaxis during the process of fertilization in the ascidians Ciona savignyi and Ciona intestinalis. Dev Biol. 1993;157:497–506. doi: 10.1006/dbio.1993.1152. [DOI] [PubMed] [Google Scholar]
- Yoshida M, et al. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc Natl Acad Sci U S A. 2002;99:14831–6. doi: 10.1073/pnas.242470599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.