Abstract
Background
Difficulty with impulse control is heightened in children with a family history of alcohol use disorders and is a risk factor for later substance problems. Cross-sectional fMRI studies have shown altered impulse control processing in family history positive adolescents, yet developmental trajectories have yet to be examined.
Methods
Longitudinal fMRI was conducted in children of alcoholic (FH+; n=43) and control families (FH−; n=30) starting at ages 7-12yr. Participants performed a go/no-go task during fMRI at 1- to 2-yr intervals, with 2-4 scans per subject. We implemented a repeated-measures linear model fit across all subjects to conduct a whole-brain search for developmental differences between groups.
Results
Performance improved with age in both groups and there were no performance differences between groups. Significant between-group differences in linear age-related activation changes were found in the right caudate, middle cingulate, and middle frontal gyrus. Post-hoc analyses revealed significant activation decreases with age in the caudate and middle frontal gyrus for FH− subjects, and a significant increase with age in middle cingulate activation for the FH+ group. Group differences were evident as early as age 7-12yr, even in alcohol and drug naïve participants, with the FH+ group showing significantly blunted activation compared to FH− subjects at baseline.
Conclusions
Differences in response inhibition circuitry are visible as early as childhood in FH+ individuals; this continues into adolescence, displaying trajectories that are inconsistent with normal response inhibition development. These patterns precede problem drinking and may be a contributing factor for subsequent substance problems.
Keywords: response inhibition, alcoholism, development, adolescence, caudate, cingulate
Children of alcoholics are at heightened risk for alcohol-use disorders (AUDs) and alcohol-related problems later in life (1,2). Emotional and behavioral traits can be identified in these children prior to the onset of drinking that are early predictors of later problem use. One such trait is behavioral undercontrol (3-5), or an inability, unwillingness, or failure to inhibit impulses or responses, even when faced with adverse repercussions.
Poor response inhibition is a key mechanism underlying behavioral undercontrol generally, and vulnerability to disinhibitory psychopathology, such as substance abuse, more specifically (6). Response inhibition can be assessed using tasks that require a prepotent response to be withheld, such as the go/no-go paradigm, where individuals respond to frequent “go” stimuli while inhibiting responses to infrequent “no-go” stimuli. Imaging studies have found that a right-hemisphere network, including cortical and subcortical regions known to be involved in executive and motor control, support response inhibition during this task (7-13).
Evidence suggests that weaknesses in this network may be a risk factor for substance problems. Heitzeg et al. (14) found abnormal caudate activation during response inhibition in 16-22 year-olds with a family history of AUD compared to controls, while Schweinsburg et al. (15) observed a decrease in left middle frontal gyrus activity during response inhibition in 12-14 year-old family history positive youth. Silveri and colleagues (16) reported greater recruitment of frontal regions, including right middle frontal and cingulate gyri, in 8-19 year-olds with parental AUDs. Further studies have used follow-up data to retrospectively classify participants (who were not recruited based on family history) by whether they began using substances after fMRI scanning to provide a more definitive connection between neural activation patterns and risk. Norman et al. (17) examined 12-14 year-olds with limited substance-use histories and used subsequent interviews to later categorize them into heavy drinkers or controls. The youth who transitioned into heavy drinking showed blunted activation during no-go trials at baseline, prior to the onset of use, in the frontal cortices and striatum; this was the first demonstration of atypical activation patterns predicting future substance use. More recently, Mahmood et al. (18) investigated whether brain responses during no-go trials in 16-19 year-olds predicted substance use 18 months later. Decreased activation in ventromedial prefrontal cortex and increased activation in regions such as the left angular gyrus was reported in participants who later became high frequency substance users.
Although evidence indicates that abnormal response inhibition is a risk factor for later substance problems, no consistent picture has emerged with respect to over- or under-reactivity for regions within this network. One reason for this may be the variability in developmental periods during which subjects were scanned in cross-sectional studies. The ability to successfully inhibit a response increases throughout youth and continues into early adulthood (19-24), concomitant with the maturation of inhibitory control (25,26). Cross-sectional fMRI studies of response inhibition have shown that prefrontal activation is bilateral in children, but lateralizes to the right hemisphere and becomes more focal in adults, while striatal activation continues to increase with age (27-30). Other studies have found greater posterior activation in children compared to adults (31). Therefore, significant and diverse developmental changes are occurring in response inhibition circuitry during the time when risk for alcohol/drug initiation is sharply increasing. Because of this, identifying specific neural abnormalities in this circuitry represents a moving target when viewed from childhood to early adulthood, as the neural differences representing risk are likely quite different at ages 12-14 versus 16-19. Furthermore, the use of broad age ranges may mask important developmental differences.
The present study was designed to identify differences in inhibition circuitry development from childhood to adolescence in those with and without a family history of alcoholism. A longitudinal study was conducted, with at least 2 and as many as 4 scans per subject, where data collection occurred at 1- to 2-yr intervals starting at ages 7-12yr and covered the range of 7-16.9yr. Additionally, a repeated-measures linear model fit across all subjects to conduct a whole-brain search for developmental differences between groups was implemented. Based on prior cross-sectional work investigating familial risk, we expected to find developmental group differences in the prefrontal cortices and striatum. Based on prior cross-sectional work investigating development, we tentatively hypothesized that emerging differences would represent either a delay or failure in the specialization of inhibitory control in the right prefrontal cortex of those with a family history of alcoholism.
Methods and Materials
Participants
Seventy-three right-handed participants (32) aged 7-12yr were recruited from the Michigan Longitudinal Study (MLS), an ongoing, prospective study of families with high levels of parental AUD and a contrast sample of nonalcoholic families (33). Families in which the target child displayed evidence of fetal alcohol effects were excluded.
Participants performed a go/no-go task during fMRI at 1- to 2-yr intervals. Included participants had at least two fMRI scans, covering the age range of 7-16.9yr. Participants were divided into two groups: those with at least one parent who had an AUD diagnosis during the child’s lifetime (FH+; n=43; scans=113), and those with no parental AUD history within 2 years of the child’s birth or during the child’s lifetime (FH−; n=30; scans=85). AUD diagnosis was calculated by a clinical psychologist based on Diagnostic Interview Schedule – Version 4 (34-36), Health History, and Drinking & Drug Use answers (Supplementary Information).
Characteristics of these groups are summarized in Table 1
Table 1.
Subject characteristics and task performance.
| All participants | FH− | FH+ | |
|---|---|---|---|
| N | 73 | 30 | 43 |
| Males/females | 51/22* | 25/5 | 26/17 |
| Total scans | 198 | 85 | 113 |
| N with at least 2 scans | 73 | 30 | 43 |
| N with at least 3 scans | 39 | 19 | 20 |
| N with 4 scans | 14 | 6 | 8 |
| Age range (min/max) | 7.58/16.83 | 7.58/16.83 | 7.85/16.74 |
| IQ (mean ±sd) | 103.60 ±14.45) | 105.36 ±13.90 | 102.46 ±14.86 |
|
Family history AUD:
Father/mother/both |
23/3/17 | 0/0/0 | 23/3/17 |
| ADHD Diagnosis (n) | 6 | 2 | 4 |
| CD Diagnosis (n) | 0 | 0 | 0 |
| CBCL at scan 1 (mean ±sd) | |||
| Aggressive behavior | 5.13 ±9.12 * † | 3.06 ±6.01 | 6.90 ±10.97 |
| Delinquent behavior | 1.07 ±1.90 † | 0.53 ±0.90 | 1.52 ±2.38 |
| Externalizing total | 6.19 ±10.86 * † | 3.59 ±6.73 | 8.43 ±13.20 |
| Alcohol/drug use | |||
| Baseline (scan 1) | |||
| Alcohol use | 4 | 0 | 4 |
| Marijuana use | 2 | 0 | 2 |
| Illicit drug use | 1 | 0 | 1 |
| Total subjects reporting any use | 5 | 0 | 5 |
| Follow-up (scans 2, 3, or 4) a | |||
| Alcohol use | 8* | 1 | 7 |
| Marijuana use | 8 | 3 | 5 |
| Illicit drug use | 5 | 0 | 5 |
| Total subjects reporting any use | 13 | 3 | 10 |
AUD: alcohol use disorder; ADHD: attention deficit/hyperactivity disorder; CD: conduct disorder; CBCL: child behavior checklist
Follow-up use was the report of alcohol/drug use at any time after the first scan was completed.
indicates p<0.05.
indicates p<0.05 with gender as a covariate.
Exclusionary criteria were neurological, acute, uncorrected, or chronic medical illness; current/recent (within 6 months) treatment with centrally active medications; and history of psychosis or schizophrenia in first-degree relatives. Participants were given a multidrug urine screen before scanning starting at age 15. The presence Axis I psychiatric or developmental disorders was exclusionary, except for conduct and attention deficit/hyperactivity disorders or prior substance use disorder, as exclusion of these would preferentially eliminate part of the phenomena of interest. Diagnosis was determined using the Diagnostic Interview Schedule– Child (37). Participants who were on medication for ADHD were required to stop taking them at least 48 hours prior to their scan.
All participants and at least one parent gave written assent, as approved by the local institutional review board.
Participant Measures
Alcohol or drug use, as well as the number of drinking- and/or drug-related problems, were assessed using the self-report Drinking and Drug History Form for Children (38,39), administered yearly between 11-17yr as part of the MLS. At ages 6-10, basic information about alcohol and drug use was collected in which children were asked if they had ever had more than a sip of alcohol and/or used marijuana or other drugs. Externalizing behavior was assessed using the Child Behavior Checklist (CBCL) (40), collected between 7-14yr; the measure collected closest to the baseline fMRI scan was used to calculate means.
fMRI Task
A go/no-go task was used to probe response inhibition and impulse control, using events and timing based on Durston et al. (30). Participants were instructed to respond to target stimuli by pressing a button (go trials; all letters except “X”) but make no response to infrequent non-target stimuli (no-go trials; letter “X”). Stimulus duration was 500ms, followed by 3500ms of fixation. There were 5 separate runs of 49 trials, each lasting 3.5min with short breaks in between, for a total of 60 no-go trials out of 245 total trials (details in Supplementary Information). Before scanning, participants practiced on a desktop computer. False alarms (FA; inhibitory response failure to a no-go trial), hit accuracy (Hit; correct response to targets), and hit reaction times (HitRT) were calculated as performance measures.
MRI Data Acquisition
Whole-brain blood oxygenated level-dependent images were acquired on a 3.0 Tesla GE Signa scanner (Milwaukee, WI) using a T2*-weighted single-shot combined spiral in-out sequence (41) with the following parameters: TR=2000ms; T=30ms; flip angle=90°; FOV=200mm; 64×64 matrix; slice thickness=4mm; 29 slices. A high-resolution anatomical T1 scan was obtained for spatial normalization (three-dimensional spoiled gradient-recalled echo, TR=25ms; min TE; FOV=25cm; 256×256 matrix; slice thickness=1.4mm).
Data Analysis
Behavioral Data
Linear mixed model analyses were conducted in SPSS (Version 19.0, Armonk, NY: IBM Corp) with scan (1st, 2nd, etc) as a repeated measure, subject as a random factor, family history as a fixed factor, and age and gender as fixed-effect covariates. Main effects of age, gender, group, and age-by-group interactions were investigated for each performance measure. Differences in CBCL scores (Table 1) were determined using a univariate general linear model analysis (n=64; 26 FH−, 38 FH+).
fMRI Data
Functional images were linearly combined and reconstructed using an iterative algorithm(42,43). Subject head motion was corrected using FSL 5.0.2.2 (FMRIB, Oxford, UK) (44); runs exceeding 3mm translation or rotation in any direction were excluded (see Supplemental Information). All remaining statistical analyses were completed using SPM8 (Wellcome Institute of Cognitive Neurology, London, UK). Functional images were spatially normalized to a standard stereotaxic space as defined by the Montreal Neurological Institute. A 6mm full-width half-maximum Gaussian spatial smoothing kernel was applied to improve signal-to-noise ratio and account for anatomical differences.
Individual analyses were completed using a general linear model. Three regressors of interest (correct no-go, failed no-go, and all go) were convolved with the canonical hemodynamic response function, with event durations of 4s from stimulus presentation. Motion parameters and white matter signal intensity were modeled as nuisance regressors to remove residual motion artifacts and capture non-task-related noise. The main contrast of interest was correct no-go versus correct go trials, as described previously (14,30,45,46). Parameter estimates were linearly combined over all runs. Test-retest reliability for the main contrast is reported in Supplemental Information.
Task effect was determined for all participants across all scans and for each group, using gender as a covariate. Areas of activation were deemed significant if they reached a cluster-level FDR-corrected threshold of p<0.05, where a cluster-forming threshold of uncorrected p<0.001 was used.
Differences in developmental trajectories between groups were tested in SPM using a second-level multiple regression analysis that accounted for subject age. Contrast images were entered into the model with the following covariates: group, gender, and age at time of scan (centered, squared, cubed; polynomials were orthogonalised); subject intercepts were added to approximately account for repeated-measures correlation. Statistical maps thresholds were p<0.005; differences between groups for linear, quadratic, and cubic age effects were deemed significant if activation reached a cluster-level FDR-corrected statistical threshold of p<0.05. Effect sizes for significant clusters were extracted using MarsBaR Region of Interest (ROI) toolbox (47).
Extracted values were imported into SPSS and linear mixed model analyses were conducted with scan (1st, 2nd, etc) as a repeated measure, subject as a random factor, and family history as a fixed factor. Schwarz’s Bayesian information criteria (BIC) (48) were used to determine the best-fitting variance-covariance structure (scaled identity). To confirm SPM results, age and gender were entered as fixed-effect covariates and main effects (age, gender, family history) and interactions were tested for each ROI. To investigate relationships between brain activation and performance, separate mixed model analyses were run with each performance measure as the dependent variable, and extracted ROI values and gender as fixed-effect covariates. Group activation differences at scan 1 were determined using a univariate general linear model analysis with brain activation as the dependent variable, family history as a fixed factor, and gender as a covariate.
To determine whether group differences existed prior to alcohol and drug use, an independent samples t-test was run on baseline scans for each extracted ROI using only subjects who had not used alcohol or drugs by scan 1 (n=68). We also removed all participants who reported alcohol or drug use at any point during the study and reran the linear mixed model analysis in SPSS with gender as a covariate (n=60).
A supplementary analysis was conducted to investigate whether age-related differences found across the entire age range of 7-16.9yr in all 73 participants with repeated scans could be observed within subjects from childhood to adolescence. Individuals with an initial scan between 7-12.9yr and a follow-up scan between 13-16.9yr were identified (n=46), and a repeated-measures ANOVA was run with time point (childhood vs. adolescence) as a within-subjects factor and family history and gender as between-subjects factors.
Results
Subject characteristics
There were no significant differences between groups for IQ (F=0.04, p=0.237), mean age (F=0.26, p=0.741), drinking/drug use at first scan (Table 1), presence of an ADHD diagnosis (χ(1)=0.29, p=0.590), or for age at each scan (Table S1). There was a significant difference between groups for drinking initiation (FH+>FH−; (1)=6.07, p=0.014), and a trend for illicit drug use (χ(1)=3.62, p=0.057) at follow-up (Table 1).
There was a significant gender difference between groups (χ(1)=11.02, p=0.001), with more females in the FH+ group (Table 1). Because females are known to be less impulsive than males (49,50), analyses were run using gender as a covariate.
Significant differences between the FH− and FH+ groups at scan 1 were found for aggressive (F=11.05, p=0.002), delinquent (F=4.56, p=0.037) and externalizing (F=11.77, p=0.001) behaviors when using gender as a covariate, where FH+ mean scores were higher for all three measures (Table 1). There was a main effect of gender for delinquent behavior (F=5.53, p=0.022); post-hoc analyses revealed this was due to males, who had greater mean delinquency scores (1.85 ±2.88) compared to females (1.00 ±1.20).
Performance
Linear mixed model analysis revealed a significant effect of age for Hit (F=14.80, p=0.001), HitRT (F=7.62, p=0.007), and FA (F=41.72, p=0.001) with more correct responses to targets, decreased reaction times, and less FA with age. A significant effect of gender was found for FA (F=12.44, p<0.001); post-hoc tests revealed that this was because females made fewer false alarms than males (females: 32.5% ±1.8sd; males: 46.1% ±1.9sd).
fMRI results
Task effect
To ensure the task worked effectively, we looked at task effects across all scans for all participants and within each group (Table 2). These regions are in agreement with previous studies that have investigated the neural correlates of response inhibition (7-13). There were no significant differences in activation between groups.
Table 2.
Task activation for correct reject across scans for all participants at a cluster-corrected threshold of p=0.05 (FDR), 35 voxels.
| Location | All scans (n=198) |
FH− scans (n=85) |
FH+ scans (n=113) |
|||
|---|---|---|---|---|---|---|
| x y z | t | x y z | t | x y z | t | |
|
R Inferior Frontal
Gyrus |
50 22 0 | 8.92 | ||||
|
L Middle Frontal
Gyrus |
−26 44 22 | 4.69 | −30 42 24 | 4.77 | ||
| R Insula | 44 16 −2 | 12.37 | 44 16 −2 | 9.27 | ||
| L Insula | −40 18 −6 | 8.96 | −40 18 −6 | 8.30 | −34 22 6 | |
| R Angular Gyrus | 46 −54 36 | 9.38 | ||||
|
R Supramarginal
Gyrus |
56 −46 26 | 11.97 | 56 −44 24 | 10.18 | ||
|
L Supramarginal
Gyrus |
−62 −54 32 | 8.90 | −60 −54 30 | 7.35 | ||
| R Precuneus | 10 −68 38 | 7.60 | 10 −68 38 | 5.94 | ||
|
L Inferior Parietal
Gyrus |
−48 −54 36 | 6.21 | ||||
| L Putamen | −28 10 −4 | 5.65 | ||||
| L Caudate | −10 8 8 | 4.27 | ||||
| L Pallidum | −16 2 −4 | 3.35 | ||||
|
R Middle
Temporal Gyrus |
58 −26 −8 | 11.59 | 64 −34 −2 | 9.61 | ||
|
R Inferior
Temporal Gyrus |
46 2 −40 | 7.68 | 46 0 −40 | 5.13 | ||
|
L Superior
Temporal Gyrus |
−64 52 22 | 7.01 | ||||
|
L Middle
Temporal Gyrus |
−58 −28 −6 | 5.72 | −70 −28 −6 | 5.77 | ||
| R Fusiform | 32 2 −36 | 3.81 | ||||
R=right hemisphere; L=left hemisphere
Longitudinal
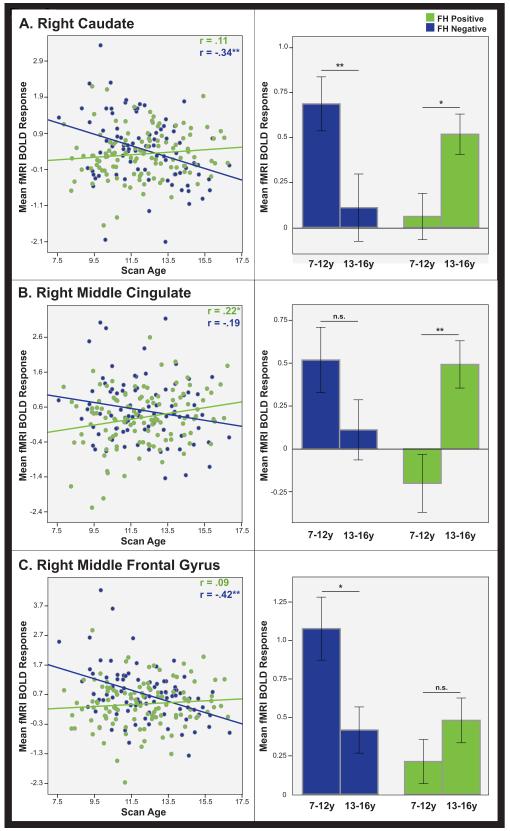
Significant group differences for linear age effects were found in the right caudate, middle frontal gyrus, and a cluster centered on the middle cingulate extending into the supplementary motor area and anterior cingulate (Figure 1; Table 3). No significant differences were found between groups for quadratic or cubic age-related effects.
Figure 1.
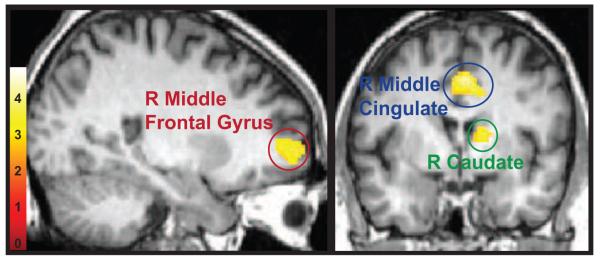
Whole-brain longitudinal results. Regions displaying a significant group difference for linear age changes during response inhibition displayed at a statistical threshold of p<0.005, 500 voxel extent. Three regions of interest passed the criteria of FDR cluster correction of p<0.05, 35 voxel extent: the right caudate (green), the right middle cingulate (blue), and the right middle frontal gyrus (red).
Table 3.
Significant ROIs that showed linear age-related changes in activation from (A) SPM whole brain analysis at FDR cluster correction of p<0.05, and (B) SPSS linear model analysis. Interaction column denotes significant interaction between family history and age-centered. P-values for SPSS analysis are for age-centered changes in each group.
| ROI | (A) SPM (n=198) |
(B) SPSS | ||||||
|---|---|---|---|---|---|---|---|---|
| x y z | k | t | Interaction | FH− (n=85) |
FH+ (n=113) |
|||
| F | p | F | p | |||||
| R Caudate | 10 0 6 | 603 | 3.96 | 0.001* | 16.45 | 0.001* | 1.43 | 0.236 |
| R Middle Cingulate/SMA |
2 4 42 | 526 | 4.01 | 0.004* | 2.36 | 0.131 | 6.96 | 0.010* |
| R Middle Frontal Gyrus |
40 46 16 | 1022 | 4.62 | 0.001* | 12.74 | 0.001* | 1.60 | 0.210 |
R = right hemisphere
SPSS linear mixed model analyses confirmed the group-by-age interaction for each ROI (Figure 2; Table 3). Post-hoc analyses revealed significant activation increases with age in the middle cingulate for the FH+ group. In the FH− group, significant decreases with age were found in the caudate and middle frontal gyrus.
Figure 2.
Regions that showed a significant group difference in the whole-brain longitudinal analysis (outlined in Figure 1), plotted for FH+ (green) and FH− (blue) groups in the (A) right caudate, (B) right middle cingulate, and (C) right middle frontal gyrus. Longitudinal linear age effects are depicted in the scatterplots on the left, illustrating changes in mean fMRI activation response across time. Bar graphs on the right depict repeated measures ANOVA analysis between initial scans (ages 7-12.9) and a follow-up scan (ages 13-16.9) that confirm that the pattern of longitudinal effects are evident within subjects. Error bars represent ±1 standard error; ** indicates p<0.01; * indicates p<0.05; n.s.= non-significant.
When examining whether group differences existed at baseline, a significant difference between groups was found for all ROIs. In all cases, the group of non-using FH+ had lower activation means than the FH− group at baseline (Table 4). When repeating the linear mixed model after removing participants with any reported drug or alcohol use, the findings remained significant, indicating that the inclusion of these subjects did not drive our results (Supplementary Information).
Table 4.
Mean activation at scan 1 in regions that showed a significant longitudinal group difference.
| ROI | F | p | FH− (n=30) |
FH+ (n=43) |
|---|---|---|---|---|
| Mean ±sd | Mean ±sd | |||
| R Caudate | 9.35 | 0.003* | 0.78 (1.03) | 0.13 (0.71) |
| R Middle Cingulate/SMA | 16.21 | 0.001* | 0.78 (0.94) | −0.07 (0.85) |
| R Middle Frontal Gyrus | 17.15 | 0.001* | 1.28 (1.06) | 0.23 (0.89) |
R=right hemisphere.
indicates p<0.05
The repeated-measures ANOVA (n=46) confirmed longitudinal effects within subjects. All ROIs showed a significant time by family history interaction (caudate: F=15.33, p<0.001; middle cingulate: F=10.10, p=0.003; middle frontal gyrus: F=6.77, p=0.013; Figure 2).
Association between brain activation and performance
There was a significant interaction between the middle cingulate response and family history for FA (F=18.49, p=0.001); post-hoc analysis revealed this was driven by the FH− group, where decreased middle cingulate activation was associated with fewer FA (F=13.90, p<0.001). This relationship approached significance in the FH+ group (F=3.75, p=0.056), where increased middle cingulate activation was associated with better performance. There was also a significant interaction between the caudate response and HitRT (F=4.25, p=0.041), where increased caudate activation was associated with slower reaction times in the FH+ group (F=6.64, p=0.012).
Discussion
The purpose of this study was to identify differences in the neural development of response inhibition in individuals with and without a parental history of AUD. Here we find that activation differences are visible as early as childhood in FH+ individuals and continue into adolescence, displaying patterns of change inconsistent with the FH− group and normal response inhibition development. These patterns precede problem drinking and may contribute to later alcohol/drug abuse and dependence.
Across all participants, performance improved with age, consistent with research illustrating the ability to quickly and accurately inhibit prepotent responses improves from childhood into adulthood (20-24,27,28). Additionally, individuals at risk for AUD achieve results that are similar to controls in simple measures of response inhibition, such as the go/no-go task (14,17). While there were no performance group differences, there was a significant main effect of gender for FA. This result, however, was found to be driven by females, who made less FA than males and are often less impulsive (49,50).
In contrast to performance, there were significant differences in the neural patterns associated with successful response inhibition between groups, with FH− individuals displaying significant decreases in activation with increasing age in the right middle frontal gyrus and caudate. These developmental trajectories differed significantly from the FH+ group, where no considerable variation in activation manifested. The changes observed in FH− individuals are thought to reflect improvements in response inhibition network specialization and organization as behavioral control evolves, and are in parallel with developmental studies highlighting inhibitory control increases that occur with maturation in healthy children (20,51,52). Younger subjects are thought to use inefficient strategies when inhibiting a response; thus, brain activity in these individuals differs with respect to magnitude, location, and distribution compared to adults. Both activation increases (27,29) and decreases (53) have been reported in key response inhibition regions across development when comparing adolescents to adults, purportedly due to local differences within the frontal lobe (i.e. some regions increase their response while others decrease; [28]) or to task-dependent differences between studies (54). Additionally, previous studies illustrate a greater engagement of prefrontal and parietal regions, and/or shift of activation, during response inhibition in children compared to adults, thought to indicate the need for increased executive control to compensate for weak anatomical connections between inhibition regions (25,30,31,46). During childhood and adolescence, substantial anatomical maturation occurs (55)—particularly in the frontal lobes—along with heightened anatomical and functional connectivity between prefrontal and posterior regions, concomitant with improved response inhibition (56). These studies, however, were conducted in healthy maturing individuals with no family history of AUD. The present study is the first to investigate maturational differences in high-risk children and adolescents. The age-related decreases observed here in FH− individuals are within the limits of normal development and provide an implicit within-study comparison to the high-risk, FH+ group.
While the FH− group follows the expected developmental trajectory, the FH+ group displays a disparate pattern as there was a significant increase in activation with age in the right middle cingulate during successful inhibition. Despite these aberrant age-related response patterns, the FH+ group was still able to match the go/no-go performance of FH− individuals. Therefore, it is possible that the FH+ group requires the recruitment of this region to successfully perform the task. The middle cingulate cluster of activation extends partially into the anterior cingulate and supplementary motor area, which are well-documented to be involved in response inhibition—more specifically, the implementation of executive control (57,58), suggesting that for FH+ individuals successful inhibition may require more effort to effectively override a prepotent response. Further disparities in impulse control circuitry are discernible in the regions that show no significant change with age in the FH+ group: the middle frontal gyrus and caudate. The middle frontal gyrus is a key part of a prefrontal network responsible for inhibiting a response to inappropriate stimuli by overriding the motor system’s automatic response tendency (54,57-59). The caudate has been implicated in task switching and preventing an action from being automatically executed (13,62); both activations and deactivations of this region have been described during successful response inhibition (26). A prior study reported blunted caudate deactivation during response inhibition in FH+ compared with FH− individuals aged 16-21 (14). The present report gives a developmental perspective across earlier ages, illustrating that the identification of specific neural abnormalities in this circuitry represents a moving target when viewed from childhood to early adulthood. As can be seen in Figure 2, although activation in the caudate is greater in FH− than FH+ participants at earlier ages, at later ages, activation patterns approach those observed in Heitzeg et al. (14). The unique contribution of each region to successful response inhibition combined with disparate age-related changes (or absence of change) in the FH+ compared to the FH− group implies a lack of maturational organization and specialization in these regions individually, and conceivably in their connections with one another.
Further support for divergent neural response inhibition comes from the interplay between brain activity and behavioral responses over time. A significant group difference was found in the relationship between middle cingulate activation and inhibitory failure. In the FH− group, fewer FAs were significantly associated with less middle cingulate activation. As these children aged, there was no significant change in middle cingulate response; however, FA rates significantly decreased. Thus, the middle cingulate appears to be important in the early development of response inhibition in FH− individuals. Portions of the cingulate cortex, particularly the anterior cingulate, are involved in error detection and performance monitoring; heightened activation in FH− children could signify an increased need for performance monitoring that becomes less essential across development as cognitive control improves. In contrast, there was no significant relationship between FA and middle cingulate activation in the FH+ group; however, there was a significant decrease in FA, as well as a significant increase in middle cingulate response, with age. This suggests that this region remains “online” well into adolescence and is continually needed for successful inhibition, perhaps to compensate for weak connectivity between other portions of response inhibition circuitry that are responsible for top-down executive control (26). However, since this study did not probe connectivity, future work directly investigating connections between response inhibition regions in those at risk for AUD is needed.
Differences in group activation are not only evident across development, but also when analyzing only baseline scans, completed at ages 7-12, where the FH+ group shows less activation than FH− in all ROIs. Because this analysis did not include subjects using alcohol/drugs at the time of this scan, it can be concluded that these differences are in place prior to substance use. This extends previous findings showing activation differences between at-risk adolescents and young adults despite similar task performance (14,15). Furthermore, widespread blunted activation during no-go trials at ages 12-14 has been found in those who later transition into heavy alcohol use, lending evidence to the hypothesis that an attenuated inhibitory response early in adolescence is one developmental manifestation in a trajectory that leads to reduced cognitive control (17). Combined with the current data, this suggests that attenuated response inhibition manifests quite early in at-risk children and continues to exacerbate with age.
It is unclear from this study the extent to which these differences reflect genetic versus environmental risk factors. Based on the definition of FH+ used for the present study, which included only those who had one or more parents with an AUD during the child’s lifetime, we expect that environmental factors are more heavily at play. Environmental factors can certainly increase risk in an individual who is also genetically at risk, or may have little effect on a person without a genetic risk. Perry et al. (63) demonstrated that a high rate of positive life events was protective for men with a high-risk GABRA2 genotype, but not for men with the low-risk genotype. Dick et al. (64) demonstrated how a genetic effect can be strong in the absence of certain supportive environmental factors such as parental monitoring, but weak when these factors are present. Such studies that probe the interaction between genetic risk and environmental stressors with respect to substance abuse are becoming more prevalent (65) however, it is an area that requires extensive exploration before definitive conclusions can be made.
There are some limitations worth noting. First, there are more male participants in both groups. Given that males are known to be more impulsive than females, the developmental trajectory of response inhibition could differ based on gender, as could its relationship with risk. Further work is necessary to understand possible gender differences in vulnerability related to impulse control development. Second, the maximum age here is approximately 17yr; therefore, it is unknown what neural changes occur with respect to both groups as the prefrontal cortex continues to develop, given that this continues into early adulthood. Third, pre-scan drug screens were not performed until age 15, so it is unknown whether any subjects who were scanned prior to 15yrs had drugs in their system at the time of scanning. However, analyses were also conducted without those reporting any lifetime substance use, and these results were consistent with those analyses that included all participants. Fourth, at present there are no neuroimaging analysis tools that can handle unbalanced repeated measures data: SPM requires an assumption of whole-brain homogeneity of the repeated-measures correlation, while FSL assumes balanced designs with compound symmetric correlation. Therefore, we used an SPM model that best approximated subject intercepts to account for repeated-measures correlation. The post-hoc SPSS linear mixed model tests support and validate this approach.
In summary, children with a family history of AUD display response inhibition patterns inconsistent with normal development. Even though FH+ and FH− groups perform similarly with respect to task behavior, the FH+ group likely relies on increased right middle cingulate activation to successfully inhibit prepotent responses, possibly to compensate for general response inhibition network weaknesses. This pattern manifests early in development and continues with age, indicating that differences precede problem drinking and may contribute to later substance use problems.
Supplementary Material
Acknowledgements
This work was supported by R01 DA027261 to MMH, JKZ, and RAZ; R01 AA12217 to RAZ and MMH; R37 AA07065 to RAZ; K01 DA031755 to BJW; K01 DA020088 to MMH; and by the Phil F. Jenkins Research Fund (JKZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures The authors report no biomedical financial interests or potential conflicts of interest relevant to the content of this manuscript.
References
- 1.Russell M. Prevalence of alcoholism among children of alcoholics. In: Windle M, Searles J, editors. Children of Alcoholics: Critical Perspectives. Guilford; New York: 1990. pp. 9–38. [Google Scholar]
- 2.National Institute on Alcohol Abuse and Alcoholism . Alcohol Involvement over the Life Course. NIAAA. Tenth Special Report to the US Congress on Alcohol and Health: Highlights from Current Research. Department of Health and Human Services; Bethesda, MD: 2000. pp. 28–53. [Google Scholar]
- 3.Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, subject use and abuse, and psychopathology. J Abnorm Psychol. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- 4.Zucker RA. Alcohol use and alcohol use disorders: a developmental-biopsychosocial systems formulation covering the life course. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. 2nd ed Wiley; Hoboken, NJ: 2006. pp. 620–656. [Google Scholar]
- 5.Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol/disinhibition pathway to substance use disorders: a multilevel developmental problem. Child Dev Perspect. 2011;5:248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neuroscience. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nachev P, Wydell H, O’Neill K, Husain M, Kennard C. The role of the pre supplementary motor area in the control of action. Neuroimage. 2007;36:T155–63. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neuroscience. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- 11.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li CS, Yan P, Sinha R, Lee TW. Sub-cortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heitzeg MM, Nigg JT, Yau WW, Zucker RA, Zubieta JK. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol Psychiatry. 2010;68:287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, et al. An fMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- 16.Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during Stroop performance. Alcohol Clin Exp Res. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmood OM, Goldenberg D, Thayer R, Migliorini R, Simmons AN, Tapert SF. Adolescents fMRI activation to a response inhibition task predicts future substance use. Addict Behav. 2013;38:1435–1441. doi: 10.1016/j.addbeh.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schachar R, Logan GD. Impulsivity and inhibitory control in normal development and childhood psychopathology. Dev Psychol. 1990;26:710–720. [Google Scholar]
- 20.Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- 21.Band GP, van der Molen MW, Overtoom CC, Verbaten MN. The ability to activate and inhibit speeded responses: separate developmental trends. J Exp Child Psychol. 2000;75:263–290. doi: 10.1006/jecp.1999.2538. [DOI] [PubMed] [Google Scholar]
- 22.Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Dev Neuropsychol. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- 23.Cragg L, Nation K. Go or no-go? Developmental improvements in the efficiency of response inhibition in mid-childhood. Dev Sci. 2008;11:819–827. doi: 10.1111/j.1467-7687.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 24.Tottenham N, Hare TA, Casey Bj. Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Front Psychol. 2011;2:39. doi: 10.3389/fpsyg.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J Cognitive Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 28.Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, et al. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- 30.Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Sci. 2002;5:F9–16. [Google Scholar]
- 31.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 33.Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, Ellis DA. The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy. In: Fitzgerald HE, Lester BM, Zucker RA, editors. Children of Addiction: Research, Health and Policy Issues. RoutledgeFalmer; New York: 2000. pp. 109–141. [Google Scholar]
- 34.Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) Washington University School of Medicine; St. Louis, MO: 2000. [Google Scholar]
- 35.Robins L, Helzer J, Croughan J, Ratcliff KS. The NIMH Diagnostic Interview Schedule: Its history, characteristics and validity. Washington University School of Medicine; St. Louis, MO: 1980. [Google Scholar]
- 36.Robins LM, Helzer JH, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Arch Gen Psychiat. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 37.Costello A, Edelbrook C, Dulcan M, Kalas R, Klanc S. Development and Testing of the NIMH Diagnostic Interview Schedule for Children in a Clinic Population. Center for Epidemiological Studies, National Institute of Mental Health; Rockville, MD: 1984. [Google Scholar]
- 38.Zucker RA, Fitzgerald HE. Drinking and drug history form for children. University of Michigan Department of Psychiatry, Addiction Research Center; Ann Arbor: 1994. [Google Scholar]
- 39.Zucker RA, Fitzgerald HE, Noll R. Drinking and Drug History. rev. ed., Version 4 University of Michigan Department of Psychiatry, Addiction Research Center; Ann Arbor: 1990. [Google Scholar]
- 40.Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington: 2001. [Google Scholar]
- 41.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 42.Sutton BP, Noll DC, Fessler JA. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Trans Med Imaging. 2003;22:178–188. doi: 10.1109/tmi.2002.808360. [DOI] [PubMed] [Google Scholar]
- 43.Noll DC, Fessler JA, Sutton BP. Conjugate phase MRI reconstruction with spatially variant sample density correction. IEEE Trans Med Imaging. 2005;24:325–336. doi: 10.1109/tmi.2004.842452. [DOI] [PubMed] [Google Scholar]
- 44.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 45.Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, Halperin JM. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related FMRI study. Am J Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- 46.Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 47.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract]. Neuroimage; Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2-6, 2002; 2002. Available on CD-ROM in. [Google Scholar]
- 48.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;2:461–464. [Google Scholar]
- 49.Labouvie EW, McGee CR. Relation of personality to alcohol and drug use in adolescence. J Consult Clin Psychol. 1986;54:289–293. doi: 10.1037//0022-006x.54.3.289. [DOI] [PubMed] [Google Scholar]
- 50.Campbell A, Muncer S. Can ‘risky’ impulsivity explain sex differences in aggression? Pers Indiv Differ. 2009;47:402–406. [Google Scholar]
- 51.Greenberg LM, Waldman ID. Developmental normative data on the test of variables of attention (T.O.V.A.) J Child Psychol Psychiatry. 1993;34:1019–1030. doi: 10.1111/j.1469-7610.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 52.Ridderinkhof KR, Band GPH, Logan GD. A study of adaptive behavior: effects of age and irrelevant information on the ability to inhibit one’s actions. Acta Psychol. 1999;101:315–337. [Google Scholar]
- 53.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 54.Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescent: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 56.Rubia K. Functional brain imaging across development. Eur Child Adoles Psy. 2012;22:719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turken AU, Swick D. Response selection in the human anterior cingulate cortex. Nat Neurosci. 1999;2:920–924. doi: 10.1038/13224. [DOI] [PubMed] [Google Scholar]
- 58.Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci USA. 2002;99:16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- 60.Sharp DJ, Bonnelle V, De Boissezon X, Beckman CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci USA. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vink M, Kahn RS, Raemaekers M, van den Jeuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp. 2005;25:336–344. doi: 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perry BL, Pescosolido BA, Bucholz K, Edenberg H, Kramer J, Kuperman S, et al. Gender-specific gene-environment interaction in alcohol dependence: the impact of daily life events and GABRA2. Behav Genet. 2013;43:402–414. doi: 10.1007/s10519-013-9607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dick DM, Pagan JL, Viken R, Purcell S, Kaprio J, Pulkkinen L, et al. Changing environmental influences on substance use across development. Twin Res Hum Genet. 2007;10:315–326. doi: 10.1375/twin.10.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Enoch MA. The influence of gene-environment interactions on the development of alcoholism and drug dependence. Curr Psychiatry. 2012;14:150–158. doi: 10.1007/s11920-011-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.