Abstract
Cell microencapsulation has been utilized for decades as a means to shield cells from the external environment while simultaneously permitting transport of oxygen, nutrients, and secretory molecules. In designing cell therapies, donor primary cells are often difficult to obtain and expand to appropriate numbers, rendering stem cells an attractive alternative due to their capacities for self-renewal, differentiation, and trophic factor secretion. Microencapsulation of stem cells offers several benefits, namely the creation of a defined microenvironment which can be designed to modulate stem cell phenotype, protection from hydrodynamic forces and prevention of agglomeration during expansion in suspension bioreactors, and a means to transplant cells behind a semi-permeable barrier, allowing for molecular secretion while avoiding immune reaction. This review will provide an overview of relevant microencapsulation processes and characterization in the context of maintaining stem cell potency, directing differentiation, investigating scalable production methods, and transplanting stem cells for clinically relevant disorders.
Keywords: alginate, bioprocessing, cell therapy, micro-encapsulation, stem cells
Introduction
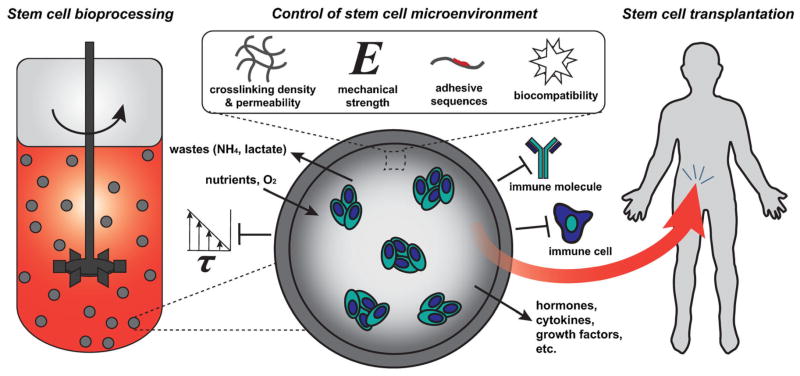
In the early 1960s, Thomas M. S. Chang drew inspiration from naturally occurring aqueous partitions, such as cells and organelles, to develop a method for microencapsulation of biological material in natural polymers (Chang, 1964). Since that time, cell microencapsulation has been widely researched, particularly in the field of cell transplantation for the treatment of endocrine disorders, such as diabetes, in which a continual regulation and response to physiologic stimuli is required. Encapsulation facilitates allogeneic and xenogeneic cell transplantation because the semi-permeable membrane of the capsule protects the enclosed cells from the host immune system. However, donor primary cells are often difficult to obtain and expand to sufficient numbers for therapeutic efficacy, rendering stem cells an attractive alternative due to their dual capacity for self-renewal and differentiation. In addition to the benefits of microencapsulation for transplantation, the technology offers several potential advantages for stem cell expansion and differentiation. The modification of encapsulation material properties, such as the polymer species, type of coating, mechanics, and permeability, can be used to control the stem cell microenvironment, allowing for either the maintenance of potency or directed differentiation toward a desired lineage. In addition, encapsulated stem cells can be expanded in scalable suspension bioreactor systems without being damaged by the presence of hydrodynamic shear forces. Consequently, microencapsulation can play an important role throughout the pipeline of production and delivery of stem cell therapies (Fig. 1).
Figure 1.
Microencapsulation of stem cells permits mass transport of nutrients and secretory products while restricting the passage of immune molecules and cells and shielding from physical forces. Modification of various capsule parameters can modulate stem cell response(s) while simultaneously enabling expansion in scalable suspension bioreactors for bioprocessing and transplantation in vivo.
Stem cells are now known to be found in nearly all tissues throughout the body and are defined by the signature characteristics of self-renewal and differentiation capacity. Pluripotent stem cells include embryonic stem cells (ESCs), derived from the inner cell mass of the pre-implantation blastocyst (Evans and Kaufman, 1981; Martin, 1981; Thomson et al., 1998), and induced pluripotent stem cells (iPSCs), obtained by reprogramming somatic cells to a pluripotent state (Takahashi and Yamanaka, 2006; Yu et al., 2007). Both ESCs and iPSCs can expand indefinitely in vitro and differentiate into all three germ lineages, consequently giving rise to cells from all tissue types. Pluripotent cells differ functionally from multipotent stem cells that are more restricted in their differentiation capacity. Common multi-potent stem cells include mesenchymal stem cells (MSCs), which can be derived from multiple sources including bone marrow (Pittenger et al., 1999) and adipose tissue (Zuk et al., 2002), and hematopoietic stem cells (HSCs), found in bone marrow (Till and McCulloch, 1961) and cord blood (Broxmeyer et al., 1989). Because most multipotent stem cells can be readily obtained from adults, autologous, and allogeneic cell therapies are feasible for several clinical applications; however, ex vivo expansion of multipotent stem cells is limited when compared to the capacity for indefinite self-renewal that defines pluripotent stem cells. Thus, ESCs and iPSCs may represent a more practical solution for large-scale production of a broader range of cell therapy products. Although iPSCs have the potential theoretically for use in autologous therapies, efficient non-viral reprogramming methods and a more complete biological understanding of reprogramming effects on epigenetic state will likely be required prior to widespread clinical use, in addition to the establishment of certified stocks of iPSC clones (Wu and Hochedlinger, 2011; Yamanaka, 2012).
Microencapsulation for Cell Therapies
Stem cells are often considered for cell therapies owing to their ability to differentiate into cells that can repair and replace damaged tissues. Mature primary cells, either autologous or allogeneic, are not ideal for use in such applications, as they are challenging to expand ex vivo and have reduced regenerative capacity (Gage, 1998). Therefore, significant effort has been put forth to develop robust and reproducible methods to differentiate stem cells into relevant therapeutic cell types. In addition to replacing damaged cells with differentiated progeny, stem cells can secrete various trophic factors, which lead to improved tissue function and regeneration (Baraniak and McDevitt, 2010). While transplantation of autologous cells is a generally favored approach to avoid a negative immune response, it is often difficult to obtain somatic cell numbers sufficient for exogenous repair, expansion, and transplantation. Therefore, transplantation of allogeneic cells may emerge as a more practical approach than autologous cell transplants due to the capacity to expand the cells to the necessary numbers, to remain within the current structure of the biopharmaceutical industry, and to ensure proper product validation (Freimark et al., 2010).
In an effort to prevent immunological rejection, transplanted cells can be encapsulated behind a semi-permeable biocompatible material, which might obviate the need for immunosuppressant drug use. The permeability of encapsulation materials should permit the passage of essential nutrients, oxygen, and most cell secreted factors while restricting the passage of larger molecules, such as antibodies, and immune cells (Uludag et al., 2000). By modifying material properties, such as the polymer species, concentration, and type of coating, the transport of molecules into and out of the material barrier can be tightly controlled. In addition to immune protection, encapsulation also provides a more consistent microenvironment for the enclosed cells with greater long-term mechanical stability (Paul et al., 2009). Microencapsulation typically refers to the creation of spherical capsules in the range of 100–1,500 μm in diameter, while macroencapsulation usually describes larger constructs with a planar or cylindrical geometry, such as flat sheet or hollow-core fiber configurations (Hernández et al., 2010). Microcapsules have a higher surface area-to-volume ratio, allowing for more durable capsule mechanical stability and enhanced mass transfer, leading to heightened cell viability through enhanced nutrient and waste transport in addition to faster secretory response to external stimuli.
Transplanted cell capsules provide a continuous and concentrated means of efficiently delivering secreted molecules locally, as opposed to systemic administration of single cell suspensions (Rabanel et al., 2009). Cell microencapsulation is particularly useful to treat dysfunction of metabolic or secretory tissues, including diseases such as diabetes, Parkinson’s, hypoparathyroidism, and hemophilia (Hasse et al., 1997; Hortelano et al., 1996; Sajadi et al., 2006). Secretory disorders are often among the most difficult to address, as a continual regulation and response to stimuli is required. In 1980, pancreatic islets encapsulated in alginate with a poly-L-lysine (PLL) coating were transplanted into diabetic rats, and the encapsulated cells remained viable and reduced diabetic symptoms for 3 weeks, compared to only 6–8 days for unencapsulated islets, indicating that the protection offered by enclosure in microcapsules increased the time of functionality (Lim and Sun, 1980). In the 1990s, the first in-human study found that encapsulated islets from cadaveric donors were able to reduce the required dose of both immunosuppressants and exogenous insulin in a diabetic patient (Soon-Shiong et al., 1994), though this approach has not attained widespread clinical use due primarily to the continued limited availability of donor tissue. When compared to treatment with a small molecule or protein therapeutic drug, encapsulated cells provide a more localized and sustained delivery that is capable of better mimicking native physiologic conditions.
Microencapsulation Process Technologies
The technologies used to microencapsulate cells have been reviewed extensively elsewhere (Brun-Graeppi et al., 2011; Murua et al., 2008; Rabanel et al., 2009; Uludag et al., 2000; Zimmermann et al., 2007), therefore the following discussion will focus on those processes deemed most relevant for stem cell microencapsulation. The general encapsulation process involves the formation of cell-containing droplets, the cross-linking of droplets, and often the coating of droplets with a stabilizing membrane. Hydrogels are the most common material used in encapsulation for several reasons. First, hydrogels generally have high porosity, leading to high permeability and minimal mass transfer limitations. Second, hydrogel materials tend to be soft and flexible, reducing mechanical friction on adjacent tissues upon transplantation. Third, the high water content leads to hydrophilic interactions, which reduce interfacial tension, protein adsorption, and cellular adhesion while enhancing biocompatibility. Natural polymeric materials are used more often than synthetic polymers because they are more biocompatible and require milder cross-linking processes (i.e., those that take place under aqueous and physiological conditions without the presence of reactive species). However, a reduction in the stability of the microcapsule can occur using natural polymers when compared to results obtained with synthetic polymers (Uludag et al., 2000).
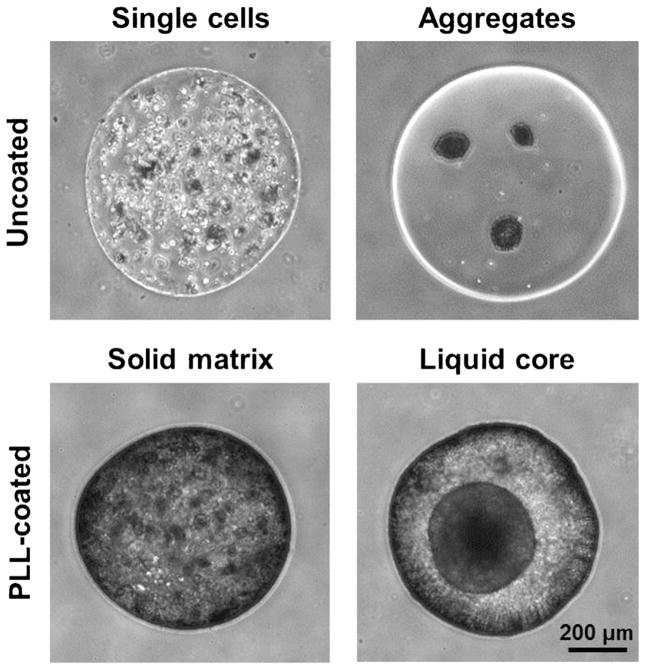
The general formulations of microcapsules include solid matrix beads, solid matrix beads with an external coating, liquid core capsules, and direct conformal coating (Fig. 2). Liquid core capsules are fashioned by liquefying the center of a solid matrix bead as the outer membrane is stabilized by a thin polyion coating (Rabanel et al., 2009). Conformal coated microcapsules are formed by constructing a thin membrane surrounding individual cells or preformed cell aggregates, thereby minimizing the empty capsule volume (Wilson and Chaikof, 2008). In some cases, encapsulated single cells will aggregate into spheroids within a capsule. The fashion in which cells form aggregates within microcapsules is based on the mechanical restriction of the hydrogel (Helmlinger et al., 1997). In solid matrix beads, encapsulated cells are more physically constrained and therefore proliferate to form smaller spheroids with multiple foci. In liquid core capsules, cell movement and proliferation are less restricted, and the cells can therefore aggregate and proliferate as a single large spheroid (Huang et al., 2012). As cells proliferate in liquid core capsules, the capsules tend to swell, but excessive swelling can be restricted with the addition of a polymeric surface coating (Pajić-Lijaković et al., 2007).
Figure 2.
Microencapsulation methods facilitate several configurations of cell entrapment, including the initial encapsulation of single cells or aggregates, the addition of a coating such as poly-L-lysine (PLL), and/or liquefaction and hollowing of the capsule core. Murine ESCs were encapsulated in alginate in each of these four configurations. In the PLL-coated capsules, the creation of a liquid core led to self-aggregation of the encapsulated single cells, while the solid matrix inhibited large aggregate formation. Scale bar =200 μm.
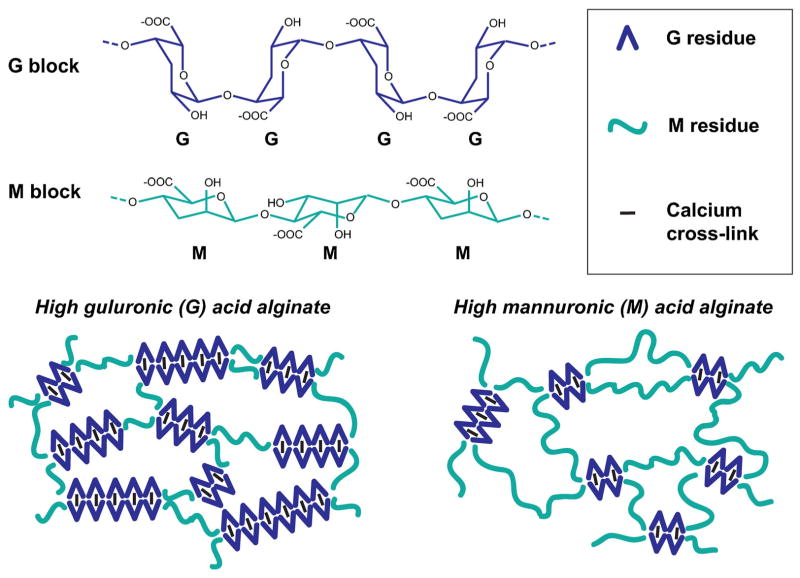
Alginate, a natural polymer purified from brown seaweed, is the most common material used for microencapsulation due to its abundance, biocompatibility, and gentle cross-linking procedure. Alginate is made up of two anionic monomers, α-L-guluronic acid (G) and β-D-mannuronic acid (M), which are arranged in both homopolymeric regions (GG blocks and MM blocks) and mixed monomeric regions (MG blocks) (Haug et al., 1966). Alginate can be cross-linked by several different divalent cations, including calcium and barium. Calcium (Ca++) cross-links only G residues, therefore, alginates with higher G content and longer GG blocks produce stiffer, more durable capsules when cross-linked with Ca++ than alginates with greater M content (Fig. 3). Alternatively, barium cross-links alginate by binding to both G and M residues, thus yielding stronger and more uniform cross-linking than Ca++ alone can provide (Mørch et al., 2006). The molecular weight of alginate also impacts its physical properties, with higher molecular weight alginate leading to capsules with greater mechanical stability than lower molecular weight alginate (Uludag et al., 2000). However, the relative impact on the mechanical stability with increasing molecular weight is not as profound as increasing either the G content or concentration of alginate (Huang et al., 2012). The stability of alginate capsules generally weakens over time due to exposure to certain species in the cell culture medium or in vivo, including chelating agents (e.g. sodium citrate, EDTA, phosphate) or monovalent ions (e.g. sodium), which can displace the ionic interactions created by the divalent cross-linkers (Darrabie et al., 2006). Although alginate has many favorable properties for cell encapsulation, native isoforms are not able to directly interact with cells since alginate lacks adhesive moieties. Therefore, it is primarily the mechanical properties of native alginate that impact its relationship with cells. The mechanical properties (e.g. elastic modulus, viscosity, osmotic tolerance/swelling) are generally based on the chemical properties of the alginate (e.g. G vs. M composition, molecular weight, concentration). Thus, when deciding on an alginate for microencapsulation, the physiochemical properties should be carefully considered depending on the intended function(s) of the system.
Figure 3.
Alginate, the most common polymer used for microencapsulation, is made up of guluronic acid (G) and mannuronic acid (M) residues. The ratio of G:M residues and block lengths of each species impact the mechanics and permeability of the hydrogel material. When using calcium as the cross-linking ion, the calcium only cross-links G residues, leading to an “egg box” configuration (Simpson et al., 2004). Therefore, alginates with higher G content yield stiffer, less flexible hydrogels that maintain mechanical stability for a longer period of time.
Alginate encapsulation alone is usually insufficient to completely shield cells from the immune system in vivo because of mechanical and chemical instability in addition to poor resistance to osmotic swelling, which often results in cell escape. Therefore, coating the exterior of alginate capsules to reduce the permeability to immune cells and proteins while simultaneously increasing the mechanical stability and biocompatibility of the capsule is often performed by polyelectrolyte complexation, where two oppositely charged polymers are complexed to form a thin membrane layer with a thickness on the order of 10–100 μm (Strand et al., 2003). Since alginate is a polyanion, the coating materials are usually polycations, with PLL being the most common since Lim and Sun introduced the alginate-PLL system in 1980 (Lim and Sun, 1980). PLL binds to both the M and G blocks through ionic interactions, though it typically binds more tightly to alginate with higher M content as there are more alginate moieties available for binding, creating a coating that is thicker and less permeable (Vandenbossche et al., 1993). To improve biocompatibility, the positive PLL charges on the exterior of the capsule are sometimes neutralized through incubation with additional alginate, forming “APA” (alginate-PLL-alginate) beads. Chitosan, polyethylene glycol (PEG), and other polycations are sometimes used as alternatives to PLL; however, PLL remains the most commonly used polymer due to its historical prevalence and creation of mechanically resistant capsules (De Castro et al., 2005).
While alginate is the most prevalent hydrogel used traditionally, agarose has also been investigated in the context of stem cell microencapsulation. Agarose, derived from red algae, is made up of β-D-galactopyranosyl and 3,6-anhydro-α-L-galactopyranosyl units and exhibits a temperature-dependent solubility in which gelation occurs below a threshold temperature (usually 15–30°C), avoiding the need for addition of cross-linking molecules. Blending agarose with collagen or gelatin promotes cell survival and proliferation by providing adhesive motifs for anchorage-dependent cells (Jain et al., 1995; Sakai et al., 2007). In general, agarose beads are not as mechanically robust as alginate beads, with agarose gel strength decreasing approximately 25% over the course of 60 days (Shoichet et al., 1996), indicating that agarose may be more appropriate for short-term applications. To prevent issues with cell protrusion and escape, agarose beads are usually coated with an additional layer of agarose in lieu of coating with a polycation in order to create a thicker barrier (Jain et al., 1995).
Regardless of the specific material, the two most common methods for hydrogel bead formation are emulsification and extrusion. The emulsification process involves creating a dispersion of a polymeric aqueous phase in an immiscible phase (often oil), followed by gelation through addition of a cross-linking agent or cooling of the mixture. There are several challenges with this approach, including the presence of shear forces during mixing that may harm cells and the insolubility of some gelling agents (e.g., CaCl2) in the immiscible phase. The extrusion process involves forcing a cell-polymer solution through a small aperture, such as a needle or an area of small-diameter tubing, into a solution containing the cross-linker to stabilize bead formation. Solely adding droplets to a cross-linking bath generates large particles (on the order of millimeters) and is feasible only for low viscosity solutions. In order to form smaller, more uniform beads with higher viscosity solutions, a voltage gradient between the needle and cross-linking solution can be applied to overcome droplet surface tension and therefore produce large quantities of smaller beads (Willaert and Brüssel, 1996). Alternatively, a constant vibration can be applied to a laminar liquid jet to produce small, uniform capsules (Serp et al., 2000; Whelehan and Marison, 2011). A coaxial gas flow can also be used to form beads by shearing droplets with compressed gas that flows around the needle exit. An additional extrusion approach is the co-flowing of two immiscible liquid streams in a coaxial tube, one in the aqueous phase containing the cells/polymer and the other a liquid paraffin solution (Sakai et al., 2004). The paraffin solution, present in the outer coaxial tube, shears the polymer drops as they are extruded through a nozzle. Recently, microfluidics and microlithography have been introduced as systematic processes for more homogeneous and controlled capsule formation, though they currently have lower throughput than the aforementioned macro-scale methods (Rabanel et al., 2009).
Microcapsule Characterization
Three primary characteristics of microcapsules, permeability, mechanical stability, and biocompatibility, impact the functionality of the encapsulated system and can be varied based on the intended application. The permeability of the capsule needs to be taken into account to ensure that the enclosed cells receive adequate levels of oxygen and nutrients to survive, that secreted factors are able to diffuse to the exterior, and that large immune molecules are incapable of penetrating the capsule. There are two components that determine the capsule permeability: the molecular weight cut-off (MWCO) and the diffusivity of the material. In order to determine the permeability of microcapsules, one common technique is to equilibrate the capsules with a solute of interest and measure either the entry (ingress) or exit (egress) of the solute as a function of time. The permeability (P) can then be estimated by multiplying the mass transfer rate (U), determined through a mass balance through the capsule wall, by the distance traveled (d) (Crooks et al., 1990). The MWCO can be determined by size exclusion chromatography with dextran molecules of different molecular weights run over a column packed with microcapsules (Wang et al., 1997).
The permeability of capsule materials can be modified by changing the type and concentration of the membrane coating in addition to the species and concentration of polymer used. For example, an MWCO between 20 and 300 kDa can be achieved by varying the PLL composition, concentration, and incubation time (King et al., 1987; Vandenbossche et al., 1993), though it is unclear from previous literature how precise this cut-off is. Inhomogeneous alginate beads, in which the alginate concentration decreases when moving toward the center of the bead, can be created through rapid cross-linking which occurs when using high cross-linker concentrations (e.g. > 100 mM CaCl2). The resulting alginate concentration gradient can be used to modify the capsule permeability in a similar manner to PLL coating (Thu et al., 1996a, b). The transport of nutrients and wastes through the capsules is critically dependent on the size of the capsule and the rate of consumption/production by the entrapped cells. For example, more proliferative cells generally require greater permeability to oxygen than more quiescent cell types. If cell spheroids are present within the microcapsule, the spheroids typically present greater mass transfer limitations than the capsule material itself, lessening the role the capsule permeability plays in the overall transport scheme (Mota et al., 2001).
Microcapsules are often exposed to mechanical forces that can lead to deformation or dissolution, which may cause release of or damage to the inner cellular contents. Because of the possibility of capsule failure and the additional challenge of the sensitivity of stem cell populations to their mechanical microenvironment (Engler et al., 2006), the mechanical strength and stability of capsules are important properties to assess and engineer. One challenge of engineering microcapsules is increasing the strength and stability to reduce the probability of mechanical failure without negatively impacting the permeability. Common methods to test the mechanical stability of capsules include exposing them to a well-defined shear flow (Lee and Chu, 1997) or uniaxial compression (Matthew et al., 1993). Osmotic pressure testing, in which the capsules swell as they are exposed to solutions of increasing osmotic strength until they burst, is also a commonly performed means of analysis (Van Raamsdonk and Chang, 2001). Compression and micro-deformation testing can determine the elastic modulus of the microcapsules (Briscoe et al., 1998; Chan et al., 2011). Unfortunately, standard methods for determining the mechanical resistance of microcapsules have not been established due to the limitations of testing materials with spherical geometry, leading to variability in the mechanical characterization of microcapsules in the literature (De Vos et al., 2009). Because stem cell fate and function are sensitive to mechanical properties of the local microenvironment (Saha et al., 2006; Terraciano et al., 2007), an improved general characterization of microcapsule mechanical properties may lead to better definition of the surrounding environment and the resulting impact on stem cell phenotype. However, it is challenging to isolate mechanical effects from other interdependent encapsulation parameters, such as the concentration and species of the polymer and cross-linker molecules.
The biocompatibility of the microcapsule material is an essential property when considering the survival of the enclosed cells and the host response post-implantation. Biocompatibility is typically determined by examining the viability of the enclosed cells, the presence of fibrosis on the capsule exterior, and the extent of the host inflammatory response (De Vos et al., 2009). Small perturbations in the material composition have been observed to drastically change the biocompatibility; for instance, changing the concentration of the cross-linker poly-methylene-co-guanidine from 1.8% to 1.0% dramatically increased the incidence of surface fibrosis in an alginate/cellulose encapsulation scheme (Wang et al., 1997). There is some evidence that the ratio of G to M residues in alginate can impact biocompatibility, though disparities in the literature (De Vos et al., 1997; Kulseng et al., 1999; Otterlei et al., 1991) may be based on the lack of a consensus definition of what constitutes “high G” or “high M” alginate, in addition to variable purity of different alginates (Hernández et al., 2010).
Microencapsulation for Regulating Stem Cell Phenotype
Encapsulation of stem cells in hydrogel capsules provides a defined microenvironment that can impact cell fate decisions. In vivo, stem cells reside in a specific niche defined by the combination of soluble factors, cell–cell adhesions, and matrix interactions required for self-renewal and, when necessary, differentiation and tissue regeneration (Morrison and Spradling, 2008; Scadden, 2006). Considerable effort has been put forth to recapitulate the stem cell niche in vitro through the development of biomaterial scaffolds, the addition of chemical cues, and the application of mechanical forces (Burdick and Vunjak-Novakovic, 2009; Discher et al., 2009). Microencapsulation offers a unique approach for manipulation of the stem cell environment to either maintain the pluripotent/multipotent state or induce differentiation toward a specific lineage(s). In addition, directing the differentiation of stem cells within micro-capsules could streamline the transition to cell transplantation by producing cells in an immune-protected format.
Maintenance of Stem Cell Viability and Potency
The ability to culture several types of microencapsulated stem cells while maintaining viability and potency has been achieved in a variety of manners, serving as a starting point for future work in stem cell microencapsulation (Table I). Maintaining potency in an encapsulated format is desired when trying to expand undifferentiated stem cell populations or transplant undifferentiated cells to take advantage of their paracrine effects. MSCs encapsulated in collagen-agarose and RGD-modified alginate yielded >75% cell viability after 8 days and >80% viability after 15 days, respectively (Batorsky et al., 2005; Markusen et al., 2006). Further studies of MSCs in alginate microcapsules found that multipotency can be maintained for up to 2 months (Goren et al., 2010) and that the MSC secretory profile is not altered by encapsulation (Penolazzi et al., 2010). The addition of a PLL coating on alginate microcapsules slightly reduced MSC viability, leading to lower total levels of secreted bFGF compared to non-coated microcapsules (Trouche et al., 2010). Proliferation of encapsulated MSCs may depend on the specific material utilized, as MSCs continued to proliferate during encapsulation in alginate-PLL capsules (Goren et al., 2010) but not in RGD-modified alginate capsules (Markusen et al., 2006). Altering capsule properties to maintain MSC viability and naïve phenotype within microcapsules will be useful for applications in which undifferentiated trophic factor secretion profiles are desired.
Table I.
Maintenance of viability and/or potency within microcapsules.
| Cell type | Capsule material | References |
|---|---|---|
| MSCs | Alginate | Goren et al. (2010), Penolazzi et al. (2010), Trouche et al. (2010) |
| Alginate w/RGD sites | Markusen et al. (2006) | |
| Agarose-collagen | Batorsky et al. (2005) | |
| Agarose-chitosan-PEG | Paul et al. (2010) | |
| Agarose w/fibronectin | Karoubi et al. (2009) | |
| ESCs | Alginate | Maguire et al. (2005), SitiIsmail et al. (2008), Wang et al. (2006) |
| Agarose | Dang et al. (2004) | |
| Agarose w/alginate core | Sakai et al. (2008) | |
| NSCs | Alginate | Li et al. (2006), Purcell et al. (2009) |
The maintenance of viability and pluripotency of microencapsulated ESCs is desirable for large-scale expansion of undifferentiated ESCs. Agarose microencapsulation prevents embryoid body (EB) agglomeration in culture, allowing for higher density cultures without the formation of aggregates with large necrotic cores (Dang et al., 2004). Murine ESCs (mESCs) were observed to retain >90% viability after 20 days in alginate-PLL capsules (Maguire et al., 2005), with liquid core capsules leading to enhanced proliferation and viability compared to unliquefied capsules (Wang et al., 2006). An altered liquid core configuration in which smaller alginate microcapsules containing mESCs were encapsulated in larger agarose capsules, followed by liquefaction of the alginate core, found that the enclosed mESCs formed EB-like spheroids and stained heterogeneously for alkaline phosphatase, suggesting a mixture of undifferentiated cells and differentiated progeny (Sakai et al., 2008). An additional study found that expression of the pluripotency markers OCT-4, SSEA-1, and alkaline phosphatase were maintained by mESCs for 2 weeks in vitro in APA liquid core capsules, though a decrease in pluripotency marker expression was observed once the capsules were implanted in vivo (Wang et al., 2006). More recently, human ESCs (hESCs) were maintained in alginate-gelatin microcapsules for up to 260 days without experiencing significant decreases in viability or pluripotency (Siti-Ismail et al., 2008), thereby supporting future endeavors in the large-scale production of hESCs.
Microencapsulation of neural stem cells (NSCs), multi-potent cells that can differentiate into neurons, oligodendrocytes, and astrocytes, has also been examined as a means to improve expansion for applications in nerve repair. Alginate microcapsules containing murine NSCs permitted neurosphere formation while maintaining multipotency and viability (Li et al., 2006). Additional studies with murine NSCs determined that proliferation and viability were not significantly affected by alginate composition or the presence of a PLL coating; however, alginates with high M composition and a PLL coating were found to be less mechanically stable than uncoated high G composition alginates, which permitted the highest level of neurotrophic factor secretion (Purcell et al., 2009). Such studies support the use of microencapsulation as a platform for efficient ex vivo NSC expansion.
Directed Differentiation
For therapies requiring a differentiated cell product, microencapsulation provides a defined environment in which to control cell fate. Cell types of various lineages have been generated, including osteogenic, chondrogenic, cardiac, pancreatic, hepatocytic, adipogenic, neural, and hematopoietic, from different types of stem cells (Table II). Because many delivery methods already employ microencapsulation as a means of immune protection, differentiating stem cells within microcapsules can improve process efficiency by making the transition from production to transplantation a one-step process. Differentiation strategies within microcapsules include adding external signals, genetically modifying cellular behavior, and modulating the physical and chemical properties of capsules.
Table II.
Directed differentiation within microcapsules.
| Differentiation lineage | Starting cell type | Capsule material | References |
|---|---|---|---|
| Osteogenic | MSCs | Alginate | Ding et al. (2007), Penolazzi et al. (2010) |
| Collagen | Chan et al. (2010) | ||
| ESCs | Alginate | Hwang et al. (2009), Tang et al. (2012) | |
| ADSCs | Alginate | Abbah et al. (2006) | |
| Chondrogenic | MSCs | Alginate | Babister et al. (2008), Endres et al. (2010) |
| Collagen | Hui et al. (2008), Li et al. (2011) | ||
| Cardiac | ESCs | Alginate | Jing et al. (2010) |
| Agarose | Bauwens et al. (2005) | ||
| Pancreatic | ESCs | Alginate | Chayosumrit et al. (2010), Wang et al. (2009) |
| Hepatocytic | ESCs | Alginate | Fang et al. (2007), Maguire et al. (2005) |
| Adipogenic | ADSCs | Alginate-gelatin | Yao et al. (2012) |
| Neural | ESCs | Alginate | Li et al. (2011b) |
| Hematopoietic | ESCs | Agarose | Dang et al. (2004), Rahman et al. (2010) |
The manipulation of external elements, such as soluble factor addition or changes in oxygen tension, within microencapsulated cell culture systems are used in an identical manner to unencapsulated differentiation protocols. Many of the biochemical cues necessary to direct differentiation can be provided by addition directly to the culture media. Additionally, reducing the oxygen tension can mimic the native microenvironment and be used to more efficiently differentiate cells toward certain lineages (Dang et al., 2004). In some instances, extracellular cues can be integrated into microencapsulated systems, such as the combination of alginate-encapsulated ESC-derived MSCs with a chitosan-calcium phosphate paste, which can promote osteogenic differentiation and mineral synthesis (Tang et al., 2012), or controlling the calcification of alginate beads containing adipose-derived stem cells (ADSCs) (Lee et al., 2010).
Genetic modification of cells within microcapsules to produce a specific protein has been a traditional approach for local and sustained protein delivery to exogenous cells; however, genetic modifications can also be used to influence differentiation of encapsulated cells. Rat MSCs transfected to constitutively express bone morphogenic protein-2 (BMP-2) and cultured in alginate (APA) microcapsules induced osteogenic differentiation when co-cultured with unencapsulated non-transfected MSCs (Ding et al., 2007). In another study, Sox-9 transfected human MSCs (hMSCs) encapsulated in an alginate “bead-in-bead” format, where microcapsules containing Sox-9 transfected hMSCs were secondarily encapsulated in an outer alginate bead containing non-transfected hMSCs, exhibited increased areas of cartilaginous matrix when compared histologically to the capsules with non-transfected cells (Babister et al., 2008), demonstrating that genetic engineering of cells within microcapsules can successfully induce tissue formation.
Modifying the material properties of microcapsules can influence stem cell differentiation by mimicking elements of the native microenvironment and/or through specific functionalization with relevant protein molecules. For example, alginate-gelatin microcapsules with functional properties more akin to the native adipogenic microenvironment led to higher proliferation and more efficient adipogenic differentiation of entrapped ADSCs when compared to those encapsulated in alginate without gelatin (Yao et al., 2012). Additionally, functionalizing agarose with vascular endothelial growth factor (VEGF) yielded more efficient differentiation of microencapsulated mESCs to blood progenitors than soluble treatment with VEGF (1.5-fold higher expression of CD34+CD31+ cells compared to the soluble control) (Rahman et al., 2010). Increasing collagen concentrations and/or initial cell seeding density of hMSCs encapsulated in collagen beads promoted chondrogenesis, determined using a 1,9-dimethylmethylene blue (DMMB) assay (P <0.001) (Hui et al., 2008). Conversely, the differentiation of encapsulated cells can also affect material properties, such as for hMSCs in collagen microcapsules whereby an increase in the reduced elastic modulus can indicate the extent of chondrogenic differentiation due to deposition of glycosaminoglycans and type II collagen, the incidence of dense collagen bundles, and the reduction of type I collagen (Li et al., 2011). The modification and monitoring of microcapsule material properties offer unique strategies for stem cell differentiation, either alone or in tandem with more traditional approaches that include soluble factor addition or genetic modification.
Stem Cell Bioprocessing
The production of stem cell therapies on a large scale requires the development of efficient and scalable bioprocesses. Most industrial bioprocessing relies upon suspension bioreactors, and stem cells have been successfully cultured in suspension as aggregates, on microcarriers, or encapsulated within microcapsules (Kehoe et al., 2010; Serra et al., 2012). Advantages of microencapsulation are that the capsules prevent excessive cell agglomeration, preserve 3D cell–cell and cell–matrix interactions, and protect the cells from hydrodynamic forces. Because stem cells are sensitive to hydrodynamic forces, which are created in agitated culture systems such as bioreactors (Kinney et al., 2011, 2012; Liu et al., 2006; Sargent et al., 2010), the physical protection afforded by encapsulation provides more consistency of the extracellular stem cell environment. Additionally, dissolving the microcapsules during downstream processing can greatly simplify cell retrieval procedures compared to microcarrier-based systems, which require enzymatic treatment (i.e. trypsin) for cell harvesting. In addition to use in scalable bioreactor systems, microencapsulation can also efficiently produce uniform EB populations, or to examine the heterotypic interactions between cells in co-culture systems. Furthermore, encapsulation can protect cells during cryopreservation, a process necessary for the stable storage and broad distribution of stem cell products.
Bioreactor Systems
Several bioreactor systems have been employed for experimental studies of microencapsulated stem cells (Table III), including spinner flasks, which simulate larger volume stirred tank bioreactors. Ex vivo expansion of APA encapsulated bone marrow HSCs using spinner flasks with continuous media exchange yielded a 12- to 24-fold multilineage expansion within 19 days (Levee et al., 1994). In addition to ex vivo expansion of HSCs, the differentiation of mESCs to hematopoietic progenitors while encapsulated in agarose microcapsules was also performed in spinner flasks (Dang et al., 2004). Cardiac differentiation protocols, which usually require an EB suspension culture step, have likewise been developed for microencapsulated mESCs in spinner flasks (Bauwens et al., 2005; Jing et al., 2010). Spinner flask culture of microencapsulated hESCs found that while encapsulation of single hESCs led to poor viability, encapsulation of hESC aggregates and hESCs on microcarriers allowed for maintenance of viability and pluripotency for up to 2 weeks in suspension culture (Serra et al., 2011). In addition to stirred tank bioreactors, other reactor configurations have been investigated, including the high aspect ratio vessel (HARV), a rotary microgravity reactor that operates under the laminar flow regime to lessen the impact of mechanical forces, which was used with mESC-containing alginate microcapsules to create mineralized constructs for bone tissue engineering (Hwang et al., 2009). A fixed bed reactor in which CellBeads, a commercially available product consisting of hMSC aggregates in alginate microcapsules, were packed and perfused with culture medium was able to maintain viability and induce adipogenic differentiation with similar results to stirred suspension controls (Weber et al., 2007). Other bioreactor configurations have been developed for the direct assembly of tissue engineered constructs, including a tubular perfusion system of aggregated alginate beads containing hMSCs (Yeatts et al., 2011). The initial results obtained from bioreactor studies suggest that the development of novel bioreactor systems may lead to improved bioprocess efficiency through better maintenance of viability or more efficient directed differentiation than can be obtained with static cultures.
Table III.
Bioreactor configurations for microencapsulated cell culture.
| Reactor configuration | Cell type | Capsule material | References |
|---|---|---|---|
| Spinner flask/stirred suspension | ESCs | Alginate | Jing et al. (2010), Serra et al. (2011) |
| Agarose | Bauwens et al. (2005) | ||
| HSCs | Alginate | Levee et al. (1994) | |
| High aspect ratio vessel (HARV) | ESCs | Alginate | Consolo et al. (2012), Hwang et al. (2009) |
| Fixed bed | MSCs | Alginate | Weber et al. (2007) |
| Tubular perfusion | MSCs | Alginate | Yeatts et al. (2011) |
Aggregate Formation and Culture
Three-dimensional spherical aggregates are used in stem cell cultures to promote spontaneous differentiation through recapitulation of developmental processes. Additionally, aggregates can be cultured in suspension, allowing for straightforward translation to scalable bioreactors. EBs, pluripotent stem cell aggregates, play pivotal roles in many differentiation protocols and can be used as a platform for directed differentiation (Bratt-Leal et al., 2009). Traditional methods for EB formation include hanging drop, static and stirred suspension formation, methylcellulose culture, and microwell forced aggregation (Kurosawa, 2007; Ungrin et al., 2008). However, each of these techniques suffers from limitations in the aggregate size control and/or throughput of EBs that can be attained. The formation of multicellular aggregates using microencapsulation techniques provides a well-controlled process for constructing EBs of a desired size in a high-throughput manner (on the order of 103 EB-generating capsules generated per minute). In addition, encapsulated ESC aggregates can be immediately introduced into a suspension bioreactor without concern for hydrodynamic damage. The encapsulation of mESCs in solid core alginate sometimes produces “lens-shaped” aggregates (Magyar et al., 2001; Wang et al., 2006), a morphology that depends on the physical properties of the type of alginate used. To provide a thicker coating and reduce the chance of cell escape, a “double encapsulation” method in which cell-containing alginate capsules are liquefied following a second agarose encapsulation step has been used for the creation of mESC spheroids (Sakai et al., 2008). Microfluidic droplet-generating devices can create liquid core alginate beads in which a shell is created around an aqueous phase in one step, rather than requiring multiple independent processing steps of solid capsule formation, coating, and liquefaction of the center; using this method with embryonic carcinoma cells led to compact aggregate formation in 80% of the capsules (Kim et al., 2011). Further development of microencapsulation methods for stem cell aggregate formation, including those for MSC mesenspheres or NSC neurospheres in addition to those for EBs, will be useful for bioprocesses by improving the homogeneity of aggregate size, the throughput of formation, and the creation of a physical barrier to shield against hydrodynamic forces and inhibit aggregate agglomeration.
Co-Culture Systems
Microencapsulation-based co-culture configurations are advantageous in bioprocessing to improve the viability, proliferation, and/or differentiation of an unencapsulated cell population through the support of secreted factors from a different encapsulated cell type, particularly in cases where direct contact is not essential and downstream separation of the two cell populations is required. One primary example is the co-culture of MSCs with HSCs to improve the ex vivo expansion of HSCs (Robinson et al., 2006), which are notoriously difficult to expand (Karlsson, 2004). Because stromal cells like MSCs are present in the HSC niche, they are thought to secrete factors that promote cell survival and proliferation. Co-culture of HSCs with microencapsulated cell types found in the bone marrow niche, including MSCs (Fujimoto et al., 2007; Liu et al., 2009) and osteoblasts (Song et al., 2009), was found to significantly promote hematopoietic expansion when compared to cultures of HSCs alone. This concept could be employed for other applications in stem cell expansion or differentiation; for example, ESCs could be co-cultured with microencapsulated cardiac fibroblasts to induce differentiation to cardiomyocytes (Ou et al., 2011) or with encapsulated endothelial cells to promote pancreatic differentiation (Talavera-Adame et al., 2011).
The impact of direct contact between two cell populations can also be examined with microencapsulation technologies by co-encapsulating two different cell types together. As discussed earlier, the creation of “bead-in-bead” micro-capsules with Sox-9 transfected hMSCs provided spatio-temporal division of two cell types (transfected hMSCs and non-transfected hMSCs) and direct examination of cartilage matrix formation (Babister et al., 2008). Microfluidic technologies can tightly control encapsulation processes of more than one cell type and give rise to high-throughput screening of the interactions between cell populations. For example, the seeding ratio of two cell types can impact viability, as demonstrated in studies with two blood progenitor cell lines (MBA2 and M07e), which have a factor-dependent and responsive relationship (Tumarkin et al., 2011). By combining multiple streams containing different cell populations in controlled ratios, emulsifying the mixture, and cross-linking the intermingled cells into microcapsules, the specific cellular composition of individual capsules can be tightly controlled. Direct co-culture systems can be used to further examine the impact of niche supportive cells on the resident stem cell populations, leading to the development of bioprocesses that better recapitulate the signals provided within the in vivo microenvironment.
Cryopreservation
Cell preservation technology must advance as stem cell bioprocessing evolves to allow for storage and transportation of cell products (Karlsson and Toner, 1996). Cells in suspension, such as erythrocytes, have been successfully stored using cryopreservation for many years; however, less progress has been made in the cryopreservation of adherent cells and 3D tissues. The shortcomings are primarily due to the inability of cryoprotectants to fully diffuse through thick tissues and the cells’ sensitivity to the osmotic and mechanical stresses that occur during cryopreservation (He, 2011; Zimmermann et al., 2007b). Reduced viability and increased tendency toward differentiation have been observed in stem cells after cryopreservation and thawing (Reubinoff et al., 2001). However, entrapping cells in a 3D gel matrix has been found to improve cell viability and function post-thaw for multiple cell types, including stem cells, by maintaining cell–cell contacts and providing better protection from damage during ice crystal formation (Malpique et al., 2009; Zimmermann et al., 2007a). The addition of 10% dimethylsulfoxide (DMSO), a common cryoprotectant, was found to be sufficient for successful cryopreservation and thawing of neurospheres in alginate microcapsules (Malpique et al., 2010). Additional work with alginate microcapsules determined that the water within the capsule can undergo preferential vitrification, a method of cryopreservation in which the formation of intracellular and extracellular ice is prevented through the creation of an amorphous, glassy state, using a low concentration of cryoprotectants (10% DMSO), therefore maintaining the capsule structure and improving the viability of the encapsulated cells post-thaw by reducing the risk of disturbing cell–cell and cell–matrix contacts (Zhang et al., 2010). Cryopreservation of hESCs adherent to microcarriers in alginate microcapsules with 10% DMSO yielded a threefold improvement in cell viability post-thaw (compared to unencapsulated controls) and maintained pluripotency, improvements which were likely due to inhibiting the disruption of cell–cell and/or cell–matrix interactions (Serra et al., 2011). Likewise, cryopreservation of MSCs encapsulated in PEG microcapsules preserved cell viability and osteogenic differentiation potential (Mumaw et al., 2012). The success of microencapsulated stem cell cryopreservation could lead to the development of more efficient preservation techniques and improved availability of off-the-shelf stem cell products.
Transplantation of Microencapsulated Stem Cells
The primary intent of original microencapsulation technologies was to provide a physical separation between transplanted and host cells to minimize immunogenic rejection. Since being developed initially for the treatment of diabetes, microencapsulated cell systems have been explored for a number of other therapeutic applications, including liver disease, renal failure, anemia, hemophilia, hypoparathyroidism, cancer, neurological diseases, musculoskeletal defects, and heart diseases (Hernández et al., 2010; Hunt and Grover, 2010; Orive et al., 2003). To lay the groundwork for clinical translation of stem cell therapies, a number of studies have been performed to examine both the safety and therapeutic efficacy of transplanting microencapsulated stem cells in vivo.
Safety and Microcapsule Characterization Studies
Initial studies with transplanted microencapsulated stem cells were performed to validate safety and to examine cell and capsule behavior in an in vivo setting. Alginate microcapsules containing mESCs were implanted intraperitoneally into 129/SVG mice, and cell growth was found to occur at a faster rate (with no lag phase) when compared to in vitro controls, while pluripotency marker expression decreased with time in vivo (Wang et al., 2006). Injection of alginate microcapsules containing mESCs or hESCs into the peritoneal cavity of SCID mice found that encapsulation delayed teratoma formation for up to 4 weeks for hESCs and up to 3 months for mESCs (Dean et al., 2006). In vivo, encapsulated mESCs formed aggregates and were more likely to differentiate toward an ectodermal lineage, whereas hESCs remained as single cells and small clumps that tended to differentiate toward endodermal lineages. Further studies with transplantation of microencapsulated pluripotent stem cells is required to examine teratoma formation; additionally, the differences in cell growth and differentiation patterns observed in vivo suggest that in vitro characterization of microencapsulated cell function is not sufficient to predict behavior post-transplantation.
Subcutaneous transplantation of hMSCs in collagen microcapsules into NOD/SCID mice determined that the encapsulated cells remained viable for 14 days and the microcapsules remained undamaged and localized to the site of implantation (Chan et al., 2007). Similarly, ADSCs in alginate microcapsules implanted percutaneously into athymic mice retained viability and proliferated for 21 days, with microcapsules remaining intact for up to 3 months (Moyer et al., 2010). Transplantation of microencapsulated rat MSCs under the renal capsule was found to have no negative impact on renal function, and the APA capsules retained their structure for up to 25 days (Trouche et al., 2010). The success of initial safety and characterization studies of transplanted MSCs and ADSCs in microcapsules provides further evidence for the feasibility and efficacy of clinical therapies with these cells.
Therapeutic Efficacy of Microencapsulated Stem Cells In vivo
Several different disease and injury animal models have been examined to evaluate the potential clinical translation of microencapsulated stem cell therapies. In an attempt to stimulate in vivo bone formation, MSCs were genetically engineered with a Tet-off expression system for BMP-2 (a common osteoinductive factor), encapsulated in APA microcapsules, and implanted into a bone defect in C3H/HeN mice. The mice that received doxycycline (and therefore were not producing recombinant BMP-2) exhibited only stromal tissue formation surrounding the implanted microcapsules. On the other hand, mice that did not receive doxycycline (and therefore produced recombinant BMP-2) exhibited bone formation at the defect site, and the cells within the microcapsules displayed a chondrogenic phenotype (Zilberman et al., 2002). Umbilical cord blood (UCB) derived cells, which contain both HSC and MSC populations, were encapsulated in APA micro-capsules and transplanted intraperitoneally into KM mice 24 h after CCl4-induced liver injury. The UCB cells exhibited increased hepatocytic behavior with time in vivo, as determined by alpha-fetoprotein (AFP) expression, albumin expression, and urea synthesis. Repair of the liver damage was also observed through decreased levels of serum amino-transferases and examination of histological structure (Li et al., 2009). Genetically modified MSCs have also been used for the development of cancer therapies. Alginate microcapsules containing hMSCs modified to express hemopexin-like protein, an angiogenesis inhibitor, were transplanted subcutaneously adjacent to gliobastoma tumors in nude mice and reduced tumor volume and weight significantly compared to wild type hMSCs and empty microcapsule controls (Goren et al., 2010).
Transplantation of microencapsulated MSCs into infarcted myocardium has been performed to examine the capacity for promoting cardiac repair. hMSCs encapsulated in an RGD-modified alginate were transplanted into a rat model of ischemia reperfusion myocardial infarction (MI) 1 week after injury. Compared to PBS controls, alginate-hMSC transplantation led to decreased infarct area, increased angiogenesis, improved cell survival, and maintenance of left ventricular geometry and function (Yu et al., 2010). In another study, acute MI was induced in Sprague-Dawley rats and APA microcapsules containing rat MSCs in combination with Schwann cells, which are known to produce VEGF, were injected intra-myocardially at four sites within and around the infarct region 30 min after acute MI induction. The greatest increase in vessel density was observed when MSCs and Schwann cells were transplanted together, with an approximate 1.6-fold increase over encapsulated MSCs alone and an approximate 1.2-fold increase over encapsulated Schwann cells alone (P <0.01), indicating that co-transplanting the microencapsulated cells can improve vascularization post-MI (Wang et al., 2012). Though the number of studies performed with transplanted stem cell microcapsules has been rather limited, initial studies indicate that microencapsulation is an effective approach to stimulate in vivo tissue repair, with functional improvement often surpassing what is seen commonly observed for unencapsulated cells. The improvement may be explained by the enhanced localization and persistence of transplanted cells, the prevention or delay of immunological rejection, and/or the mechanical support provided to host tissues by implanting miniature load-bearing scaffolds.
Conclusions and Future Trends
The current body of work in stem cell microencapsulation has laid the groundwork for directed differentiation, bioprocessing, and transplantation protocols. However, additional innovation in material design, microcapsule formation methods, and modeling of encapsulated systems could benefit the further development and eventual translation of microencapsulated stem cell technologies.
Microcapsule materials have been engineered to contain adhesive moieties to facilitate cell attachment and migration (Bidarra et al., 2011; Evangelista et al., 2007; Karoubi et al., 2009; Markusen et al., 2006), modified with growth factors, such as VEGF, to modulate differentiation (Rahman et al., 2010), or tailored to degrade with regulated rates to enable controlled release of the cellular contents (Ashton et al., 2007; Fonseca et al., 2011; Zhou and Xu, 2011). Because stem cells are often sensitive to their surrounding mechanical microenvironment, the development of hydrogels with variable, well-defined mechanical properties could also be used to regulate cell viability, proliferation, and differentiation. Several methods to create capsules with a range of mechanical properties have been previously developed, including the use of photocross-linkable alginates (Jeon et al., 2009) or microfluidics to precisely control concentrations of the cell/polymer and cross-linking solutions (Kumachev et al., 2011). Engineered materials which provide a more controlled release of stem cell secreted factors could be used to regulate the size range of molecules allowed to enter or leave the capsule; for example, the development of dual capsules, in which a secondary material provides additional control over mass transfer processes, may provide a platform for future advances (Zhang et al., 2008). Likewise, the incorporation of molecules with specific binding characteristics, such as the affinity of glycosaminoglycans for positively charged growth factors, could be used to further modulate the types of molecules captured by and/or permitted to pass through a capsule. In addition to modifying encapsulation materials, microparticles with diverse material properties can be incorporated within aggregates (Bratt-Leal et al., 2011) that could then be encapsulated to provide spatially localized cues for differentiation, and also potentially impact secretory properties of the cells.
Microfluidic and microlithography methods are also being investigated to achieve more precise formation of stem cell microcapsules. Microfluidic-based methods can improve the efficiency and precision of encapsulation by reducing exposure to cytotoxic cross-linking agents (Kim et al., 2009), by facilitating the homogeneous formation of cellular aggregates within liquid core capsules (Kim et al., 2011), or by combining two cell populations in a precise ratio (Tumarkin et al., 2011). Microfluidic chips can also be utilized for culture of encapsulated stem cells, allowing for temporally controlled delivery of chemical cues, real-time imaging, and on-chip phenotypic analyses (Kim et al., 2012). The formation of microcapsules using microlithography, in which gels are cast in 3D patterned microwells, can be used to create homogeneously sized capsules of different shapes (Qiu et al., 2007), though current methods are relatively low-throughput. The development of novel systems with micro-level control of capsule formation and analysis will facilitate more precise encapsulation methods (e.g., exposure time and concentration of cross-linker, cell seeding ratios), more systematic analysis of capsule parameters (e.g., mechanical stability), and precise evaluation of cellular phenotype and response (e.g., differentiation status, secretory response to stimuli).
Computational modeling of encapsulated cell systems is currently an underutilized approach to provide additional insight into efficient differentiation and manufacturing processes where experimental systems alone often fall short. Mass transfer modeling of oxygen diffusion is important to ensure cell viability and proliferation, as well as to confirm exposure of cells to appropriate oxygen levels for directed differentiation (Gross et al., 2007; Mohyeldin et al., 2010). Additionally, computational fluid dynamic models of microencapsulated cells within bioreactors can elucidate estimates of hydrodynamic forces and concentration gradients present in the system that are challenging to measure directly (Consolo et al., 2012).
In summary, microencapsulation provides a robust platform for manipulating stem cell expansion, differentiation, and transplantation. The significant knowledge gained from the history of microencapsulation for the production of enzymes and isolation of primary cells can be leveraged to accelerate the development of stem cell products. The ability of microencapsulated environments to simultaneously control cell phenotype by varying material properties, prevent detrimental agglomeration and shield hydrodynamic forces in large bioreactors, and allow for allogeneic stem cell transplantation with reduced immunogenic risk renders microencapsulation an important and integral technology for the future of stem cell research, biomanufacturing, and clinical therapies.
Acknowledgments
The authors are supported by funding from the NIH (EB010061). J.L.W. is currently supported by a GAANN Fellowship (Department of Education P200A090099) and previously by an NSF IGERT (DGE 0965945). Our apologies to any investigators whose important works we were unable to cite due to space constraints.
Contract grant sponsor: NIH
Contract grant number: EB010061
Contract grant sponsor: GAANN Fellowship Department of Education
Contract grant number: P200A090099
Contract grant sponsor: NSF IGERT
Contract grant number: DGE 0965945
References
- Abbah SA, Lu WW, Chan D, Cheung KMC, Liu WG, Zhao F, Li ZY, Leong JCY, Luk KDK. In vitro evaluation of alginate encapsulated adipose-tissue stromal cells for use as injectable bone graft substitute. Biochem Biophys Res Commun. 2006;347:185–191. doi: 10.1016/j.bbrc.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Ashton RS, Banerjee A, Punyani S, Schaffer DV, Kane RS. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture. Biomaterials. 2007;28:5518–5525. doi: 10.1016/j.biomaterials.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Babister JC, Tare RS, Green DW, Inglis S, Mann S, Oreffo ROC. Genetic manipulation of human mesenchymal progenitors to promote chondrogenesis using “bead-in-bead” polysaccharide capsules. Biomaterials. 2008;29:58–65. doi: 10.1016/j.biomaterials.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regener Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batorsky A, Liao J, Lund AW, Plopper GE, Stegemann JP. Encapsulation of adult human mesenchymal stem cells within collagen-agarose microenvironments. Biotechnol Bioeng. 2005;92:492–500. doi: 10.1002/bit.20614. [DOI] [PubMed] [Google Scholar]
- Bauwens C, Yin T, Dang S, Peerani R, Zandstra PW. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: Oxygen-mediated enhancement of cardiomyocyte output. Biotechnol Bioeng. 2005;90:452–461. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- Bidarra SJ, Barrias CC, Fonseca KB, Barbosa MA, Soares RA, Granja PL. Injectable in situ crosslinkable RGD-modified alginate matrix for endothelial cells delivery. Biomaterials. 2011;32:7897–7904. doi: 10.1016/j.biomaterials.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Bratt-Leal AM, Carpenedo RL, McDevitt TC. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25:43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt-Leal AM, Carpenedo RL, Ungrin MD, Zandstra PW, McDevitt TC. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011;32:48–56. doi: 10.1016/j.biomaterials.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe BJ, Liu KK, Williams DR. Adhesive contact deformation of a single microelastomeric sphere. J Colloid Interface Sci. 1998;200:256–264. [Google Scholar]
- Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun-Graeppi AKAS, Richard C, Bessodes M, Scherman D, Merten O-W. Cell microcarriers and microcapsules of stimuli-responsive polymers. J Control Release. 2011;149:209–224. doi: 10.1016/j.jconrel.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng A. 2009;15:205–219. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BP, Hui TY, Yeung CW, Li J, Mo I, Chan GCF. Self-assembled collagen-human mesenchymal stem cell microspheres for regenerative medicine. Biomaterials. 2007;28:4652–4666. doi: 10.1016/j.biomaterials.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Chan BP, Hui TY, Wong MY, Yip KHK, Chan GCF. Mesenchymal stem cell-encapsulated collagen microspheres for bone tissue engineering. Tissue Eng C. 2010;16:225–235. doi: 10.1089/ten.tec.2008.0709. [DOI] [PubMed] [Google Scholar]
- Chan E-S, Lim T-K, Voo W-P, Pogaku R, Tey BT, Zhang Z. Effect of formulation of alginate beads on their mechanical behavior and stiffness. Particuology. 2011;9:228–234. [Google Scholar]
- Chang TMS. Semipermeable microcapsules. Science. 1964;146:524–525. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- Chayosumrit M, Tuch B, Sidhu K. Alginate microcapsule for propagation and directed differentiation of hESCs to definitive endoderm. Biomaterials. 2010;31:505–514. doi: 10.1016/j.biomaterials.2009.09.071. [DOI] [PubMed] [Google Scholar]
- Consolo F, Bariani C, Mantalaris A, Montevecchi F, Redaelli A, Morbiducci U. Computational modeling for the optimization of a cardiogenic 3D bioprocess of encapsulated embryonic stem cells. Biomech Model Mechanobiol. 2012;11:261–277. doi: 10.1007/s10237-011-0308-0. [DOI] [PubMed] [Google Scholar]
- Crooks CA, Douglas JA, Broughton RL, Sefton MV. Microencapsulation of mammalian cells in a HEMA-MMA copolymer: Effects on capsule morphology and permeability. J Biomed Mater Res. 1990;24:1241–1262. doi: 10.1002/jbm.820240908. [DOI] [PubMed] [Google Scholar]
- Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275–282. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- Darrabie MD, Kendall WF, Opara EC. Effect of alginate composition and gelling cation on micro-bead swelling. J Microencapsul. 2006;23:29–37. doi: 10.1080/02652040500286144. [DOI] [PubMed] [Google Scholar]
- De Castro M, Orive G, Hernández RM, Gascón AR, Pedraz JL. Comparative study of microcapsules elaborated with three polycations (PLL, PDL, PLO) for cell immobilization. J Microencapsul. 2005;22:303–315. doi: 10.1080/026520405000099893. [DOI] [PubMed] [Google Scholar]
- De Vos P, Haan BD, Schilfgaarde RV. Effect of the alginate composition on the biocompatibility of alginate-polylysine microcapsules. Biomaterials. 1997;18:273–278. doi: 10.1016/s0142-9612(96)00135-4. [DOI] [PubMed] [Google Scholar]
- De Vos P, Bucko M, Gemeiner P, Navrátil M, Svitel J, Faas M, Strand BL, Skjak-Braek G, Morch YA, Vikartovská A, Lacík I, Kolláriková G, Orive G, Poncelet D, Pedraz JL, Ansorge-Schumacher MB. Multiscale requirements for bioencapsulation in medicine and biotechnology. Biomaterials. 2009;30:2559–2570. doi: 10.1016/j.biomaterials.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Dean SK, Yulyana Y, Williams G, Sidhu KS, Tuch BE. Differentiation of encapsulated embryonic stem cells after transplantation. Transplantation. 2006;82:1175–1184. doi: 10.1097/01.tp.0000239518.23354.64. [DOI] [PubMed] [Google Scholar]
- Ding HF, Liu R, Li BG, Lou JR, Dai KR, Tang TT. Biologic effect and immunoisolating behavior of BMP-2 gene-transfected bone marrow-derived mesenchymal stem cells in APA microcapsules. Biochem Biophys Res Commun. 2007;362:923–927. doi: 10.1016/j.bbrc.2007.08.094. [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Wenda N, Woehlecke H, Neumann K, Ringe J, Erggelet C, Lerche D, Kaps C. Microencapsulation and chondrogenic differentiation of human mesenchymal progenitor cells from subchondral bone marrow in Ca-alginate for cell injection. Acta Biomater. 2010;6:436–444. doi: 10.1016/j.actbio.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Evangelista MB, Hsiong SX, Fernandes R, Sampaio P, Kong H-J, Barrias CC, Salema R, Barbosa MA, Mooney DJ, Granja PL. Upregulation of bone cell differentiation through immobilization within a synthetic extracellular matrix. Biomaterials. 2007;28:3644–3655. doi: 10.1016/j.biomaterials.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fang S, Qiu Y-D, Mao L, Shi X-L, Yu D-C, Ding Y-T. Differentiation of embryoid-body cells derived from embryonic stem cells into hepatocytes in alginate microbeads in vitro. Acta Pharmacol Sin. 2007;28:1924–1930. doi: 10.1111/j.1745-7254.2007.00713.x. [DOI] [PubMed] [Google Scholar]
- Fonseca KB, Bidarra SJ, Oliveira MJ, Granja PL, Barrias CC. Molecularly designed alginate hydrogels susceptible to local proteolysis as three-dimensional cellular microenvironments. Acta Biomater. 2011;7:1674–1682. doi: 10.1016/j.actbio.2010.12.029. [DOI] [PubMed] [Google Scholar]
- Freimark D, Pino-Grace P, Pohl S, Weber C, Wallrapp C, Geigle P, Pörtner R, Czermak P. Use of encapsulated stem cells to overcome the bottleneck of cell availability for cell therapy approaches. Transfus Med Hemother. 2010;37:66–73. doi: 10.1159/000285777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto N, Fujita S, Tsuji T, Toguchida J, Ida K, Suginami H, Iwata H. Microencapsulated feeder cells as a source of soluble factors for expansion of CD34(+) hematopoietic stem cells. Biomaterials. 2007;28:4795–4805. doi: 10.1016/j.biomaterials.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Gage FH. Cell therapy. Nature. 1998;392:18–24. [PubMed] [Google Scholar]
- Goren A, Dahan N, Goren E, Baruch L, Machluf M. Encapsulated human mesenchymal stem cells: A unique hypoimmunogenic platform for long-term cellular therapy. FASEB J. 2010;24:22–31. doi: 10.1096/fj.09-131888. [DOI] [PubMed] [Google Scholar]
- Gross JD, Constantinidis I, Sambanis A. Modeling of encapsulated cell systems. J Theor Biol. 2007;244:500–510. doi: 10.1016/j.jtbi.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasse C, Klöck G, Schlosser A, Zimmermann U, Rothmund M. Parathyroid allotransplantation without immunosuppression. Lancet. 1997;350:1296–1297. doi: 10.1016/S0140-6736(05)62473-7. [DOI] [PubMed] [Google Scholar]
- Haug A, Larsen B, Smidsrod O. A study of the constitution of alginic acid by partial acid hydrolysis. Acta Chem Scand. 1966;20:183–190. [Google Scholar]
- He X. In: Preservation of embryonic stem cells, methodological advances in the culture, manipulation and utilization of embryonic stem cells for basic and practical applications. Atwood C, editor. InTech; 2011. Available from: http://www.intechopen.com/books/methodological-advances-in-the-culture-manipulation-and-utilization-of-embryonic-stem-cells-for-basic-and-practical-applications/preservation-of-embryonic-stem-cells. [DOI] [Google Scholar]
- Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- Hernández RMA, Orive G, Murua A, Pedraz JL. Microcapsules and microcarriers for in situ cell delivery. Adv Drug Deliv Rev. 2010;62:711–730. doi: 10.1016/j.addr.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Hortelano G, Al-Hendy A, Ofosu FA, Chang PL. Delivery of human factor IX in mice by encapsulated recombinant myoblasts: A novel approach towards allogeneic gene therapy of hemophilia B. Blood. 1996;87:5095–5103. [PubMed] [Google Scholar]
- Huang X, Zhang X, Wang X, Wang C, Tang B. Microenvironment of alginate-based microcapsules for cell culture and tissue engineering. J Biosci Bioeng. 2012;114:1–8. doi: 10.1016/j.jbiosc.2012.02.024. [DOI] [PubMed] [Google Scholar]
- Hui TY, Cheung KMC, Cheung WL, Chan D, Chan BP. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: Influence of cell seeding density and collagen concentration. Biomaterials. 2008;29:3201–3212. doi: 10.1016/j.biomaterials.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Hunt NC, Grover LM. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol Lett. 2010;32:733–742. doi: 10.1007/s10529-010-0221-0. [DOI] [PubMed] [Google Scholar]
- Hwang Y-S, Cho J, Tay F, Heng JYY, Ho R, Kazarian SG, Williams DR, Boccaccini AR, Polak JM, Mantalaris A. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials. 2009;30:499–507. doi: 10.1016/j.biomaterials.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Jain K, Yan H, Cai B-R, Haque B, Hurvitz A, Diehl C, Miyata T, Smith B, Stenzel K, Suthanthiran M, Rubin A. Retrievable, replaceable, macroencapsulated pancreatic islet xenografts. Transplantation. 1995;59:319–324. [PubMed] [Google Scholar]
- Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30:2724–2734. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Jing D, Parikh A, Tzanakakis ES. Cardiac cell generation from encapsulated embryonic stem cells in static and scalable culture systems. Cell Transplant. 2010;19:1397–1412. doi: 10.3727/096368910X513955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S. Stem cell expansion: Success and complexities. Blood. 2004;104:2210–2211. [Google Scholar]
- Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: Critical issues. Biomaterials. 1996;17:243–256. doi: 10.1016/0142-9612(96)85562-1. [DOI] [PubMed] [Google Scholar]
- Karoubi G, Ormiston ML, Stewart DJ, Courtman DW. Single-cell hydrogel encapsulation for enhanced survival of human marrow stromal cells. Biomaterials. 2009;30:5445–5455. doi: 10.1016/j.biomaterials.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Kehoe DE, Jing D, Lock LT, Tzanakakis ES. Scalable stirred-suspension bioreactor culture. Tissue Eng A. 2010;16:405–421. doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Lee KS, Kim YE, Lee K-J, Lee SH, Kim TS, Kang JY. Rapid exchange of oil-phase in microencapsulation chip to enhance cell viability. Lab Chip. 2009;9:1294–1297. doi: 10.1039/b819044e. [DOI] [PubMed] [Google Scholar]
- Kim C, Chung S, Kim YE, Lee KS, Lee SH, Oh KW, Kang JY. Generation of core-shell microcapsules with three-dimensional focusing device for efficient formation of cell spheroid. Lab Chip. 2011;11:246–252. doi: 10.1039/c0lc00036a. [DOI] [PubMed] [Google Scholar]
- Kim C, Bang JH, Kim YE, Lee JH, Kang JY. Stable hydrodynamic trapping of hydrogel beads for on-chip differentiation analysis of encapsulated stem cells. Sens Actuators B. 2012;166–167:859–869. [Google Scholar]
- King GA, Dougulis AJ, Faulkner P, Goosen FA. Alginate-polylysine microcapsules of controlled membrane molecular weight cutoff for mammalian cell culture engineering. Biotechnol Prog. 1987;3:231–240. [Google Scholar]
- Kinney MA, Sargent CY, McDevitt TC. The multiparametric effects of hydrodynamic environments on stem cell culture. Tissue Eng B. 2011;17:249–262. doi: 10.1089/ten.teb.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney MA, Saeed R, McDevitt TC. Systematic analysis of embryonic stem cell differentiation in hydrodynamic environments with controlled embryoid body size. Integr Biol. 2012;4:641–650. doi: 10.1039/c2ib00165a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulseng B, Skjak-Braek G, Ryan L, Andersson A, King A, Faxvaag A, Espevik T. Transplantation of alginate microcapsules: Generation of antibodies against alginates and encapsulated porcine islet-like clusters. Transplantation. 1999;67:978–984. doi: 10.1097/00007890-199904150-00008. [DOI] [PubMed] [Google Scholar]
- Kumachev A, Greener J, Tumarkin E, Eiser E, Zandstra PW, Kumacheva E. High-throughput generation of hydrogel microbeads with varying elasticity for cell encapsulation. Biomaterials. 2011;32:1477–1483. doi: 10.1016/j.biomaterials.2010.10.033. [DOI] [PubMed] [Google Scholar]
- Kurosawa H. Methods for inducing embryoid body formation: In vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- Lee C-S, Chu I-M. Characterization of modified alginate-poly-L-lysine microcapsules. Artif Organs. 1997;21:1002–1006. [PubMed] [Google Scholar]
- Lee CSD, Moyer HR, Gittens RAI, Williams JK, Boskey AL, Boyan BD, Schwartz Z. Regulating in vivo calcification of alginate microbe-ads. Biomaterials. 2010;31:4926–4934. doi: 10.1016/j.biomaterials.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levee MG, Lee G-M, Pack S-H, Palsson B. Marrow cultures: A potential culture system for the clonal outgrowth of hematopoietic progenitor cells. Biotechnol Bioeng. 1994;43:734–739. doi: 10.1002/bit.260430807. [DOI] [PubMed] [Google Scholar]
- Li X, Liu T, Song K, Yao L, Ge D, Bao C, Ma X, Cui Z. Culture of neural stem cells in calcium alginate beads. Biotechnol Prog. 2006;22:1683–1689. doi: 10.1021/bp060185z. [DOI] [PubMed] [Google Scholar]
- Li S, Sun Z, Lv G, Guo X, Zhang Y, Yu W, Wang W, Ma X. Microencapsulated UCB cells repair hepatic injure by intraperitoneal transplantation. Cytotherapy. 2009;11:1032–1040. doi: 10.3109/14653240903121278. [DOI] [PubMed] [Google Scholar]
- Li C-H, Chik T-K, Ngan AHW, Chan SCH, Shum DKY, Chan BP. Correlation between compositional and mechanical properties of human mesenchymal stem cell-collagen microspheres during chondrogenic differentiation. Tissue Eng A. 2011a;17:777–788. doi: 10.1089/ten.TEA.2010.0078. [DOI] [PubMed] [Google Scholar]
- Li L, Davidovich AE, Schloss JM, Chippada U, Schloss RR, Langrana NA, Yarmush ML. Neural lineage differentiation of embryonic stem cells within alginate microbeads. Biomaterials. 2011b;32:4489–4497. doi: 10.1016/j.biomaterials.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- Liu H, Lin J, Roy K. Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells. Biomaterials. 2006;27:5978–5989. doi: 10.1016/j.biomaterials.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu T, Ma X, Fan X, Bao C, Cui Z. Effects of encapsulated rabbit mesenchymal stem cells on ex vivo expansion of human umbilical cord blood hematopoietic stem/progenitor cells. J Microencapsul. 2009;26:130–142. doi: 10.1080/02652040802193014. [DOI] [PubMed] [Google Scholar]
- Maguire T, Novik E, Schloss R, Yarmush M. Alginate-PLL microen-capsulation: Effect on the differentiation of embryonic stem cells into hepatocytes. Biotechnol Bioeng. 2005;93:581–591. doi: 10.1002/bit.20748. [DOI] [PubMed] [Google Scholar]
- Magyar JP, Nemir M, Ehler E, Suter N, Perriard JC, Eppenberger HM. Mass production of embryoid bodies in microbeads. Ann N Y Acad Sci. 2001;944:135–143. doi: 10.1111/j.1749-6632.2001.tb03828.x. [DOI] [PubMed] [Google Scholar]
- Malpique R, Ehrhart F, Katsen-Globa A, Zimmermann H, Alves PM. Cryopreservation of adherent cells: Strategies to improve cell viability and function after thawing. Tissue Eng C. 2009;15:373–386. doi: 10.1089/ten.tec.2008.0410. [DOI] [PubMed] [Google Scholar]
- Malpique R, Osório LM, Ferreira DS, Ehrhart F, Brito C, Zimmermann H, Alves PM. Alginate encapsulation as a novel strategy for the cryopreservation of neurospheres. Tissue Eng C. 2010;16:965–977. doi: 10.1089/ten.TEC.2009.0660. [DOI] [PubMed] [Google Scholar]
- Markusen JF, Mason C, Hull DA, Town MA, Tabor AB, Clements M, Boshoff CH, Dunnill P. Behavior of adult human mesenchymal stem cells entrapped in alginate-GRGDY beads. Tissue Eng. 2006;12:821–830. doi: 10.1089/ten.2006.12.821. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew HW, Salley SO, Peterson WD, Klein MD. Complex coacervate microcapsules for mammalian cell culture and artificial organ development. Biotechnol Prog. 1993;9:510–519. doi: 10.1021/bp00023a010. [DOI] [PubMed] [Google Scholar]
- Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Mørch YA, Donati I, Strand BL, Skjåk-Braek G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7:1471–1480. doi: 10.1021/bm060010d. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota M, Teixeira JA, Yelshin A. Immobilized particles in gel matrix-type porous media. Homogeneous porous media model. Biotechnol Prog. 2001;17:860–865. doi: 10.1021/bp010064t. [DOI] [PubMed] [Google Scholar]
- Moyer HR, Kinney RC, Singh KA, Williams JK, Schwartz Z, Boyan BD. Alginate microencapsulation technology for the percutaneous delivery of adipose-derived stem cells. Ann Plast Surg. 2010;65:497–503. doi: 10.1097/SAP.0b013e3181d37713. [DOI] [PubMed] [Google Scholar]
- Mumaw J, Jordan ET, Sonnet C, Olabisi RM, Olmsted-Davis EA, Davis AR, Peroni JF, West JL, West F, Lu Y, Stice SL. Rapid heterotrophic ossification with cryopreserved poly(ethylene glycol-) microencapsulated BMP2-expressing MSCs. Int J Biomater. 2012;2012:1–11. doi: 10.1155/2012/861794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murua A, Portero A, Orive G, Hernández RM, de Castro M, Pedraz JL. Cell microencapsulation technology: Towards clinical application. J Control Release. 2008;132:76–83. doi: 10.1016/j.jconrel.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Orive G, Gascón AR, Hernández RM, Igartua M, Luis Pedraz J. Cell microencapsulation technology for biomedical purposes: Novel insights and challenges. Trends Pharmacol Sci. 2003;24:207–210. doi: 10.1016/S0165-6147(03)00073-7. [DOI] [PubMed] [Google Scholar]
- Otterlei M, Ostgaard K, Skjak-Braek G, Smidsrod O, Soon-Shiong P, Espevik T. Induction of cytokine production from human monocutes stimulated with alginate. J Immunother. 1991;10:286–291. doi: 10.1097/00002371-199108000-00007. [DOI] [PubMed] [Google Scholar]
- Ou D-B, He Y, Chen R, Teng J-W, Wang H-T, Zeng D, Liu X-T, Ding L, Huang J-Y, Zheng Q-S. Three-dimensional co-culture facilitates the differentiation of embryonic stem cells into mature cardiomyocytes. J Cell Biochem. 2011;112:3555–3562. doi: 10.1002/jcb.23283. [DOI] [PubMed] [Google Scholar]
- Pajić-Lijaković I, Bugarski D, Plavšić M, Bugarski B. Influence of microenvironmental conditions on hybridoma cell growth inside the alginate-poly-L-lysine microcapsule. Process Biochem. 2007;42:167–174. [Google Scholar]
- Paul A, Ge Y, Prakash S, Shum-Tim D. Microencapsulated stem cells for tissue repairing: Implications in cell-based myocardial therapy. Regener Med. 2009;4:733–745. doi: 10.2217/rme.09.43. [DOI] [PubMed] [Google Scholar]
- Paul A, Shum-Tim D, Prakash S. Investigation on PEG integrated alginate-chitosan microcapsules for myocardial therapy using marrow stem cells genetically modified by recombinant baculovirus. Cardiovasc Eng Technol. 2010;1:154–164. [Google Scholar]
- Penolazzi L, Ph D, Tavanti E, Vecchiatini R, Lambertini E, Vesce F, Gambari R, Mazzitelli S, Mancuso F, Luca G, Nastruzzi C, Piva R. Encapsulation of mesenchymal stem cells from Wharton’s Jelly in alginate microbeads. Tissue Eng C. 2010;16:141–155. doi: 10.1089/ten.TEC.2008.0582. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multi-lineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Purcell EK, Singh A, Kipke DR. Alginate composition effects on a neural stem cell-seeded scaffold. Tissue Eng C. 2009;15:541–550. doi: 10.1089/ten.tec.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Chen M, Yan H, Wu H. Generation of uniformly sized alginate microparticles for cell encapsulation by using a soft-lithography approach. Adv Mater. 2007;19:1603–1607. [Google Scholar]
- Rabanel J-M, Banquy X, Zouaoui H, Mokhtar M, Hildgen P. Progress technology in microencapsulation methods for cell therapy. Biotechnol Prog. 2009;25:946–963. doi: 10.1002/btpr.226. [DOI] [PubMed] [Google Scholar]
- Rahman N, Purpura KA, Wylie RG, Zandstra PW, Shoichet MS. The use of vascular endothelial growth factor functionalized agarose to guide pluripotent stem cell aggregates toward blood progenitor cells. Biomaterials. 2010;31:8262–8270. doi: 10.1016/j.biomaterials.2010.07.040. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- Robinson SN, Ng J, Niu T, Yang H, McMannis JD, Karandish S, Kaur I, Fu P, Del Angel M, Messinger R, Flagge F, de Lima M, Decker W, Xing D, Champlin R, Shpall EJ. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Ji L, de Pablo JJ, Palecek SP. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126–137. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- Sajadi A, Bensadoun J-C, Schneider BL, Lo Bianco C, Aebischer P. Transient striatal delivery of GDNF via encapsulated cells leads to sustained behavioral improvement in a bilateral model of Parkinson disease. Neurobiol Dis. 2006;22:119–129. doi: 10.1016/j.nbd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Sakai S, Kawabata K, Ono T, Ijima H, Kawakami K. Preparation of mammalian cell-enclosing subsieve-sized capsules (<100 micron) in a coflowing stream. Biotechnol Bioeng. 2004;86:168–173. doi: 10.1002/bit.20006. [DOI] [PubMed] [Google Scholar]
- Sakai S, Hashimoto I, Kawakami K. Agarose-gelatin conjugate for adherent cell-enclosing capsules. Biotechnol Lett. 2007;29:731–735. doi: 10.1007/s10529-007-9312-y. [DOI] [PubMed] [Google Scholar]
- Sakai S, Hashimoto I, Kawakami K. Production of cell-enclosing hollow-core agarose microcapsules via jetting in water-immiscible liquid paraffin and formation of embryoid body-like spherical tissues from mouse ES cells enclosed within these microcapsules. Biotechnol Bioeng. 2008;99:235–243. doi: 10.1002/bit.21624. [DOI] [PubMed] [Google Scholar]
- Sargent CY, Berguig GY, Kinney MA, Hiatt LA, Carpenedo RL, Berson RE, McDevitt TC. Hydrodynamic modulation of embryonic stem cell differentiation by rotary orbital suspension culture. Biotechnol Bioeng. 2010;105:611–626. doi: 10.1002/bit.22578. [DOI] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Serp D, Cantana E, Heinzen C, Von Stockar U, Marison IW. Characterization of an encapsulation device for the production of monodisperse alginate beads for cell immobilization. Biotechnol Bioeng. 2000;70:41–53. [PubMed] [Google Scholar]
- Serra M, Correia C, Malpique R, Brito C, Jensen J, Bjorquist P, Carrondo MJT, Alves PM. Microencapsulation technology: A powerful tool for integrating expansion and cryopreservation of human embryonic stem cells. Ed. Christina Chan. PLoS ONE. 2011;6:e23212. doi: 10.1371/journal.pone.0023212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Brito C, Correia C, Alves PM. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012;30:350–359. doi: 10.1016/j.tibtech.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Shoichet MS, Li RH, White ML, Winn SR. Stability of hydrogels used in cell encapsulation: An in vitro comparison of alginate and agarose. Biotechnol Bioeng. 1996;50:374–381. doi: 10.1002/(SICI)1097-0290(19960520)50:4<374::AID-BIT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Simpson NE, Stabler CL, Simpson CP, Sambanis A, Constantinidis I. The role of the CaCl2–guluronic acid interaction on alginate encapsulated βTC3 cells. Biomaterials. 2004;25:2603–2610. doi: 10.1016/j.biomaterials.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Siti-Ismail N, Bishop AE, Polak JM, Mantalaris A. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials. 2008;29:3946–3952. doi: 10.1016/j.biomaterials.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Song K, Zhao G, Liu T, Zhang L, Ma X, Liu J, Cui Z. Effective expansion of umbilical cord blood hematopoietic stem/progenitor cells by regulation of microencapsulated osteoblasts under hypoxic condition. Biotechnol Lett. 2009;31:923–928. doi: 10.1007/s10529-009-9961-0. [DOI] [PubMed] [Google Scholar]
- Soon-Shiong P, Heintz R, Merideth N, Yao Q, Yao Z, Zheng T, Murphy M, Moloney M, Schmehl M, Harris M, Mendez R, Sandford PA. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343:950–951. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Strand BL, Mørch YA, Espevik T, Skjåk-Braek G. Visualization of alginate-poly-L-lysine-alginate microcapsules by confocal laser scanning microscopy. Biotechnol Bioeng. 2003;82:386–394. doi: 10.1002/bit.10577. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Talavera-Adame D, Wu G, He Y, Ng TT, Gupta A, Kurtovic S, Hwang JY, Farkas DL, Dafoe DC. Endothelial cells in co-culture enhance embryonic stem cell differentiation to pancreatic progenitors and insulin-producing cells through BMP signaling. Stem Cell Rev Rep. 2011;7:532–543. doi: 10.1007/s12015-011-9232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Chen W, Weir MD, Thein-Han W, Xu HHK. Human embryonic stem cell encapsulation in alginate microbeads in macro-porous calcium phosphate cement for bone tissue engineering. Acta Biomater. 2012;8:3436–3445. doi: 10.1016/j.actbio.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, Elisseeff J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25:2730–2738. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thu B, Bruheim P, Espevik T, Smidsrød O, Soon-Shiong P, Skjåk-Braek G. Alginate polycation microcapsules I. Interaction between alginate and polycation. Biomaterials. 1996a;17:1031–1040. doi: 10.1016/0142-9612(96)84680-1. [DOI] [PubMed] [Google Scholar]
- Thu B, Bruheim P, Espevik T, Smidsrød O, Soon-Shiong P, Skjåk-Braek G. Alginate polycation microcapsules. II. Some functional properties. Biomaterials. 1996b;17:1069–1079. doi: 10.1016/0142-9612(96)85907-2. [DOI] [PubMed] [Google Scholar]
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;175:145–149. doi: 10.1667/rrxx28.1. [DOI] [PubMed] [Google Scholar]
- Trouche E, Girod Fullana S, Mias C, Ceccaldi C, Tortosa F, Seguelas MH, Calise D, Parini A, Cussac D, Sallerin B. Evaluation of alginate microspheres for mesenchymal stem cell engraftment on solid organ. Cell Transplant. 2010;19:1623–1633. doi: 10.3727/096368910X514297. [DOI] [PubMed] [Google Scholar]
- Tumarkin E, Tzadu L, Csaszar E, Seo M, Zhang H, Lee A, Peerani R, Purpura K, Zandstra PW, Kumacheva E. High-throughput combinatorial cell co-culture using microfluidics. Integr Biol. 2011;3:653–662. doi: 10.1039/c1ib00002k. [DOI] [PubMed] [Google Scholar]
- Uludag H, De Vos P, Tresco PA. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000;42:29–64. doi: 10.1016/s0169-409x(00)00053-3. [DOI] [PubMed] [Google Scholar]
- Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS ONE. 2008;3:e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Chang PL. Osmotic pressure test: A simple, quantitative method to assess the mechanical stability of alginate microcapsules. J Biomed Mater Res. 2001;54:264–271. doi: 10.1002/1097-4636(200102)54:2<264::aid-jbm14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Vandenbossche GM, Van Oostveldt P, Demeester J, Remon JP. The molecular weight cut-off of microcapsules is determined by the reaction between alginate and polylysine. Biotechnol Bioeng. 1993;42:381–386. doi: 10.1002/bit.260420316. [DOI] [PubMed] [Google Scholar]
- Wang T, Lacik I, Brissova M, Anilkumar A, Prokop A, Hunkeler D, Green R, Shahrokhi K, Powers A. An encapsulation system for the immunoisolation of pancreatic islets. Nat Biotechnol. 1997;15:358–362. doi: 10.1038/nbt0497-358. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang W, Ma J, Guo X, Yu X, Ma X. Proliferation and differentiation of mouse embryonic stem cells in APA microcapsule: A model for studying the interaction between stem cells and their niche. Biotechnol Prog. 2006;22:791–800. doi: 10.1021/bp050386n. [DOI] [PubMed] [Google Scholar]
- Wang N, Adams G, Buttery L, Falcone FH, Stolnik S. Alginate encapsulation technology supports embryonic stem cells differentiation into insulin-producing cells. J Biotechnol. 2009;144:304–312. doi: 10.1016/j.jbiotec.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang G, Hou Y, Chen J, Wang J, Zou C, Li D, Li H, Zhang Q, Wang A, Fan Q. Transplantation of microencapsulated Schwann cells and mesenchymal stem cells augment angiogenesis and improve heart function. Mol Cell Biochem. 2012;366:139–147. doi: 10.1007/s11010-012-1291-1. [DOI] [PubMed] [Google Scholar]
- Weber C, Pohl S, Pörtner R, Wallrapp C, Kassem M, Czermak P. Cultivation and differentiation of encapsulated hMSC-TERT in a disposable small-scale syringe-like fixed bed reactor. Open Biomed Eng J. 2007;1:64–70. doi: 10.2174/1874120700701010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelehan M, Marison IW. Microencapsulation using vibrating technology. J Microencapsul. 2011;28:669–688. doi: 10.3109/02652048.2011.586068. [DOI] [PubMed] [Google Scholar]
- Willaert RG, Brüssel B. Gel entrapment and micro-encapsulation: Methods, applications and engineering principles. Rev Chem Eng. 1996:12. [Google Scholar]
- Wilson JT, Chaikof EL. Challenges and emerging technologies in the immunoisolation of cells and tissues. Adv Drug Deliv Rev. 2008;60:124–145. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. Induced pluripotent stem cells: Past, present, and future. Cell Stem Cell. 2012;10:678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Yao R, Zhang R, Luan J, Lin F. Alginate and alginate/gelatin microspheres for human adipose-derived stem cell encapsulation and differentiation. Biofabrication. 2012;4:025007. doi: 10.1088/1758-5082/4/2/025007. [DOI] [PubMed] [Google Scholar]
- Yeatts AB, Gordon CN, Fisher JP. Formation of an aggregated alginate construct in a tubular perfusion system. Tissue Eng C. 2011;17:1171–1178. doi: 10.1089/ten.tec.2011.0263. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu J, Du KT, Fang Q, Gu Y, Mihardja SS, Sievers RE, Wu JC, Lee RJ. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials. 2010;31:7012–7020. doi: 10.1016/j.biomaterials.2010.05.078. [DOI] [PubMed] [Google Scholar]
- Zhang X, He H, Yen C, Ho W, Lee LJ. A biodegradable, immuno-protective, dual nanoporous capsule for cell-based therapies. Biomaterials. 2008;29:4253–4259. doi: 10.1016/j.biomaterials.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yang G, Zhang A, Xu LX, He X. Preferential vitrification of water in small alginate microcapsules significantly augments cell cryo-preservation by vitrification. Biomed Microdevices. 2010;12:89–96. doi: 10.1007/s10544-009-9363-z. [DOI] [PubMed] [Google Scholar]
- Zhou H, Xu HHK. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials. 2011;32:7503–7513. doi: 10.1016/j.biomaterials.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman Y, Turgeman G, Pelled G, Xu N, Moutsatsos IK, Hortelano G, Gazit D. Polymer-encapsulated engineered adult mesenchymal stem cells secrete exogenously regulated rhBMP-2, and induce osteogenic and angiogenic tissue formation. Polym Adv Technol. 2002;13:863–870. [Google Scholar]
- Zimmermann H, Ehrhart F, Zimmermann D, Müller K, Katsen-Globa A, Behringer M, Feilen PJ, Gessner P, Zimmermann G, Shirley SG, Weber MM, Metze J, Zimmermann U. Hydrogel-based encapsulation of biological, functional tissue: Fundamentals, technologies and applications. Appl Phys A. 2007a;89:909–922. [Google Scholar]
- Zimmermann H, Shirley SG, Zimmermann U. Alginate-based encapsulation of cells: Past, present, and future. Curr Diabetes Rep. 2007b;7:314–320. doi: 10.1007/s11892-007-0051-1. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, Ugarte DAD, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]