Abstract
Over-expression of the protein Dickkopf-1 (Dkk1) has been associated with multiple myeloma bone disease. Previous reports with the use of anti-Dkk1 neutralizing Ab directed strategies have demonstrated a pro-anabolic effect with associated anti-myeloma activity in 2 in vivo mouse models. However new insights on the role of the wnt pathway in osteoclasts (OC) are emerging and the potential effect of a neutralizing Ab to Dkk1 in osteoclastogenesis remains to be elucidated. In order to better define the effect of an anti-Dkk1 neutralizing Ab on osteoclastogenesis and myeloma, we studied a novel anti-Dkk1 monoclonal Ab in our preclinical models. In vivo data confirmed the pro-anabolic and anti-MM effect. In vitro data in part confirmed the in vivo observation, suggesting an indirect anti-MM effect secondary to inhibition of osteoclastogenesis and thus the interaction between MM and bone microenvironment. However, when studies on osteoclastogenesis were extended to samples derived from MM patients, we observed a variable response to anti-Dkk1 treatment without correlation to expression of surface receptors for Dkk1 in OCs suggesting potential heterogeneity in efficacy of such a strategy. In conclusion, Dkk1 is a promising target for the treatment of both MM and bone disease, and ongoing clinical studies will help elucidate its efficacy.
Keywords: Dkk1, wnt, multiple myeloma, osteoclast, osteoblast
INTRODUCTION
Ninety percent of multiple myeloma (MM) patients manifest with osteolytic bone lesions. These can evolve to pathological fractures in 40-50% of the cases [1], spinal cord compression or require radiation therapy. Several studies have demonstrated that bone damage is consequent to the uncoupling of osteoclasts (OC) and osteoblasts (OB), ultimately leading to enhanced OC activity and blunted OB function with a consequent imbalance favoring bone resorption. A wide range of cytokines have been implicated in increased OC formation, including interleukin-6 (IL-6), IL-3, macrophage inflammatory protein-1 alpha (mip1α), upregulation of Receptor Activator for Nuclear Factor κ B Ligand (RANKL), and decreased expression of osteoprotegerin (OPG) [2]. In addition to enhanced bone destruction, bone deposition is also impaired secondary to OB dysfunction. Several factors affect osteoblastogenesis in MM patients including increased IL-3, IL-7, hepatocyte growth factor (HGF) and proteins involved in the wingless/int (wnt) pathway: secreted frizzled related proteins (sFRPs) and Dickkopf-1 (Dkk1) protein [3].
Dkk1 is one of 4 proteins that negatively regulates canonical wnt/β-catenin signaling. While activation of wnt pathway mediates the translocation of nonphosphorylated β-catenin into the nucleus with consequent activation of T-cell transcription factor (TCF)/lymphoid enhancer-binding factor (LEF), the binding of Dkk1 to LRP5/6 and kremen receptors ultimately induces β-catenin degradation by the proteasome [4]. Wnt pathway is active both in embryonic as well as in adult life. It is involved in cell proliferation, motility and apoptosis [5] and in embryos it regulates the development of head and limbs [6] as well as heart formation [7]. Wnt pathway is also implicated in regulation of the hematopoietic stem cell niche [8]; and in adult life it maintains bone homeostasis and bone mass, enhancing OB differentiation and function [4]. Dkk1 not only affects osteoblastogenesis through the modulation of OPG/RANKL ratio, but also affects osteoclastogenesis indirectly [4]. More interestingly, given that OC and OB are an integral part of the bone marrow microenvironment, the effect of wnt/Dkk1 antagonism may affect tumor growth negatively via impact on the BM milieu.
Deregulation of wnt pathway is implicated in oncogenesis and metastasis of several types of cancers [5]. Overexpression of Dkk1 has been described as a negative prognostic factor in patients with non-small cell lung cancer and esophageal squamous cell carcinoma [9], and aberrant activation of wnt pathway is involved in hepatocellular carcinoma [10], breast cancer [5], and other types of solid tumors. In MM patients, Dkk1 has been related to the extent of bone involvement. In 2003, Tian and colleagues [11] demonstrated that a subset of MM patients had increased serum levels of Dkk1 compared to MGUS, which correlated with the extent of bone disease. These observations have been confirmed by others [12,13], and gone further to show decreased levels of serum Dkk1 in MM patients responsive to anti-myeloma treatment [14,15].
Since Dkk1/wnt pathway is involved in cancer and bone pathophysiology, and the interaction of cancer and bone microenvironment is crucial to the progression of MM, Dkk1 may represent a potential target for treatment. Previous publications [16-18] demonstrated that neutralization of Dkk1 in in vivo mouse models of MM had a bone anabolic effect, with inconsistent results on OC and myeloma growth. Moreover wnt/Dkk1 pathway has recently been confirmed to be active in OC [19] although its role in osteoclastogenesis is poorly understood. The aim of this study was to evaluate a novel anti-Dkk1 neutralizing monoclonal Ab and to elucidate its effect in MM and the bone microenvironment, in particular in OCs.
MATERIALS AND METHODS
Anti-Dkk1 neutralizing Ab
A mouse variable domain/rat IgG1 kappa chimeric monoclonal neutralizing Dkk1 Ab and isotype control were provided by Eli Lilly and Company [20]. The Ab cross reacts with both human and mouse Dkk1 protein.
Severe combined immunodeficient-hu mouse model (SCID-hu)
The SCID-hu MM mouse model was generated as previously described [18], in accordance with the animal protocol approved by the Institutional Animal Care and Use Committee. A total of 8 CB-17 severe combined immunodeficient (SCID) mice (Taconic) were implanted subcutaneously with human fetal long bone grafts. After 4 weeks, 2.5 × 106 human INA-6 MM cells were injected into bone implants in 7 of 8 (negative control) animals. Blood was collected weekly to monitor the serum levels of soluble human interleukin 6 receptor (shuIL-6R), a marker of tumor burden. Following detection of shuIL-6R by ELISA (R&D System), animals were treated with anti-Dkk1 monoclonal Ab (5 mg/Kg, 2x/week) or isotype control (5 mg/Kg, 2x/week) for 4 weeks. Animals were sacrificed at the end of treatment. Human bone implants as well as mice femurs were collected and fixed in 10% formalin for 48h, washed, and preserved in 70% ethanol for further analysis.
Micro-computed tomography and Bone Volume Fraction quantification
After fixation, human bone implants were scanned using micro-computed tomography for 3-D reconstruction, as previously described [21]. Mice femurs were scanned for Bone Volume Fraction (BVF) quantification, as previously described [22].
Histologic analysis
After microCT analysis, human bone implant and mice femurs were decalcified using 10% EDTA, processed, and embedded in paraffin. Five μm sections were obtained and stained with hematoxylin and eosin (H&E) following standard procedures.
Mice femur sections were subjected to Masson Trichrome staining. Slides were deparaffinized and rehydrated in water following standard procedures, and incubated in Boutin solution overnight at room temperature. After incubation the slides were washed in running water and stained in Weigert’s Hematoxylin for 10 minutes, washed, and stained in Biebrich Scarlet-Acid Fuchsin solution for 9 minutes and rinsed in water again. Slides were then placed in phosphotungstic-phosphomolybdic acid for 12-15 minutes, followed by aniline blue for 5 minutes, and then rinsed in water, followed by 1% acetic acid solution for 10 seconds. For the quantification of the Bone Area (BA) over the Tissue Area (TA), serial pictures of the trabecular region above 1 mm from the growth plates were obtained by photocamera SPOT, Insight QE (Diagnostic Instruments, Inc, US) mounted on microscope NIKON Eclipse TS100. Images were assembled using Adobe Photoshop CS, and bone area was quantified at primary and secondary spongiosa, by Image J software (http://rsbweb.nih.gov/ij/download.html). Sections of mice femurs were deparaffinized, rehydrated, and stained for Tartrate Resistant Acid Phosphatase (TRAP) staining. Adequate amount of TRAP substrate solution (Takara, Cat. #MK300) was added to the slides following manufacturer’s instructions, and incubated for 45 minutes at 37°C. At the end of incubation TRAP substrate was removed, washed 3 times with distilled water, and analyzed by microscopy. Images were acquired as described above, and OC numbers were counted along the trabeculae in the primary and secondary spongiosa, as described above. The same procedure was applied to OB and osteocytes on hematoxylin eosin (H&E) sections.
In vitro generation of human primary osteoclasts from myeloma patients and functional assays
OC were generated from the mononuclear fraction of peripheral blood and bone marrow aspirates of MM patients. Samples were collected following the approval of the Institution Review Committee. OC from peripheral mononuclear cells were generated as previously described [23].
Differentiation of OC from bone marrow
Cells were isolated by Ficoll-Paque gradient separation and incubated overnight in cell culture flasks with Minimum Essential Medium, Alpha 1x (αMEM) containing 20% Fetal Bovine Serum (FBS), 2% penicillin-streptomycin (P/S) and 1% L-glutamine. After incubation, the non-adherent cell component was plated in 96 well plates (5×105/well), in the presence of OC differentiation medium (1x αMEM, 10% FBS, 2% P/S, 1% L-glutamine, 25 ng/ml of RANKL (PeproTech) and 25ng/ml M-CSF (R&D System). Anti-Dkk1 neutralizing Ab or isotype control were added to the OC medium starting from the day of differentiation for 7-28 days. Cultured medium was replaced twice a week till the day of the analysis.
TRAP staining
At the time points indicated, culture medium was removed, and cells were washed once with Phosphate Buffered Saline (PBS), fixed, and stained for Tartrate-Resistant Acid Phosphatase (TRAP), according to the manufacturer’s instructions (TRACP & ALP Double-Stain kit; Takara, Tokyo, Japan). TRAP positive cells containing 3 or more nuclei/cell were enumerated using a 10x magnification lens microscope (NIKON Eclipse TS100, Japan). Each experiment was performed at least 3 times using cells from different donors.
Pit formation assay
OC were generated from BM samples of MM patients as described above. OC were cultured for 4 weeks on dentin slices (Immunodiagnostic Systems, Boldon, United Kingdom) in 96-well plates as per the manufacturer’s guidelines. At the end of the fourth week, pit formation quantification was performed as previously described [22].
ELISA
Supernatant from OB cultures was collected after 6 and 13 days of differentiation, and Osteoprotegerin (OPG) concentration was quantified by ELISA (R&D System). Dkk1 levels were measured in the supernatant of MM cells (ELISA developed by Eli Lilly pharmaceutical company).
Cell viability and proliferation assay
Seven MM cell lines (MM.1S, MM.1R, Dox-40, LR5, OPM2, RPMI 8266 and INA-6) were provided by the Jerome Lipper Center and LeBow Institute for Myeloma Therapeutics at Dana Farber Cancer Institute. Supernatant was collected and tested by ELISA for the expression of Dkk1. MM cell lines were tested with two doses of anti-Dkk1 monoclonal Ab (100 and 1000 ng/ml) or isotype control. Cell viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (Sigma, Catalog #:M2128-500MG), after 24 and 48h of treatment as previously described [24]. MM1.S and INA-6 MM cells were treated with anti-Dkk1 monoclonal Ab or isotype control, and cell proliferation was evaluated by tritiate thymidine uptake (3H-TdR; Perkin Elmer, Boston, MA), as previously described [23]. To evaluate the effect of anti-Dkk1 monoclonal Ab in the presence of growth factors, IL-6 (10 ng/ml) was added in the cell culture, and cell proliferation was evaluated at 24 and 48h. MM1.S and INA6 cells were co-cultured with bone marrow stromal cells (BMSC), OB and OC derived from MM patients and co-cultured for 24 and 48h; cell proliferation was evaluated after 24 and 48h of incubation.
Reverse transcription polymerase chain reaction (RT-PCR)
RNA samples were purified from MM cell lines (MM1.S and INA-6) and OC differentiated as described above. Total cellular RNA was isolated using RNeasy® Micro kit (Qiagen). The total cellular RNA concentration was determined using a spectrophotometer. Reverse transcription was performed using TaqMan® Reverse Transcription Reagents (Life Technologies Corporation) with oligo-dT (16mer) primer. Then cDNAs were amplified using GoTaq® Green Master Mix (Promega) with a pair of gene-specific primers according to GoTaq® specification. Primer sequences for the analyzed genes were as follows: LRP5: 5’-CGGCCGCAGTGGACTTCCAGTTT-3’ and 5’-CCACCTTCTGCCGGAACGAGC-3’; LRP6: 5’-GGCGGGCAAACGGCCTAACT-3’ and 5’-GCACGCTCAATGCTGCGTCG-3’; Kremen-1: 5’-GTGTCCTAGCCCGCTTCCACG-3’ and 5’-AGGGAGAGGAGGGGAAGAGTCTGTC-3’; Kremen-2: 5’- ATGGGCGGCTGGGCGTCTAT-3’ and 5’- GCCAGCAGACAGCTCCCCCAATC-3’; Frizzled-1: 5’-GGAGAGTTGCGCTCTCTACGGG-3’ and 5’-GGCCCGGGACTTCTTAGGCG-3’; Frizzled-2: 5’-AGAACCACTCCGAGGACGGAGCT-3’ and 5’-GGCCTACGAAGCACACGCCG-3’; Frizzled-3: 5’-TGAAGGAGCCCCAGTGGCAGT-3’ and 5’-GCCCCATGCACTGGCGTGA-3’; Frizzled-4; 5’ -CGCCTGCAGCCATGACCCTC-3’ and 5’-CCCCAGGCTGGATGGGGGTT-3’; Frizzled- 5: 5’-ATGTGCCGCTGCCACGGGTAC-3’ and 5’-CGAAGCCGTACTGGCGCATCA-3’; Frizzled-6; 5’-CCGTCTCAGGTCCCTGGGGG-3’ and 5’-ATGTTGGGGCATGGAGGCGC-3’; Frizzled-7; 5’-TCTCCCAACCGCCTCGTCGC-3’ and 5’-GCACCGTGCACCGGGAAGTT-3’; Frizzled-8; 5’-GCAACCCTGACACGCTGTGCAT-3’ and 5’-GCAGCGCGCAGTTAGCGATC-3’; Frizzled-9: 5’-GGACCGCTGAGCCCGAGTGAG-3’ and 5’-GCCATCCAGACCAGCGCGAA-3’; Frizzled-10: 5’-TCGAAACAGCTGCCGGCTGGT-3’ and 5’-AACTGGATGGCTGCCTCGCG-3’. mRNA was purified from OC, and cDNA was obtained as previously described. Quantitative (qPCR) for the expression of cathepsin k was performed using the specific primer sequence cathepsin K 5’-TGCCCTGGAGGGCCAACTCA-3’ (forward) and 5’-AGCCCCCTCCACAGCCATCA-3’ (reverse), and primer for TRAP was purchased from SA Biosciences (cat. # PPH07056A).
Expression level of target genes was normalized to GAPDH expression.
Statistical analysis
Statistical analysis of the experiments was obtained using Graph-prism pad and Excel for Student’s T-test.
RESULTS
Anti-Dkk1 neutralizing antibody inhibits tumor growth in a SCID-hu mouse model with anabolic effect in mice femurs
To address the effect of a novel anti-Dkk1 neutralizing Ab on both tumor burden and bone structure, SCID-hu mice were implanted with human fetal bone chips and injected with INA6 human MM cell line. Seven of eight mice (3 in the control group, and all 4 mice in the treated group) developed tumor, as demonstrated by an increase in shuIL6R levels. shuIL6R levels decreased after 4 weeks of treatment, suggesting an inhibitory effect of the anti-Dkk1 monoclonal Ab on INA6 MM cell growth (30.9±10.5 vs 14± 1.7 ng/ml) in the treated group compared to control (P=0.02) (Fig. 1a). To study the effect of the Dkk1 neutralizing Ab on bone, 3D reconstruction of microCT scans of fetal bone implants were obtained. The bone implants with human MM cells showed decreased myeloma infiltrate in the treated samples compared with control. The trabecular and cortical bone of the treated samples appeared thicker, and bone destruction by MM cells was less pronounced in the samples treated with the Dkk1 neutralizing Ab (Fig.1b, upper panel). The same observations were confirmed by microscopy in the H&E stained sections (Fig.1b, lower panel). However, CT quantification of the effect of treatment on bone implants was not possible due to variable structure of the bone implant. Further analysis was therefore extended to non-tumor bearing femurs of mice allowing us to determine if the effect observed on bone was direct or consequent to inhibition of tumor growth. Bone Volume Fraction (BVF) was quantified in distal femurs and midshaft to evaluate the impact of Dkk1 Ab on trabecular and cortical bone, respectively. At the distal femur, bone volume over tissue volume (BV/TV) confirmed an anti-catabolic effect in the treatment group compared with control (P<0.05) which was related to increased trabecular thickness (Tb.Th) (P<0.05), with a less pronounced effect on trabecular number (Tb.N.) (P=0.17) and trabecular separation (Tb.Sp.) (P=0.16) (Fig.2a,b,c,d). The analysis of the femur midshaft showed an anti-catabolic effect of Dkk1 Ab, as evidenced by an increase in BV/TV and cortical thickness (Cort.Th.) (P 0.08 and 0.08), respectively (Fig.2e,f), but less pronounced compared with the effect on trabecular bone. The observations were confirmed by static histomorphometry at the primary and secondary spongiosa of mice femurs, using microscopic analysis. The quantification of bone trabecular area (0.19±0.02 vs 0.47±0.11) and bone perimeter (10.94±2.81 vs 17.89±2.28) confirmed the BVF data, showing improvement in treated samples compared with controls (Fig.3a and 3b). The anti-catabolic effect was secondary to a decrease of OC/OB ratio (0.28± 0.16 vs 0.20± 0.04). OB number/mm were slightly reduced by treatment (21.05±3.79 vs 16.7±2.19) and OC/mm were equally impaired (5.57±2.09 vs 3.44±0.72), although these data did not reach statistical significance (Fig.3c, 3d and 3e).
Fig.1. (a) Anti-Dkk1 neutralizing Ab inhibits myeloma growth and induces an anti-catabolic effect in SCID-hu myeloma mouse model.

SCID mice were implanted with human fetal bone chips and locally injected with the myeloma cell line INA-6 (MM). Animals were treated with isotype control (CNT) or anti-Dkk1 Ab for 4 weeks (Dkk1). Panel (a) shows levels of soluble interleukin 6 receptor (sIL6R) at baseline (0) and during the 4 weeks of treatment. Treatment with anti-Dkk1 Ab inhibited tumor growth compared with control as seen by decrease in sIL6R levels. In panel (b) the 3D reconstruction of the μCT (upper panel) and the microphotograph of H&E staining (lower panel) of the human bone implant show preserved bone architecture in the treated samples compared with osteolytic disease in the controls.
Fig.2. Treatment with anti-Dkk1 monoclonal Ab has an anti-catabolic effect in mice femurs.
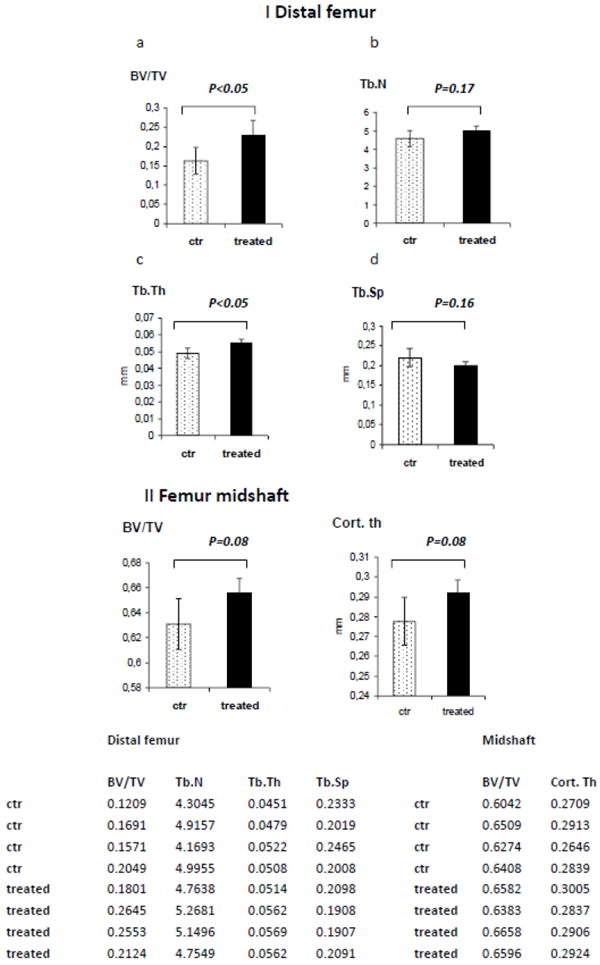
(Panel I) The Bone Volume Fraction (BVF) analysis of the mice distal femurs demonstrates a bone anti-catabolic effect induced by treatment with anti-Dkk1 Ab compared with control as expressed by increased Bone Volume over Tissue Volume (BV/TV) (a) consequent to increased Trabecular Thickness (Tb.Th) (b), and not due to increase in Trabecular Number (Tb.N) (c) or Trabecular Separation (Tb.Sp) (d). (Panel II) The analysis of the BVF in the mice femur midshaft confirms the anti-catabolic effect on the cortical bone (e,f). Raw data are reported in the table at the bottom.
Fig.3. Histomorphometry of the mice distal femurs confirms the anti-catabolic effect observed by μCT, and is associated with decreased osteoclasts/osteoblasts ratio.
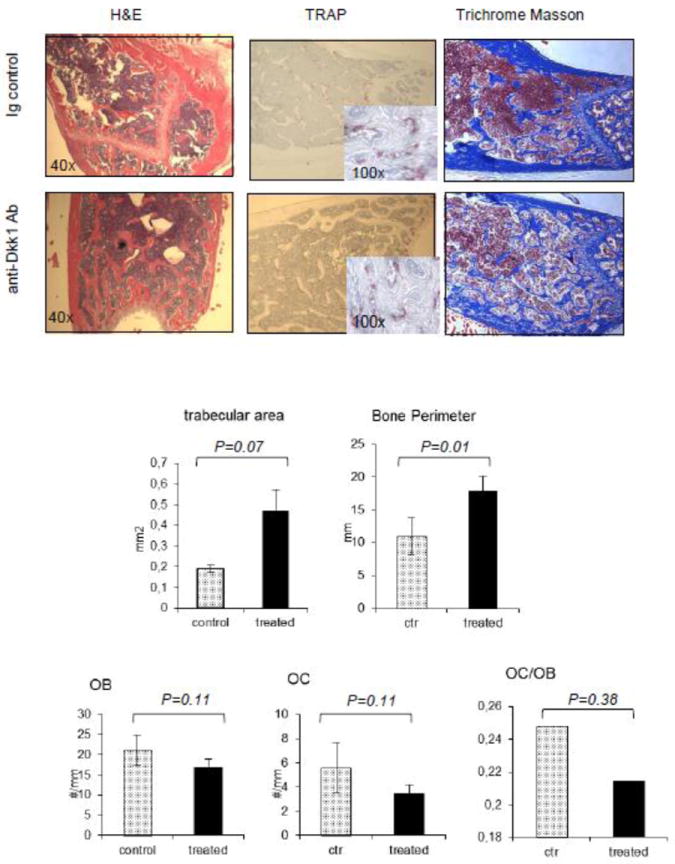
(Upper Panel) Microphotographs of mice femurs demonstrating hematoxylin eosin staing (H&E), tartrate resistant acid phosphatase (TRAP) and trichrome Masson staining. (Lower panel) Histomorphometry was applied to the primary and secondary spongiosa of mice femurs, confirming observations of the Bone Volume Fractions, showing an anti-catabolic effect induced by treatment with anti-Dkk1 Ab. This is demonstrated by increased trabecular area and bone perimeter (a,b). OB/mm were slightly reduced with a more evident reduction of OC/mm (c,d). The decreased ratio of OC/OB confirms the pro-anabolic effect induced by the anti-Dkk1 Ab (e).
Anti-Dkk1 monoclonal Ab affects OC differentiation in vitro
To better define the effect of anti-Dkk1 Ab treatment on osteoclastogenesis, OC derived from MM patients were differentiated in vitro. Cells were treated with anti-Dkk1 monoclonal Ab during 3 weeks of differentiation. At the end of the third week, cells were fixed and stained for TRAP, and trinucleate TRAP positive cells were counted. We observed a dose dependent reduction of OC number per well in treated samples compared with isotype control (Fig.4a), although the reduction was not statistically significant due to high inter-experiment variability. Pit formation assay showed a marginal inhibitory effect on OC function (Fig.4c and d). It has previously been reported [26,27] that wnt mediates bone resorption via increased OPG expression. We therefore evaluated the concentration of OPG in the supernatant of OB culture. OPG levels were increased in treated samples compared with control, suggesting a possible secondary inhibition of osteoclastogenesis (Fig.4e). In order to better understand the observations on OC, qPCR for TRAP and cathepsin k was performed in OC derived from 4 patients (Fig 5a and b). Treatment response was patient dependent, as evidenced by inhibition of osteoclastogenesis in 2 patients and enhancement in the other 2. In order to find a possible explanation to such a high variability of response, RT-PCR for the expression of Dkk1 receptors on the same OC samples was performed. We confirmed, as previously described [19,25] that patients’ expression of surface cell receptors is highly variable (Fig.5c). In particular we observed variable expression of co-receptor LRP5 and kremen 1 and treatment with isotype control or anti-Dkk1 monoclonal Ab did not significantly alter receptor expression (Fig.5d). However the variable receptor expression (LRP5/& and kremen) was not predictive of response to treatment.
Fig.4. In vitro treatment of OC with anti-Dkk1 Ab showed overall inhibition of OC differentiation, with high patient heterogeneity.
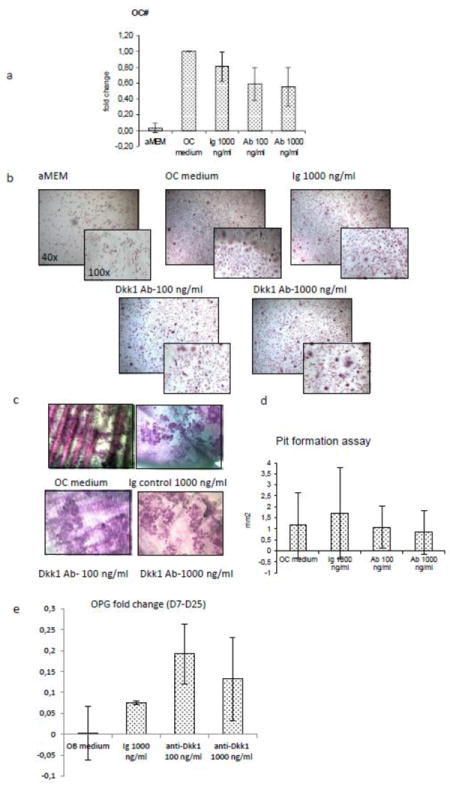
OC derived from myeloma patients were differentiated for 11-17 days in the presence of isotype control (Ig) or anti-Dkk1 neutralizing Ab. Treatment with anti-Dkk1 neutralizing Ab impairs OC number (a,b) as shown by TRAP staining. The pit formation assay (c,d) shows a decrease in OC function albeit with high variability among patients. The inhibitory effect of anti-Dkk1 Ab observed on osteoclastogenesis is at least partly mediated by an increase in osteoprotegerin (OPG) levels in OB supernatant as expressed by the fold change between day 7 and 25 of differentiation (e).
Fig.5. OC derived from MM patients show high heterogeneity of response to anti-Dkk1 treatment.
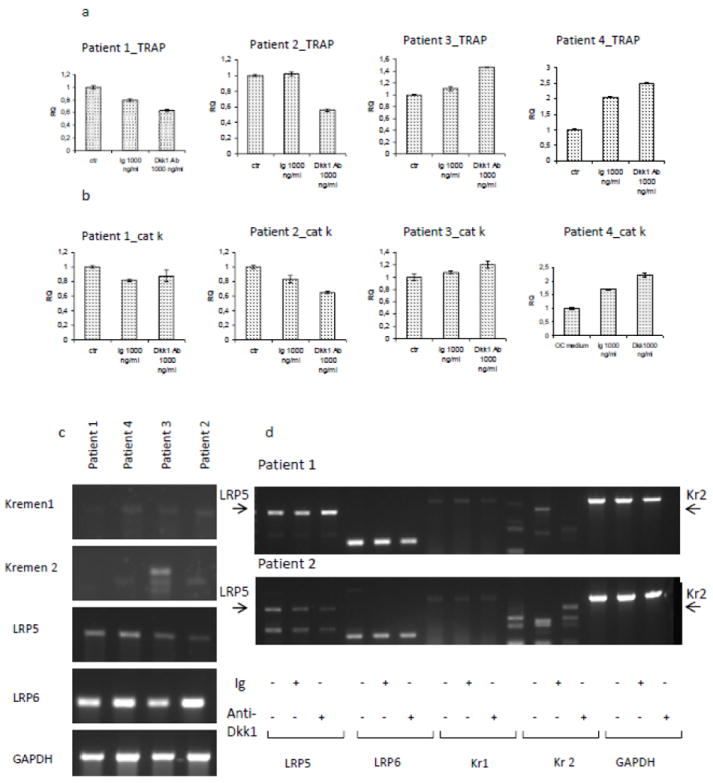
OC were derived from 4 myeloma patients. qPCR for tartrate resistant acid phosphatase (TRAP) and cathepsin k (cat k) was performed after 9-13 days of differentiation (a,b). Results show that treatment induced variable responses, either inhibiting or enhancing OC differentiation. To better understand if the effect was mediated by the expression of the wnt/Dkk1 receptors, RT-PCR for kremen 1, kremen 2, LRP5 and LRP6 was performed in the same 4 patients. Results show heterogeneity of expression of kremen2 receptor. However, treatment did not induce any major change in receptor expression (figure shows gels of two of the patients) (d) and no relation between receptor expression and response to treatment was observed.
The anti-MM effect is mediated by the indirect effects in the context of the bone microenvironment by ant-Dkk1 Ab
One of the major concerns regarding the use of anti-Dkk1 neutralizing Ab and other agents affecting the wnt pathway is the potential activation of wnt and consequent stimulation of cancer cell growth. Our in vivo data, however, confirmed previous reports showing an anti-MM effect induced by the anti-Dkk1 Ab in mice. To better define the effect of anti-Dkk1 neutralizing Ab on MM cells, we tested the Ab in in vitro experiments. We first evaluated the production of Dkk1 in 7 human MM cell lines. Supernatant from cell cultures of MM.1S, MM.1R, Dox-40, LR5, OPM2, RPMI 8266 and INA-6 was collected, and Dkk1 expression was evaluated by ELISA assay. Only INA6, an IL-6 dependent cell line, produced detectable concentrations of Dkk1 (4.1 ng/ml). We next tested the cytotoxicity of the Ab by MTT assay. Cells were treated with 100 and 1000 ng/ml of anti-Dkk1 monoclonal Ab or isotype control (Ig control) 1000 ng/ml for 24 and 48 hours. MTT assay did not show direct cytotoxic effect of the monoclonal Ab, either in cells expressing Dkk1 (INA6) or in cell lines negative for Dkk1 expression (Fig.6a). To determine if Dkk1 production affected MM cells via an autocrine loop, we next studied the RNA expression of the receptors and co-receptors (LRP5 and 6 and kremen1 and 2) involved both in the activation and inhibition of wnt canonical pathway. Primers specific for human receptor FZD isoforms 1 to 10, co-receptor LRP5 and LRP6, and receptor Kremen 1 and 2 were generated. RNA expression was evaluated in one cell line positive for Dkk1 (INA6) and 1 cell line negative for Dkk1 production (MM1s) by RT-PCR. Data showed that both MM1s and INA6 express LRP5 and LRP6 and FZD3, -6 and FZD5 (Fig.6b). However, both cell lines did not express Kremen 1 and 2. We next evaluated the anti-proliferative potential of the anti Dkk1 Ab by [H3] thymidine up-take. Proliferation of MM.1S was not affected by treatment with the Ab, both when cultured alone or in co-culture with either BMSC or cytokines (IGF1 or IL6). To elucidate the role played by wnt pathway in the context of the bone microenvironment, INA6 cells were co-cultured with BMSC, OB, and OC derived from MM patients, or with IL6. Treatment of INA6 cells in co-culture with BMSC and OB with anti-Dkk1 Ab did not affect MM cell proliferation at 24 and 48h. Interestingly, in 1 of 3 cases, treatment of INA6 in co-culture with OC inhibited MM cell growth, consistently, both at 24 and 48h (Fig.6c), suggesting a possible role played by the anti-Dkk1 Ab in the OC-MM interaction. Since IL6 is a key factor in MM and OC cell growth, and given the observation that anti-Dkk1 Ab reduced the expression of IL6 level in OC supernatant, we next tested the effect of the treatment of anti-Dkk1 Ab in INA-6 cells, in the presence of IL6. Data showed that treatment of INA6 with anti-Dkk1 monoclonal Ab 1000 ng/ml was able to reduce INA6 cell proliferation, partially overcoming the advantage conferred by IL6 (Fig.6d).
Fig.6. Treatment of INA6 cell line with anti-Dkk1 Ab has an indirect anti-proliferative effect mediated by the bone microenvironment.
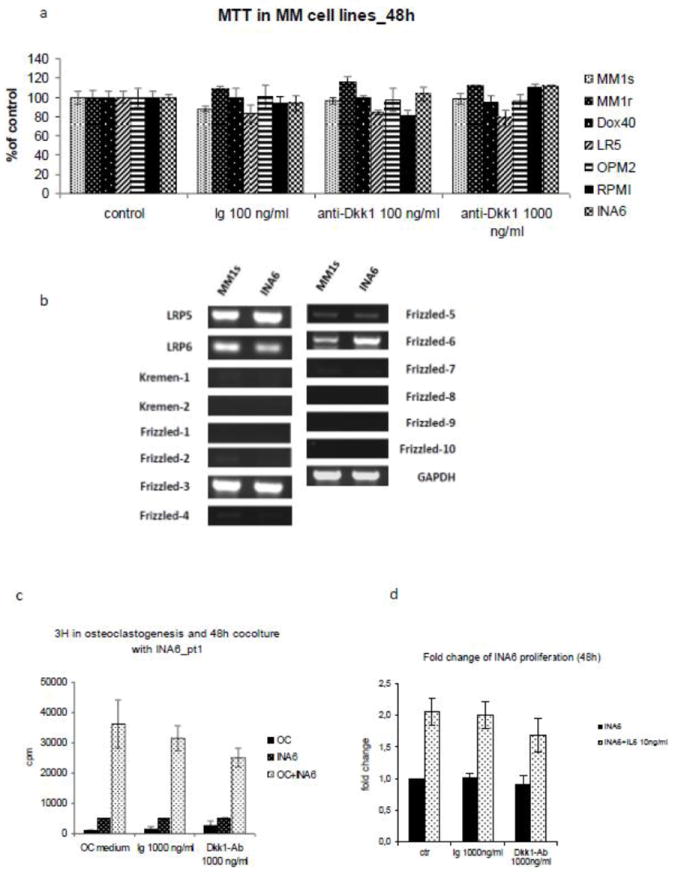
(a) MTT assay with anti-Dkk1 Ab in 7 MM cell lines treated for 48h did not show any direct cytotoxic effect. (b) In order to determine if MM cell lines were directly affected by Dkk1, and consequently by the antibody, RT-PCR for the expression of wnt/Dkk receptors and co-receptor was performed in one cell line negative for Dkk1 expression (MM.1S) and one cell line positive for Dkk1 production (INA-6). Both MM1.S and INA6 cell lines express LRP5 and LRP6, and the wnt receptors Frizzled-3, 5 and 6. However kremen 1 and 2 expression was not be detected. (c) Since the treatment did not induce direct cytotoxic effect, we evaluated the potential anti-proliferative effect of the Ab on INA6 cell line in conditions mimicking the bone microenvironment. INA6 myeloma cell line was treated with isotype control (Ig) or anti-Dkk1 Ab, in the presence of IL6 (10 ng/ml) or in a co-culture with OC. Treatment with anti-Dkk1 Ab partially inhibited myeloma cell growth in the presence of IL-6 and in co-culture with OC derived from one myeloma patient (c,d) supporting the role played by anti-Dkk1 Ab in the context of the bone microenvironment.
CONCLUSION AND DISCUSSION
MM is a treatable but incurable hematological cancer associated with high morbidity related to bone involvement. The characteristic osteolytic lesions are the result of enhanced osteoclastogenesis and impaired osteoblastogenesis; moreover OC and OB, as part of the bone microenvironment also play a pivotal role in MM growth and progression. The observation that overexpression of Dkk1 in a subset of MM patients is associated with bone disease makes Dkk1 a promising target to improve bone quality and disrupt the cross talk between MM and bone.
Previous publications have reported on the effects of two different Dkk1-neutralizing Ab (R&D and BHQ880 by Novartis) in three mouse-models, confirming a pro-anabolic effect in bone and in some cases prevention of progression of osteolysis [16,17], with variable results on osteoclastogenesis and myeloma progression [16-18]. Yaccoby and Fulciniti [16,18] described an anti-MM effect in SCID-rab and SCID-hu mouse models bearing human MM cells. Heath et al, on the other hand, did not observe any reduction in tumor burden in the 5T2MM mouse myeloma model [17]. Yaccoby et al. showed that the use of an anti-Dkk1 neutralizing Ab (R&D) reduced OC number/mm [16], while BHQ880 monoclonal Ab did not show any effect on OC [17,18]. In our in vivo study using a SCID-hu mouse model, anti-Dkk1 neutralizing Ab (Lilly) confirmed both an anti-catabolic effect and anti-MM activity. The anti-catabolic effect was observed in human bone implant as well as in mouse femurs. Of note, the absolute number of OB per section was significantly increased in the treated samples compared with control. Importantly, we also observed a reduction of OC/mm in this model.
The role played by wnt and Dkk1 in osteoblastogenesis is well known, however data on Dkk1 in the context of OC are controversial and the role played by wnt and Dkk1 in osteoclastogenesis remains to be determined. In a mouse model overexpressing Dkk1, Guo and colleagues observed increased OC number in the tibial primary spongiosa associated with increased levels of mRNA encoding TRAP, MMP9, and RANKL as well as decreased expression of OPG, hypothesizing that OC may play a role in the impaired trabecular bone of Dkk1 mice [28]. Similar observations were reported by Wang and colleagues [29], who noted that the use of a Dkk1 antisense oligonucleotide had a positive effect on OB numbers and OB surface, while reducing OC numbers. This effect of wnt/Dkk1 on OC was mainly considered to be mediated by OB. Evidence of direct effects on OC are now being considered. For example, Spencer et al. [30] observed that activation of wnt signalling downregulated RANKL and RANKL promoter activity. Moreover, OC formation was inhibited by activation of wnt signalling by wnt3a and lithium chloride (LiCl) in a co-culture system with mice OB [30]. In addition, Modarresi et al. [31] observed that the activation of wnt/β-catenin and up-regulation of β-catenin inhibits osteoclastogensis. More recently, Qiang confirmed that wnt/β-catenin signalling is active in OC derived from MM patients, although not implicated in regulation of OC differentiation, survival and proliferation [19].
These observations highlight that the role played by wnt and Dkk1 in OC is very complex, involving the canonical and the non canonical pathway; the effect may be mediated by either indirect effects on the OB or by direct activity on OC, and may vary in different phases of osteclastogenesis. This is likey because OC progenitors and mature OC have variable expression of the wnt co-receptors (negative for LRP5 and positive for LRP6) [30]. Our in vitro studies confirmed that increasing concentrations of anti-Dkk1 Ab reduced the number of OCs, with modest effects on OC function, albeit not statistically significant. Politou data also showed inter-patient variability on osteoclastogenesis [13], consitent with Tian et al. [11] who demonstrated overexpression of Dkk1 in a subset of patients in different phases of the disease, reinforcing the concept of high patient heterogeneity.
Our data showed higher concentrations of OPG in supernatants on antiDkk1 treated OB, confirming the observations by Glass et al. that activation of wnt pathway induces increased OPG expression, thereby subsequently impairing osteoclastogenesis [26,27]. Furthermore we confirmed that the use of the antiDkk1 Ab decreases the concentration of IL6 in the supernatant from OC cultures (data not shown), another possible contributing factor for the inhibition of osteoclastogenesis and tumor growth.
The use of anti-Dkk1 neutralizing Ab in MM cell lines in vitro did not show any direct cytotoxic effect, and more importantly, proliferation assays did not reveal that anti-Dkk1 Ab conferred any proliferative advantage to MM cells alone. Qiang similarly tested the proliferative potential mediated by the stimulation of wnt pathway in MM cell lines in vitro, without observing any proliferative advantage [32].
Based on the hypothesis that the anti-MM effect of Dkk-1 Ab is mediated by the bone microenvironment, an anti-proliferative effect of Dkk-1 Ab was observed in the treated samples compared with control in the context of IL-6. More importantly, treatment with the Dkk-1 Ab showed anti-proliferative effects on MM cells in co-culture with OC (1/3), reinforcing the concept that the effect of the Ab is indirect and mediated by OC. This has been further confirmed by studies of the effect of LiCl in mice bearing medullary or extramedullary plasmacytomas where activated wnt pathway had anti-MM effect in the context of the bone, whereas it induced tumor growth at extra-medullary sites [33].
In conclusion, while the in vivo data clearly demonstrated a benefit of Dkk-1 Ab treatment, confirmation of these same effects in vitro was difficult and results were variable. This discrepancy may be related at least in part to the use of primary cells from MM patients, as well as the fact that in vitro experiments do not accurately reflect the complex interactions between OB-OC-MM and the bone marrow microenvironment. Our data however confirmed that anti-Dkk1 monoclonal Ab reduced tumor growth and induced a bone anti-catabolic effect in vivo in the SCID-hu mouse model. In vitro experiments excluded a direct cytotoxic effect of Ab on MM cell lines, suggesting that the anti-MM effect observed in vivo are likely mediated by the bone microenvironment, and in particular by IL6 and OC. Moreover, we show that patient MM cells have variable expression of Dkk1 and wnt/Dkk1 receptors and coreceptors (LRP5/6 and kremen), which may be associated with variable responses to treatment. Finally, we also confirm that wnt regulates bone mass, via the upregulation of OPG which is a target gene of β-catenin [26,27].
Highlights.
A novel anti-Dkk1 neutralizing antibody inhibits myeloma cells growth and inhibits bone resorption in a SCID-hu mouse model
Anti-Dkk1 monoclonal Ab affects OC differentiation in vitro.
The anti-MM effect is mediated by the effect of anti-Dkk1 Ab in the context of the bone microenvironment
Acknowledgments
SZ is the recipient of the Brian Durie International Myeloma Foundation junior fellow award. NR is a LLS Clinical Scholar and recipient of the Claflin Distinguished Scholar Award.
Footnotes
CONFLICTS OF INTEREST
S.K. and L.S. are employees at Eli Lily and Company, Indianapolis; N.R is on the advisory board of Celgene, Amgen, Millenium and Novartis. NR has research funding from Acetylon.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raje N, Roodman GD. Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res. 2011;17:1278–86. doi: 10.1158/1078-0432.CCR-10-1804. [DOI] [PubMed] [Google Scholar]
- 2.Roodman GD. Pathogenesis of myeloma bone disease. J Cell Biochem. 109(2):283–91. doi: 10.1002/jcb.22403. [DOI] [PubMed] [Google Scholar]
- 3.Giuliani N, Mangoni M, Rizzoli V. Osteogenic differentiation of mesenchymal stem cells in multiple myeloma: identification of potential therapeutic targets. Exp Hematol. 2009;37:879–86. doi: 10.1016/j.exphem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113:517–25. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi-Yanaga F, Kahn M. Targeting, Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153–62. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 6.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–62. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 7.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–27. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–83. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 2010;70:5326–36. doi: 10.1158/0008-5472.CAN-09-3879. [DOI] [PubMed] [Google Scholar]
- 10.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–92. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser M, Mieth M, Liebisch P, Oberlander R, Rademacher J, Jakob C, et al. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol. 2008;80:490–4. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 13.Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, et al. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer. 2006;119:1728–31. doi: 10.1002/ijc.22033. [DOI] [PubMed] [Google Scholar]
- 14.Heider U, Kaiser M, Mieth M, Lamottke B, Rademacher J, Jakob C, et al. Serum concentrations of DKK-1 decrease in patients with multiple myeloma responding to anti-myeloma treatment. Eur J Haematol. 2009;82:31–8. doi: 10.1111/j.1600-0609.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 15.Lemaire O, Attal M, Bourin P, Laroche M. DKK1 correlates with response and predicts rapid relapse after autologous stem cell transplantation in multiple myeloma. Eur J Haematol. 2010;84:276–7. doi: 10.1111/j.1600-0609.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 16.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–11. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD, Jr, Evans HR, et al. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. 2009;24:425–36. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 18.Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–9. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiang YW, Chen Y, Brown N, Hu B, Epstein J, Barlogie B, et al. Characterization of Wnt/beta-catenin signalling in osteoclasts in multiple myeloma. Br J Haematol. 2010;148:726–38. doi: 10.1111/j.1365-2141.2009.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agholme F, Isaksson H, Kuhstoss S, Aspenberg P. The effects of Dickkopf-1 antibody on metaphyseal bone and implant fixation under different loading conditions. Bone. 2011;48:988–96. doi: 10.1016/j.bone.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Fulciniti M, Hideshima T, Vermot-Desroches C, Pozzi S, Nanjappa P, Shen Z, et al. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15:7144–52. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozzi S, Vallet S, Mukherjee S, Cirstea D, Vaghela N, Santo L, et al. High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin Cancer Res. 2009;15:5829–39. doi: 10.1158/1078-0432.CCR-09-0426. [DOI] [PubMed] [Google Scholar]
- 23.Vallet S, Raje N, Ishitsuka K, Hideshima T, Podar K, Chhetri S, et al. MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood. 2007;110:3744–52. doi: 10.1182/blood-2007-05-093294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raje N, Kumar S, Hideshima T, Roccaro A, Ishitsuka K, Yasui H, et al. Seliciclib (CYC202 or R-roscovitine), a small-molecule cyclin-dependent kinase inhibitor, mediates activity via down-regulation of Mcl-1 in multiple myeloma. Blood. 2005;106:1042–7. doi: 10.1182/blood-2005-01-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dun X, Jiang H, Zou J, Shi J, Zhou L, Zhu R, et al. Differential expression of DKK-1 binding receptors on stromal cells and myeloma cells results in their distinct response to secreted DKK-1 in myeloma. Mol Cancer. 2010;9:247. doi: 10.1186/1476-4598-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Glass DA, 2nd, Karsenty G. Canonical Wnt signaling in osteoblasts is required for osteoclast differentiation. Ann N Y Acad Sci. 2006;1068:117–30. doi: 10.1196/annals.1346.015. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ, et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11:161–71. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang FS, Ko JY, Lin CL, Wu HL, Ke HJ, Tai PJ. Knocking down dickkopf-1 alleviates estrogen deficiency induction of bone loss. A histomorphological study in ovariectomized rats. Bone. 2007;40:485–92. doi: 10.1016/j.bone.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–96. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 31.Modarresi R, Xiang Z, Yin M, Laurence J. WNT/beta-catenin signaling is involved in regulation of osteoclast differentiation by human immunodeficiency virus protease inhibitor ritonavir: relationship to human immunodeficiency virus-linked bone mineral loss. Am J Pathol. 2009;174:123–35. doi: 10.2353/ajpath.2009.080484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiang YW, Endo Y, Rubin JS, Rudikoff S. Wnt signaling in B-cell neoplasia. Oncogene. 2003;22:1536–45. doi: 10.1038/sj.onc.1206239. [DOI] [PubMed] [Google Scholar]
- 33.Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–42. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]


