Abstract
In general, human pathogen-related small circular deoxyribonucleic acid (DNA) molecules are bacterial plasmids and a group of viral genomes. Plasmids are extra-chromosomal small circular DNAs that are capable of replicating independently of the host, and are present throughout a variety of different microorganisms, most notably bacteria. While plasmids are not essential components of the host, they can impart an assortment of survival enhancing genes such as for fertility, drug resistance, and toxins. Furthermore, plasmids are of particular interest to molecular biology especially in relation to gene-cloning. Among viruses, genomes of anelloviruses, papillomaviruses, and polyomaviruses consist of small circular DNA. The latter two virus families are known for their potential roles in a number of pathogenic processes. Human papillomaviruses (HPV) are now widely recognised to be associated with a greatly increased risk of cervical cancer, especially oncogenic strains 16 and 18. On the other hand, human cells may contain several types of small circular DNA molecules including mitochondrial DNA (mtDNA). The mitochondrial genome consists of 37 genes that encode for proteins of the oxidation phosphorylation system, transfer ribonucleic acids (tRNAs), and ribosomal RNAs (rRNAs). Though mitochondria can replicate independently of the host; nuclear DNA does encode for several mitochondrial proteins. Mutations in mtDNA contribute to some well characterised diseases; mtDNA is also implicated in several diseases and malignancies with poorly elucidated aetiologies. Furthermore, mtDNA can function as a diagnostic tool. Other extra-chromosomal circular DNAs are usually detected in cancer. This review article is intended to provide an overview of four broad categories of small circular DNAs that are present in non-eukaryotic (plasmids and relevant viral genomes) and eukaryotic (mtDNA and other extra-chromosomal DNAs) systems with reference to human diseases, particularly cancer. For this purpose, a literature search has been carried out mainly from PubMed. Improved understanding of the significance of small circular DNA molecules is expected to have far reaching implications in many fields of medicine.
Keywords: episomes, cancer, HPV, mitochondrial DNA, plasmids, polyomavirus
Introduction
Our cellular environment can harbour several forms of small circular deoxyribonucleic acid (DNAs) such as in mitochondria, during viral infection, and gene therapy. The size distribution of small circular DNA complexes shows a heterogeneous characteristic and varies from 0.1 μm to more than 2 μm (1). Pathologically, multiple systemic, autoimmune, and malignant conditions have potential correlations with small circular DNAs. Viruses with small circular DNA genomes include single-stranded circular DNA viruses (like anelloviruses and circoviruses) as well as double-stranded circular DNA viruses (such as human polyomaviruses and papillomaviruses). In addition, bacterial plasmid DNA has been found to be associated with various mechanisms of drug resistance. This review paper will discuss some areas related to small circular DNAs in health and disease states with special reference to cancer as well as therapeutic approaches relevant to such DNA molecules.
Normally, mitochondrial DNA (mtDNA) molecules are present in the matrix of each mitochondrion and the number varies depending on the cellular metabolic activities. The mitochondrial genome comprises a circular, histone-free DNA, present in one or more copies in every mitochondrion (2). Due to its histone-free nature, mtDNA has limited repair ability. Furthermore, mitochondrial inheritance is cytoplasmic and usually maternal, thus the maintenance/evolution is asexual. It is believed that mitochondria originated from free-living eubacterium that were endosymbiotically taken up by a nucleus-containing eukaryotic cell more than a billion years ago. Interestingly, the mitochondrion matrix is the site for a number of enzymes and for production of reactive oxygen species (ROS). Therefore, the above mentioned factors such as maternal inheritance, poor repair mechanisms or aberrant release of ROS can cause mutations in mtDNA and different mitochondrial dysfunctions. Diseases like Kearns–Sayre syndrome, Leber hereditary optic neuropathy, and mitochondrial encephalomyopathies (like MELAS and MERRF) are a few important pathological conditions caused by defects in mtDNA.
A major health problem that we commonly encounter is associated with a group of small, circular, extra-chromosomal DNAs called plasmids, which can replicate independently of the host (3). The most frequent type of bacterial resistance to antibiotics is acquired and transmitted horizontally via the conjugation of a plasmid. Plasmids can confer a variety of survival enhancing genes to the host microorganisms including fertility, drug resistance, and toxins. Alternatively, plasmids can be used therapeutically in both DNA vaccines and gene therapy. DNA vaccines consist of a closed circular bacterial plasmid that encodes the antigen of interest under the control of a strong eukaryotic promoter (4). Following transfection, the plasmid DNA is taken up by host cells (e.g., muscle cells after intramuscular injection) and monocytes, which subsequently express the antigen. On the other hand, gene therapy is a novel design for treatment; and the main targets of this approach include monogenic diseases, cardiovascular disorders, and cancer. Gene carriers such as viral vectors or plasmids are used to administer the therapeutic genes into target tissues. However, unlike viral vector-related problems like toxicity and immunity, plasmid-based gene transfer is very safe - although its efficiency is relatively low (5). To overcome this problem, few enhancement techniques such as ultrasound with microbubbles or electroporation have been suggested to augment the plasmid DNA gene transfer efficiency.
Gene therapy is a promising direction for the treatment of cancer patients. Plasmids bearing genes that encode cytotoxic proteins, e.g., diphtheria toxin, Pseudomonas exotoxin A, streptococcal streptolysin, etc., can kill cancer cells and hinder tumour growth (6). Interestingly, plasmids encoding tumour-associated antigens can induce both humoral and cellular immune responses. Similarly, plasmids encoding cytokines could enhance immune responses to vaccination in infectious diseases and cancer (7). Overall, it is obvious that plasmids are associated with two conflicting aspects of therapeutic interests, i.e., drug resistance and DNA-based treatment.
In this review, four categories of small circular DNAs have been emphasised. At the outset, an overview of medical aspects of microbial small circular DNAs, e.g., bacterial plasmids, and genomes of human anelloviruses, polyomaviruses, and human papillomaviruses (HPV), are provided. Subsequently, this paper presents a brief description of mtDNA-the small circular DNA that exists most frequently and naturally in different human cells. Finally, other small circular DNAs and relevant health issues are mentioned.
Bacterial Plasmid DNA
Plasmids are autonomously replicating, double-stranded, extra-chromosomal DNA molecules, which are prevalent in several kingdoms of life such as bacteria, archaea, and fungi; most bacterial plasmids investigated so far are circular. Plasmids serve a central role in mechanisms of bacterial antibiotic resistance (Table 1) (8–14) that include; target protection, target substitution, antibiotic detoxification, and blockage of intracellular antibiotic accumulation. A recent report on trends in antibiotic resistance in intensive care units (ICUs) across Europe showed a continent-wide emergence of infections caused by multi-resistant Gram-negative bacteria/Enterobacteriaceae, especially Escherichia coli (E. coli) and Klebsiella pneumoniae, with easily exchangeable resistance genes located on plasmids producing enzymes such as extended spectrum β-lactamases and carbapenamases (15). Of note, there are more than 890 plasmid-encoded β-lactamases among Gram-negative bacteria, the most important are: AmpC cephalosporinases (encode AmpC enzymes, confer resistance against penicillins, cephamycins, and oxyimino-β-cephalosporins), extended-spectrum β-lactamases (i.e. CTX-M enzymes that hydrolyse monobactams and cephalosporins), and carbapenemases (i.e. metallo-β-lactamases, seen in organisms with other β-lactamases, conferring resistance to all β-lactams except aztreonam) (16). Moreover, recent evidence shows a widespread resistance of the Enterobacteriaceae to quinolone antibacterial drugs like nalidixic acid and ciprofloxacin. Since the first detection of resistance in 1998, three kinds of plasmid-mediated quinolone resistance (PMQR) determinants have been identified: qnr genes, aac(6’)-Ib-cr (inactivation of ciprofloxacin), and qepA (inactivation of ciprofloxacin and norfloxacin) (17).
Table 1.
Few important multidrug resistance plasmids and their biological features
| Natural plasmids | Present in bacteria | Resistance to antibiotics | Brief description of genetic and biochemical nature |
|---|---|---|---|
| pIP501 | Escherichia coli, Streptococcus pneumoniae, and others | MLS group of antibiotics like macrolides (erythromycin), chloramphenicol | A 30 kb plasmid originated from Streptococcus agalactiae with the broadest known host range amongst Gram-positive bacteria; the first conjugative plasmid originating from Gram-positive bacteria that can stably replicate in Gram-negative bacteria. Mechanisms: transmethylase (erm) gene encodes for an RNA methylase, resulting in a reduced affinity between the antibiotics and ribosome, leading to the resistance |
| pIP816 | Enterococcus faecium | vancomycin, teicoplanin | The first VanA-type plasmid (34 kb) reported; harbours transposon Tn1546, encoding for VanA, a ligase catalysing peptide and ester bond formation. In addition, vanH gene that is located upstream of VanA encodes for vanH dehydrogenase, leading to synthesis of D-2-hydroxybutyrate, a preferred substrate for VanA. Finally, the affinity of the antibiotics decreases by over 1,000 fold |
| pAM830 | Staphylococcus aureus | vancomycin, erythromycin | The prototypical plasmid (45 kb) of transferring Tn1546 to methicillin-resistant strains of S. aureus (MRSA) |
| NR1 (R100 or 222) | Shigella flexneri | tetracycline, chloramphenicol, streptomycin, spectinomycin, sulfonamide | This 94.5 kb plasmid includes several genes. Tn10 encodes for TetA, a tetracycline/metal-proton antiporter in the cytoplasmic membrane. Tn21 has genes for streptomycin, spectinomycin (str/spc) and sulfonamide (sulf) resistance. A catA1 gene encodes for a chloramphenicol acetyltransferase, which binds to and sequesters chloramphenicol |
| pSK41 | Staphylococcus aureus | aminoglycosides, bleomycin, disinfectants | A 46.4 kb conjugative plasmid that has been implicated in the development of a vancomycinresistant S. aureus strain. The gene aacA-aphD confers resistance against gentamicin, tobramycin and kanamycin and is located on a Tn4001-IS257 hybrid structure. The small plasmid pUB110 integrates its genes aacA-aphD and aacA-aphD, conferring resistance to 4’-hydroxyl containing neomycin and bleomycin respectively |
| R1033 | Pseudomonas aeruginosa | aminoglycosides, chloramphenicol, sulphonamide, penicillin, tetracycline | R1033 (molecular weight 45 × 106) encodes for neomycin phosphotransferase 1 and gentamicin acetyltransferase 1 resulting in resistance to gentamicin, kanamycin, and sisomicin. This plasmid determines the production of a TEM β-lactamase; and contains TN1696 associated with the mercury resistance |
| pIP1202 | Yersinia pestis | streptomycin, tetracycline, chloramphenicol, sulfonamides, trimethoprim, ampicillin, spectinomycin | This 182913 bp plasmid has a blaSHV-1 gene that mediates the hydrolysis of the beta-lactam ring conferring resistance to beta-lactam antibiotics. The genes strAB, aadA, and aphA encode for aminoglycosides resistance; pIP1202 also contains tetRAclass D, cat, qacEdelta1, sul1 and sul2 genes |
Bacterial plasmids are responsible for various toxin productions. Plasmids in several E. coli pathotypes, e.g., enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), and enterohemorrhagic E. coli (EHEC), encode toxins. For instance, plasmids of EAEC strains, which cause travelers' diarrhea, encode toxins such as the plasmid-encoded toxins (Pet) and EAST1 (18). Pet, a serine protease, disrupts the organisation of the actin cytoskeleton, which finally results in cell detachment from the substratum; and EAST1 activates guanylate cyclase causing secretion of ions. Similarly, Bacillus anthracis, the causative agent for anthrax, has two large plasmids that are essential for toxicity: pXO1, which contains the toxin genes, and pXO2, which encodes proteins required for capsule synthesis (19). The potent neurotoxin and several associated components of Clostridium botulinum, responsible for the serious paralytic illness botulism, are often plasmid-borne (20).
The bacterial core genome is interspersed with groups of acquired genes-mostly originated from integration events of mobile genetic elements, such as insertion sequences, transposons, phages, and plasmids. This horizontal gene transfer is thought to be associated with bacterial toxin– antitoxin (TA) systems that probably favour the maintenance of plasmids or such genetic elements by post-segregational killing of the cell that loses these genes (21). Five types of TA systems have been proposed thus far, and under normal growth conditions the activity of the toxin protein or its translation is counteracted by an antitoxin protein or non-coding RNA (22). Recent interest in TA systems has increased dramatically, as the exploitation of TA systems as a potential strategy against drug-resistant bacteria might be feasible via artificial activation of the toxin (23).
Viruses with Small Circular DNA Genomes
Anelloviridae
The anelloviridae have been suggested as causative and exacerbating factors in a number of pathological processes in different species. The human anelloviruses vary in genome size ranging from 3.8–3.9 kb for Torque Teno Virus (TTV), an intermediate 3.2 kb for Torque Tenolike Midi Virus (TTMDV), and a smaller 2.8–2.9 kb for Torque Teno-like Mini Virus (TTMV). In 1997, TTV was first isolated from a patient with post-transfusion hepatitis of unknown aetiology (24). However, the real impact of TTV on liver diseases remains uncertain to date (25). Similarly, anelloviruses/TTV have been epidemiologically suggested to be associated with various pathologies including respiratory disorders, haematological diseases, lupus, cancer, and even fever, though there is no substantial evidence for correlations between the viral agents and specific clinical entities (26–28). Nevertheless, several characteristics of anelloviruses/TTV are highly debatable due to their high prevalence in various populations, extreme genetic diversity, poorly understood natural history, as well as implication in human health (29).
Polyomaviridae
Viruses in this family are non-enveloped and have small, circular, double-stranded, supercoiled DNA genomes of approximately 5 kb, measuring 40.5–44 nm in diameter (30). Polyomavirus is able to induce tumours in its natural host as well as transform cells in cultures. The initial findings on polyomaviruses, i.e., mouse polyomavirus (mPyV) and simian virus 40 (SV40), have provided many insights into the signalling of cell transformation and tumourigenesis (31). Viruses that are mainly associated with human diseases include BK virus (BKV), John Cunningham virus (JC virus/JCV), and Merkel cell polyomavirus (MCPyV). Generally, persistent and asymptomatic presence of BKV and JCV occurs from early childhood; the viruses remain latent in healthy individuals, but can be reactivated in immunocompromised individuals. JCV causes progressive multifocal leukoencephalopathy (PML) in cases of severe immunodeficiency, whereas BKV causes nephropathy in patients who have undergone renal transplantation (30). Furthermore, oncogenic potentials of both BKV and JCV have been observed in animal models; however, their neoplastic role in humans remains unclear. Few reports have suggested an association between BKV infection and the development of renal and bladder cancers in renal transplant recipients (32). Similarly, some researchers have documented an association of JCV with human cancers including brain tumours, colorectal cancers, and cancers of the gastrointestinal tract (33). In regards to this connection, a brief account of BKV- and JCVassociated pathologies has been presented in Table 2 (32–44). In immunosuppressed conditions, the involvement of these two polyomaviruses in different diseases, particularly the role of JCV in PML, is an active area of research that might lead to new insights into immune function and host antiviral defense, as well as to potential new therapies (45).
Table 2.
Recent reports on BK virus and JC virus associated pathologies in humans and animal models
| Investigators | Study/Clinical setup | Patients’ information | Disease processes |
|---|---|---|---|
| Saundh et al. 2013 (34) | The study was conducted in Leeds, UK., from 2006 to 2009 | BKV-positive renal transplant recipients: n = 37; age – 48.4 ± 14.3; M – 26, F – 11. BKV-negative renal transplant recipients: n = 75; age – 46.1 ± 14.3; M – 61, F – 14 | 37 were positive for BKV viruria, of whom 12 developed BKV viremia, and 2 progressed to BKV-associated nephropathy |
| Neirynck et al. 2012 (32) | Comprehensive overview of the literature | M: n = 7, age – 35.6 ± 21.7; and F: n = 3, age – 45.0 ± 10.4 | BKV-associated renal and urothelial carcinoma in renal allograft patients |
| Chung et al. 2012 (35) | Renal transplant recipients in Seoul, Korea, from 2001 to 2008. The study had two parts – retrospective and prospective cohorts | Retrospective cohort: n = 376; age – 43.5 ± 16.5; M – 205 (54.5%). Prospective cohort: n = 145; age – 44.9 ± 10.7; M – 78 (53.8%) | BKV-associated nephropathy developed in 3.7% of patients (14/376) in the retrospective cohort; of the 14 cases, allograft failure developed in 28.6% (4/14). The rate of BKV-associated nephropathy in the prospective cohort was only 1 (0.6% vs. 3.7% of retrospective cohort) |
| Mateen et al. 2012 (36) | Multicentric study – data from the United States and Netherlands, from 1988 to 2008 | Cases of progressive multifocal leukoencephalopathy (PML) among transplant recipients: n = 69; M – 40, age – 47.5 ± 14.4; and F – 29, age – 44.7 ± 11.8 | 44 solid organ and 25 bone marrow cases of post-transplantation PML were found |
| Haines et al. 2011 (37) | Performed during 2007 to 2010 at Cincinnati Children’s Hospital, Ohio, US | Hematopoietic stem cell transplantation (HSCT) cases: n = 314 | 35 HSCT patients (11%) had documented haemorrhagic cystitis. Urine was tested for BKV in 33 of these 35 patients, and was positive in 30 (91%) |
| Geetha et al. 2011 (38) | Adult patients with repeat transplantation after previous loss of allograft due to BKVassociated nephropathy from six US centres | 31 patients underwent retransplantation after a median of 6 months after failure of the first allograft. The mean age at transplantation was 47 ± 17 years; M – 20 and F – 11 | After repeat transplant, 11 (35%) had BKV replication in urine and plasma with two patients experiencing BKVassociated nephropathy. Seven had acute rejection |
| Huang et al. 2010 (39) | Conducted in China from 2006 to 2007 | Renal transplant recipients: n = 90; M – 66 and F – 24; Age – 39.0 ± 11.8 Overall, BKV+ cases were 41 (age ― 36.6 ± 11.9; M – 33 and F – 8) and BKV(-) cases were 49 (age ― 41.0 ± 11.5; M – 33 and F – 16) | By one post-transplant year, urinary decoy cells, BKV viruria, BKV viremia, and BKV-associated nephropathy occurred in 42.2% (n = 38), 45.6% (n = 41), 22.2% (n = 20), and 5.6% (n = 5) of patients, respectively |
| Maginnis and Atwood 2009 (33) | Reviewed reports postulated that JCV infection is linked with various cancers | Human cancers including gastrointestinal cancers, brain cancers, and lung cancer | Experimental studies on small rodents and non-human primates confirmed that JCV can induce tumour formation |
| Dharnidharka et al. 2009 (40) | Kidney transplant recipients from the US Organ Procurement Transplant Network database, during 2003 to 2006 | Transplant recipients: n = 48,292; BKV infection: n = 1474; M – 1025, F – 449; age up to 17: 126, 18–34: 186, 35–55: 577, more than 55: 585 | The study determined the rates of BKV infection within 24 months posttransplant time; 1474 transplants reported this problem |
| Smith et al. 2007 (41) | North American Pediatric Renal Trials and Collaborative Studies database, between 2000 and 2004 | Paediatric renal transplant recipients: n = 542; BKVassociated nephropathy: n = 25 (4.6%); M – 17; F – 8 | Among cases who received a transplant, BKV-associated nephropathy was reported in 25 patients (within first year: 1, between 2-5 yrs: 5, 6-12 yrs: 7, ≥ 13 yrs: 12) |
| Shitrit et al. 2005 (42) | PML in transplant recipients | Cases: n = 24 (renal-9, bone marrow-6, liver-4, heart-3, and lung-2); M – 13, age – 43.2 ± 12.8; F – 11, age – 46.1 ± 13.2 | Lesions usually occurred within 17 months of transplantation, with a tendency for a later onset in renal compared with other transplant recipients |
| Schmid et al. 2005 (43) | 15 European transplant centres; renal biopsies were analysed for presence of BKV and JCV | Transplant biopsies: n = 302; Controls (kidney biopsies like different glomerulopathies): n = 60; BKV DNA + cases: n = 8 (M – 5, age – 42.6 ± 11.6; F – 3, age – 62.0 ± 7.8) | BKV DNA was detected in 8 of the 302 (2.6%) biopsies obtained for transplant dysfunction, but in none of the controls |
| Hurault de Ligny et al. 2000 (44) | 6 French transplantation centres from 1996 to 1999 | Kidney transplants: n = 647; BKV + cases: n = 9, and JCV +: n = 1 | 10 were diagnosed as having polyomavirus-induced acute tubulointerstitial nephritis (1.5%) |
BKV: BK virus, JCV: JC virus, M: male, F: female, n: number of patients, age: in years.
Recently, MCPyV has been implicated in 80% of cases of Merkel cell carcinoma, which is a rare and potentially aggressive neuroendocrine skin cancer affecting older white people and younger immunosuppressed individuals (46,47). Infection with MCPyV is prevalent in the human population and the virus is detected in various tissues, most commonly in the skin. A majority of tumours contain clonally integrated viral DNA, express viral T antigen transcripts and protein, as well as exhibit an addiction to the viral large T and small T antigen oncoproteins (48). Although it is commonly believed that Merkel cell carcinoma arises from Merkel cells located in the basal layer of the epidermis, the question of the cell of origin in tumour formation remains unresolved (49). A precise knowledge in this field could be helpful to develop more specific therapies that target the particular cells.
Papillomaviridae
Human papillomavirus (HPV) genome consists of small, circular, double-stranded DNA and is approximately 8 kb in length; this non-enveloped virus has an icosahedral capsid of about 55 nm in size (50). The viral genome encodes six early proteins (E1, E2, E4, E5, E6, and E7) which play roles in virus replication and cell transformation, and two late (L1 and L2) proteins that form the capsid. More than 100 HPV types have been identified and they are broadly divided into low-risk and high-risk types according to their aetiological role in cutaneous/mucosal benign and malignant lesions, respectively. In general, the low-risk HPV6 and HPV11 provoke genital warts, while the high-risk HPV16 and HPV18 can cause cancers (51). Persistent high-risk HPV infections are associated with a risk for developing cancer of the uterine cervix (cervical cancer) (52–54). Furthermore, HPV has been implicated in nongenital cancers, e.g., a subgroup of head and neck cancers, particularly squamous cell cancer of the oropharynx and anal cancer (54,55).
Cervical cancer is a leading female cancer; women of lower socioeconomic status are mainly affected by this disease (56). Reports show that the two most common high-risk types- HPV 16 and 18 are involved in 70% or more of cases of cervical cancers worldwide (52,53,57). The E6 and E7 gene products of HPV are thought to play oncogenic roles: E6 promotes the degradation of tumour suppressor p53, whereas E7 inactivates the retinoblastoma protein (pRB), another important tumour suppressor (52).
Considering the oncogenic role of HPV, it is rational to believe that prevention of infection through vaccination could hinder the pathological processes of the cervical pre-cancerous stage and invasive cancer. Currently, two prophylactic HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18, and a bivalent vaccine against types 16 and 18. Both vaccines contain virus-like particles (VLPs) produced by self-assembly of recombinant major capsid protein L1 derived from each of the concerned HPV types (58,59). VLPs are empty viral capsids that contain no viral DNA inside and thus are non-infectious, which can induce type specific humoral immune response in hosts.
There are several concerns surrounding the current HPV vaccines such as expense, type restriction, and the requirement of proper refrigeration: many of these issues are major obstacles for developing countries where incidence of cervical cancer is high. To overcome these limitations, efforts are underway to develop the second-generation vaccines like bacterial vectors for HPV L1 capsid protein delivery and the use of HPV L2 capsid protein (60). Implementation of current HPV vaccines decreases the risk of infection, but the effect on rate of cervical cancer has yet to be confirmed in long-term prospective clinical and epidemiological studies (56).
Mitochondrial Genome
Mitochondrial dysfunction can be secondary to mutations in nuclear DNA (nDNA) that can be inherited autosomally or primarily by defects of mtDNA that are maternally inherited; however, both types of mutations can occur sporadically. Since mitochondrial proteins are predominantly coded by nDNA, mutations in nDNA are a major cause of mitochondrial dysfunction (Table 3) (61–66). These mutations can alter the structure of the mitochondria and impair mitochondrial function as well as cause depletion of mtDNA since nDNA codes for proteins involved in replication, transcription, translation, and maintenance of mtDNA. Diseases caused by nDNA mutations that code for mitochondrial proteins include neoplastic, neuropathic, degenerative, metabolic, and other systemic disorders (which will not be discussed here).
Table 3.
Common mitochondrial diseases, their origins and clinical features
| Diseases | Clinical features in brief | |
|---|---|---|
| Origin from mtDNA | Kearns-Sayre syndrome (KSS) | The most common disease caused by large-scale mtDNA deletions, with an incidence of 1 to 3 per 1000 00. Presents with a triad of: onset before age 20, pigmentary retinopathy, and chronic progressive ophthalmoplegia. In addition to one of the following: cardiac conduction block, cerebellar ataxia, and/or a cerebrospinal fluid protein greater than 100 mg/dL. Muscle biopsy displaying ‘ragged red fibers’ (RRFs) is diagnostic |
| Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) | Exact incidence is unknown. Childhood onset of intermittent hemicranial headache, vomiting and proximal limb weakness. With pathognomonic focal brain lesions, which are frequently localised to the parieto-occipital lobes leading to stroke-like episodes. These atypical strokes occur in patients less than 40 years of age and do not generally follow large vessel distribution. The most prominent biochemical finding is complex I dysfunction of the respiratory chain complexes in the mitochondria | |
| Myoclonic epilepsy with ragged-red fibres (MERRF) | Incidence unknown. Defined by the clinical triad of: myoclonus, epilepsy, and ataxia; along with numerous RRFs on muscle biopsy. Can also manifest with muscle weakness, muscle wasting, encephalomyopathy, deafness, dementia, and cerebellar ataxia. Most commonly accompanied by complex IV deficiency | |
| Leber's hereditary optic neuropathy (LHON) | Incidence of approximately 1 in 300 00 to 500 00. Pathognomonic features are axonal loss in the papillomacular bundle, leading to temporal atrophy of the optic disc, culminating in rapid, painless unilateral central vision loss, usually followed by the other eye within days/weeks. The accompanied ocular fundus changes consist of: circumpapillary telangiectatic microangiopathy with tortuous peripapillary arterioles, pseudoedema of the nerve fiber layer around a hyperaemic optic disc, no leakage on fluorescein angiography. No RRFs on biopsy. Biochemically, complex I functions are affected | |
| Origin from nDNA | Isolated Complex I deficiency (Mitochondrial complex I deficiency) | This autosomal recessive disorder is due to disturbance of the oxidative phosphorylation system, comprising more than 30% of patients. A child presents with Leigh disease, an early-onset neurodegenerative disorder of symmetrical necrotic lesions and capillary proliferation in the central nervous system, which is fatal. The main clinical manifestations are trunk hypotonia, encephalopathy, failure to thrive, cardiomyopathy and liver failure |
| Mitochondrial neurogastro-intestinal encephalomyopathy (MNGIE) | A rare, autosomal recessive disorder with mutations in the gene encoding for thymidine phosphorylase (TP). A toxic build up of thymidine and deoxyuridine occurs, which alters the deoxynucleoside triphosphate (dNTP) pool. Disruption of the intramitochondrial dNTP pool leads to mtDNA deletions, depletion and increases mtDNA mutagenesis. Juvenile onset of severe gastrointestinal dysmotility is pathognomonic, leads to cachexia and death by 37 years of age. Other features are progressive external ophthalmoplegia, ptosis, peripheral neuropathy, myopathy and leukoencephalopathy (diffuse on MRI). Evidence of histologically dysfunctional mitochondria is present | |
| Autosomal-dominant external ophthalmoplegia (adPEO) | Incidence estimated to be 1:1000 00. Early adulthood onset of painless bilateral ptosis, with the cardinal feature of progressive weakness of the extraocular muscles. May progress to paresis in all extraocular muscles resulting in complete ophthalmoplegia. RRFs are observed in skeletal muscle and a mild reduction in respiratory chain enzyme activities may be present | |
| Alpers syndrome (Infantile hepatocerebral syndrome) | Incidence of approximately 1 in 1000 00. Autosomal recessive mtDNA depletion disorder. Manifests from birth to 3 years of age with: progressive spastic quadriparesis, progressive cerebral degeneration leading to mental deterioration and seizures, and liver failure. Severe developmental delay and cortical blinding are seen in older patients. Children and young adults die of fatal brain and liver disease |
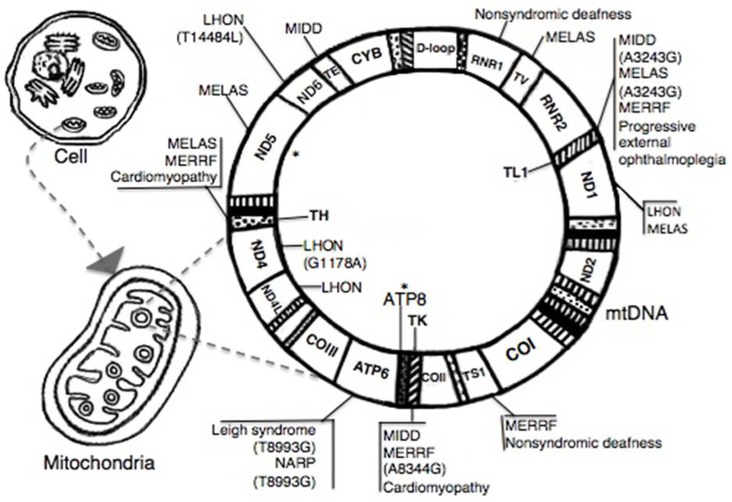
Mitochondria are the main producers of superoxide ion formation in the body, which results in higher oxidation rates of mtDNA compared to nDNA. Furthermore, mitochondria lack histone proteins to protect mtDNA and have a poor repair system to correct for oxidative damage. It is not surprising that mtDNA has a higher incidence of harmful alterations than nDNA (67,68) and therefore they are more susceptible to somatic mutations resulting in diseases. Many diseases have been reported to be linked to mtDNA alterations including a number of well established pathologies (Figure 1) as well as some other diseases of special interest, such as neurodegenerative disorders like Parkinson's disease and Alzheimer's disease, diabetes, and cancer (69,70). In regards to this connection, two leading cancer pathologies, i.e., breast and prostate carcinomas, have been discussed below.
Figure 1.
Some well established illnesses related to mtDNA alterations MELAS = mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes; MIDD = maternally inherited diabetes and deafness; MERRF = myoclonic epilepsy with ragged red fibres; LHON = Leber hereditary optic neuropathy; NARP = neuropathy, ataxia, and retinitis pigmentosa; * = m8470_13446del4977 (Kearns-Sayre syndrome).
Breast cancer: Carcinoma of the breast has been shown to be associated with different alterations of mtDNA including D-loop and the copy number of mtDNA (71). According to a recently conducted study, reduced mtDNA was found in 82% of breast cancer tissues and was associated with hormone receptor status, so it may prove useful to determine the prognosis of breast cancer (72). Similarly, breast cancer patients with D-loop mutations had significantly poorer disease free survival (71). Furthermore, breast tissue surrounding some samples of ductal carcinoma in-situ and invasive carcinoma were found to have similar mutations in the D310 region (within the D-loop) as the tumour part and could represent a potential marker for clonal expansion of premalignant breast cancer cells (73). It may be worthwhile to mention that new primary tumours from true recurrences are associated with poorer prognoses and often require more aggressive treatment. For determining cell lineages (clonality) of primary and secondary breast tumours, one group of investigators examined gene alterations of the mtDNA D-loop region and found it to be more precise than the conventional X-chromosomelinked human androgen receptor analysis method (74). Interestingly, it has been demonstrated that hormone-resistant breast cancer cells derived from MCF-7 cell-line showed depletion of mtDNA and can become sensitive again after inserting mtDNA into the cells (75). Currently, resistance to hormonal therapy poses a serious challenge in clinical practice; a clear understanding of the pathological processes would be of great benefit to help formulate new therapeutic strategies (76).
Prostate cancer: A recent report on prostate cancer samples has revealed a greatly elevated mutation rate in the mitochondrial genome compared to the autosomal chromosomes (77). Prostate cancer cells have shown alterations in various regions of mtDNA; common mutations are within the D-loop (52%), RNR2 (14%), and ND4 (13%) regions (78). There may be racial discrepancy with respect to mtDNA mutations, particularly in the region of cytochrome c oxidase subunit 1 (COI) between Caucasian and African American prostate cancer patients (79,80). Nevertheless, it has been demonstrated that several prostate carcinoma cell lines displayed depletion of mtDNA and significantly decreased COI; mutations in mtDNA might confer survival and migratory advantage to prostate cancer cells (81). Another study, which analysed mtDNA mutations in prostate cancer tissue, found that the presence of somatic mutations in transfer RNAs was associated with elevated prostatespecific antigen (PSA) levels (82). To overcome the unpredictable diagnostic procedures of prostate cancer, such as blood PSA-related ambiguity, it has been shown that the neoplastic process was significantly associated with a 3.4 kb mitochondrial genome deletion, which occurred in regions like ND4, ND4L, and ND5 (83).
Mutations in the mitochondrial genome have been reported in cancers of various sites. Although it is debatable whether the mutations are causative or reactive to the pathology, an increased production of ROS and resultant oxidative stress may play an important role in the disease course. Higher levels of oxidative stress in the tumour environment could provide or accelerate the aggressive behaviour of cancers. More research needs to be done with respect to the relationship between specific mtDNA alterations and different onco-pathological conditions.
Extra-Chromosomal Elements in Human Cells
Both prokaryotic and eukaryotic cells may harbour several forms of extra-chromosomal DNA such as transposons, integrons, and episomes. In humans, apart from mitochondrial DNA, other extra-chromosomal elements can be present in every category of cells. These are small circular DNA structures and are physically separated from chromosomes. Some of the clinically important extra-chromosomal elements are episomes, small polydispersed circular DNA (spcDNA), double minute chromosomes (dmin or DM), and small, circular DNAs generated during B- and T-cell development (84). Although the presence of extra-chromosomal elements is ubiquitous, generally their occurrence has been suggested to be connected with genetic instability and related phenomena like aging and cancer.
Physiologically, small circular DNA molecules are evolved as a byproduct during assembly among variable (V), diversity (D), and joining (J) DNA segments in immunocompetent B- and T-cells (85,86). This V(D)J recombination is dependent on the recombination activating rag genes; and the generated circular DNAs are called B- or T-cell receptor excision circles (84,87). Interestingly, evaluation of T-cell receptor excision circles (TRECs), which signify the status of newly formed T-cells, may indicate disease conditions associated with thymic dysfunction like severe combined immunodeficiency (88,89). Furthermore, TREC quantification might be helpful to evaluate the impact of hormones and endocrine disorders upon thymic function (90).
Two types of micronuclei may exist in cells. The chromosome-type micronuclei are formed from chromosomal material. Their presence reflects the damage of genomic materials or abnormal mitosis. Therefore, these micronuclei may be used as a biomarker for the genotoxic stress induced by drugs or environmental influences (91). Different agents such as clastogens like mitomycin C, cyclophosphamide, or γ-irradiation as well as spindle poisons like paclitaxel and vinblastine can produce the chromosome-type micronuclei. On the other hand another type of micronuclei, the double minutes (DMs), are formed from extra-chromosomal elements. DMs are small, paired chromatin bodies that lack a centromere and represent a form of extrachromosomal gene amplification (92). Although they have been found in a variety of solid tumours, their presence in haematological malignancies is rare.
DMs have been suggested to be one of the principal genetic structures on which specific oncogenes are located and DM-mediated oncogene amplification or over-expression contributes to their carcinogenic role (93). Analyses of DMs have shown involvement of various genes such as c-myc, mdm2, and EGFR (94–96), which are intimately connected to neoplastic processes (97,98). However, c-myc has been observed to be the most frequently amplified gene in DMs. It is worth noting that over-expression of c-myc in tumours is associated with a poor prognosis. In addition, c-myc–dependent generation of extrachromosomal elements also has been described (99).
In general, the broad category of extrachromosomal elements includes DMs and episomes (100). Episomes are often considered precursors of DMs (101). Nevertheless, episomes predominantly carry extra-chromosomal amplification of oncogenes (102) or drug resistance genes (103). In contrast, smaller circular molecules that carry repetitive DNA sequences are called spcDNA (104). Although the function of spcDNA is currently unclear, it is present in all investigated human cells yet the amount is higher in cancer cells (105).
Extra-chromosomal small circular DNA molecules indisputably confer plasticity to genetic information that was initially thought to be relatively rigid. A precise knowledge of the formation and function of these molecules is definitely helpful in understanding the cellular mechanisms both in physiological and pathological conditions. Moreover, many studies observed that the reduction in the number of DMs in cancer cells resulted in the attenuation of the pathological processes (91). Consequently, removal of DMs from malignant cells may render a favourable situation in the management of different cancers.
Conclusion
Genomes are widely diverse with variations intertwined in cellular processes of both physiological and pathological conditions. Although there is current scientific interest in this field, the complex structures and mechanisms of extra-chromosomal DNAs are not completely understood. For instance, the mitochondrion is postulated as the evolutionary descendant of endosymbiotic eubacterium, and its genome, i.e., mtDNA, is the most common physiological small circular DNA in human cells. It has been conserved for more than half a billion years and is easily affected in different pathologies (106). Apart from well-established diseases, mtDNA has a perplexing association with numerous situations such as: endocrine properties of adipose tissue, renal function, intrauterine growth retardation, diabetes, cancer, etc. Characteristics of mtDNA are helpful in anthropology, like studies of human dispersal and forensic genetics; alterations could be useful in the diagnosis of some pathological conditions. Furthermore, another interesting small circular DNA molecule is TTV-genome; the viruses are ubiquitous in the majority of adults worldwide without any established pathogenicity, resembling commensal organisms. Similar to mtDNA, a precise understanding of TTVs could lead to the exploitation of these single stranded circular DNA molecules as potential tools such as in forensic medicine. Generally, small circular DNA viruses stay in the healthy body as non-infectious agents due to the homeostasis of immune mechanisms. Ineffective immunity may play a major role in clinical manifestations of both polyomavirus and HPV infections. Therefore, the prevalence of cervical cancer among women with lower socioeconomic status probably denotes a defective immune system, due to under-nutrition and poor hygienic conditions. Currently, expensive HPV vaccines are unfeasible for the underprivileged; logically HPV-related carcinogenesis is expected to maintain a similar trend in developing countries, impeded from a beneficial impact (107). Plasmid DNA encoding tumour antigens could be an alternative affordable immunization method. Overall, an improved knowledge of extra-chromosomal DNAs in a tumour microenvironment might allow for the rational design of molecules to block the pathological processes.
Acknowledgments
We are grateful to Dr Claude-Bernard lliou, Professor and Dean, Saint James School of Medicine (SJSM), Anguilla, for his constant supports. In addition, we would like to thank Monica Ficek, Kirolos Iskander, Azim Hemani, and Paul Nosa-Ovoasu, who were members of a Research in Health and Medicine (RHM3) group at SJSM, which is where we first delved into our research.
Footnotes
Conflict of interest
None.
Funds
No financial supports were received.
Authors’ Contributions
Conception and design, collection and assembly of data, final approval of the article: AR
Analysis and interpretation of the data, critical revision of the article for the important intellectual content: AR, SB
Drafting of the article: SB, AR, MU
Administrative, technical or logistic support: SB
References
- 1.Kunisada T, Yamagishi H, Sekiguchi T. Intracellular location of small circular DNA complexes in mammalian cell lines. Plasmid. 1983;10(3):242–250. doi: 10.1016/0147-619x(83)90038-0. doi: 10.1016/0147-619X(83)90038-0 . [DOI] [PubMed] [Google Scholar]
- 2.Jansen RP. Origin and persistence of the mitochondrial genome. Hum Reprod. 2000;15(Suppl 2):1–10. doi: 10.1093/humrep/15.suppl_2.1. doi: 10.1093/humrep/15.suppl_2.1 . [DOI] [PubMed] [Google Scholar]
- 3.Ray A. Varanasi (India): Banaras Hindu University; 1990. Analysis of plasmid DNA in enteric bacteria with reference to drugs resistant markers [M.D.- thesis] [Google Scholar]
- 4.Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines. 2012;11(2):189–209. doi: 10.1586/erv.11.188. doi: 10.1586/erv.11.188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniyama Y, Azuma J, Kunugiza Y, Iekushi K, Rakugi H, Morishita R. Therapeutic option of plasmid-DNA based gene transfer. Curr Top Med Chem. 2012;12(15):1630–1637. doi: 10.2174/156802612803531342. doi: 10.2174/156802612803531342 . [DOI] [PubMed] [Google Scholar]
- 6.Glinka EM. Eukaryotic expression vectors bearing genes encoding cytotoxic proteins for cancer gene therapy. Plasmid. 2012;68(2):69–85. doi: 10.1016/j.plasmid.2012.05.003. doi: 10.1016/j.plasmid.2012.05.003 . [DOI] [PubMed] [Google Scholar]
- 7.Ferrone CR, Perales MA, Goldberg SM, Somberg CJ, Hirschhorn-Cymerman D, Gregor PD, et al. Adjuvanticity of plasmid DNA encoding cytokines fused to immunoglobulin Fc domains. Clin Cancer Res. 2006;12(18):5511–5519. doi: 10.1158/1078-0432.CCR-06-0979. doi: 10.1158/1078-0432.ccr-06-0979 . [DOI] [PubMed] [Google Scholar]
- 8.Schaberg DR, Zervos MJ. Intergeneric and interspecies gene exchange in gram-positive cocci. Antimicrob Agents Chemother. 1986;30(6):817–822. doi: 10.1128/aac.30.6.817. doi: 10.1128/aac.30.6.817 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goessweiner-Mohr N, Grumet L, Pavkov-Keller T, Birner-Gruenberger R, Grohmann E, Keller W. Crystallization and preliminary structure determination of the transfer protein TraM from the Gram-positive conjugative plasmid pIP501. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013;69(Pt 2):178–183. doi: 10.1107/S1744309113000134. doi: 10.1107/s1744309113000134 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arthur M, Molinas C, Courvalin P. The VanSVanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1992;174(8):2582–2591. doi: 10.1128/jb.174.8.2582-2591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Womble DD, Rownd RH. Genetic and physical map of plasmid NR1: comparison with other IncFII antibiotic resistance plasmids. Microbiol Rev. 1988;52(4):433–451. doi: 10.1128/mr.52.4.433-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg T, Firth N, Apisiridej S, Hettiaratchi A, Leelaporn A, Skurray RA. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J Bacteriol. 1998;180(17):4350–4359. doi: 10.1128/jb.180.17.4350-4359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DI, Lus RG, Rubio Calvo MC, Datta N, Jacob AE, Hedges RW. Third type of plasmid conferring gentamicin resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1975;8(3):227–230. doi: 10.1128/aac.8.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welch TJ, Fricke WF, McDermott PF, White DG, Rosso ML, Rasko DA, et al. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One. 2007;2(3):e309. doi: 10.1371/journal.pone.0000309. doi: 10.1371/journal.pone.0000309 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Duijn PJ, Dautzenberg MJ, Oostdijk EA. Recent trends in antibiotic resistance in European ICUs. Curr Opin Crit Care. 2011;17(6):658–665. doi: 10.1097/MCC.0b013e32834c9d87. doi: 10.1097/mcc.0b013e32834c9d87 . [DOI] [PubMed] [Google Scholar]
- 16.Bush K. Bench-to-bedside review: The role of β-lactamases in antibiotic-resistant gram-negative infections. Crit Care. 2010;14(3):224. doi: 10.1186/cc8892. doi: 10.1186/cc8892 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev. 2009;22(4):664–689. doi: 10.1128/CMR.00016-09. doi: 10.1128/cmr.00016-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson TJ, Nolan LK. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol Mol Biol Rev. 2009;73(4):750–774. doi: 10.1128/MMBR.00015-09. doi: 10.1128/mmbr.00015-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolstø AB, Tourasse NJ, Økstad OA. What sets Bacillus anthracis apart from other Bacillus species? Annu Rev Microbiol. 2009;63:451–476. doi: 10.1146/annurev.micro.091208.073255. doi: 10.1146/annurev.micro.091208.073255 . [DOI] [PubMed] [Google Scholar]
- 20.Skarin H, Segerman B. Horizontal gene transfer of toxin genes in Clostridium botulinum: Involvement of mobile elements and plasmids. Mob Genet Elements. 2011;1(3):213–215. doi: 10.4161/mge.1.3.17617. doi: 10.4161/mge.1.3.17617 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Melderen L, Saavedra De Bast M. Bacterial toxinantitoxin systems: more than selfish entities? PLoS Genet. 2009;5(3):e1000437. doi: 10.1371/journal.pgen.1000437. doi: 10.1371/journal.pgen.1000437 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuster CF, Bertram R. Toxin-Antitoxin systems are ubiquitous and versatile modulators of prokaryotic cell fate. FEMS Microbiol Lett. 2013;340(2):73–85. doi: 10.1111/1574-6968.12074. doi: 10.1111/1574-6968.12074 . [DOI] [PubMed] [Google Scholar]
- 23.Williams JJ, Hergenrother PJ. Artificial activation of toxin-antitoxin systems as an antibacterial strategy. Trends Microbiol. 2012;20(6):291–298. doi: 10.1016/j.tim.2012.02.005. doi: 10.1016/j.tim.2012.02.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241(1):92–97. doi: 10.1006/bbrc.1997.7765. doi: 10.1006/bbrc.1997.7765 . [DOI] [PubMed] [Google Scholar]
- 25.Law J, Jovel J, Patterson J, Ford G, O'keefe S, Wang W, et al. Identification of hepatotropic viruses from plasma using deep sequencing: a next generation diagnostic tool. PLoS One. 2013;8(4):e60595. doi: 10.1371/journal.pone.0060595. doi: 10.1371/journal.pone.0060595 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hino S, Miyata H. Torque teno virus (TTV): current status. Rev Med Virol. 2007;17(1):45–57. doi: 10.1002/rmv.524. doi: 10.1002/rmv.524 . [DOI] [PubMed] [Google Scholar]
- 27.Okamoto H. History of discoveries and pathogenicity of TT viruses. Curr Top Microbiol Immunol. 2009;331(1):20. doi: 10.1007/978-3-540-70972-5_1. [DOI] [PubMed] [Google Scholar]
- 28.McElvania Tekippe E, Wylie KM, Deych E, Sodergren E, Weinstock G, Storch GA. Increased prevalence of anellovirus in pediatric patients with Fever. PLoS One. 2012;7(11):e50937. doi: 10.1371/journal.pone.0050937. doi: 10.1371/journal.pone.0050937 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biagini P, de Micco P. Anelloviruses (TTV and variants): state of the art 10 years after their discovery. Transfus Clin Biol. 2008;15(6):406–415. doi: 10.1016/j.tracli.2008.10.003. doi: 10.1016/j.tracli.2008.10.003 . [DOI] [PubMed] [Google Scholar]
- 30.Delbue S, Comar M, Ferrante P. Review on the relationship between human polyomavirusesassociated tumors and host immune system. Clin Dev Immunol. 2012;2012:542092. doi: 10.1155/2012/542092. doi: 10.1155/2012/542092 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng J, DeCaprio JA, Fluck MM, Schaffhausen BS. Cellular transformation by Simian Virus 40 and Murine Polyoma Virus T antigens. Semin Cancer Biol. 2009;19(4):218–228. doi: 10.1016/j.semcancer.2009.03.002. doi: 10.1016/j.semcancer.2009.03.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neirynck V, Claes K, Naesens M, De Wever L, Pirenne J, Kuypers D, et al. Renal cell carcinoma in the allograft: what is the role of polyomavirus? Case Rep Nephrol Urol. 2012;2(2):125–134. doi: 10.1159/000341917. doi: 10.1159/000341917 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maginnis MS, Atwood WJ. JC virus: an oncogenic virus in animals and humans? Semin Cancer Biol. 2009;19(4):261–269. doi: 10.1016/j.semcancer.2009.02.013. doi: 10.1016/j.semcancer.2009.02.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saundh BK, Baker R, Harris M, Welberry Smith MP, Cherukuri A, Hale A. Early BK polyomavirus (BKV) reactivation in donor kidney is a risk factor for development of BKV-associated nephropathy. J Infect Dis. 2013;207(1):137–141. doi: 10.1093/infdis/jis642. doi: 10.1093/infdis/jis642 . [DOI] [PubMed] [Google Scholar]
- 35.Chung BH, Hong YA, Kim HG, Sun IO, Choi SR, Park HS, et al. Clinical usefulness of BK virus plasma quantitative PCR to prevent BK virus associated nephropathy. Transpl Int. 2012;25(6):687–695. doi: 10.1111/j.1432-2277.2012.01480.x. doi: 10.1111/j.1432-2277.2012.01480.x . [DOI] [PubMed] [Google Scholar]
- 36.Mateen FJ, Muralidharan R, Carone M, van de Beek D, Harrison DM, Aksamit AJ, et al. Progressive multifocal leukoencephalopathy in transplant recipients. Ann Neurol. 2011;70(2):305–322. doi: 10.1002/ana.22408. doi: 10.1002/ana.22408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haines HL, Laskin BL, Goebel J, Davies SM, Yin HJ, Lawrence J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(10):1512–1519. doi: 10.1016/j.bbmt.2011.02.012. doi: 10.1016/j.bbmt.2011.02.012 . [DOI] [PubMed] [Google Scholar]
- 38.Geetha D, Sozio SM, Ghanta M, Josephson M, Shapiro R, Dadhania D, et al. Results of repeat renal transplantation after graft loss from BK virus nephropathy. Transplantation. 2011;92(7):781–786. doi: 10.1097/TP.0b013e31822d08c1. doi: 10.1097/TP.0b013e31822d08c1 . [DOI] [PubMed] [Google Scholar]
- 39.Huang G, Chen LZ, Qiu J, Wang CX, Fei JG, Deng SX, et al. Prospective study of polyomavirus BK replication and nephropathy in renal transplant recipients in China: a single-center analysis of incidence, reduction in immunosuppression and clinical course. Clin Transplant. 2010;24(5):599–609. doi: 10.1111/j.1399-0012.2009.01141.x. doi: 10.1111/j.1399-0012.2009.01141.x . [DOI] [PubMed] [Google Scholar]
- 40.Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87(7):1019–1026. doi: 10.1097/TP.0b013e31819cc383. doi: 10.1097/tp.0b013e31819cc383 . [DOI] [PubMed] [Google Scholar]
- 41.Smith JM, Dharnidharka VR, Talley L, Martz K, McDonald RA. BK virus nephropathy in pediatric renal transplant recipients: an analysis of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry. Clin J Am Soc Nephrol. 2007;2(5):1037–1042. doi: 10.2215/CJN.04051206. doi: 10.2215/cjn.04051206 . [DOI] [PubMed] [Google Scholar]
- 42.Shitrit D, Lev N, Bar-Gil-Shitrit A, Kramer MR. Progressive multifocal leukoencephalopathy in transplant recipients. Transpl Int. 2005;17(11):658–665. doi: 10.1007/s00147-004-0779-3. doi: 10.1111/j.1432-2277.2004.tb00491.x . [DOI] [PubMed] [Google Scholar]
- 43.Schmid H, Nitschko H, Gerth J, Kliem V, Henger A, Cohen CD, et al. Polyomavirus DNA and RNA detection in renal allograft biopsies: results from a European multicenter study. Transplantation. 2005;80(5):600–604. doi: 10.1097/01.tp.0000173385.45918.39. [DOI] [PubMed] [Google Scholar]
- 44.Hurault de Ligny B, Etienne I, Francois A, Toupance O, Buchler M, Touchard G, et al. Polyomavirus-induced acute tubulo-interstitial nephritis in renal allograft recipients. Transplant Proc. 2000;32(8):2760–2761. doi: 10.1016/s0041-1345(00)01869-8. doi: 10.1016/s0041-1345(00)01869-8 . [DOI] [PubMed] [Google Scholar]
- 45.Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 2012;25(3):471–506. doi: 10.1128/CMR.05031-11. doi: 10.1128/cmr.05031-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rockville Merkel Cell Carcinoma Group Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol. 2009;27(24):4021–4026. doi: 10.1200/JCO.2009.22.6605. doi: 10.1200/jco.2009.22.6605 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pink R, Ehrmann J, Molitor M, Tvrdy P, Michl P, Pazdera J, et al. Merkel cell carcinoma. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012;156(3):213–217. doi: 10.5507/bp.2012.033. doi: 10.5507/bp.2012.033 . [DOI] [PubMed] [Google Scholar]
- 48.Spurgeon ME, Lambert PF. Merkel cell polyomavirus: A newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118–130. doi: 10.1016/j.virol.2012.09.029. doi: 10.1016/j.virol.2012.09.029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tilling T, Moll I. Which are the cells of origin in merkel cell carcinoma? J Skin Cancer. 2012;2012:680410. doi: 10.1155/2012/680410. doi: 10.1155/2012/680410 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alp Avcı G. Genomic organization and proteins of human papillomavirus. Mikrobiyol Bul. 2012;46(3):507–515. [PubMed] [Google Scholar]
- 51.Prétet JL, Charlot JF, Mougin C. Virological and carcinogenic aspects of HPV. Bull Acad Natl Med. 2007;191(3):611–623. [PubMed] [Google Scholar]
- 52.Narisawa-Saito M, Kiyono T. Mechanisms of high-risk human papillomavirus-induced cervical carcinogenesis. Nihon Rinsho. 2009;67(1):53–61. [PubMed] [Google Scholar]
- 53.Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110(3 Suppl 2):S4–7. doi: 10.1016/j.ygyno.2008.07.045. doi: 10.1016/j.ygyno.2008.07.045 . [DOI] [PubMed] [Google Scholar]
- 54.Ray A, Barreto SC, Armstrong E, Dogan S. Pathobiology of cancer and clinical biochemistry. J Pediatr Biochem. 2013;3(4):187–201. doi: 10.3233/jpb-130092 . [Google Scholar]
- 55.Zandberg DP, Bhargava R, Badin S, Cullen KJ. The role of human papillomavirus in nongenital cancers. CA Cancer J Clin. 2013;63(1):57–81. doi: 10.3322/caac.21167. doi: 10.3322/caac.21167 . [DOI] [PubMed] [Google Scholar]
- 56.Mocarska A, Starosławska E, Zelazowska-Cieślińska I, Łosicki M, Stasiewicz D, Kieszko D, et al. Epidemiology and risk factors of the cervical squamous cell carcinoma. Pol Merkur Lekarski. 2012;33(194):101–106. [PubMed] [Google Scholar]
- 57.Sharma BK, Ray A, Murthy NS. Prevalence of serum antibodies to synthetic peptides to HPV16 epitopes among Indian women with cervical neoplasia. Eur J Cancer. 1996;32(5):872–876. doi: 10.1016/0959-8049(96)00005-6. [DOI] [PubMed] [Google Scholar]
- 58.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012; 30;(Suppl 5)(F123):138. doi: 10.1016/j.vaccine.2012.04.108. doi: 10.1016/j.vaccine.2012.04.108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin XW, Lipold L, Sikon A, Rome E. Human papillomavirus vaccine: Safe, effective, underused. Cleve Clin J Med. 2013;80(1):49–60. doi: 10.3949/ccjm.80a.12084. doi: 10.3949/ccjm.80a.12084 . [DOI] [PubMed] [Google Scholar]
- 60.Gersch ED, Gissmann L, Garcea RL. New approaches to prophylactic human papillomavirus vaccines for cervical cancer prevention. Antivir Ther. 2012;17(3):425–434. doi: 10.3851/IMP1941. doi: 10.3851/imp1941 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeviani M, Di Donato S. Mitochondrial disorders. Brain. 2004;127(Pt 10):2153–2172. doi: 10.1093/brain/awh259. doi: 10.1093/brain/awh259 . [DOI] [PubMed] [Google Scholar]
- 62.Rötig A. Genetic bases of mitochondrial respiratory chain disorders. Diabetes Metab. 2010;36(2):97–107. doi: 10.1016/j.diabet.2009.11.002. doi: 10.1016/j.diabet.2009.11.002 . [DOI] [PubMed] [Google Scholar]
- 63.Chinnery PF, Hudson G. Mitochondrial genetics. Br Med Bull. 2013;106:135–159. doi: 10.1093/bmb/ldt017. doi: 10.1093/bmb/ldt017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finsterer J. Overview on visceral manifestations of mitochondrial disorders. Neth J Med. 2006;64(3):61–71. [PubMed] [Google Scholar]
- 65.Zhang L, Chan SS, Wolff DJ. Mitochondrial disorders of DNA polymerase γ dysfunction: from anatomic to molecular pathology diagnosis. Arch Pathol Lab Med. 2011;135(7):925–934. doi: 10.1043/2010-0356-RAR.1. doi: 10.1043/2010-0356-rar.1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6(5):389–402. doi: 10.1038/nrg1606. doi: 10.1038/nrg1606 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neiman M, Taylor DR. The causes of mutation accumulation in mitochondrial genomes. Proc Biol Sci. 2009;276(1660):1201–1209. doi: 10.1098/rspb.2008.1758. doi: 10.1098/rspb.2008.1758 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lagouge M, Larsson NG. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J Intern Med. 2013;273(6):529–543. doi: 10.1111/joim.12055. doi: 10.1111/joim.12055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci. 2012;322(1-2):254–262. doi: 10.1016/j.jns.2012.05.030. doi: 10.1016/j.jns.2012.05.030 . [DOI] [PubMed] [Google Scholar]
- 70.Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion. 2013;13(5):481–92. doi: 10.1016/j.mito.2012.10.011. doi: 10.1016/j.mito.2012.10.011 . [DOI] [PubMed] [Google Scholar]
- 71.Tseng LM, Yin PH, Chi CW, Hsu CY, Wu CW, Lee LM, et al. Mitochondrial DNA mutations and mitochondrial DNA depletion in breast cancer. Genes Chromosomes Cancer. 2006;45(7):629–638. doi: 10.1002/gcc.20326. doi: 10.1002/gcc.20326 . [DOI] [PubMed] [Google Scholar]
- 72.Fan AX, Radpour R, Haghighi MM, Kohler C, Xia P, Hahn S, et al. Mitochondrial DNA content in paired normal and cancerous breast tissue samples from patients with breast cancer. J Cancer Res Clin Oncol. 2009;135(8):983–989. doi: 10.1007/s00432-008-0533-9. doi: 10.1007/s00432-008-0533-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu C, Tran-Thanh D, Ma C, May K, Jung J, Vecchiarelli J, et al. Mitochondrial D310 mutations in the early development of breast cancer. Br J Cancer. 2012;106(9):1506–1511. doi: 10.1038/bjc.2012.74. doi: 10.1038/bjc.2012.74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masuda S, Kadowaki T, Kumaki N, Tang X, Tokuda Y, Yoshimura S, et al. Analysis of gene alterations of mitochondrial DNA D-loop regions to determine breast cancer clonality. Br J Cancer. 2012;107(12):2016–2023. doi: 10.1038/bjc.2012.505. doi: 10.1038/bjc.2012.505 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naito A, Carcel-Trullols J, Xie CH, Evans TT, Mizumachi T, Higuchi M. Induction of acquired resistance to antiestrogen by reversible mitochondrial DNA depletion in breast cancer cell line. Int J Cancer. 2008;122(7):1506–1511. doi: 10.1002/ijc.23235. doi: 10.1002/ijc.23235 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ray A, Ficek M. Immunomodulatory effects of antiestrogenic drugs. Acta Pharm. 2012;62(2):141–155. doi: 10.2478/v10007-012-0012-3. doi: 10.2478/v10007-012-0012-3 . [DOI] [PubMed] [Google Scholar]
- 77.Lindberg J, Mills IG, Klevebring D, Liu W, Neiman M, Xu J, et al. The mitochondrial and autosomal mutation landscapes of prostate cancer. Eur Urol. 2013;63(4):702–708. doi: 10.1016/j.eururo.2012.11.053. doi: 10.1016/j.eururo.2012.11.053 . [DOI] [PubMed] [Google Scholar]
- 78.Gómez-Zaera M, Abril J, González L, Aguiló F, Condom E, Nadal M, et al. Identification of somatic and germline mitochondrial DNA sequence variants in prostate cancer patients. Mutat Res. 2006;595(1–2):42–51. doi: 10.1016/j.mrfmmm.2005.10.012. doi: 10.1016/j.mrfmmm.2005.10.012 . [DOI] [PubMed] [Google Scholar]
- 79.Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci U S A. 2005;102(3):719–724. doi: 10.1073/pnas.0408894102. doi: 10.1073/pnas.0408894102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray AM, Zuhlke KA, Levin AM, Douglas JA, Cooney KA, Petros JA. Sequence variation in the mitochondrial gene cytochrome c oxidase subunit I and prostate cancer in African American men. Prostate. 2009;69(9):956–960. doi: 10.1002/pros.20943. doi: 10.1002/pros.20943 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moro L, Arbini AA, Yao JL, di Sant'Agnese PA, Marra E, Greco M. Mitochondrial DNA depletion in prostate epithelial cells promotes anoikis resistance and invasion through activation of PI3K/Akt2. Cell Death Differ. 2009;16(4):571–583. doi: 10.1038/cdd.2008.178. doi: 10.1038/cdd.2008.178 . [DOI] [PubMed] [Google Scholar]
- 82.Kloss-Brandstätter A, Schäfer G, Erhart G, Hüttenhofer A, Coassin S, Seifarth C, et al. Somatic mutations throughout the entire mitochondrial genome are associated with elevated PSA levels in prostate cancer patients. Am J Hum Genet. 2010;87(6):802–812. doi: 10.1016/j.ajhg.2010.11.001. doi: 10.1016/j.ajhg.2010.11.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maki J, Robinson K, Reguly B, Alexander J, Wittock R, Aguirre A, et al. Mitochondrial genome deletion aids in the identification of false- and true-negative prostate needle core biopsy specimens. Am J Clin Pathol. 2008;129(1):57–66. doi: 10.1309/UJJTH4HFEPWAQ78Q. doi: 10.1309/ujjth4hfepwaq78q . [DOI] [PubMed] [Google Scholar]
- 84.Kuttler F, Mai S. Formation of non-random extrachromosomal elements during development, differentiation and oncogenesis. Semin Cancer Biol. 2007;17(1):56–64. doi: 10.1016/j.semcancer.2006.10.007. doi: 10.1016/j.semcancer.2006.10.007 . [DOI] [PubMed] [Google Scholar]
- 85.Iwasato T, Shimizu A, Honjo T, Yamagishi H. Circular DNA is excised by immunoglobulin class switch recombination. Cell. 1990;62(1):143–149. doi: 10.1016/0092-8674(90)90248-d. doi: 10.1016/0092-8674(90)90248-d . [DOI] [PubMed] [Google Scholar]
- 86.Toda M, Fujimoto S, Iwasato T, Takeshita S, Tezuka K, Ohbayashi T, et al. Structure of extrachromosomal circular DNAs excised from T-cell antigen receptor alpha and delta-chain loci. J Mol Biol. 1988;202(2):219–231. doi: 10.1016/0022-2836(88)90453-6. doi: 10.1016/0022-2836(88)90453-6 . [DOI] [PubMed] [Google Scholar]
- 87.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. doi: 10.1016/0092-8674(92)90030-g . [DOI] [PubMed] [Google Scholar]
- 88.Punwani D, Gonzalez-Espinosa D, Comeau AM, Dutra A, Pak E, Puck J. Cellular calibrators to quantitate T-cell receptor excision circles (TRECs) in clinical samples. Mol Genet Metab. 2012;107(3):586–591. doi: 10.1016/j.ymgme.2012.09.018. doi: 10.1016/j.ymgme.2012.09.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwan A, Church JA, Cowan MJ, Agarwal R, Kapoor N, Kohn DB, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: Results of the first 2 years. J Allergy Clin Immunol. 2013;132(1):140–150.e7. doi: 10.1016/j.jaci.2013.04.024. doi: 10.1016/j.jaci.2013.04.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Geenen V, Poulin JF, Dion ML, Martens H, Castermans E, Hansenne I, et al. Quantification of T cell receptor rearrangement excision circles to estimate thymic function: an important new tool for endocrine-immune physiology. J Endocrinol. 2003;176(3):305–311. doi: 10.1677/joe.0.1760305. doi: 10.1677/joe.0.1760305 . [DOI] [PubMed] [Google Scholar]
- 91.Shimizu N. Molecular mechanisms of the origin of micronuclei from extrachromosomal elements. Mutagenesis. 2011;26(1):119–123. doi: 10.1093/mutage/geq053. doi: 10.1093/mutage/geq053 . [DOI] [PubMed] [Google Scholar]
- 92.Thomas L, Stamberg J, Gojo I, Ning Y, Rapoport AP. Double minute chromosomes in monoblastic (M5) and myeloblastic (M2) acute myeloid leukemia: two case reports and a review of literature. Am J Hematol. 2004;77(1):55–61. doi: 10.1002/ajh.20151. doi: 10.1002/ajh.20151 . [DOI] [PubMed] [Google Scholar]
- 93.Jin Y, Liu Z, Cao W, Ma X, Fan Y, Yu Y, et al. Novel functional MAR elements of double minute chromosomes in human ovarian cells capable of enhancing gene expression. PLoS One. 2012;7(2):e30419. doi: 10.1371/journal.pone.0030419. doi: 10.1371/journal.pone.0030419 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villa O, Salido M, Pérez-Vila ME, Ferrer A, Arenillas L, Pedro C, et al. Blast cells with nuclear extrusions in the form of micronuclei are associated with MYC amplification in acute myeloid leukemia. Cancer Genet Cytogenet. 2008;185(1):32–36. doi: 10.1016/j.cancergencyto.2008.04.014. doi: 10.1016/j.cancergencyto.2008.04.014 . [DOI] [PubMed] [Google Scholar]
- 95.Meddeb M, Valent A, Danglot G, Nguyen VC, Duverger A, Fouquet F, et al. MDM2 amplification in a primary alveolar rhabdomyosarcoma displaying a t(2;13) (q35;q14) Cytogenet Cell Genet. 1996;73(4):325–330. doi: 10.1159/000134368. [DOI] [PubMed] [Google Scholar]
- 96.Vogt N, Lefèvre SH, Apiou F, Dutrillaux AM, Cör A, Leuraud P, et al. Molecular structure of doubleminute chromosomes bearing amplified copies of the epidermal growth factor receptor gene in gliomas. Proc Natl Acad Sci U S A. 2004;101(31):11368–11373. doi: 10.1073/pnas.0402979101. doi: 10.1073/pnas.0402979101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol. 2013;25(4):398–406. doi: 10.1097/CCO.0b013e3283622c1b. doi: 10.1097/CCO.0b013e3283622c1b . [DOI] [PubMed] [Google Scholar]
- 98.Naik DS, Sharma S, Ray A, Hedau S. Epidermal growth factor receptor expression in urinary bladder cancer. Indian J Urol. 2011;27(2):208–214. doi: 10.4103/0970-1591.82839. doi: 10.4103/0970-1591.82839 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith G, Taylor-Kashton C, Dushnicky L, Symons S, Wright J, Mai S. c-Myc-induced extrachromosomal elements carry active chromatin. Neoplasia. 2003;5(2):110–120. doi: 10.1016/s1476-5586(03)80002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gaubatz JW. Extrachromosomal circular DNAs and genomic sequence plasticity in eukaryotic cells. Mutat Res. 1990;237(5–6):271–292. doi: 10.1016/0921-8734(90)90009-g. doi: 10.1016/0921-8734(90)90009-g . [DOI] [PubMed] [Google Scholar]
- 101.Carroll SM, DeRose ML, Gaudray P, Moore CM, Needham-Vandevanter DR, Von Hoff DD, et al. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol Cell Biol. 1988;8(4):1525–1533. doi: 10.1128/mcb.8.4.1525. doi: 10.1128/mcb.8.4.1525 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maurer BJ, Lai E, Hamkalo BA, Hood L, Attardi G. Novel submicroscopic extrachromosomal elements containing amplified genes in human cells. Nature. 1987;327(6121):434–437. doi: 10.1038/327434a0. [DOI] [PubMed] [Google Scholar]
- 103.Ruiz JC, Choi KH, von Hoff DD, Roninson IB, Wahl GM. Autonomously replicating episomes contain mdr1 genes in a multidrug-resistant human cell line. Mol Cell Biol. 1989;9(1):109–115. doi: 10.1128/mcb.9.1.109. doi: 10.1128/mcb.9.1.109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Regev A, Cohen S, Cohen E, Bar-Am I, Lavi S. Telomeric repeats on small polydisperse circular DNA (spcDNA) and genomic instability. Oncogene. 1998;17(26):3455–3461. doi: 10.1038/sj.onc.1202250. [DOI] [PubMed] [Google Scholar]
- 105.Schmidt H, Taubert H, Lange H, Kriese K, Schmitt WD, Hoffmann S, et al. Small polydispersed circular DNA contains strains of mobile genetic elements and occurs more frequently in permanent cell lines of malignant tumors than in normal lymphocytes. Oncol Rep. 2009;22(2):393–400. doi: 10.3892/or_00000450 . [PubMed] [Google Scholar]
- 106.Boczonadi V, Horvath R. Mitochondria: Impaired mitochondrial translation in human disease. Int J Biochem Cell Biol. 2014;48:77–84. doi: 10.1016/j.biocel.2013.12.011. doi: 10.1016/j.biocel.2013.12.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Kriekinge G, Castellsagué X, Cibula D, Demarteau N. Estimation of the potential overall impact of human papillomavirus vaccination on cervical cancer cases and deaths. Vaccine. 2014;32(6):733–739. doi: 10.1016/j.vaccine.2013.11.049. doi: 10.1016/j.vaccine.2013.11.049 . [DOI] [PubMed] [Google Scholar]