Abstract
Background:
Humoral and cellular immune responses are associated with protection against extracellular and intracellular pathogens, respectively. In the present study, we evaluated the effect of receiving human secretory immunoglobulin A (hsIgA) on the histopathology of the lungs of mice challenged with virulent Mycobacterium tuberculosis.
Methods:
The hsIgA was purified from human colostrum and administered to Balb/c mice by the intranasal route prior to infection with M. tuberculosis or in a pre-incubated formulation with mycobacteria, with the principal aim to study its effect on qualitative pulmonary histopathology.
Results:
The intranasal administration of hsIgA and the pre-incubation of mycobacteria with this preparation was associated with the presence of organised granulomas with signs of immune activation and histological features related to efficient disease control. This effect was highly evident during the late stage of infection (60 days), as demonstrated by numerous organised granulomas with numerous activated macrophages in the lungs of treated mice.
Conclusion:
The administration of hsIgA to mice before intratracheal infection with M. tuberculosis or the pre-incubation of the bacteria with the antibody formulation induced the formation of well-organised granulomas and inflammatory lesions in lungs compared with non-treated animals which correlates with the protective effect already demonstrated by these antibody formulations.
Keywords: colostrums, Mycobacterium tuberculosis, secretory immunoglobulin A
Introduction
It is known that humoral immunity is associated with protection against extracellular pathogens, whereas cellular immune responses are primarily associated with protection against intracellular pathogens (1). Thus, in the case of tuberculosis (TB), in both man and mouse, protective immunity is mediated by activated macrophages and Th1 lymphocytes (2). These cells are the most important constituents of granulomas, which are essential elements in the confinement and control the bacilli growth. These structures are produced by mice and humans with some differences (2). However, it is currently thought that humoral and cell-mediated immune responses collaborate in the protection against intracellular microbial pathogens (1,3–5). Most vaccines that are licensed and used against bacterial infections in humans are aimed at inducing a protective antibody response that allows the elimination of the microorganisms, including intracellular pathogens such as Salmonella typhi (6). Antibodies have the ability to control intracellular pathogens. Indeed, the prevalence of antibody susceptibility in the extracellular phase has been documented for certain categories of microbes (7,8). Secretory antibodies trap exogenous antigens and exclude immune complexes with the assistance of a variety of innate mucosal defence mechanisms (9). SIgA is a highly stable antibody that can preserve its activity for prolonged periods of time even in hostile environments such as the gut lumen (10) and oral cavity (11). In particular, the function of sIgA is most likely enhanced by its high level of cross-reactive activity and its presence in human secretions (12). SIgA is not only present in external secretions but also has antimicrobial properties in epithelial cells during its transport across the epithelium. SIgA is the primary immunoglobulin type found in external secretions at a welldefined quantity, which provides specific immune protection for all mucosal surfaces by blocking the penetration of pathogens into the body (13).
Additional work is needed to understand the molecular mechanisms behind the IgAmediated inhibition of pulmonary infection caused by M. tuberculosis. Reljic et al. proposed that the principal mechanism is the enhancement of the bactericidal functions of IgA on infected macrophages (14,15). IgA may lead to activationdependent apoptosis causing bacterial death, or the blockage of bacterial interaction with the phagosomal membrane mediated by IgA/Gal-3, which is required for the inhibition of phagolysosome fusion (15,16). In a previous work, the protective effect of hsIgA administered to mice, including significant decreases in pneumonic areas by morphometric evaluation, was demonstrated (17). To complement these previous results, we present a morphometric evaluation of granuloma areas and perivenular infiltrates as well as a detailed qualitative histopathological study of the effect of hsIgA on tissue damage in the lungs of Balb/c mice challenged with virulent M. tuberculosis.
Materials and Methods
HsIgA purification
HsIgA was purified from human colostrum donated by healthy mothers 3 to 5 days after delivery. The purification was performed by anion exchange chromatography and subsequent gel filtration, using DEAE Sepharose Fast Flow and preparative-grade Superose 6 (Pharmacia, Sweden), respectively. The presence of IgA in the chromatographic fractions was identified by dot blot analysis and the purity of hsIgA was verified by 12.5% sodium dodecylsulfate-polyacrylamide gel electrophoresis sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The purity of hsIgA was confirmed by the detection of only IgA heavy and light chains with a migration pattern corresponding to their molecular weights (18). After purification, the reactivity of hsIgA with M. tuberculosis antigens was demonstrated (17).
Mycobecterium tuberculosis culture
M. tuberculosis H37Rv (ATCC 27294) was grown to early mid-log phase in Middlebrook 7H9 medium (Difco, Detroit, MI) supplemented with albumin-dextrose-catalase (BBL, Cockeysville, MD) and 0.05% Tween 80 (Sigma Chemical Co., St. Louis, MO). Cultures were incubated at 37 °C with 5% CO2 and shaken continuously for 28 days. The bacteria were harvested by centrifugation at 5000 × g for 15 minutes, resuspended in saline solution, dispensed in aliquots containing 106 bacteria/mL, and stored at –70 °C until use.
Inoculation and infection schedule
Three groups (n = 15 in each group) of male Balb/c mice of eight weeks of age were used as follows for inoculation and infection: the non-treated (NT) group, consisting of mice that were intratracheally infected with 2.5 × 105 CFU of M. tuberculosis in 100 μL of saline solution; the HsIgA-treated group, consisting of mice intranasally inoculated with purified hsIgA (1 mg of hsIgA in 50 μL of saline solution, 25 μL in each nostril) and intratracheally challenged with 2.5 × 105 CFU of M. tuberculosis 2 h after inoculation with the antibody; and the preincubated hsIgA group (Preinc), consisting of mice challenged intratracheally with 2.5 × 105 CFU M. tuberculosis previously incubated with 1 mg of the purified hsIgA for 4 h at room temperature. Five mice from each group were sacrificed on days 1, 7 and 60 after M. tuberculosis challenge and lungs were perfused with 10% formaldehyde dissolved in Phospahate Buffer Saline (PBS) and extracted for histopathological analysis. Infected mice were housed in individual micro-isolator cages in a Biosafety Level 3 (BL3) animal facility. All experimental procedures with animals were performed in a laminar flow cabinet in the BL3 facility, under anaesthesia and according to the guidelines approved by the Animal Ethics Committee of the National Institute of Medical Sciences and Nutrition, Mexico.
Tissue preparation, morphometric evaluation, and lung histopathology
The right lungs of each mouse were fixed with 10% formaldehyde dissolved in PBS solution, dehydrated in alcohol, cleared with xylol, and embedded in paraffin. Lung tissues were sectioned at 3 μm and stained with hematoxylin and eosin using standard techniques. All lung tissues were mounted in the same orientation and sagittal sections.
For quantitative purpose, three sections with 100 μm of distance separating them were obtained from each mouse. Granulomas were defined as well-delimited nodular structures constituted by lymphocytes and macrophages. Well-formed granulomas were those lesions that included both cellular types, with the latter activated, as defined by the presence of large cells with large and compact cytoplasms and peripheral nuclei with finely dispersed chromatin. In each section, all the granulomas were measured with a determination of their surface area in square microns at 200× magnification using automated morphometry equipment (Leica Microsystems Imaging Solutions LTD, Cambridge, UK). Regarding blood vessels, all venules in transversal sections from 80–100 μm of diameter in each section were considered, with the area occupied by the inflammatory cells around these blood vessels measure in square microns. The data were analysed using one-way ANOVA and a post hoc Tukey multiple comparison procedure. P values under 0.05 were considered statistically significant. All data were analysed using GraphPad Prism 4 software.
Results
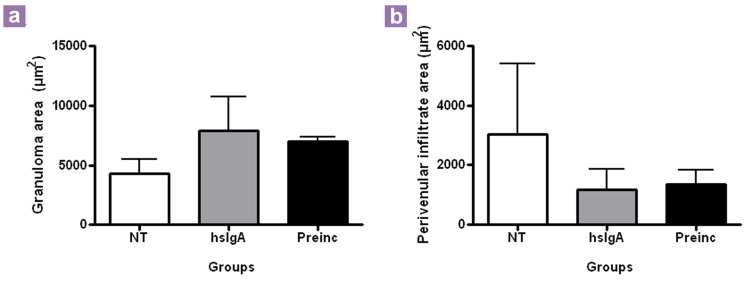
Histopathological analysis revealed no qualitative differences in the lungs of mice from the different groups at days 1 and 7 after infection. Sixty days after infection, however, there were pneumonic areas constituted by abundant macrophages with cytoplasmic vacuoles (foamy macrophages) and lymphocytes that occupied the alveolar lumen, with some areas of focal necrosis and large perivascular cuffs of lymphocytes in the non-treated group (Figure 1). At this time point, IgA treatment resulted in fewer lung areas affected by pneumonia, and lesions that consisted of predominantly abundant large activated macrophages (Figure 1). More organised granulomas in the Preinc and hsIgA groups were observed, with these lesions constituted by activated macrophages with large and compact cytoplasms and large nuclei with disperse chromatin; these cells were surrounded by numerous lymphocytes (Figure 1). In comparison, the control group showed smaller granulomas constituted predominantly by lymphocytes and activated macrophages. Both groups treated with IgA showed granulomas with similar cellular constitution and reduced perivascular inflammation from a qualitative point of view, but from the statistical point of view, the morphometry of perivascular inflammation and the total area occupied by granulomas were not significantly different (Figure 2).
Figure 1:
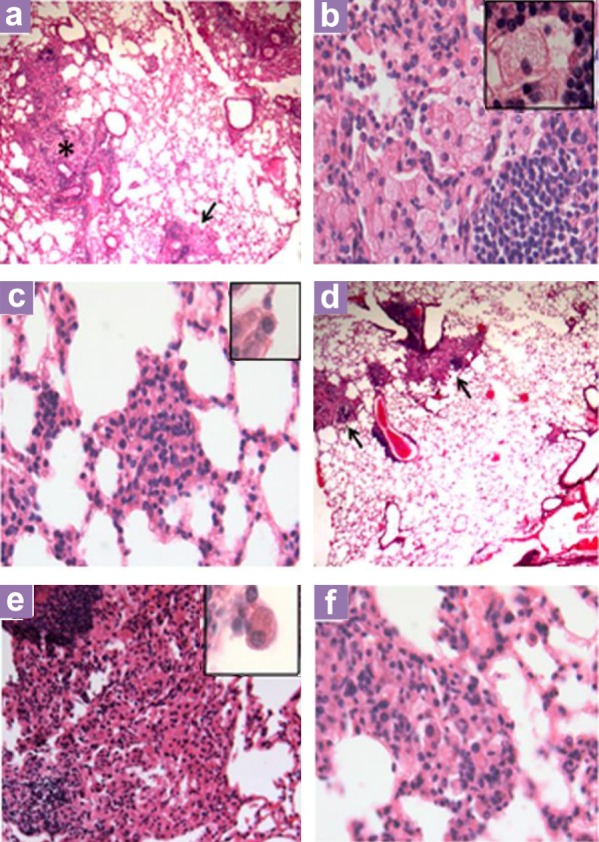
Representative histopathology of the lungs of mice treated with hsIgA in comparison with control nontreated tuberculous mice at day 60 of infection. (a) Low power micrograph shows extensive areas of pneumonia (asterisk) and focal necrosis (arrow) in the lung of a control mouse. (b) A high-power micrograph of these pneumonic areas reveals numerous vacuolated macrophages (inset). (c) A granuloma from the lung of a control mouse comprises small activated macrophages (inset). (d) In contrast, reduced pneumonic areas (arrow) are seen after 60 days of infection in mouse treated with hsIgA. (e) High power of this pneumonic area shows numerous activated macrophages with compact cytoplasms and large nuclei (inset). (f) Granuloma from the lung of mouse treated with hsIgA show numerous activated macrophages and abundant lymphocytes (low power micrograph 25× magnification, high power micrograph 200× magnification, inset 1000× magnification).
Figure 2:
Determination of the granuloma (a) and perivenular inflammation (b) area in the lungs of both non-treated mice and mice treated with hsIgA 1, 7 and 60 days after inoculation with M. tuberculosis. NT: non-treated group; hsIgA: mice receiving human secretory IgA intranasally and intratracheally challenged with M. tuberculosis; Preinc: mice intratracheally receiving hsIgA preincubated with M. tuberculosis. Granulomas and perivenular infiltrate areas were measured, and the data were analysed using one-way ANOVA and a post-hoc Tukey multiple comparison procedure. P < 0.05 were considered statistically significant. No significant results between the groups.
Discussion
The qualitative histopathological study showed marked differences in the morphology and cellular composition of pulmonary granulomas between the control non-treated and treated mice with hsIgA. In mice treated with IgA monoclonal antibodies against 16 kDa protein of M. tuberculosis, more organised granulomas were reported in the treated group than in the non-treated control group or animals receiving a non related monoclonal antibody (18). Mice challenged with M. tuberculosis coated with an IgM monoclonal antibody against mycolylarabinogalactan-peptidoglycan complex also showed more organised granulomas than nontreated mice (19). At the same time, bacteria were present only in granulomatous lesions in treated animals compared with controls which showed a random presence of the bacteria in lung tissue (19). In a previous study with hsIgA there was correlation between the decrease bacterial load in lungs and the pneumonic area 60 days after the administration of hsIgA and M. tuberculosis preincubated with the same antibody formulation compared with non-treated animals (16). These results were confirmed and extended in the present study by the demonstration of different cellular composition in IgA treated animals that showed predominant activated macrophages in the pneumonic areas and granulomas, while in control animals the predominant cells in pneumonia were vacuolated macrophages. Foamy or vacuolated macrophages contain numerous bacilli and they show little immunostaining for the immunoprotective factors tumor necrosis factor alpha (TNF-a) and the induced isoform of nitric oxide synthase (iNOS); but strong immunoreactivity to the immunosuppressive cytokine transforming growth factor beta (TGF) (20,21). Thus, foamy macrophages are related to disease worsening, while activated macrophages show the inverse immunostaining cytokine profile and are associated to efficient bacilli growth control. In comparison with control mice, animals treated with IgA showed also bigger granulomas. In this mice model large granulomas are related to protection (22), and lesser inflammation around airways and blood vessels is also related to efficient bacilli growth control. Thus, IgA treatment well correlated with histological features related with efficient disease control.
It has been suggested that there are essentially two mechanisms by which antibodies mediate protection against M. tuberculosis infection: the first is the opsonisation of mycobacteria which improves the processes of phagocytosis and intracellular killing by neutrophils and macrophages, and the second is the activation induced by immunoglobulins in antigen-presenting cells that can enhance the response of specific T cells against mycobacteria (24). Secretory IgA antibodies (sIgA) can operate by other protective mechanisms, such as the inhibition of bacterial or antigen adherence to mucosal surfaces by the properties conferred by the secretory component (25). Other additional mechanisms include the acceleration of immune complex elimination through respiratory ciliary movement and intestinal peristalsis, antibodydependent cellular cytotoxicity (26), and the stimulation of antigen presenting cells for activation of T cells (27). Indeed, IgA protects the mucosal epithelial barrier through different mechanisms (28). sIgA antibodies interact with antigens at the stromal side of the epithelium, and immune complexes are engulfed and eliminated by phagocytic cells or are incorporated into the vascular system or passed through the epithelium associated with the polymeric immunoglobulin receptor (pIgR) (29,30). This latter process is called sIgA immune exclusion and is able to react to various antigens, including those expressed by bacteria, blocking adherence and microbial penetration of the epithelium and thereby providing an effective means of protection (28). sIgA also prevents mucosal infections by inhibiting the initial pathogen colonisation and eliminating epithelial cells without tissue damage during its transit to the lumen mediated by pIgR (31). In contrast to IgG, IgA is considered an anti-inflammatory element in secretions due to its minimal activating effect on the complement system (31).
The protective effect of IgA in experimental tuberculosis has been previously demonstrated by the intranasal inoculation of monoclonal TBA61 IgA antibodies against the α-crystallin (acr1) antigen of M. tuberculosis (32,33). However, it offered only a short duration of protection, which could be prolonged by the administration of IFN-γ three days before infection and further administration of IgA at 2 h before and two and seven days after aerosol infection with M. tuberculosis H37Rv (32). Rodriguez et al showed that IgA-deficient mice immunised with the mycobacterium cell surface antigen PstS-1 were more susceptible to intranasal infection with BCG than wildtype non-targeted littermate controls (34). Recently, it was shown that the combined intranasal administration of a novel human IgA monoclonal antibody (2E9IgA1) and recombinant mouse IFN-γ significantly reduced lung infection induced by M. tuberculosis H37Rv in CD89 transgenic mice but not in CD89-negative controls, indicating that 2E9IgA1-mediated protection largely depends on its interaction with CD89 (35). Our results on the qualitative evaluation of histopathological lesions after the administration of hsIgA correlate with other reports of the association between granuloma organisation and the presence of activated macrophages with the protective capacity of other antibody formulations (1,15,19).
Conclusion
Human secretory IgA antibodies purified from colostrum can interact with antigens and inhibit the adherence of diverse microrganisms to mucosal surfaces. In comparison with control non-treated mice, the protective role of hsIgA is notable due to the formation and consolidation of well-constituted granulomas with numerous activated macrophages in the lungs of infected mice pre-treated with purified hsIgA or infected with M. tuberculosis preincubated with purified hsIgA.
Acknowledgments
None.
Footnotes
Conflict of interest
None.
Funds
This work was jointly supported by the Ministry of Higher Education, Malaysia LRGS Grant (203. PSK.6722001), Conacyt contract 84456 and the Ministry of Science and Technology, Cuba.
Authors’ contributions
Conception and design and final approval of the article: NA, NMN, MES, RHP, AA
Analysis and interpretation of the data: NA, JFI, MES, RHP, AA
Drafting of the article: NA, JFI, MMH
Critical revision of the article for the important intellectual content: NMN, MES, RHP, AA
Final approval of the article: NA, NMN, MES, RHP, AA
Provision of study materials or patient: DM, JB, RHP
Obtaining of funding and administrative, technical or logistic support: NMN, MES, RHP, AA
Collection and assembly of data: NA, RB
References
- 1.Glatman-Freedman A. The role of antibody-mediated immunity in defense against Mycobacterium tuberculosis: Advances towards a novel vaccine strategy. Tuberculosis. 2006;86(3–4):191–197. doi: 10.1016/j.tube.2006.01.008. doi: 10.1016/j.tube.2006.01.008 . [DOI] [PubMed] [Google Scholar]
- 2.Rook GAW, Hernández Pando R. The pathogenesis of tuberculosis. Annual Rev Microbiol. 1996;50:259–284. doi: 10.1146/annurev.micro.50.1.259. doi: 10.1146/annurev.micro.50.1.259 . [DOI] [PubMed] [Google Scholar]
- 3.Mazanec MB, Kaetzel CS, Lam ME, Fletcher D. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89(15):6901–6905. doi: 10.1073/pnas.89.15.6901. doi: 10.1073/pnas.89.15.6901 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mineo JR, Khan IA, Kassper LH. Toxoplasma gondii: a monoclonal antibody that inhibit intracellular replication. Exp Parasitol. 1994;79(3):351–361. doi: 10.1006/expr.1994.1097. doi: 10.1006/expr.1994.1097 . [DOI] [PubMed] [Google Scholar]
- 5.Saanford JE, Lupan DM, Schlangeter AM, Kozel TR. Passive immunization against Cryptococcus neoformans with an isotype-swich family of monoclonal antibodies reactive with criptoccocal polysaccharide. Infect Immun. 1990;58(6):1919–1923. doi: 10.1128/iai.58.6.1919-1923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins JB, Schneerson R, Szu SC. Hypothesis: how licensed vaccines confer protective immunity. Adv Exp Med Biol. 1996;397:169–182. doi: 10.1007/978-1-4899-1382-1_22. [DOI] [PubMed] [Google Scholar]
- 7.Casadevall A, Pirofski LA. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 2003;24(9):474–478. doi: 10.1016/s1471-4906(03)00228-x. doi: 10.1016/S1471-4906(03)00228-X . [DOI] [PubMed] [Google Scholar]
- 8.Casadevall A. Antibody-mediated immunity against intracellular pathogens: two-dimensional thinking comes full circle. Infect Immun. 2003;71(8):4225–4228. doi: 10.1128/IAI.71.8.4225-4228.2003. doi: 10.1128/IAI.71.8.4225-4228.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller CA, Autenrieth IB, Peschel A. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci. 2005;62(12):1297–1307. doi: 10.1007/s00018-005-5034-2. doi: 10.1007/s00018-005-5034-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haneberg B. Immunoglobulins in feces from infants fed human or bovine milk. Scand J Immunol. 1974;3(2):191–197. doi: 10.1111/j.1365-3083.1974.tb01247.x. doi: 10.1111/j.1365-3083.1974.tb01247.x . [DOI] [PubMed] [Google Scholar]
- 11.Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, et al. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med. 1998;4(5):601–606. doi: 10.1038/nm0598-601. doi: 10.1038/nm0598-601 . [DOI] [PubMed] [Google Scholar]
- 12.Quan CP, Berneman A, Pires R, Avrameas S, Bouvet JP. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun. 1997;65(10):3997–4004. doi: 10.1128/iai.65.10.3997-4004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corthesy B, Spertin F. Secretory immunoglobulin A: from mucosal protection to vaccine development. Biol Chem. 1999;380(11):1251–1262. doi: 10.1515/BC.1999.160. doi: 10.1515/BC.1999.160 . [DOI] [PubMed] [Google Scholar]
- 14.Reljic R, Crawford C, Challacombe S, Ivanji J. Mouse monoclonal IgA binds to the galectin-3/Mac-2 lectin from mouse macrophage cell lines. Immunol Lett. 2004;93(1):51–56. doi: 10.1016/j.imlet.2004.01.015. doi: 10.1016/j.imlet.2004.01.015 . [DOI] [PubMed] [Google Scholar]
- 15.Reljic R, Williams A, Ivanyi J. Mucosal immunotherapy of tuberculosis: is there a value in passive IgA? Tuberculosis. 2006;86(3–4):179–190. doi: 10.1016/j.tube.2006.01.011. doi: 10.1016/j.yube.2006.01.011 . [DOI] [PubMed] [Google Scholar]
- 16.de Chastellier C, Thilo L. Phagosome maturation and fusion with lysosomes in relation to surface property and size of the phagocytic particle. Eur J Cell Biol. 1997;74(1):49–62. [PubMed] [Google Scholar]
- 17. Alvarez N, Otero O, Camacho F, Borrero R, Tirado Y, Puig A, et al. Passive administration of purified secretory IgA from human colostrum induces protection against Mycobacterium tuberculosis in a murine model of progressive pulmonary infection. BMC Immunology. 2013;14(Suppl 1):S3. doi: 10.1186/1471-2172-14-S1-S3. doi: 10.1186/1471-2172-14-S1-S3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez N, Otero O, Falero-Diaz G, Cadiz A, Marcet R, Carbonell AE, et al. Purificacion de inmunoglobulina A secretora a partir de calostro humano. Vaccimonitor. 2010;19(3):26–29. [Google Scholar]
- 19.Lopez Y, Yero D, Falero-Diaz G, Olivares N, Sarmiento ME, Sifontes S, et al. Induction of a protective response with an IgA monoclonal antibody against Mycobacterium tuberculosis 16 kDa protein in a model of progressive pulmonary infection. Int J Med Microbiol. 2009;299(6):447–452. doi: 10.1016/j.ijmm.2008.10.007. doi: 10.1016/j.ijmm.2008.10.007 . [DOI] [PubMed] [Google Scholar]
- 20.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci USA. 1998;95(26):15688–15693. doi: 10.1073/pnas.95.26.15688. doi: 10.1073/pnas.95.26.15688 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez Pando R, Orozco EH, Arriaga K, Sampieri A, Larriva SJ, et al. Analysis of the local kinetics and localization of interleukin 1 alpha, tumor necrosis factor alpha and transforming growth factor beta, during the course of experimental pulmonary tuberculosis. Immunology. 1997;90(4):607–616. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez Pando R, Schon T, Orozco EH, Serafin M, Estrada GI. Expression of nitric oxide synthase and nitrotyrosine during the evolution of experimental pulmonary tuberculosis. Exp Toxicol Pathol. 2001;53(4):257–265. doi: 10.1078/0940-2993-00182. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez Pando R, Pavon L, Arriaga K, Orozco EH, Madrid MV, Rook GAW. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacteria saprophyte before infection. Infect Immun. 1997;65((8):3317–3327. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Valliere S, Abate G, Blazevic A, Heuertz RM, Hoft DF. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun. 2005;73(10):6711–6720. doi: 10.1128/IAI.73.10.6711-6720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phalipon A, Corthesy B. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2003;24(2):55–58. doi: 10.1016/s1471-4906(02)00031-5. doi: 10.1016/S1471-4906(02)00031-5 . [DOI] [PubMed] [Google Scholar]
- 26.Tagliabue A, Boraschi D, Villa L, Keren DF, Lowell GH, Rappouli R, et al. IgA-dependent cellmediated activity against enteropathogenic bacteria: distribution, specificity and characterization of the effector cells. J Immunol. 1984;133(2):988–992. [PubMed] [Google Scholar]
- 27.Arulanandam BP, Raeder RH, Nedrud JG, Bucher DJ, Le J, Metzger DW. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J Immunol. 2001;166(1):226–231. doi: 10.4049/jimmunol.166.1.226. [DOI] [PubMed] [Google Scholar]
- 28.Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci USA. 1991;88(19):8796–8800. doi: 10.1073/pnas.88.19.8796. doi: 10.1073/pnas.88.19.8796 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson JK, Blanchard TG, Levine AD, Emancipator SN, Lamm ME. A mucosal IgA-mediated excretory immune system in vivo. J Immunol. 2001;166(6):3688–3692. doi: 10.4049/jimmunol.166.6.3688. [DOI] [PubMed] [Google Scholar]
- 30.Brandtzaeg P. Role of secretory antibodies in the defense against infections. Int J Med Microbiol. 2003;293(1):3–15. doi: 10.1078/1438-4221-00241. doi: 10.1078/1438-4221-00241 . [DOI] [PubMed] [Google Scholar]
- 31.Snoeck V, Peters IR, Coz E. The IgA system: a comparison of structure and function in different species. Vet Res. 2006;37(3):455–467. doi: 10.1051/vetres:2006010. doi: 10.1051/vetres:2006010 . [DOI] [PubMed] [Google Scholar]
- 32.Reljic R, Clark SO, Williams A, Falero-Diaz G, Singh M, Challacombe S, et al. Intranasal IFN-γ extends passive IgA antibody protection of mice against Mycobacterium tuberculosis lung infection. Clin Exp Immunol. 2006;143(3):467–473. doi: 10.1111/j.1365-2249.2006.03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams A, Reljic R, Naylor I, Clark SO, Falero-Diaz G, Singh M, et al. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111(3):328–333. doi: 10.1111/j.1365-2567.2004.01809.x. doi: 10.1111/j.1365-2567.2004.01809 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez A, Tjarnlund A, Ivanji J, Singh M, Garcia I, Williams A, et al. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine. 2005;23(20):2565–2572. doi: 10.1016/j.vaccine.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 35.Balu S, Reljic R, Lewis MJ, Pleass RJ, McIntosh R, van Kooten C, et al. A novel human IgA monoclonal antibody protects against tuberculosis. J Immunol. 2011;186(5):3113–3119. doi: 10.4049/jimmunol.1003189. doi: 10.4049/jimmunol.1003189 . [DOI] [PMC free article] [PubMed] [Google Scholar]